Universidade de Trás-os-Montes e Alto Douro
Contribution of different pack members to a wolf chorus howl:
Influence of sex, age, social status, and pack
Dissertação de Mestrado em Biologia
Master thesis by Barbara Marti Domken
Orientadores: Vicente Palacios and Aurora Carmen Monzón y Capape
Universidade de Trás-os-Montes e Alto Douro
Contribution of different pack members to a wolf chorus howl:
Influence of sex, age, social status, and pack
Dissertação de Mestrado em Biologia
Barbara Martí Domken
Orientadores: Vicente Palacios Sanchez and Aurora Carmen Monzón
Composição do Júri:
Presidente: Doutora Sandra Mariza Veiga Monteiro Orientador: Doutora Aurora Monzón y Capape
Arguente: Doutor Francisco José Petrucci Guterres da Fonseca
I
Acknowledgements
We thank F. Petrucci-Fonseca and S. Pinho (C.R.L.I., Malveira, Portugal), J. Escudero (La Dehesa), P. España and E. Sánchez (Cañada Real, Madrid, Spain), D. Weigend (Lobo Park, Málaga, Spain), and Lori Schmidt (International Wolf Center, Minnesota) for allowing us to obtain recordings from the captive wolves under their care.
I personally thank Vicente Palacios for the great orientation, knowledge, and advice he has given me during this entire process. I am especially grateful for the time spent side by side with my colleague and friend Sara Roque, who shared her enthusiasm and knowledge with me. I want to mention the great support of my family, especially my father, who has always the best words to encourage me.
III
Abstract
Wolves live in packs with a complex social structure, for which acoustic communication plays an important role in their behaviour. Wolf packs perform group vocalisations called chorus howls. These acoustic signals have a complex structure and could be involved in functions such as strengthening of social bonds, territory advertisement or spacing packs. We analysed video recordings of 43 chorus howls emitted by wolves held in captivity, in order to investigate whether sex, age, social status, pack, or individual influence the way wolves participate in a chorus. We found that, during a chorus, wolves vocalised 71 % of the time, being howl the most performed vocalisation (39 % of the chorus duration), followed by woa-woa howls (20 %), other vocalisations (9.5 %), and bark-like sounds (2.5 %). Individual and age were the principal factors affecting the wolf acoustic behaviour in a chorus. The time spent vocalising, howling and emitting woa-woa howls were the variables that better discriminated among individuals. Young wolves vocalised less, and uttered shorter acoustic signals, than adults during a chorus. Regarding types of vocalisations, adults emitted 46 % of howls, and 18.6 % of woa howls, while subadults only howled a 4.7 %, and emitted woa-woa howls a 2.4 % of the time. By contrast, sex, social status, and pack had few effect on wolf’s vocal behaviour in a chorus. This study is the first attempt to address how the individuals of a pack of wolves, participate in a chorus howl. Further research is needed to investigate more deeply the contribution of wolves to a chorus howl, and the information encoded in this group vocalisations.
Keywords: wolf, acoustic communication, group vocalisation, chorus howl, individual differences, age
IV
Resumo
O lobo vive em alcateias com uma estrutura social complexa na qual a comunicação acústica tem um papel importante no comportamento. As alcateias emitem vocalizações de grupo chamados coros de uivos. Estes sinais acústicos têm uma estrutura complexa e podem estar envolvidos em funções como fortalecer os laços sociais, a delimitação de território ou o distanciamento entre alcateias. Nós analisámos gravações de vídeo de 43 coros de uivos emitidos por lobos em cativeiro para investigar se o sexo, a idade, o estatuto social, a alcateia ou o indivíduo influenciam o modo como os lobos participam num coro. Nós observámos que, durante um coro, os lobos vocalizaram 71 % do tempo, o uivo foi a vocalização mais executada (39 % da duração de coro), seguida por uivos de woa-woa (20 %), outras vocalizações (9.5 %) e ou ladrido (2.5 %). O indivíduo e a idade foram os principais factores que afectam o comportamento acústico dos lobos num coro. O tempo gasto emitindo vocalizações, uivando e emitindo uivos de woa-woa foram as variáveis que melhor discriminaram indivíduos. Os lobos juvenis vocalizaram menos e também emitiram sinais acústicos mais curtos durante um coro. Com respeito às categorias das vocalizações, os adultos emitiram 46 % de uivos e 18.6 % de uivos de woa-woa, enquanto que os subadultos só uivaram 4.7 % e emitiram uivos de woa-woa 2.4 % do tempo. Pelo contrário, não encontrámos diferenças entre o sexo, o estatuto social e a alcateia. Este estudo é a primeira tentativa de analisar como os indivíduos de uma alcateia participam num coro de uivos. Torna-se também crucial continuar a desenvolver linhas de investigação para o entendimento da contribuição dos lobos num coro de uivos e a informação codificada neste tipo de vocalizações grupais.
V
Table of Contents
ACKNOWLEDGEMENTS ... I ABSTRACT ... III RESUMO ... IV TABLE OF CONTENTS ... V LIST OF FIGURES ... VII LIST OF TABLES ... VII1. INTRODUCTION ... 1
1.1.ANIMAL COMMUNICATION ... 1
1.2.ACOUSTIC COMMUNICATION ... 1
1.3.THE WOLF ... 3
1.4.WOLF ACOUSTIC COMMUNICATION ... 4
1.4.1. Close-range vocalisations ... 5 1.4.1.1. Noisy sounds ... 5 Bark-like sounds ... 5 Growl-like sounds ... 5 1.4.1.2. Harmonic sounds ... 5 Whine-like vocalisations ... 5 Yelp-like vocalisations ... 5 Moan-like vocalisations ... 6
1.4.2. Long distance vocalisations ... 7
Howl ... 7 Chorus howls ... 8 1.5. OBJECTIVES ... 8 2. METHODOLOGY ... 11 2.1.RECORDINGS ... 11 2.2.VIDEO ANALYSES ... 11 2.3.STATISTICAL ANALYSES ... 15 3. RESULTS ... 17
3.1.SEX, AGE, AND STATUS DIFFERENCES ... 17
3.2.INDIVIDUAL DIFFERENCES ... 19
3.2.1. Cañada real ... 19
3.2.2. International Wolf Centre ... 21
3.2.3. Lobo Park ... 22
3.3.FACTORS EXPLAINING THE CONTRIBUTION OF WOLVES IN A CHORUS ... 23
4. DISCUSSION ... 25
5. CONCLUSIONS ... 29
VII
List of Figures
Figure 1. Iberian wolf and pack of North American wolves ... 4
Figure 2. Spectogram of different types of close range vocalisation of the wolf’s vocal repertoire ... 6
Figure 3. Spectrogram of a wolf howl ... 7
Figure 4. Spectrogram of a) a howl, b) a woa-woa howl, c) a bark, and d) others, e.g. whine. ... 13
Figure 5. Percentage of time the wolves vocalised (Vocal) and emitted different vocal types (Howl, Woa, Bark, and Others) in a chorus howl ... 17
Figure 6. Boxplots illustrating the time spent vocalising (Vocal) and emitting different types of vocalisations measured in this study according to age (Adults and subadults) ... 19
Figure 7. Boxplots illustrating individual differences regarding the time spent vocalising (Vocal) and emitting different vocal types for the Cañada real pack ... 20
Figure 8. Boxplots illustrating individual differences regarding the time spent vocalising (Vocal) and emitting different vocal types for the International Wolf Centre pack. ... 22
Figure 9. Boxplots illustrating individual differences regarding the time spent vocalising (Vocal) and emitting different vocal types for the Lobo Park pack ... 23
List of Tables
Table 1. Location, composition and structure of the wolf packs in this study. ... 12Table 2. Descriptions of physical characteristics performed during the emission of a howl and a woa-woa howl. ... 14
Table 3. Results of Wilcoxon test, regarding the factors: sex, status, and age. ... 18
Table 4. Results of Tukey and Kramer (Nemenyi) post hoc tests for the Cañada real pack.. ... 20
Table 5. Results of Tukey and Kramer (Nemenyi) post hoc tests for the International Wolf Centre pack. ... 21
Table 6. Results of Tukey and Kramer (Nemenyi) post hoc tests for the Lobo park pack. ... 22
Table 7. Results of PERMANOVA and SIMPER analyses carried out to analyse how different factors influence the contribution of a wolf in a chorus howl. ... 24
1
1. Introduction
1.1. Animal communication
Communication in animals is a major area of focus within biology, for instance, in behaviour, evolution, ecology, neurobiology and physiology. There hasn’t been a consensus about the exact definition for communication, although, it seems, the disagreement of scientists on this issue, is the acquaintance of both, the signal sender and the receiver (Slater 1983; Bradbury and Vehrencamp 2011). More recent studies defined that any act that elicits a behavioural reaction from the receiver and lasts over time, is because a message is transferred to the receiver (Carazo and Font 2010).
Animals exchange information of multiple meaning in different sensory modalities (Alcock 1989). Scent marking appears to play a paramount role in air and water diffusion (Bossert and Wilson 1963), and is driven by different motivations, such as territorial defence or recognition of kin (Johnson 1973; Johnston 2008). Visual and tactile displays might hold strong social details, and encourage group cohesion and structure (Schenkel 1967; Waal 2003). Acoustic communication demands a high energy investment, for example, nightingale males (Luscinia megarhynchos) can lose 5-10 per cent of his body mass by singing loudly during a brief period of time (Thomas 2002). However, this behaviour is beneficious, thus it is instantaneous, and can achieve long distances in heterogeneous environments (Catchpole and Slater 2008).
1.2. Acoustic communication
The communication through sound production is generated by the oscillation of the vocal chords in the larynx (vocal sounds) and other mechanisms (non-vocal sounds), that emit all kind of vertebrate and invertebrate animals (Peters and Wozencraft 1989; Bradbury and Vehrencamp 2011). This behaviour is shown in both solitary and social contexts, and may signal the physiological state, stimulus, and intentions of the sender (Peters and Wozencraft 1989).
Animals can produce tonal, atonal, and mixed vocal and nonvocal signals, with notable fluctuation in frequency, amplitude, and time parameters (Peters and Wozencraft 1989). Researchers interested in bioacoustics, study conspecific and interspecific communication by
2
means of vocalisations, that provide valuable information about the sender. For example, sex-related differences have been described in the acoustic structure of chacma baboon Papio
cynocephalus ursinus (Ey et al. 2007), red deer Cervus elaphus (Volodin et al. 2016), and the
kittiwake seabird Rissa tridactyla (Aubin et al. 2007). In addition, calls that contain information about the sender individual identity have been reported in greater spear-nosed bats Phyllostomus hastatus (Boughman and Wilkinson 1998), and artic foxes Alopex lagopus (Frommolt et al. 2003). Deer (Reby and Mccomb 2003) and cattle (Watts and Stookey 2000) use a wide diversity of calls, that ranges from alarm to mating calls, along with other contexts that include social contact, and mother-young interactions. Social animals may perform vocalisations for other reasons than lone animals, for example, for location of group members, group reunion or territory advertisement (Lehner and Rodd 1982). Individual recognition of acoustic signals is a widespread phenomenon in animal communication (Stoddard 1996). It has been described in African elephants Loxodonta africana (McComb et al. 2000), coyotes Canis latrans (Mitchell et al. 2006), hyenas Crocuta crocuta (Mathevon et al. 2010), and wolves Canis lupus (Tooze et al. 1990; Palacios et al. 2007). Moreover, hyenas are capable of vocal identification, and numerical assessment of group vocalisations, providing them with valuable information about rival packs (Holekamp et al. 1999; Benson-amram et al. 2011). Sex and individual recognition systems vary among species and can have various functions in different contexts (Aubin et al. 2007). Mother-offspring recognition has been studied in a variety of species, for example in sheep (Searby and Jouventin 2003). Sex and status-specific differences have been reported in hyenas (Mathevon et al. 2010), and in the vocal repertoire of adult red-capped mangabeys Cercocebus torquatus (Bouchet et al. 2010).
A number of birds and mammal species live in groups of diverse sizes, and emit group vocalisations or choruses (Mech 1970; Lehner and Rodd 1982; Foote et al. 2011). To study which type of information is encoded in these group vocalisations is important to understand communication in social animals. Baker (2004), for example, suggested that the laughing kookaburra Dacelo novaeguineae, could use choruses for identification and location of the group, while interactions with neighbouring families, besides of territory defence. Whereas a great deal of research has been carried out on acoustic signalling by individuals in the context of kin or stranger discrimination, few studies have examined the acoustic properties and meaning of group choruses.
3
1.3. The wolf
The wolf is a social canid that lives at low densities, between 0.3 and 4.3 wolves/100 km2
(Boitani 2000), and in wide territories, varying from 33 to 6,272 km2 (Mech and Boitani
2003). These carnivores are present throughout all the Northern Hemisphere and tolerate a wide range of environmental conditions (Mech and Boitani 2003). Wolves live in packs with a complex social structure (Fig.1). Essentially, the pack size ranges between 3 and 11 individuals (Fuller et al. 2003), and comprises a breeding pair and their offspring, which function as a family unit during most of the year (Mech and Boitani 2003). Social behaviour in wolves, can be ruled by linear hierarchy, e.g. age-graded dominance, as well as sex dependent (Packard 2003). The breeding season occurs during the summer, and the wolf packs spend most of their energy raising the pups. The places occupied by the pack during this time, are called home sites: the den site (comprising the early life of the pups) and the rendezvous sites (later development of pups) (Packard 2003). The coordination and assistance of all the pack members is crucial to raise the pups successfully (Mech and Boitani 2003). These carnivores spend an average of 30 % of their time hunting, and travel long distances within their territory in doing so, e.g. about 50 km/day (Mech 1970). Wolves feeding habits vary from scavenging to predation of large ungulates (for example, deer). Kill sites can be revisit several times after a hunt (Mech 1970). Wolves of the same pack can forage and search for food sources separately (Demma and Mech 2009; Palacios and Mech 2011). Moreover, wolves are territorial and encounters between neighbouring packs often result in highly aggressive contests (Mech and Boitani 2003). It has been reported, that scent marking and acoustic signals are used for boundary defence. (Mech and Boitani 2003).
4
Figure 1. Iberian wolf (picture taken in the Centro de Recuperaçao do Lobo Iberico) and pack of North American
wolves (picture obtained from http://publicradio1.wpengine.netdna-cdn.com/todays-question/files/2013/06/isle_royale_wolves_33.jpg).
1.4. Wolf acoustic communication
Acoustic communication plays an important role in wolves' behaviour. The wolf's vocal repertoire has been described by some authors, although there is no consensus about the number of broad categories of vocal types (Harrington and Mech 1978; Schassburger 1993; Palacios 2015). Harrington and Mech (1978) grouped the wolf's vocal repertoire into four broad categories: growls, barks, whines (all of them close range vocalisations) and howls (long distance vocalisations). Schassburger (1993) identified 11 vocal types, classifying the
5
vocalisations into close range, and long-distance vocalisations, as well as into noisy or harmonic sounds. According to the most recent description of wolf's vocal repertoire (Palacios 2015), sounds emitted by wolves can be grouped into:
1.4.1. Close-range vocalisations
1.4.1.1. Noisy sounds
Bark-like sounds
Bark-like sounds are noisy, short (less than a second of duration), and explosive sounds (Harrington and Mech 1978), emitted alone or with other vocalisations such as growls and howls (Schassburger 1993; Holt 1998). It is a vocalisation with a low frequency, between 320 to 904 Hz (Tembrock 1976) with emphasis near 500 Hz (Harrington and Mech 1978) (Fig.2). Growl-like sounds
Growls are produced during long breathing exhalations (Palacios 2015). Growls are vocalisations with variable duration (from tenths of a second to 4.5 s) and dominant frequencies about 500 Hz (Fig.2).
1.4.1.2. Harmonic sounds
Whine-like vocalisationsTwo whine-like vocal classes have been defined, whines and whimpers. Whines are harmonic sounds, from tenths of a second to 1 s in duration, with a mean fundamental frequency about 500-600 Hz (Fig.2). Whimpers are short whine-like sounds emitted consecutively in bouts. Yelp-like vocalisations
Yelp-like vocalisations are short (tenths of a second) with fundamental frequencies ranging from 800 to 1400 Hz, emitted in agonistic interactions (Fig.2).
6 Moan-like vocalisations
Vocalisations lasting from tenths of a second to several seconds, lower in pitch than whines (fundamental from 50 to 500 Hz), emitted in social interactions (e.g. agonistic or mating, Fig.2)
Figure 2. Spectogram of different types of close range vocalisation of the wolf’s vocal repertoire. 2048 FFT
7
1.4.2. Long distance vocalisations
Howl
Wolf howling unlike other acoustic signals of carnivores is one of the best-studied mammalian vocalisations (Theberge and Falls 1967; Tembrock 1976; Harrington and Mech 1978; Harrington and Asa 2003; Palacios 2015). Wolves howl to communicate over long distances (Joslin 1967). The range of detection of a howl stands between 1.6 km and 6.4 km (Joslin 1967). Depending on the habitat structure, wolves can detect the howl of another wolf, even at greater distances, for example as far as 16 km on tundra (Harrington and Asa 2003). The howl has been described as a long harmonic sound, often lower pitched, with a fundamental frequency from 150 Hz to more than 1,000 Hz for adults (Harrington and Asa 2003, Fig.3). Scientific literature has attributed specific functions to howling. Among others, howls could convey information about pack members (e.g. location), and potential mates (Harrington and Mech 1979). A study across multiple species of canids stated that howling is a signal with species-specific information (Kershenbaum et al. 2016). Moreover, experimental evidence in Iberian wolves showed, familiar discrimination based on the acoustic structure of howls (Palacios et al. 2007, 2015). The possibility of individual recognition would allow wolves to identify packmates from outsiders (Harrington and Asa 2003).
Figure 3. Spectrogram of a wolf howl. 2048 FFT (Fast Fourier Transform) points, Hann window (adapted from
8 Chorus howls
Wolf packs perform group vocalisations called chorus howls, that can last 30-120 seconds (Joslin 1967; Harrington 1989). Group size could be responsible for the duration of the chorus (Harrington 1989). It has been suggested that chorus howls could be involved in a number of functions. For example, wolves howl when separated from their group, more likely to reunite with the pack (Harrington and Asa 2003). Another function could be the strengthening of social bonds, as wolves usually engage in social activity before and after the chorus, although this is generally associated with general arousal. Observations by Rutter and Pimlott (1968), and Harrington and Mech (1979) of packs, eluding one another territories after vocal interactions, supports the theory of spacing between packs. Wolves advertise their presence over an area ranging from at least 7.8 km² to nearly 129.5 km² (Joslin 1967).
Furthermore, the increased aggressiveness toward intruders and high hormone levels during the breeding season, is known to elevate the vocal response rate (Harrington and Mech 1979). The presence of pups in a chorus howl, can be detected based on their acoustic energy distribution, suggesting that wolves could use this information (Palacios et al. 2016).
Chorus howls are complex vocalisations. Howls being part of a chorus vary more than elicited by lone individuals (McCarley 1978; Harrington 1989; Holt 1998). Furthermore, besides howls, other vocalisations often occur in a chorus, such as growls, barks, and squeaks (Holt 1998; Harrington and Asa 2003, Fragnaud 2014; Palacios 2015, Passilongo et al 2017). Some authors mention another vocal type, that is found to be performed only in group howling, called the woa-woa howl (Holt 1998, Fragnaud 2014, Palacios 2015). The woa-woa howl is characterized by being more modulated and at higher frequency than howls (Fragnaud 2014), which could be used to encode different information than other howls. It has been suggested that choruses become discordant and change their structure when wolves are in proximity of group mates, because of physiological arousal or to enforce group cohesion (Theberge and Falls 1967; Harrington and Mech 1979; Harrington 1987).
1.5. Objectives
Despite being the hallmark of wolf acoustic communication and one of the most studied vocalisations, how different individuals contribute to a chorus is completely unknown. We
9
hypothesise that factors such as sex, age, social status, individual, or pack, affect the vocal behaviour of wolves in a chorus. This is the first attempt to study whether these attributes influence the acoustic structure of the resulting chorus. The objectives of the thesis are: 1. To analyse whether all the individuals of the pack participate equally in a chorus howl. 2. To describe differences regarding sex, age, social status, and pack.
3. To describe the use of different vocal types included in a chorus according to sex, age, social status, and pack.
11
2. Methodology
2.1. Recordings
We analysed video recordings of chorus howls emitted by wolves held in captivity at four locations: Cañada Real (Madrid, Spain), Centro de Recuperação do Lobo Ibérico (Malveira, Portugal), Lobo Park (Málaga, Spain), and the International Wolf Centre (Minnesota, USA). Recordings were obtained during March 2005, summer of 2007 and 2008, and March 2017, using a digital video camera Sony Handycam DCR-HC54.
The researcher simulated howls to encourage wolves to respond. Taking in consideration their highest activity point, simulations were performed during dawn and dusk. Howls were conducted from at least 5 to 40 m, often in full sight of the animals. Wolves were habituated to human presence. We analysed chorus howls from 26 wolves, from five different subspecies, of 8 different packs comprising 2-6 members (Table 1).
2.2. Video analyses
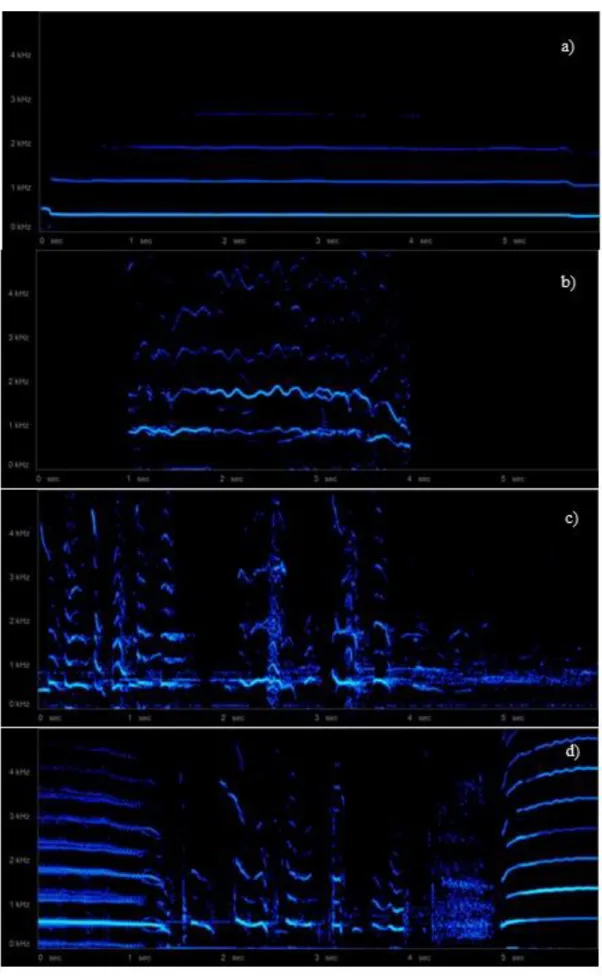
To analyse behaviours from the video recordings, we used Solomon software (Péter 2011). We conducted a focal-animal sampling (one individual is the focus of observations, Lehner 1996), analysing every individual participating in a chorus. Previous inspection of video recordings and the repertoire of vocalisations described for the wolf (Schassburger 1993; Holt 1998; Fragnaud 2014; Palacios 2015) were used to define four sound type categories: howls, woa-woa howls, barks (bark-like vocalisations), and other vocalisations (whines, growls, whistles, and short sounds that could not be identified by the researcher, Fig. 4). Wolves performed mixed sounds, and sometimes it was difficult to identify the type of vocalisation emitted, especially when combining howl and woa-woa howl, as a continuous action. To distinguish between howling and woa-woa howling, we considered, besides the sound, changes in the body positioning, that were performed by the animals during the vocalisation of those two sound types (Table 2). If visual observations of the individuals were not enough to identify the vocal type, we ratified it by the visualization of the spectrogram.
12
Table 1. Location, composition and structure of the wolf packs in this study. F: female, M: male, PP: parental
pair, Other: other type of status, A: adult, S: subadult, NA: not available, P: pup, IB: Iberian wolf, EU: European wolf, RM: Rocky mountain wolf, GP: Great plane wolf, AR: Artic wolf.
Location Pack Group
composition N. of analysed chorus Sex Social status Age class Individual Subspecies Centro de Recuperaçao do Lobo Iberico A* 2 2 F PP A IB B 2 3 M F PP PP A A Lobo Park C 3 7 F PP A EU M PP A M O A D 3 2 F PP A RM M PP A F O A E 4 1 M PP A IB F PP A NA O S NA O S Cañada Real F 4 15 M PP A IB F PP A M O A M O A G 3 1 M PP A IB F PP A F O A International Wolf Centre H 6 12 M PP A AR F PP A GP M O A GP M O A AR M O P RM M O P RM
13
Figure 4. Spectrogram of a) a howl, b) a woa-woa howl, c) a bark, and d) other type of vocalisations, e.g. whine. 2048 FFT (Fast Fourier Transform) points, Hann window.
14
Table 2. Descriptions of physical characteristics performed during the emission of a howl and a woa-woa howl.
Snapshots taken from video recordings of the present study.
Howl
• muzzle slightly pointing upwards/overhead • ears directed backwards lying close to the
head
• mouth slightly opened and fixed position • under canine teeth usually visible
• normally static body position during the entire howl extend
Woa-woa howl
• muzzle slightly pointing upwards/overhead • ears directed backwards lying close to the
head
• mouth wide open • retracted lips
• modulating mouth aperture from slow to fast
• normally body position unsteady and changeable throughout the woa-woa howl extend
For each individual participating in a chorus we measured five variables: 1) time spend vocalizing (vocal), 2) time emitting howls 3) time emitting woa-woa howls (woa), 4) time barking (bark), and 5) time doing other sound types. Wolves were at times beyond the visual range of observations, therefore, only data with animals in full sight at least a 50 % of the time were considered.
Information of sex, age, and social status was obtained from the animal handlers. All individuals were categorized into three age classes (adult: ≥ 2 years old, yearling: 1 year old, pups: <1-year-old), but due to the small sample size, pups and yearlings were grouped into subadults. For the social status category, we considered that dominants could only be adult
15
animals. In consequence, only data from wolves over 2 years were utilised, defining two social rank categories: alfa pair (breeding pair) and other adults.
2.3. Statistical analyses
To test whether sex, age, and status influenced the participation of the individuals in a chorus howl, we calculated the mean value of every variable for each wolf, avoiding pseudoreplication. As the variables were not distributed normally (Shapiro-Wilk normality test), we conducted non-parametrical tests (Wilcoxon test). The significance level was adjusted using the Bonferroni method (dividing p by the number of tests, Sokal and Rohlf 1995).
We selected all the data from the three wolf packs with biggest sample size to evaluate individual differences within the same social group (Kruskal-Wallis test). For each wolf pack, we performed Tukey and Kramer (Nemenyi) post-hoc tests for pairwise multiple comparisons of the ranked data, as it has been used by Lisberg and Snowdon (2011).
In addition, we applied a PERMANOVA with 1000 permutations (i.e. nonparametric multivariate analysis of variance, Adonis function, VEGAN package in R; Oksanen et al. 2007), to test for differences regarding sex, age, status, pack, and individual. PERMANOVA allows distance-based tests of significance for comparing a priori groupings as in a classical partitioning. We used similarity percentage analysis (SIMPER) to determine which variables contributed to significant differences observed between categories (Clarke 1993).
17
3. Results
We analysed 43 wolf chorus howls, emitted by 8 packs comprising 26 wolves of different condition (sex, status), and age composition. The parental or alfa pair were the core component of all the packs. The chorus howls lasted a minimum of 33 s, and a maximum of 185 s, with a mean time length of 77.34 ± 36.38 s (mean±sd). During a chorus, wolves vocalised 71 % of the time. Howling was the most performed vocalisation (39 % of the chorus duration), followed by woa-woa howls (20 %), other vocalisations (9.5 %), and bark-like sounds (2.5 %) (Fig.5).
Figure 5. Percentage of time the wolves vocalised (Vocal) and emitted different vocal types (Howl, Woa,
Bark, and Others) in a chorus howl.
3.1. Sex, age, and status differences
Males and females participated equally in a chorus howl, with no significant differences between the five sound categories established for the study (Table 3). The social status of the subjects did not significantly affect how they participated in a chorus howl (Table 3). By contrast, analyses made on the age classes revealed, that subadults performed less acoustic displays, than adults (Wilcoxon test; w = 90, p < 0.001, Table 3, Fig.6). Adult wolves howled and woa-woa howled more, than younger individuals, although differences in woa-woa howls are only marginally significant (Wilcoxon test; w = 86, p = 0.003 for howls, and w = 70, p =
18
0.064 for woa-woa howls, Table 3, Fig.6). In addition, other types of vocalisations were more often emitted by subadults (Wilcoxon test; w = 17, p = 0.037, Table 3, Fig.6). Differences found for woa-woa howl and other types of vocalisations would not be significant applying Bonferroni corrected significance criterion (p < 0.0033).
Table 3. Results of Wilcoxon test, regarding the factors: sex, status, and age. Sound type categories: vocal,
howl, woa, bark, and others. Percentage of every variable measured: mean ± standard deviation. P-value adjusted according to Bonerroni: 0.0033. Wilc: Wilcoxon test.
Factors Vocal Howl Woa Bark Others
Sex ♂ N=12
% Wilc % Wilc % Wilc % Wilc % Wilc
62.5 ±20.6 w=74 p=0.169 36.7 ±19.6 w=67 p=0.382 13.7 ±14 w=62 p=0.592 2.3 ±2.6 w=45 p=0.541 9.9 ±8.2 w=34 p=0.169 ♀ N=9 ±15.5 75.1 46.9 ±26 20.7 ±22 ±4.6 2.6 ±5.6 5 Status O N=9 ±29.1 44.9 w=23 p=0.645 21.2 ±21.8 w=20 p=0.442 11.19 ±16.4 w=30.5 p=0.831 1.7 ±2.8 w=20 p=0.421 10.8 ±8.9 w=27 p=0.959 PP N=14 74.2 ±14 ±20.7 48 ±18.4 17.8 ±3.8 2.6 ±5.5 5.9 Age A N=18 ±14.5 73.2 w=90 p<0.001 46.6 ±19.1 w=86 p=0.003 18.6 ±18.3 w=70 p=0.064 2.3 ±3.5 w=50 p=0.734 5.6 ±5.1 w=17 p=0.037 S N=5 ±18.6 25.1 ±7.2 4.7 ±5.9 2.8 ±3.3 1.9 15.7 ±9.1
19
Figure 6. Boxplots illustrating the time spent vocalising (Vocal) and emitting different types of vocalisations
measured in this study according to age (Adults and subadults). *: Significant differences.
3.2. Individual differences
To test individual differences, we compared the wolves within the same group, of three different packs (Cañada Real, International Wolf Centre, and Lobo Park packs). The results had consistent differences between the animals.
3.2.1. Cañada real
We analysed 15 chorus howls emitted by the pack of Cañada Real, composed by four individuals. We found significant differences in all variables measured (Kruskal-Wallis test for: vocal, chi-squared = 46.438, df = 3, p < 0.001; howls, chi-squared = 40.915, df = 3, p < 0.001; woa-woa howl, chi-squared = 40.155, df = 3, p < 0.001; barks, chi-squared = 20.275, df = 3, p < 0.001; others, chi-squared = 28.95, df = 3, p < 0.001). The pairwise comparisons test for independent samples, revealed dissimilarities between individuals for at least one of the vocalisations (Table 4, Fig. 7).
20
Table 4. Results of Tukey and Kramer (Nemenyi) post hoc tests for the Cañada real pack. F=female,
PP=parental pair, M=male, S=subadult, A=adult. Only significant p-values are shown.
Individuals F/PP M/S M/A M/S vocal, p<0.001 howl, p=0.03 woa, p<0.001 - - M/A vocal, p=0.047 other, p=0.003 vocal, p<000.1 howl, p<0.001 woa, p<0.001 bark, p=0.002 other, p<0.001 M/PP howl, p=0.007 woa, p<0.001 vocal, p=0.007 howl, p<0.001 bark, p<0.001 other, p=0.009 vocal, p=0.003 woa, p<0.001
Figure 7. Boxplots illustrating individual differences regarding the time spent vocalising (Vocal) and emitting
different vocal types for the Cañada real pack. F=female, PP=parental pair, M=male, S=subadult, A=adult. *: Significant differences.
21
3.2.2. International Wolf Centre
We analysed 12 chorus howls emitted by the pack of International Wolf Centre pack, composed by six individuals, four adult wolves and two pups. None of the data available from one individual was included in the analyses since the time the animal could be seen in the video recording never exceeded 50 % of the chorus duration. We found differences for all the variables measured excepting barks (Kruskal-Wallis test for: vocal, chi-squared = 14.887, df = 4, p < 0.001; howls, squared = 21.343, df = 4, p < 0.001; woa-woa howl, chi-squared = 19.755, df = 4, p < 0.001; barks, chi-chi-squared = 8.276, df = 4, p-value = 0.082; others, chi-squared = 15.138, df = 4, p = 0.004). The pups of the group strongly varied from the other pack mates in their use of acoustic calls. No differences were found for the parental male in any of the variables (Table 5, Fig.8).
Table 5. Results of Tukey and Kramer (Nemenyi) post hoc tests for the International Wolf Centre pack.
F=female, PP=parental pair, M=male, Pup (1, 2) = wolves <1year, A=adult. Only significant p-values are shown.
F/PP M/A
M/A howl, p=0.002
woa, p=0.005
Pup1 vocal, p=0.054 woa, p=0.021
Pup2 vocal, p=0.035
22
Figure 8. Boxplots illustrating individual differences regarding the time spent vocalising (Vocal) and emitting
different vocal types for the International Wolf Centre pack. F=female, PP=parental pair, M=male, Pup (1, 2) = wolves <1year, A=adult. *: Significant differences.
3.2.3. Lobo Park
We analysed 7 chorus howls emitted by the pack of the Lobo Park pack, composed by three wolves. Results showed significance between individuals in vocalisation (Kruskal-Wallis test: chi-squared = 10.42, df = 2, p = 0.006) and woa-woa howl (Kruskal-Wallis: chi-squared = 12.064, df = 2, p = 0.002) (Table 6, Fig. 9).
Table 6. Results of Tukey and Kramer (Nemenyi) post hoc tests for the Lobo park pack. F=female, PP=parental
pair, M=male, A=adult. Only significant p-values are shown. F/PP
M/A vocal, p=0.032
woa, p=0.01
M/PP vocal, p=0.008
23
Figure 9. Boxplots illustrating individual differences regarding the time spent vocalising (Vocal) and emitting
different vocal types for the Lobo Park pack. F=female, PP=parental pair, M=male, A=adult. *: Significant differences.
3.3. Factors explaining the contribution of wolves in a chorus
Multivariate comparisons (PERMANOVA) considering the five variables measured (Vocal, howl, woa, bark, and others), showed significant differences regarding sex, age, status, individual, and pack (Table 7). However, only individual and age explained an important percentage of the variance in the data (R2 = 0.69 and 0.42 respectively). SIMPER analyses
revealed that the time spent howling was the most important discriminant variable for all the factors considered, and the time vocalising and emitting woa-woa howls were also important to discriminate individuals (Table 7).
24
Table 7. Results of PERMANOVA and SIMPER analyses carried out to analyse how different factors
influence the contribution of a wolf in a chorus howl.
Factor F df P R2 Most important variables
Individual 20.07 11 < 0.001 0.69 howl, vocal, woa1
Age 75.98 1 < 0.001 0.42 howl
Status 16.14 1 < 0.001 0.13 howl
Pack 7.35 2 < 0.001 0.12 howl2
Sex 6.47 1 < 0.001 0.06 howl
1: Howl was the most important variable in 45%, vocal in 39%, and woa in 15% of pairwise comparisons 2: Howl was the most important variable in all pack pairwise comparisons
25
4. Discussion
This study is the first attempt to address how the individuals of a pack of wolves participate in a chorus howl. Previous studies have focused their attention on the vocal repertoire of the chorus howl but did not measure the contribution of each individual (Harrington 1989; Holt 1998; Fragnaud 2014; Passilongo et al. 2017). Moreover, our study provides valuable information regarding the acoustic structure of chorus howls, one of the most important vocalisations emitted by wolves. Wolf choruses are long acoustic signals. Choruses overall duration has been analysed in different wolf subspecies (North American wolves, ranging from 33 to 123 s, mean of 60 s, Harrington 1989; North American wolves, ranging from 45 to 210 s, mean of 101.5 ± 44.4 s, Holt 1998; and Iberian wolves, ranging from 26 to 183 s, mean 66 ± 27, Palacios et al. 2016). These values are consistent with our results, suggesting that the chorus howl is a group vocalisation with a similar structure across wolf subspecies.
Taking into account every vocalisation included in a chorus, Harrington (1989), Holt (1998), and Passilongo et al. (2017), as the findings of this study indicate, reported that howl was the most frequent type observed. Nevertheless, instead of being named "chorus howls", these group vocalisations should not be considered only as several wolves howling simultaneously, since other vocal types are also emitted, for instance, squeaks, barks, growls, and miscellaneous vocalisations (Holt 1998). More recently, Passilongo et al. (2017) identified barks, whines, growls, whimpers, squeaks and yelps as part of this group vocalisation. So far, hardly any studies centred on vocal type descriptions in a chorus howl, offer a proper distinction between howls and woa-woa howls (but see Fragnaud 2014). Our research highlights the importance of the woa-woa howl (20 % of the chorus duration wolves emit woa-woa howls), since our analyses revealed differences between ages, and individuals. Woa-woa howls were first reported by Tooze (1987), and briefly described by Holt (1998), although neither of both authors made a clear distinction between howls and woa-woa howls. Recently, Fragnaud (2014) found significant differences between woa-woa howls and howls, in duration, frequency range, frequency modulation, and on the way, they are elicited, and therefore suggested a different category for this sound variation. Our steps proceed very much in the same way as Fragnaud (2014), regarding the importance of woa-woa howl in a chorus. More broadly, future work should target the relevance of woa-woa howls for the wolf acoustic communication. Our work offers a novel approach on the chorus howl, since our
26
results strongly suggest that, besides differences in acoustic features among vocal types, wolves used these vocalisations depending on age and individual identity.
Measuring different acoustic parameters of sounds and looking into special traits or specific context of the sender, can provide insight into how these calls are used in communication (Bradbury and Vehrencamp 2011). The present study is in line with numerous works, that found differences between immature and full-grown individuals in the vocal structure (Blumstein and Daniel 2004; Mathevon et al. 2010; Volodin et al. 2016). The results point towards the idea that adults vocalised consistently during a chorus howl, and emitted longer and more harmonic sounds, than younger ones. This could be explained by changes in body size, limited experience, naïve behaviour, and sound production learning, resulting in a scarce, shorter and more chaotic uttering of sounds by younger animals (Janik and Slater 2000). The mechanics of sound production predicts that larger, older or bigger animals, emit longer calls, with lower, and less modulated fundamental frequency, than smaller ones (Ey et al. 2007; Bowling et al. 2017). Some authors revealed that the fundamental frequencies in young individuals can be reliable proxies to discriminate pups from adults in wolves (Palacios et al. 2016). The learning process is progressive (Payne and Payne 1985), therefore we would expect young individuals to mimic the sounds of their surrounding adults, till they gain experience, and learn to use the same signals in different contexts (Coscia et al. 1991; Janik and Slater 2000). Another plausible cause for the strong differences between juveniles and adults, is the caller’s competitive ability, that should be greater in adults. As reported in baboons, quality signals imply additional costs for the sender, therefore, usually produced by adult individuals, in addition with great stamina (Fischer et al. 2004). Wolf pups and yearlings are not yet in a competitive state, meaning they do not have the physical endurance nor need to exhibit these long calls.
The most marked observation to emerge from our results, is the fact that wolves have conspicuous individual differences regarding the way they participate in a chorus howl. A fair amount of research has been carried out in this manner, searching for individual differences in specific acoustic signals across a variety of species, namely regarding the acoustic structure of vocalisations. For instance, individual distinction in calls has been reported for meerkats (Suricatta suricatta, Townsend et al. 2010), wild agile gibbon (Hylobates agilis
agilis, Oyakawa et al. 2007), horned guan (Oreophasis derbianus, González-garcía et al.
27
(2007) concluded that howls uttered by wolves have individual differences. Terrestrial animals are not the only target species with reported identity cues. Marine mammals, like the bottlenose dolphins, present stereotyped whistles that could suggest long-term individual recognition (Janik and Sayigh 2013; Luís et al. 2016). Individual differences may be used by animals for individual recognition, however not all differences found between individuals encode information for the receiver (Townsend et al. 2010). Thus, to which extend the differences we found in our study are used for wolves to recognize other pack mates, needs further research.
By contrast, we found that sex, status, and pack explained very little of the way in which wolves participated in a chorus howl. It is plausible that a number of limitations might have influenced the results obtained. First, due to the difficulty of collecting valuable data, we got a small sample size, especially the number of individuals belonging to the subadults age class. Further research including a bigger sample size is needed to increase the robustness of the results. An additional problem in viewing differences between status categories, is the fact that the wolves used for this study were raised and held in captivity. Wild wolves are known to struggle for food sources and follow harsh nature rules, which should have a direct effect on the animal condition. In captivity the wolf pack is artificially handled to secure the animal welfare. As a consequence, the physical state, and the social structure of confined packs, could differ greatly from those in the wild. Moreover, in our research sex has little importance for the contribution of wolves in a chorus howl. Although this result could be a priori unexpected, lack of sex differences in howl acoustic structure has been previously reported by Palacios (2004), suggesting that other types of information encoded in howling rather than sex, may be more important for wolf acoustic communication.
29
5. Conclusions
Chorus howls are group vocalisations that include other type of acoustic signals than howls, such as woa-woa howls, barks, and whines. Howls are the most frequent vocalisations emitted in a chorus. The second most uttered vocal type is the woa-woa howl, followed by other types (e.g. whines), and barks.
Individual identity cues are present in all wolves of the present study, considering the time the animals spend vocalizing different sound types. The most important vocalisations to point toward differences between the wolves are howling and woa-woa howling.
Subadult wolves vocalise less than adults during a chorus howl, emitting short vocalisations more often. Age seems to be a relevant factor to look into differences regarding the way wolves participate in a chorus howl.
Other factors, such as sex, social status, and pack, apparently have fewer importance for finding differences in wolves taking part in a chorus.
Further research is needed to investigate more deeply the contribution of wolves to a chorus howl, and the information encoded in this group vocalisations.
31
6. References
ALCOCK,J. 1989. Animal behaviour: an evolutionary approach. Sutherland, Massachusetts:
Sinauer Associates Publishers.
AUBIN, T., N. MATHEVON, V. STASZEWSKI, AND T. BOULINIER. 2007. Acoustic
communication in the Kittiwake Rissa tridactyla: Potential cues for sexual and individual signatures in long calls. Polar Biology 30:1027–1033.
BAKER, M. C. 2004. The Chorus Song of Cooperatively Breeding Laughing Kookaburras
(Coraciiformes, Halcyonidae: Dacelo novaeguineae): Characterization and Comparison Among Groups. Ethology 110:21–35.
BENSON-AMRAM, S., V. K. HEINEN, S. L. DRYER, AND K. E. HOLEKAMP. 2011. Numerical
assessment and individual call discrimination by wild spotted hyaenas , Crocuta
crocuta. Animal Behaviour 82:743–752.
BLUMSTEIN,D.T., AND J.C.DANIEL. 2004. Yellow-bellied marmots discriminate between the
alarm calls of individuals and are more responsive to calls from juveniles. Animal
Behaviour 68:1257–1265.
BOITANI,L. 2000. Action Plan for the conservation of the wolves (Canis lupus) in Europe.
Estrasburgo.
BOSSERT, W. H., AND E. O. WILSON. 1963. The Analysis of Olfactory Communication
Among Animals. Journal of Theoretical Biology 5:443–469.
BOUCHET,H.,A.-S.PELLIER,C.BLOIS-HEULIN, AND A.LEMASSON. 2010. Sex Differences in
the Vocal Repertoire of Adult Red-Capped Mangabeys (Cercocebus torquatus): A Multi-Level Acoustic Analysis. American Journal of Primatology 72:360–375.
BOUGHMAN,J.W., AND G.S.WILKINSON. 1998. Greater spear-nosed bats discriminate group
mates by vocalizations. Animal Behaviour 55:1717–1732.
BOWLING,D. L.,M.GARCIA,J.C.DUNN,R.RUPRECHT,A.STEWART,K. FROMMOLT,W.T.
FITCH. 2017. Body size and vocalization in primates and carnivores. Scientific
Reports:1–11.
BRADBURY,J.W., AND S.L.VEHRENCAMP. 2011. Principles of animal communication. 2nd
edition. Sinnauer Associates Publishers, Sutherland, Massachusetts.
CARAZO, P., AND E. FONT. 2010. Putting information back into biological communication.
Journal of Evolutionary Biology 23:661–669.
CATCHPOLE,C.K., AND P.J.B.SLATER. 2008. Bird song. Biological themes and variations.
32
CLARKE, K. R. 1993. Non-parametric multivariate analyses of changes in community
structure. Australian Journal of Ecology 18:117–143.
COSCIA,E.M.,D.P.PHILLIPS, AND J.C.FENTRESS. 1991. Spectral analysis of neonatal wolf
Canis lupus vocalizations. Bioacoustics:The International Journal of Animal Sound and its Recording 3:275–293.
DEMMA, D. J., AND L. D. MECH. 2009. Wolf Use of Summer Territory in Northeastern
Minnesota. Journal of Wildlife Management 73:380–384.
EY,E.,D.PFEFFERLE, AND J.FISHER. 2007. Do age- and sex-related variations reliably reflect
body size in non-human primate vocalizations ? A review. Primates 48:253–267.
FISCHER,J., D.M.KITCHEN,R.M.SEYFARTH, AND D. L. CHENEY. 2004. Baboon loud calls
advertise male quality : acoustic features and their relation to rank, age, and exhaustion.
Behavioral Ecology and Sociobiology 56:140–148.
FOOTE,J.R., L.P. FITZSIMMONS,D. J.MENNILL, AND L. M.RATCLIFFE. 2011. Male
black-capped chickadees begin dawn chorusing earlier in response to simulated territorial insertions. Animal Behaviour 81:871–877.
FRAGNAUD,H. 2014. Vocalizations emitted by iberian wolves (Canis lupus signatus) during
chorus howls. Master thesis presented at the Universite Paris Sud. Pp. 31.
FROMMOLT,K., M. E. GOLTSMAN, AND D. W. MACDONALD. 2003. Barking foxes , Alopex
lagopus : field experiments in individual recognition in a territorial mammal. Animal Behaviour:509–518.
FULLER,T.K.,L.D.MECH, AND J.F.COCHRANE. 2003. Wolf population dynamics. Wolves.
Behavior, ecology, and conservation (L. D. Mech & L. Boitani, eds.). University of Chicago Press, Chicago.
GONZÁLEZ-GARCÍA,F.,J.R.SOSA-LÓPEZ,J.F.ORNELAS,P.JORDANO,V.RICO-GRAY, AND V.
U. MOLINER. 2016. Individual variation in the booming calls of captive Horned Guans
(Oreophasis derbianus): an endangered Neotropical mountain bird. Bioacoustics:The
International Journal of Animal Sound and its Recording:1–14.
HARRINGTON,F.H. 1987. Aggressive howling in wolves. Animal behaviour 35:7–12.
HARRINGTON,F. H. 1989. Chorus howling by wolves: acoustic structure, pack size and the
beau geste effect. Bioacoustics-The International Journal of Animal Sound and its
Recording 2:117–136.
HARRINGTON, F. H., AND C. S. ASA. 2003. Wolf communication. Pp. 66–103 in Wolves:
behavior, ecology, and conservation (L. D. Mech & L. Boitani, eds.). University of Chicago Press, Chicago.
33
HARRINGTON,F. H., AND L. D. MECH. 1978. Wolf vocalization. Pp. 109–132 in Wolf and
man: evolution in parallel (R. L. Hall & H. S. Sharp, eds.). Academic Press, New York, NY.
HARRINGTON, F. H., AND L. D. MECH. 1979. Wolf howling and its role in territory
maintenance. Behaviour 68:207–249.
HOLEKAMP,K.E.,E.BOYDSTON,M.SZYKMAN,I.GRAHAM,K.J.NUTT,S.BIRCH,A.PISKIEL,
M.SINGH. 1999. Vocal recognition in the spotted hyaena and its possible implications
regarding the evolution of intelligence. Animal Behaviour 58:383–395.
HOLT, T. D. 1998. A structural description and reclassification of the wolf, Canis lupus,
chorus howl. Master thesis presented at the Dalhousie University. Pp. 100.
JANIK,V. M., AND L. S.SAYIGH. 2013. Communication in bottlenose dolphins: 50 years of
signature whistle research. Journal of Comparative Physiology A: Neuroethology,
Sensory, Neural, and Behavioral Physiology 199:479–489.
JANIK, V. M., AND P. J. B. SLATER. 2000. The different roles of social learning in vocal
communication. Animal Behaviour 60:1–11.
JOHNSON,R.P. 1973. Scent marking in mammals. Animal Behaviour 21:521–535.
JOHNSTON,R.E. 2008. Individual Odors and Social Communication : Individual Recognition,
Kin Recognition, and Scent Over ‐ Marking. Advances in the Study of Behavior 38:439– 505.
JOSLIN,P.W.B. 1967. Movements and Home Sites of Timber Wolves in Algonquin Park.
American Zoologist 7:279–288.
KERSHENBAUM,A.,H.ROOT-GUTTERIDGE,B.HABIB,J.KOLER-MATZNICK,B.MITCHELL,V.
PALACIOS,S.WALLER. 2016. Disentangling canid howls across multiple species and
subspecies : Structure in a complex communication channel. Behavioural Processes 124:149–157.
LEHNER,P.N. 1996. Handbook of ethological methods. second. Cambridge University Press,
Cambridge.
LEHNER,P.N., AND F.H.RODD. 1982. Differential vocal response of coyotes to “group howl” and “group yip-howl” playbacks. Journal of Mammalogy 63:675–679.
LISBERG,A.E., AND C.T.SNOWDON. 2011. Effects of sex , social status and gonadectomy on
countermarking by domestic dogs, Canis familiaris. Animal Behaviour 81:757–764. LUÍS, A. R., M.N. COUCHINHO, AND M. E. DOS SANTOS. 2016. Signature whistles in wild
bottlenose dolphins : long-term stability and emission rates. Acta Ethologica 19:113– 122.
34
MATHEVON,N.,A. KORALEK,M.WELDELE,S.E.GLICKMAN, AND F.E. THEUNISSEN. 2010.
What the hyena’s laugh tells: Sex, age, dominance and individual signature in the giggling call of Crocuta crocuta. BMC Ecology 10:9.
MCCARLEY, H. 1978. Vocalizations of red wolves (Canis rufus). Journal of Mammalogy
59:27–35.
MCCOMB,K.,C.MOSS,S.SAYIALEL, AND L.BAKER. 2000. Unusually extensive networks of
vocal recognition in African elephants. Animal behaviour 59:1103–1109.
MECH,L.D. 1970. The wolf: the ecology and behavior of an endangered species. The Natural
History Press, Garden City, New York.
MECH, L. D., AND L. BOITANI. 2003. Wolves: Behavior, Ecology, and Conservation.
University of Chicago Press.
MITCHELL, B., M. M. MAKAGON, M. M. JAEGER, AND R. H. BARRETT. 2006. Information
content of coyote barks and howls. Bioacoustics: The International Journal of Animal
Sound and its Recording 15:289–314.
OKSANEN,J.,R.KINDT,P.LEGENDRE,B.O'HARA,M.H.H.STEVENS,M.J.OKSANEN. 2007.
Vegan: Community Ecology Package. <http://cran.r-project.org/package=vegan>.
OYAKAWA, C., H. KODA, AND H. SUGIURA. 2007. Acoustic Features Contributing to the
Individuality of Wild Agile Gibbon (Hylobates agilis agilis) Songs. American Journal of
Primatology 69:777–790.
PACKARD, J. M. 2003. Wolf behavior: reproductive, social, and intelligent. Pp. 35–65 in
Wolves: behavior, ecology, and conservation (L. D. Mech & L. Boitani, eds.).
University of Chicago Press, Chicago.
PALACIOS, V. 2004. Estructura acústica de los aullidos del lobo ibérico. Master thesis
presented at Universidad de Valencia. Pp. 62.
PALACIOS,V. 2015. Vocal communication in the iberian wolf. Doctoral thesis presented at
the Universidad de Valencia. Pp. 244.
PALACIOS, V., E. FONT, AND R. MÁRQUEZ. 2007. Iberian wolf howls: acoustic structure,
individual variation, and a comparison with north american populations. Journal of
Mammalogy 88:606–613.
PALACIOS,V.,E. FONT, R.MARQUEZ, AND P.CARAZO. 2015. Recognition of familiarity on
the basis of howls: a playback experiment in a captive group of wolves. Behaviour 152:593 – 614.
PALACIOS,V.,J.V.LÓPEZ-BAO,L. LLANEZA,C.FERNÁNDEZ, AND E. FONT. 2016. Decoding
35 Determine Wolf Reproduction. PloS one 11:1–12.
PALACIOS,V., AND L.D.MECH. 2011. Problems with studying wolf predation on small prey
in summer via global positioning system collars. European Journal of Wildlife Research 57:149–156.
PASSILONGO, D., M. MARCHETTO, AND M. APPOLONIO. 2017. Singing in a wolf chorus : structure and complexity of a multicomponent acoustic behaviour. Hystrix, the Italian
Journal of Mammalogy 28:1–6.
PAYNE,K., AND R.PAYNE. 1985. Large scale changes over 19 years in songs of humpback
whales in Bermuda. Zeitschrift für Tierpsychologie 68:89–114. PÉTER,A. 2011. Solomon Coder: a simple solution for behavior coding.
PETERS, G., AND W. C. WOZENCRAFT. 1989. Acoustic communication by Fissiped
Carnivores. Pp. 14–49 in Carnivore behavior, ecology, and evolution (J. Gittleman, ed.).
New York University Press, Ithaca, N Y.
R DEVELOPMENT CORE TEAM. 2010. R: A language and environment for statistical
computing. Vienna, Austria.
REBY, D., AND K. MCCOMB. 2003. Vocal Communication and Reproduction in Deer.
Advances in the Study of Behavior 33:231–264.
RUTTER, R. J., AND D. H. PIMLOTT. 1968. The world of the wolf. Lippincott J.B Co.,
Philadelphia.
SCHASSBURGER, R. M. 1993. Vocal Communication in the Timber Wolf, Canis lupus,
Linnaeus: structure, motivation, and ontogeny. Advances in Ethology Series 30.
SCHENKEL,R. 1967. Submission : Its Features and Function in the Wolf and Dog. American
Zoologist 7:319–329.
SEARBY, A., AND P. JOUVENTIN. 2003. Mother-lamb acoustic recognition in sheep: a
frequency coding. Proceedings of the Royal Society of London.Series B: Biological
Sciences 270:1765–1771.
SLATER,P.J.B. 1983. The study of communication. Animal behaviour 2: communication (T.
R. Halliday & P. J. B. Slater, eds.). Blackwell, Oxford.
SOKAL,R.R., AND F.J.ROHLF. 1995. Biometry: The Principle and Practice of Statistics in
Biological Research. Third. W.H.Freeman and Company, New York, NY.
STODDARD,P.K. 1996. Vocal recognition of neighbors by territorial passerines. Pp. 356–34
in Ecology and evolution of acoustic communication in birds (D. E. Kroodsma & E. H. Miller, eds.). Cornell University Press, Ithaca, N Y.
36
THEBERGE,J.B., AND J. B. FALLS. 1967. Howling as a means of communication in timber
wolves. American Zoologist 7:331–338.
THOMAS,R.J. 2002. The costs of singing in nightingales. Animal Behaviour 63:959–966.
TOOZE,Z.J. 1987. Some aspects of the structure and function of long-distance vocalizations
of timber wolves (Canis lupus). Dalhouise University, Halifax, Nova Scotia.
TOOZE, Z. J., F. . H. HARRINGTON, AND J. C. FENTRESS. 1990. Individually distinct
vocalizations in timber wolves, Canis lupus. Animal Behaviour 40:723–730.
TOWNSEND, S. W., L. I. HOLLÉN, AND M. B. MANSER. 2010. Meerkat close calls encode
group-specific signatures, but receivers fail to discriminate. Animal Behaviour 80:133– 138.
VOLODIN,I.A.,O.VSIBIRYAKOVA, AND E.VVOLODINA. 2016. Sex and age-class differences
in calls of Siberian wapiti Cervus elaphus sibiricus. Mammalian Biology 81:10–20. WAAL,F.B.M.D. 2003. Darwin’s Legacy and the Study of Primate Visual Communication.
Annals of the New York Academy of Sciences 1000:7–31.
WATTS, J. M., AND J. M. STOOKEY. 2000. Vocal behaviour in cattle: The animal’s commentary on its biological processes and welfare. Applied Animal Behaviour Science 67:15–33.