LINKING PERSONALITY
AND
APPRAISAL MODULATORS
IN
FISH
MARCO ALEXANDRE CAVACO CERQUEIRA
Tese
Doutoramento em Ciências da Vida, do Mar, da Terra e do Ambiente Ramo de Aquacultura
(Especialidade Técnicas de produção)
Trabalho efetuado sob a orientação de:
Professora Catedrática Aposentada Maria Teresa Dinis, Faculdade de Ciências e Tecnologia, Universidade do Algarve, Faro, Portugal.
Doutor Catarina Oliveira, Centro de Ciências do Mar do Algarve (CCMAR), Faro, Portugal. Doutor Rui F. Oliveira, Instituto Superior de Psicologia Aplicada (ISPA), Lisboa, Portugal Doutor Simon Mackenzie, University of Stirling, Stirling, UK
UNIVERSIDADE DO ALGARVE
LINKING PERSONALITY AND APPRAISAL MODULATORS IN
FISH
Marco Alexandre Cavaco Cerqueira
Tese
Doutoramento em Ciências da Vida, do Mar, da Terra e do Ambiente Ramo de Aquacultura
(Especialidade Técnicas de produção)
Trabalho efetuado sob a orientação de:
Professora Catedrática Aposentada Maria Teresa Dinis, Faculdade de Ciências e Tecnologia, Universidade do Algarve, Faro, Portugal.
Doutor Catarina Oliveira, Centro de Ciências do Mar do Algarve (CCMAR), Faro, Portugal. Doutor Rui F. Oliveira, Instituto Superior de Psicologia Aplicada (ISPA), Lisboa, Portugal Doutor Simon Mackenzie, University of Stirling, Stirling, UK
Linking Personality and Appraisal modulators in fish
Declaração de autoria do trabalho
Declaro ser o autor deste trabalho, que é original e inédito. Autores e trabalhos consultados estão devidamente citados no texto e constam da listagem de referências incluída.
Copyright© Marco Alexandre Cavaco Cerqueira, 2016
A Universidade do Algarve tem o direito, perpétuo e sem limites geográficos, de arquivar e publicitar este trabalho através de exemplares impressos reproduzidos em papel ou de forma digital, ou por qualquer outro meio conhecido ou que venha a ser inventado, de o divulgar através de repositórios científicos e de admitir a sua cópia e distribuição com objetivos educacionais ou de investigação, não comerciais, desde que seja dado crédito ao autor e editor.
i
This work was funded by Fundação para a Ciência e Tecnologia (FCT) and partially supported by the European Commission under the 7th Framework Programme FP7–KBBE-2010-4 Contract n°: 265957 COPEWELL. Thanks for making this dissertation possible!
Many people have helped me in many different ways throughout last few years. To them, I would like to show my most honest appreciation and gratitude.
Firstly I would like to thanks to Catarina Martins for believe in me and support my application to FCT. Thanks for the excellent guidance, scientific discussions and valuable advices in the very beginning of my research career. Thanks also to Professor Maria Teresa Dinis for taking me as her student, for her availability and for valuable advices.
I would like to give a special word for Sandie Millot, “merci beaucoup” for the possibility to work with someone so passionate for her work, for the good moments, support and valuable scientific advices. Also, by contribute with her knowledge and technical assistance during my experiments.
A big word of appreciation for my supervisors: thank you Catarina Oliveira, for accepting to guide me during the hardest stages of this thesis, for discussions and valuable comments on manuscripts, encouraging and supporting me when the motivation was narrow. Also would like to acknowledge Simon Mackenzie, not only for give me the possibility for being his student, but as well for being possible to work directly with a outstanding researcher, relax and “cool” in so many ways. At the same time, for all the philosophical and scientific discussions, manuscripts comments, patience and friendship. A big word of appreciation to Rui Oliveira, with whom was a huge pleasure to work with, for his knowledge, brilliant comments on manuscripts, revisions, discussions and scientific advises.
To Maria Filipa for being highly professional and hardworking, for allow me to integrate some of her research, for valuable discussions, suggestions and technical assistance, but mainly for her friendship; “Obrigado”.
A special thanks to Sonia Rey, for the good moments, scientific discussions, comments on the manuscripts, technical assistance during my experiments, and for all the help in particular moments. Specially to make me feel so well abroad of my home, by offering the possibility to be one more from her “family”. Thank you for your patience and real friendship.
I am also grateful to AQUAEXCEL mobility program funded by the European Union‘s Seventh Framework Programme (FP7/2007-2013) under grant agreement n° 262336, by offer me the possibility to work in the Institute of aquaculture from the University of Stirling, valuable for meeting a new work reality, culture and to meet many other researchers from related and divergent fields.
ii
I am truly indebted to all the members of the Aquaculture Research Group from CCMAR for making the work so much easier. I would like to specifically acknowledge Lenita, Rita Cólen, Rita Teodósio, Denise, Sarita for their friendship, for sometimes just drove me away from the pressure and hard work of this thesis. A special thanks to Filipe for the important help during the fish sampling. A word to Filipa Rocha, for her friendship, valuable advices and by simply hears me when everything was breaking up.
I would also like to thanks to Miguel Garcia and João Reis from Ramalhete research station for their valuable help in the technical part of my experiments, patience and supporting me despite all the things i have break during that period.
I would like to thanks to Rui Oliveira lab team; Magda, Julia, Ana Faustino, Sara, Gonçalo, André and all the others for their valuable help, technical assistance and friendship, good discussions and good moments. I would like specifically endorse it to Ana Sofia Félix, for her patience, shared knowledge, technical assistance, for being who she is, a truly person… thank you.
Thank you to all the people from the Immunology lab, from CNC from University of Coimbra, for the precious help during the molecular work. I would like to thanks specifically to Patricia Couceiro, first for being a great sister-in-law, and secondly for her friendship, advices, technical and emotional support. To Paulo Rodrigues Santos, for give me the possibility to use their facilities, material and knowledge to accomplish one big part of this dissertation. A sincere thank you!
An honest word of appreciation to Tomé Silva, for your struggle and hard work with my data and thesis, for all the time you spent on that, for your patience and good scientific advices.
A special word to my good friends that despite the distance to some of them were so important during this PhD in so many ways: to Marisa, Chico and Lidia for the good moments, friendship or just for being present; to Paulo Frias, Sérgio Amorim, Rui Gonçalves and Pedro André for their truly friendship and awesome talks, discussions and moments.
To my family, thanks for in some step of my life, being supportive and overall accepting my options that conducted me here today… “Um sincero Muito obrigado!” A vocês, Pai e Mãe um especial obrigado pelo vosso sacrifício, carinho e amor.
iii Às mulheres da minha vida, Monalisa e Madalena, por simplesmente poder viver com a alegria de as ter ao meu lado. Pela sua compreensão, sacrifícios, carinho e amor ao longo deste período, pelo apoio, pelas palavras reconfortantes ou mesmo pelas menos reconfortantes que me fizeram lutar para querer algo mais. São o meu porto de abrigo, o meu sol, o meu oceano, o meu oásis, o meu horizonte, a minha razão para evoluir.
v
The reason why some individuals from the same fish population react so differently under similar aquaculture husbandry practices or to any other stressful situation is at the core of today’s fish welfare research. In this context, the large individual variation in the physiological or behavioural response under stressful conditions is now accepted as reflection of distinct personality traits and of divergent cognitive evaluation that the individual makes of the situation, i.e. on the way the stressor is appraised. This thesis aimed to uncover which appraisal criteria fish use, the interplay with their personality traits, the underlying neurophysiological mechanisms and the potential application of psychological modulators of the stress response to improve fish welfare. Thus, the experimental work was oriented towards: i) investigating whether pre-existing inherent traits in behaviour and physiology affect the outcome of exposure to environmental stressors in Gilthead seabream (Sparus aurata) and Atlantic seabass (Dicentrarchus labrax) (Chapter II.1 and Chapter II.2); ii) evaluating the effect of predictability on the onset of action-related responses to aversive and appetitive stimuli (Chapter III.1); iii) inferring how predictability stressor modulates the cognitive stress response (Chapter III.2); iv) investigating how controllability improves coping ability on both seabream and seabass (Chapter IV.1); v) examining the relationship between thermal choice and animal personality using Nile tilapia (Oreochromis niloticus) as model (Chapter IV.2). Differences in behaviour, physiology and brain states measurements support specific appraisal and motivations in fish, according to the prospect of reward or punishment. This seems to be, nevertheless, highly dependent of both species - and context-specificity. In addition they suggest that predictable stimuli and social support alongside with perception of control can be used as psychological modulators of the stress response to make animals more resilient and empowered under sustainable farming systems. The link between personality and appraisal deserves further investigation as evidences are narrow. The work developed in the core of this thesis has brought new insights on how to manipulate fish´s ability to cope with changes in their environment, ensuring positive outcomes in terms of welfare, fitness and survival.
Keywords: Aquaculture; Environmental Appraisal; Personality; Psychological manipulation;
vii
O motivo pelo qual alguns indivíduos pertencentes a uma mesma população de peixes, reagem de modo diferente às rotinas praticadas numa aquacultura, ou em qualquer outra situação motivadora de stress, é hoje o cerne de investigação relacionada com bem-estar animal. Neste contexto, a variabilidade individual encontrada em termos de resposta fisiológica ou comportamental sob condições de stress é presentemente aceite como sendo um reflexo de distintos traços de personalidade e da avaliação cognitiva que o indivíduo faz da mesma, i.e. o modo como o factor de stress é percepcionado/avaliado. Esta tese teve como objectivo investigar quais os critérios que os peixes usam na avaliação do seu meio envolvente, a sua interacção com os seus traços de personalidade, os mecanismos neurofisiológicos subjacentes e o potencial de aplicação de factores psicológicos para orientar a forma como os indivíduos avaliam o seu meio, contribuindo assim para o seu bem-estar. Posto isto, o presente estudo foi desenvolvido para: i) investigar se as disposições intrínsecas pré-existente no comportamento e fisiologia manifestadas pela dourada (Sparus aurata) e pelo robalo (Dicentrarchus labrax), afectam as suas acções/reacções perante a exposição a factores de stress integrados no seu ambiente (Capítulo II.1 e Capítulo II.2) ; ii) avaliar o efeito da previsibilidade na resposta a estímulos aversivos e apetitivos (Capítulo III.1); iii) avaliar de que forma a previsibilidade de um evento aversivo modifica a resposta cognitiva ao stress (Capítulo III.2); iv) investigar o efeito da controlabilidade na regulação da resposta de stress a factores aversivos em dourada e robalo de lidar com situações de stress (Capítulo IV.1); v) examinar a relação entre temperatura preferencial e personalidade na tilápia do Nilo (Oreochromis niloticus) (Capítulo
IV.2). Diferenças na expressão de comportamento, fisiologia e estados neurais evidenciam
motivações e mecanismos específicos para actuar perante o prospecto de recompensa ou de punição. Contudo, isto revela-se ser fortemente influenciado pela especificidade da espécie e do contexto em questão. Mais, sugere que estímulos previsíveis, presença de conspecíficos e controlo podem ser usados como moduladores psicológicos em situações de stress para tornar os animais mais resistentes e adaptados às actividades recorrentes numa aquacultura. A ligação entre personalidade e percepção requer ainda investigação uma vez que as evidências são parcas. O trabalho desenvolvido no âmbito desta tese oferece novas perspectivas em como manipular e melhorar a capacidade dos peixes para lidarem com
viii
Palavras-chave: Aquacultura, Percepção/avaliação do ambiente; Personalidade; Manipulação psicológica; Bem-estar animal
ix
Acknowledgments ……….. i
Summary ……….. v
Resumo ……….. vii
Chapter I: General introduction ………. 1
1. Aquaculture: definition and its importance ………. 3
1.1. Research-related in aquaculture ……….. 4
1.2. Animal Welfare: origin and definitions ………. 5
1.3. Fish culture and Welfare standards: where are we? ………. 6
1.3.1. Current issues of welfare in fish ……….. 8
1.4. Assessing welfare in aquaculture ………. 8
1.4.1. Stress responses in fish ……….. 10
1.4.1.1. Physiological response to stress ………. 12
1.4.1.2. Behavioural response to stress ……… 13
1.4.1.3. Integrating learning and preference to promote welfare … 16 1.5. Intra-individual variability of stress responses ...……….. 18
1.5.1 Personality traits in fish ………. 19
1.5.1.1. How to assess fish personality in aquaculture ………. 21
1.5.1.2. Importance of assessing personality ……… 23
1.5.1.3. Personality and appraisal ………. 24
1.5.2. Appraisal concept ……… 25
1.5.2.1. Appraisal processes in animals ………. 28
1.6. Promoting psychological welfare in cultured fish ………. 29
1.6.1. Psychological components of the stress response ………. 29
1.6.1.1. Stimuli-related components: predictability, pleasantness and social support ………. 31
1.6.1.2. Response-related modulators: Controllability ………. 34
x
II.1. Use of conditioned place preference / avoidance tests to assess affective states in fish …….……… II.2. Behavioural stress responses predict environmental perception in European sea
bass (Dicentrarchus labrax) ………. 63
Chapter III: Predictability as an appraisal modulator in fish ……… 81
III.1. Appraisal of stimulus valence and predictability induces emotion-like states in fish 85 III.2. Cognitive appraisal in fish: stressor predictability modulates the physiological and neurobehavioural stress response in seabass ……… 113
Chapter IV: Controllability as an appraisal modulator ………. 135
IV.1. Controllability over the environment increases affective stress-coping ability .…. 139 IV.2. Thermal preference predicts animal personality in Nile tilapia Oreochromis niloticus ………. 173
Chapter V: General discussion, Conclusions and Future perspectives …..………. 201
General discussion ………. 203
5.1Characterization of distinct personality traits in important commercial farmed fish ………. 203
5.1.1 Behavioural and physiological paradigms to assess personality on the focal fish ……….. 204
5.1.2 Behavioural and physiological expression characterizing distinct personalities ……….. 207
5.1.3 Consistency and repeatability across and over time ……… 208
5.2Demonstrating appraisal capability in the target species ………. 211
5.3Psychological manipulation of environmental appraisal ……….. 213
5.3.1 Predictability, pleasantness and social support modulates fish appraisal ……….. 214
5.3.2 Control over the environment increases fish fitness towards environmental challenges ……….. 219
5.4The link between personality and appraisal ……….. 221
5.5Proximate states uncover appraisal in fish ………. 224
Conclusions ……….. 225 43
xi
General Introduction
Chapter I
3
Aquaculture: definition and its importance
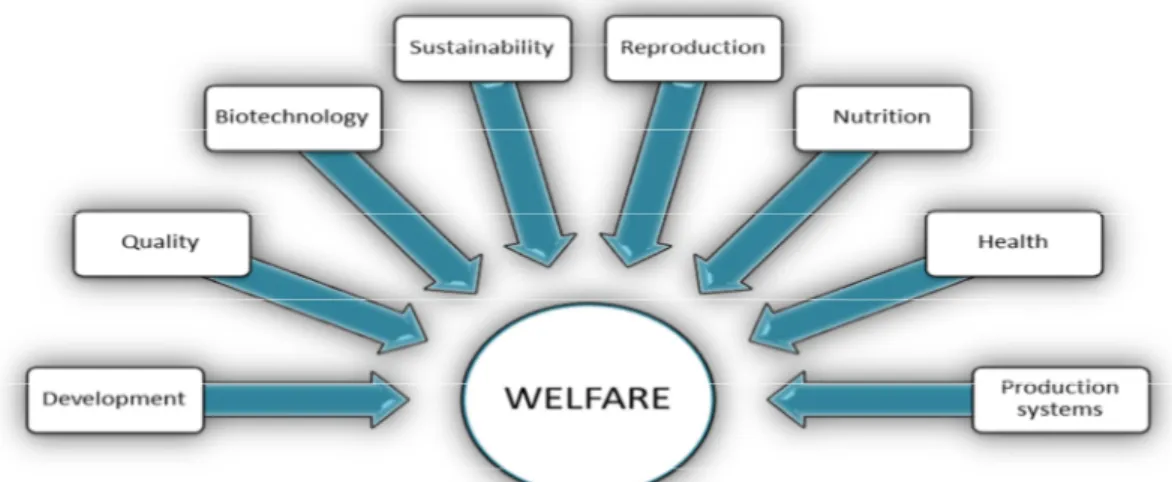
The culturing of aquatic organisms is known as aquaculture and covers breeding, rearing and harvesting of both freshwater and marine species (FAO, 2002), which includes a wide variety of animals, from fish to molluscs, crustaceans, amphibians, reptiles and algae, but with finfish representing 67% of all aquaculture production (FAO, 2014). Fish final consumption as food is not however the only purpose of fish farming. Stock management for recreational and commercial fisheries, conserving and saving threatened populations, as well the production of fish for domestic use (as in the case of ornamental fish), are also common objectives of finfish farming. Finfish also became more prominent as a research subject during the past three decades, due to their neural complexity, size and visibility of the industry, being also produced for biomedical research and for environmental impact studies. The range of applications for farmed fish is indeed wide but, ultimately, the major role of aquaculture is to sustain the increasing consumer demand for fish products and the over-exploitation of fisheries (Le Francois et al., 2010).
Figure 1.1 |World capture fisheries and aquaculture production (Source: FAO 2014).
Currently, aquaculture products are patently accepted as good replacers of wild aquatic food, since fish culture production has exceeded both fisheries productivity and the rate of human population growth (Fig. 1.1 in FAO, 2014). As the production of fish products moved beyond the provisional rearing of fish for individuals and into product marketing, demand and economics of production led to intensification of aquaculture. In line with this growth, an increasing concern by consumers, researchers and stakeholders regarding how fish are
4 being reared and kept in captivity, their health and welfare has boosted the development of aquaculture-related research areas.
1.1. Research in aquaculture
For all the positive effects aquaculture can have on food supply and conservation of wild fisheries, it can also lead to environmental risks and create sustainability challenges. Research driven by either government or academic laboratories has become essential to avoid such problems and guarantee fish quality and welfare. An assessment of the scope of aquaculture research over the last years shows that subjects such as seafood quality, nutrition, reproduction, genetic improvement, fish health and welfare are prevalent (Fig. 1.2). Nevertheless, all subject areas are related and many often overlapping in the conducted research, with the central goal of adjusting rearing conditions to each species’ biology and life cycle. For example, developing specific feeds for specific developmental stages improves the nutritional state of fish, ensuring optimal farming conditions. This topic is particularly important nowadays, given the introduction of alternative ingredient sources in fish feed formulations which, though a requirement to ensure long-term sustainability of the sector, have the potential to undermine fish nutrition, if proper care is not taken. In addition, genetic improvement can be used to develop selective breeding stocks that perform well under certain culture conditions, thus guaranteeing the biological success of the animals in captivity (Beveridge and McAndrew, 2000; Le Francois et al., 2010; Lim and Webster, 2006). Looking at the variety of species used in aquaculture, with their distinct life cycles and habitat preferences, and having in mind the increased public concern about the conditions is which fish are kept in captivity, promoting welfare is undeniably one of the goals of aquaculture-related research.
Overall, effective and sustainable fish culture demands economic production of a product that meets consumer expectations, with minimum negative impacts on fish welfare and on the environment (Le Francois et al., 2010). An interesting study by Kristian et al. (2015), demonstrated how much Norwegian population care about fish welfare. The authors showed that the public is concerned about fish welfare and that they were willing to pay a higher price for welfare-assured fish. However, they agree that the costs of welfare should be shared by the producers and the government. Alongside, fish welfare is not only
5 important for the public perception, marketing or product recognition, but also to enhance production efficiency, quality and quantity (Southgate and Wall, 2001).
Figure 1.2 |Research-driven areas within aquaculture scientific field with fish welfare as the core goal of those areas
Similar to other countries in southern Europe, research in Portugal has been driven mainly to teleost fish species with high commercial value, such as gilthead seabream (Sparus aurata), European seabass (Dicentrarchus labrax) and some flatfish, like sole (Solea senegalensis) and turbot (Psetta maxima) (FAO, 2004). The level of acceptance of cultured fish with higher nutritional value was assessed through a survey on the Portuguese population (Ramalho et al., Unpublished). Although welfare was not the focal point of this survey, positive public preference for such fish points towards the choice of fish being produced under guidelines and standards for good welfare.
1.2. Animal Welfare: origin and definitions
The scientific basis of animal welfare has been established through the “Brambell report” in 1965, in which ideas like “behaviour assessment” or “animal’s needs” were exposed (Rollin, 1989). Since then, many definitions have been proposed, either focusing on animal’s condition, how they subjectively experienced such condition or how it allows them to lead a natural life (FSBI, 2002). These divergent definitions demonstrate that welfare definition is not straightforward, but rather complex and often controversial. The most simplistic and common definition is that animal welfare is consistent with the individual’s quality of life (Appleby, 1999; Miller, 2001). Beyond the simplicity of the definition, different questions can be raised concerning is the definition of “quality of life”, if animals from different taxa
6 experience such condition, or even if it is possible to have it in other conditions than their natural environment. In 1997, the different prevailing definitions were clustered into three categories (Fraser et al., 1997): (1) feeling-based: the animal should feel well, being free from negative experiences such as pain or fear, and have access to positive experiences, such as interactions with conspecifics, in the case of social species; (2) function-based: focus on the animal’s ability to adapt to its present environment, being in good health, with its biological systems functioning properly, including physiological stress responses, and not being forced to respond beyond their capacity; (3) nature-based definitions: individuals should express their inherent biological nature, hence the animal should be able to lead a natural life and express its natural behaviour. Indeed, impairing natural behaviour is normally accompanied by health problems and negative experiences, making the different approaches often overlapping (Appleby and Sandøe, 2002). Having this into account, it is evident that no easy and single all-encompassing measure of welfare is available. Despite not being mutually exclusive, these definitions provide a more complete meaning of animal welfare and reflect what we should be focusing on when handling animals. Arguments for and against each approach, with the strengths and weakness of each, were exposed by Appleby and Sandøe (2002), highlighting a necessity for standardization. In the specific case of fish, it has been extensively debated over the last years whether this group of animals has aptitudes to consciously experience the surrounding environment, either by suffering pain or experience positive states, like other vertebrates (Braithwaite and Huntingford, 2004; Braithwaite and Boulcott, 2007; Chandroo et al., 2004; Dawkins, 1998; Rose et al., 2012; Sneddon, 2004), giving “fish welfare” a whole new connotation.
1.3. Fish culture and Welfare standards: where are we?
Fish are extremely exploited by humans (e.g. fisheries, intensive aquaculture, pet industry and scientific research); however, recent perception that fish are far more complex organisms than previously thought led this taxa to be considered within national welfare legislation in many world countries (Mejdell et al., 2007). As result, and as any other sector dealing with animals, aquaculture practices are being thoroughly examined to evaluate their impact on both the environment and the welfare of the cultured fish. In the EU, Council Directive 98/58/EC set minimum standards for the protection of animals reared or kept
7 under farming purposes, including fish. But it was mostly over the last two decades that aquaculture industry started to implement Codes of Practice for responsible aquaculture that take fish welfare into consideration (Huntingford, 2008), promoting optimal rearing conditions. The development of guidelines for responsible aquaculture by the Federation of European Aquaculture Producers (FEAP) is just one example of how welfare is a rising concern for farmers. In 2005, the Council of Europe adopted a recommendation on the welfare of farmed fish. In 2008, the World Organization for Animal Health (OIE) adopted guiding principles and policies for fish welfare and, in 2009, adopted an opinion on the general approach to achieve fish welfare and proper killing methods. The involvement of OIE in aquatic animal welfare was noted by Hastein (2007), where the scope and development of the welfare guidelines for aquatic animals were thoroughly described. To adopt these guidelines or protocols all over the aquaculture sector, worldwide and across cultured species, much work has to be undertaken as we are still in the very beginning of understanding what is really good welfare (e.g. depending on the situation, on the production regime, on the species biology or ecology requirements, etc.). Despite the considerable progress made over these last two decades, the knowledge regarding welfare or the proximate mechanisms underlying such concept is still sparse, when compared to mammals or birds (Ashley, 2007; Huntingford et al., 2006; Mejdell et al., 2007; Sneddon, 2007; Turnbull, 2006). In fact, to adopt such guidelines for fish welfare, I share the opinion of Mancuso (2013), which states that research should be the way to address these issues. Efforts should be made to decrease the stress of aquaculture routines, through multidisciplinary approaches that rely on both known and unknown indicators of well-being, behavioural, physiological, neuroendocrine, etc. One good example has been the development of proteomics as a method to obtain unbiased information regarding the impact of stressors on both plasma and organs/tissues (Alves et al., 2010; Rodrigues et al., 2012). Assessment of a wide range of responses under different conditions or situations provides an appropriate and reasonable basis to assist OIE in carrying out its work regarding the protection of fish farming.
8
1.3.1. Current issues of welfare in fish
Recent studies showing that fish are capable of environmental perception and preference, sense pain in similar ways to mammals and experience aversive states (Braithwaite and Boulcott, 2007a; b; Dunlop et al., 2006; Madaro et al., 2015; Madaro et al., 2016; Millot et al., 2014a; Millot et al., 2014b; Reilly et al., 2008; Sneddon, 2006; Sneddon, 2003; 2009; Vindas et al., 2012; Vindas et al., 2014) have brought to light a whole new perspective in terms of fish welfare, which is now being employed in terms of freedom from hunger, thirst, discomfort, pain, injury, fear, distress, disease, along with freedom to express normal behaviour (Ashley, 2007b). Despite lacking a developed neocortex, self-awareness and cognitive abilities on the same level as mammals, it is suggested that fish can certainly sense noxious stimuli and, to some level, experience both pain and fear (Sneddon, 2009). Recently, in zebrafish (Danio rerio), the capacity of expressing emotional fever (e.g. physical reaction similar to fever but triggered by a stressful situation), a trait normally used to identify consciousness in mammals (Rey et al., 2015b), has been described. Such studies, alongside with the extensive evidence of fish behavioural and cognitive talent and pain perception, justifies the need to think about the importance of welfare in aquaculture from an ethical point of view (Brown, 2014; Grigorakis, 2009), rather than only pragmatically (i.e. in terms of quality or performance improvements). Thus, new lines of research focused on understanding the welfare of a more ancient and divergent taxonomic group than mammals are being opened. Still, it is essential to go deeper in safeguarding cultured fish through concession of the same level of protection as that provided to any other vertebrate group.
1.4. Assessing welfare in aquaculture
Though it is true that, in the natural environment, fish can be subjected to many types of stressors (injuries, diseases, parasites, floods or storms, predators or larger conspecifics), they are likely to have adaptive coping mechanisms for dealing with such adverse conditions (up to certain limits, if such conditions are short-term events or otherwise avoidable). On the other hand, various aspects of standard aquaculture practices throughout all production stages can impair considerably their welfare. Often, cultured fish suffer unavoidable and prolonged or repetitive physical constraints, which can undermine their adaptive coping responses, either physiologically or behaviourally. It is thus important to mitigate those
9 effects, minimizing the avoidable stressors during all production cycle and promoting welfare (Conte, 2004; Pickering, 1993). The strategy adopted in fish to “assess or promote welfare” was based on the evaluation of five domains (e.g. conditions) in which welfare might be compromised (Fig. 1.3). Originally edited as the “five freedoms” and defined by UK Farm Animal Welfare Council, was later adapted for fish (FAWC, 1996). Within this framework, in order to achieve good welfare and health of fish in aquaculture, these five conditions should be respected, maintained and improved (Mellor and Stafford, 2001). Fish condition can be assessed by means of their behavioural, physiological, morphological or physical stress responses, which can differ among species. Thus, each species’ biology and environmental requirements must be taken in consideration. In particular, knowledge of species-specific behaviour is critical to safeguarding fish welfare (Conte, 2004). In line with the way welfare definition has been evolving, so has the number of variables used to assess the state of the fish. In addition to physiological and biochemical measures, recent knowledge about behavioural alterations and contiguous molecular states are now seen as important fish responses for welfare assessment. Thus it is reasonable to state that welfare should be viewed as a result of divergent effects (e.g. water quality, tank densities, diet composition, restricted feeding or management procedures as confinement or air exposure) known to affect fish (Branson, 2008) and subsequent individual tolerance to stress, health, aggressiveness or lethargic behaviour.
Figure 1.3 |Five domains defined by UK Farm Animal Welfare Council to assure and assess welfare of animal reared and kept under artificial conditions (adapted from FSBI, 2002).
10 The factors affecting the life of a fish and the indicators used by farmers to assess the general status of their stock have been reviewed by a number of authors (Conte, 2004; Huntingford et al., 2006; Martins et al., 2011a; Schreck et al., 1997) (Table 1.1. adapted from Silva et al., 2013).
“Welfare indicators that are relevant for inclusion in an operational welfare assessment
system should be science-based, should measure welfare over extended time periods, should be measurable on a commercial farm within a realistic framework and should be relevant as a decision support system for the farmer” (Martins et al., 2011).
Table 1.1 |Main factors affecting fish welfare and most common indicators of impaired fish welfare.
Factors impairing welfare Indicators of impaired welfare
Environment
Temperature; pH; salinity
Behavioural
Food intake; aggressiveness O2; CO2; NH3; PO4
2-Swimming pattern
Photoperiod Ventilation rate
Pollutants Reaction to novelty
Xenobiotics Other changes
Health
Pathogens
Health and performance
Injuries; malformations
Somatic/fin lesions Diseases; Changes in color
Disease treatments Impaired reproduction
Vaccination side-effects Growth and FCR
Nutrition
Food deprivation
Haematological parameters
Cortisol; Glucose; Lactate;
Malnutrition Haematocrit; free fatty acids
Anti-nutritional factors Free amino acids
Management practices
Sorting, Handling, Grading
Post-mortem dynamics
Rigor mortis; sensorial parameters
Transporting; harvesting Energy stores(ATP, glycogen)
Slauthering; Sampling Freshness indicators
Stocking densities Instrumental parameters
Social dynamics
Sorting/grading
Cellular stress indicators
Heat shock proteins
enforced social contact Antioxidant potential
Genetic factors Reactive oxygen species
Agonistic behaviors/competition Apoptosis/necrosis
1.4.1. Stress responses in fish
Stress can be defined as any disturbance of the organism homeostasis as result of an internal or external stressor. Fish respond to external or internal challenges through a series of neurological and endocrine adjustments generically called “stress responses”, often used as indicators of impaired welfare (Table 1.1). Stress response has three different stages (Barton, 2002): first an endocrine response is induced through the release of glucocorticoids to the
11 bloodstream, mostly cortisol and epinephrine in teleost fish (Wendelaar Bonga, 1997; 2011); The secondary or metabolic and tertiary or behavioural responses make fish more efficient at overcoming or avoiding the challenge, improving the short-term capacity of fish to cope with it.
Repetitive or long-term activation of endocrine responses can induce chronic stress and eventually motivate maladaptive effects such as decreased growth, low performance and well-being issues, impaired reproductive function and immunosuppression, anorexia, disease and ultimately death (Barton, 2002; Gesto et al., 2008; Iwama, 2007; Pankhurst and Van Der Kraak, 1997; Pankhurst et al., 2008; Pottinger, 2008; Schreck, 2010; Van Weerd and Komen, 1998; Wendelaar Bonga, 1997; 2011). To describe the role of the primary stress response, the concept of allostasis was introduced to support homeostasis. Hence, rather than continuously making every effort towards a static (conceptually optimal) state, organisms have “the ability to achieve stability through change” (McEwen and Wingfield, 2003), which more accurately explains the adaptive and dynamic nature of biological systems. This means that, in a naturally dynamic environment, the internal balance of fish is reshaped to accommodate different requirements and adapt to them. Under an allostatic framework, the absence of any signals or challenges from the envrionment can lead to a state of allostatic underload, which can be as detrimental for the organism as allostatic overload (achieved by the inhability to cope with cumulative disturbing challenges). This implies that insulating fish from all type of challenges would also not be in their best interest, in terms of welfare. Nevertheless, allostatic load can either have an adaptive (load type I, normally referred as “eustress”) or maladaptive value (load type II, normally referred as “distress”). An overview of the relationship between stressors and biological responses within the context of finfish aquaculture can be seen in Fig. 1.4. It should be noted that a stress response is not necessarily a direct indicator of poor welfare. Indeed, lower cortisol levels can mean that fish interregnal tissue was in an overloaded state, hence misleading an interpretation of the effective stressed condition of the individual. Due to this fact, when assessing welfare, different stress responses should be monitored, integrating behaviour, neurophysiology, pathological and molecular indicators to draw robust conclusions about the state of the individual.
12 Figure 1.4 |Overview of the relationship between stressors and both physiological and psychological stress, within an allostatic framework.
1.4.1.1. Physiological response to stress
In teleosts, cortisol is the main glucocorticoid released by hypothalamic-pituitary-interregnal tissue axis (HPI) during stress and plasma cortisol concentration is often used as an index of stress response (Barton 2002). Cortisol is a central hormone for the maintenance of allostasis as it supports other hormones during basal conditions and has a stress-induced regulatory role (Mommsen et al., 1999). Other plasma metabolites associated with stress response, such as glucose and lactate, can be also used to assess conditions of impaired welfare, however with some constraints. The response to a stressor is a very dynamic process and physiological measurements can be only a snap-shot and not be representative of the stress experienced (Barton, 2002). Furthermore, cortisol levels are normally biased as blood sampling itself constitutes a source of stress. This hormone is also highly context- and species-dependent, as along with glucose and lactate. More specifically, basal levels measured are known to be affected by environmental conditions, aquaculture procedures, feeding, maturation, season, photoperiod, sex differences and other unknown stressors (Barton, 2002). For instance, in seabass, no differences were found between fish subjected to confinement and non-stimulated fish, and alterations due to external factors was the explanation given for such results (Rotllant et al., 2003). It is generally accepted that cortisol
13 rises after the exposure to a stressor within the first 4-10 minutes and this peak lasts for a few hours (Sumpter, 1997). In seabream, plasma cortisol concentrations increased 50 fold within 30 min after air exposure, while under confinement levels increased 8 fold within the same time (Arends et al., 1999). When fish are subjected to chronic stress, plasma cortisol can be elevated for many days or even weeks, or may return to basal levels due to habituation mechanisms or impairment of the HPI axis e.g. exhaustion of the endocrine stress axis (Madaro et al., 2015). Indeed, the nature of the stressor i.e. intensity, repeatability, predictability, controllability or familiarity, is known to influence the magnitude of cortisol response. In this sense, in the field of psychological modulators of stress response, it was shown that unpredictable, uncontrollable and unfamiliar environmental contexts accentuates stress responses in salmon and rainbow trout respectively, sustained by higher levels of plasma cortisol besides behavioural alterations (Carpenter and Summers, 2009; Madaro et al., 2015; Yue and Duncan, 2006). In addition, inherent features of the individuals promote inter-individual variability in cortisol response under the same challenging situation. Two personality traits have been defined due to their consistent neuroendocrine and behavioural characteristics: the proactive (bold) and reactive (shy) coping response. Psychological stressors and personality will be addressed further down in this chapter (section 1.5).
1.4.1.2. Behavioural responses to stress
Behavioural responses are the first line of defence of the animal against environmental changes, predators or social conflicts and are often caused by the same stimuli that elicited the physiological responses. Within a more welfare functional-based approach, the fish functions that are known to be affected by stressors include foraging behaviour, swimming patterns, shoaling, thermoregulation, orientation, avoidance, ventilator frequency, chemoreception and agonistic behaviour e.g. social interactions or evasion from predators (Conte 2004). Exploratory behaviour, food anticipatory behaviour, environmental preference, learning and reward-related operant behaviour are seen as patterns within a more feeling-based approach, as reviewed by Martins et al. (2011). Due to this extensive list of fish behavioural stress responses and for the sake of briefness, only few behaviours are
14 here briefly described. The behaviours described represent the most important and most used indicators by farmers or researchers:
Freezing behaviour is the most classic response of fish towards conflicting situations by
remaining motionless on the bottom and suppressing fin movements (Vilhunen and Hirvonen, 2003), increasing vigilance and arousal over the stressor. Divergent results from several works, often under different contextual situations, showed the dichotomy of this behaviour by being interpreted as either good or poor welfare. Furthermore, as also stated, the divergent fish responses can also be explained by other factors, such as personality traits or individual cognitive appraisal of the challenge. When under rewarding situations,
swimming activity levels are normally used, rather than freezing behaviour. This behaviour
is described as a measure of fish performance, therefore an indicator of the ability of the fish to feed, evade from predators and maintain position in a current (Beaumont et al., 1996). Swimming activity can be related with different swimming behaviours, among them
swimming speed, which has been used to evaluate the physiological condition of fish
(Wolters and Arlinghaus, 2004). As for freezing behaviour, this response can be interpreted in two directions: either signalling underfeeding, thus indicating poor welfare or a foraging strategy, thus indicating good welfare. Indeed, foraging behaviour (e.g. the search and exploitation of food resources (Danchin E et al., 2008)) is one of the most used welfare indicators by fish farmers. Teleost fish can exhibit diverse strategies to improve feed intake, in order to satisfy their nutritional requirements. This can be measured by feeding intake or
feeding motivation (e.g. latency to reassume feeding), behaviours which are highly affected
by feeding regime: predictable feeding time over random schedules was shown to affect behaviour and physiology (Sánchez et al., 2009). A predictable meal thus seems to help fish readjust themselves for the incoming event and optimize feed intake. It should however be noted that predictable regimes tend to increase “anticipatory behaviour”, tuned with increased swimming activity in the feeding area or increased schooling activity (Chen and Purser, 2001). In agreement to what was previously stated, this increase can imply good or poor welfare. Folkedal et al. (2010) stated that a good anticipatory response and feed intake can be signs of high feeding motivation and welfare. Lower latency to reassume feeding was also described as good welfare, demonstrated in Nile tilapia (Oreochromis niloticus) and in rainbow trout (Martins et al., 2011b; Øverli et al., 2006b). On the other hand, it can also
15 indicate underfeeding, and here anticipatory responses lead to aggression and injuries, with repercussions even for survival. Limited resources can drive fish to agonistic encounters, recognized as responses affecting the dominance rank-based hierarchies (Jobling, 2011).
Agonistic behaviours refer to a set of fight-or-flight behaviours expressed between at least
two social partners in which one exerts dominance over the other (Martins et al., 2011). Fight-or-flight behaviours (onwards referred to as interactions between conspecifics) are a defensive mechanism with the purpose of acting over the stressor or moving away; thus includes attacks, bites or bite attempts, threatening displays such as chasing, and behaviours related to submission such as flight or immobility (Steckler, 2005). Interactions between conspecifics are normally associated with the competition for rewards, thus its absence under punishment situations does not necessarily mean good welfare, but rather a different context and consequently fish expressing other responses. Shoaling or group swimming behaviour is another behavioural response that can be used to assess hunger, stress level and health status and it relates to the spatial distribution and swimming activity of the group. The motivational state of the individuals to explore the surrounding environment will vary with their internal affective and physiological states, again resulting from inherent predispositions and from the subjective appraisal of the stressor. If one individual interprets the stressor as more positive (or less negative), or associated with the possibility to escape from an aversive condition (e.g. operant behaviour to decrease the impact of the stressor), it will probably increase the level of exploration. In this case, individual personality and subjective appraisal overrides the predisposition of the group swimming behaviour.
Ventilatory frequency or number of opercular movements provides an index of ventilatory
activity, thus offering evidences of physiological stress. Well-balanced ventilatory activity is fundamental to maintain a good oxygen supply to blood and tissues. Increased ventilatory frequency is normally related with poor welfare and can be a consequence of the several aquaculture practices or stressors previously identified. Different authors have shown the effect of such stressors and how they can compromise fish welfare (Barreto and Volpato, 2006; Barreto and Volpato, 2011; Barreto et al., 2003; Brydges et al., 2009; Scott and Sloman, 2004). Nevertheless, this indicator should be combined with other welfare indictors as it does not give any information regarding the nature and intensity of the stressor. In fact, arousal caused by positive experiences can also trigger an increase in ventilatory frequency, hence it should be interpreted with caution.
16
1.4.1.3. Integrating learning and preference to promote Welfare
Fish natural responses and learned preferences can and should be used by them in order to avoid stressful situations. One example in seabass and seabream is the behavioural response towards a microbial parasite infection known to affect fish gills (e.g. Amyloodinium
ocellatum), and which compromises oxygen trades and often leads to mortalities (Pereira et
al., 2011). Due to the positive correlation of salinity with the occurrence of this parasite, introducing inflow of fresh water to the pond make fish aggregate and adopt a synchronised swimming towards it entrance, this way decreasing the spreading of the infection.
Good welfare is attained when the difference between the current and the expected state is minimized (sensu feeling-based approach). Learning is the process by which an animal benefits from existing information from the environment, so that its behaviour becomes fit to the environmental conditions, by maximizing positive and minimizing negative states (Spruijt et al., 2001). This requires that the animals have the ability to learn about predictive stimuli, i.e. which events follows which (classical conditioning), and about the consequences of their own behaviour (operant learning). There is considerable evidence that cultured fish are able to improve performance in order to meet new challenges that are introduced (Fernö et al., 2006). Learning ability allows them to adjust behaviours and has been investigated in a wide range of contexts. In Aquaculture, different strategies are used for that purpose, such as using light to identify where and when the food will be dropped, reducing food waste and improving fish performance and productivity (Karplus et al., 2007). The ability to associate cues with relevant environmental stimuli is crucial for performance, for instance in agonistic interactions and reproduction (Hollis, 1999; Jenkins and Rowland, 1996) and for adapting to new environmental situations such as fish orientation (Braithwaite et al., 1996; Odling-Smee et al., 2006; Vargas et al., 2004), foraging (Warburton, 2003) and predator avoidance (Martins et al., 2011d).
Classical Conditioning learning
In classical conditioning, an association is made between a stimulus and a response. A conditioned stimulus (CS) acquires the ability to trigger a new response by virtue of being paired with an unconditioned stimulus (US), which by definition is biologically important and capable of triggering an innate reflex. For example, carp can learn to associate a 400 Hz pure
17 pulsed sound with food by classical conditioning (Zion et al., 2007). In the same species, known to be a strongly schooling fish, choice of food over social attraction was possible by prior light cues conditioning (Mesquita et al., 2015).
Operant conditioning learning
In operant conditioning, the consequence (positive or negative) of performing a particular behaviour alters the probability of that action being repeated. In this case, the behaviour is spontaneously emitted and not, elicited by a stimulus. For favourable consequences, animals learn to perform it in order to be rewarded. An example, is when Atlantic cod (Gadus
morhua) were trained to operate a trigger to receive food in an experiment using a
self-feeding system (Nilsson and Torgersen, 2010). Under aversive conditions, operant conditioning can take the form of punishment avoidance conditioning, in which instead of a reward, the animal receives a punishment (Bolhuis and Giraldeau, 2005): rainbow trout that learned to escape, showed lower cortisol responsiveness and latency to escape over a training period, than no learners (Carpenter and Summers, 2009).
Although learning may occur with a single trial, it often takes gradual training for effective conditioning. However, this can lead to habituation, sometimes described as one of the simpler forms of learning. Habituation is a type of non-associative learning leading to a decrement in response intensity repetition of the triggering stimulus. The first conditioning applied is seen as novel and induces a reaction, however, with the repetition such response decreases and may disappear. This can be useful in aquaculture, allowing a better adaption to daily procedures (Fernö et al., 2006). In accordance, social learning also has a pivotal role on that, as organisms are able to learn from others. As expected, this is much more common in social species. The adaptive value of social learning lies in saving time and energy in developing proper coping mechanisms by trial and error. It is also generally assumed that social learning is beneficial, because naïve individuals can acquire adaptive behaviour quickly and efficiently from more knowledgeable individuals (Brown and Laland, 2006; Bshary and Brown, 2014). As referred before, inherent predispositions of the individuals also affect learning, where proactive individuals are quicker learners but often with less awareness of the changes in the environment (Castanheira et al., 2015).
18
1.5. Intra-individual variability of stress responses
Individual behavioural and physiological variability towards environmental challenges or stressors are today a well-known and accepted phenomenon within the animal kingdom (Carere et al., 2010; Réale et al., 2010). This variation suggests that stress responses do not depend exclusively on the situation to which the individual is exposed, but also on the cognitive evaluation made of the situation, i.e. how the stressor is appraised (Lazarus, 1991). In fish, as often mentioned throughout this chapter, this individual variability is related to differences in learning, adaptation to new environments, growth and metabolism, reproduction, susceptibility to diseases and welfare, health, flesh quality, performance and any other situation that elicits a response from the fish (Ashley, 2007a; Basic et al., 2012; Castanheira et al., 2013b; Herrera et al., 2014; Ibarra Zatarain, 2015; Iguchi et al., 2001; MacKenzie et al., 2009; Rey et al., 2015a; Ruiz-Gomez et al., 2011). Understanding such differences in coping ability and environmental perception, fundamental to fitness and quality of life, is particularly pertinent under aquaculture conditions for an effective understanding of individual behavioural patterns under stressful conditions (Huntingford and Adams, 2005).
Individual response variability to any challenge is not an arbitrary variation around an optimal mean; it is consistent under a diversity of stressful conditions. Behavioural and physiological differences in response to stress, which are consistent throughout time and across situations, are collectively termed as coping styles (Koolhaas et al., 1999a). In the literature, this phenomenon is also referred to as temperament (Francis, 1990), personality (Briffa and Weiss, 2010) or behavioural syndromes (Sih et al., 2004) (see review of Castanheira et al. (2015) for differences between terminology). Despite the different terminology the core concept behind each is based on the fact that individuals consistently differ from one another in behaviour or physiological responses in such a way that these differences can be described as individual traits. The term coping styles is narrowly used and primarily defined as “a coherent set of consistent behavioural and physiological stress responses over time and across situations” (Koolhaas et al., 1999b) and has been considered in a wide range of taxa, such as birds, insects, mammals (including humans) (LaRowe et al., 2006; Reale et al., 2007; van Oers et al., 2005) and fish (Toms et al., 2010; Conrad et al., 2011; Castanheira et al., 2015). When addressing humans or other mammals, the term
19 “personality” is more often used, because it also takes account of the emotional reactiveness of the individuals e.g. feeling, thinking, cognitive appraisal, motivational states (Gosling, 2001). Indeed, several reviews regarding personality in animals in the recent past years have been addressed; non-human primates (Freeman and Gosling, 2010), avian (Groothuis and Carere, 2005), felid (Groothuis and Carere, 2005), canids (Jones and Gosling, 2005) and other species (Gosling, 2001). Furthermore, Martins et., al. (2011d) demonstrated that coping styles in fish are predictive of how stimuli are appraised, hence supporting the inclusion of emotional or affective states as a relevant component of coping styles (see below). In agreement, Coppens et al. (2010a) pinpoints that motivational reflections may explain distinct traits in reaction to challenges. We thus reserve the use of the term “personality traits” in agreement with the objectives described (see below), and will use this terminology through this dissertation, even when mentioning other researchers’ work. It should however be noted that personality in fish does not necessarily imply homology to humans or other animals, as it rather represents a more primitive or simplistic system, likely to be evolutionary conserved, thus working as a raw material for natural selection (Castanheira et al., 2015).
1.5.1 Personality traits in fish
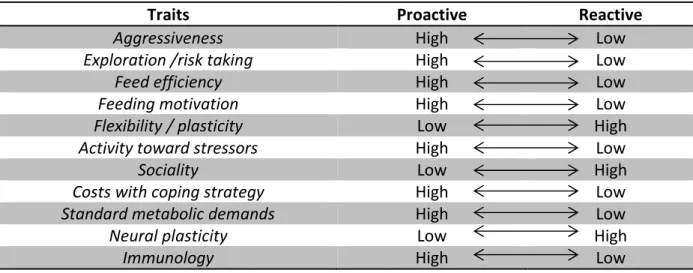
In fish, one of the first references to the existence of distinct behavioural phenotypes was published by Huntingford (1976): three-spined stickleback (Gasterosteus aculeatus) were shown to be more aggressive to conspecific intruders during the breeding season, and also more aggressive towards heterospecifics. Moreover, the most aggressive individuals in the breeding season were also the boldest when approached by a predator outside of breeding season. Besides the clear consistency over time (e.g. during and post breeding) and across situations (e.g. conspecifics and heterospecifics), consistency in different behaviours was found. Those results, even though no mention was made, satisfy the description of personality. Since then, several studies have addressed the existence of such traits in fish in different contexts, situations and species that led to specific research focus surrounding this issue. Two distinct stress response patterns have been described, reflected in both behaviour and neuro-endocrines profiles: proactive (active traits) and reactive (passive traits). Behaviourally, proactive individuals are more aggressive, bold when facing potential
20 danger or in exploring novel environments and with a tendency to develop rigid learned routines, hence lower sensitivity to environmental stressors. Physiologically, proactive individuals show lower HPI axis reactivity, hence low production of glucocorticoids (i.e. catecholamines or cortisol) and a high sympathetic activity leading to a high increase of noradrenaline and adrenaline in blood (Overli et al., 2007). In contrast, reactive individuals show lower degrees of aggressiveness and boldness, and are more flexible to changes in the environment. Moreover, reactive individuals show high attack latency and freezing behaviour under stressful situations, higher HPI axis reactivity and lower noradrenaline and adrenaline releases. A number of recent reviews pinpoint the differences between this dichotomous classification (Toms et al., 2010; Conrad et al., 2011; Mittelbach et al., 2014; Castanheira et al., 2015).
Table 1.2 |Summary of the main differences between proactive and reactive individuals (adapted from Castanheira et al., 2015)
Traits Proactive Reactive
Aggressiveness High Low
Exploration /risk taking High Low
Feed efficiency High Low
Feeding motivation High Low
Flexibility / plasticity Low High
Activity toward stressors High Low
Sociality Low High
Costs with coping strategy High Low
Standard metabolic demands High Low
Neural plasticity Low High
Immunology High Low
It is also important to note, as reported elsewhere, that the distribution of personality traits throughout captive animals is not expressed as a binomial distribution, it is rather a proactive-intermediate-reactive traits continuum (Réale et al., 2010). The existence of an intermediate group can be due to the lack of environmental demands or pressures and accommodates, in due course, the individuals whose stress response is not consistent over time and contexts (i.e. higher plasticity). In nature, this group is possibly less fit to survive, both in stable and unstable environments (Boersma, 2011).
21
1.5.1.1. How to assess fish personality in aquaculture
The concept of personalities in fish has gained an increased interest in recent years. The majority of research focused on the most important farmed species, among them Atlantic Salmon (Salmo salar) (Kittilsen et al., 2012; Vaz-Serrano et al., 2011), Nile tilapia (Martins et al. 2011b,d), common carp (Cyprinus carpio) (Huntingford et al., 2010; MacKenzie et al., 2009), rainbow trout (Laursen et al., 2011; Øverli et al., 2006a; Øverli et al., 2006b), seabass (Ferrari et al., 2014; Millot et al., 2009a; Millot et al., 2009b) and seabream (Castanheira et al., 2013a; b; Herrera et al., 2014). In addition, species with limited expression in these industry have been investigated, such as Senegalese sole (Solea senegalensis) (Ibarra Zatarain, 2015; Silva et al., 2010), Atlantic halibut (Hippoglossus hippoglossus) (Kristiansen and Fernö, 2007) and turbot (Psetta maxima) (Hermann et al., 2016).
Different behavioural screening approaches have become available to assess individual variation in fish, both individual- and group-based (for more details on each test mentioned below see Castanheira et al. 2015). Within the first set of behavioural tests, patterns of feeding behaviour (Barreto and Volpato, 2011), exploration of novel environment (Killen et al., 2011), novel object (Frost et al., 2007), resident-intruder test (Brelin et al., 2005) and net restraining (Castanheira et al., 2013b) are the most commonly used. Hypoxia (Ferrari et al., 2014; Laursen et al., 2011) and risk-taking (Castanheira et al., 2013a) are the behavioural tests normally used for mass-screening. Physiological individual-based approaches can also be used to discriminate distinct adaptive traits, for instance ventilation frequency (Barreto and Volpato, 2011) or metabolic responses (e.g. O2 consumption measured in metabolic
chambers (Herrera et al., 2014), HPI reactivity or sympathetic system activity (Overli et al., 2007)). Recently, it was shown that subtle thermal gradients are likely to impact specific physiological and behavioural processes, which is reflected as a suite of traits described by animal personality (Rey et al., 2015a). As such, thermal preference alongside with hypoxia or risk-taking, are promising tools for farmers, as they can be for mass-screening. Individual tests are often time consuming and demanding (e.g. restraining requires video recording and video analysis afterwards) and highly stressful, sometimes driving even to mortalities; as such, the development of group-based paradigms, adapted to the ecological features of the species, can be highly advantageous since it allows screening large number of fish in a shorter time and with immediate results, being highly attractive for the aquaculture
22 industry. With this, welfare can be attained within production systems, adapting optimal conditions to proactive and reactive traits (Castanheira et al., 2015). The diversity of paradigms to assess coping strategies in fish, which should be fitted to species, shows how challenging it is to assess personality traits. Behavioural responses to changes in the environment can be extremely plastic and most often context-dependent (Coppens et al., 2010b; Wolf and Weissing, 2010),and reported to be shaped by numerous factors, such as the predictability of food regime (Chapman et al. 2010), food density (Dunbrack et al., 1996) or social context (Castanheira et al., 2016). Other factors are also well recognized to affect the consistency of personality: temperature or hypoxia (Biro and Stamps, 2010; Rey et al., 2015a), predation pressure (Brown and Braithwaite, 2004; Archard et al., 2012), learning (Millot et al., 2009b), social interactions (Chapman et al., 2008), environment constancy (Brelin et al., 2008), stress (Ruiz-Gomez et al., 2008) or even the time gap between repeatable tests (Stamps and Groothuis, 2010; Ferrari et al., 2016; Castanheira et al., 2016). Indeed, in seabream, restraining escape performance was shown to be consistent between repeatable tests 14 days and 8 months apart. Nevertheless, after sexual maturation, a loss of consistency was marked. As such, measuring the stress response in different contexts would allow a more accurate characterization of species-specific personality. Analyses of the consistency of behavioural screening results between repeated tests (i.e. “the extent to which scores for behaviour in a given context at a given time are correlated across individuals with scores for the same behaviour in the same context at a later time”; Stamps and Groothuis, 2010) or different challenges (cross-context analyses) are generally carried out over periods of one to eight days (Wilson and Stevens, 2005; Øverli et al., 2007; Wilson and Godin, 2009; Wilson et al., 2010) and are reported to be important to tune the population personality screening; and to effectively discriminate personality (Castanheira et al., 2015, 2016; Ferrari et al., 2016). However, such premise is still one of the major gaps in the literature concerning the characterizations of personalities in animal, including fish, despite the recent works in that direction (Castanheira et al., 2016; Ferrari et al., 2016). In both seabream and seabass, the net restraining test was shown to be a robust approach to assess divergent personality traits (Castanheira et al., 2013a; Ferrari et al., 2014); it has been developed in the past to evaluate the stress response of seabream to air exposure (Arends et al., 1999) and adapted afterwards as a methodological approach to characterize
23 personalities in Senegalese sole (Martins et al., 2011c; Silva et al., 2010). It is representative of different aquaculture routine procedures (e.g. grading, sampling, sorting, vaccination, transport). The test relies on the escape performance of fish, known to have an ecological implication (interaction between predator and prey) and physiological repercussions (anaerobic recovery capacity of white muscle). In Nile tilapia, latency to reassume feeding and ventilation frequency (Barreto and Volpato et al., 2011) were the most common and efficient tests. In fact, Barreto and Volpato (2011) observed that ventilation frequency of Nile tilapia was correlated with the return to feeding in isolation. By assuming this correlation in stress responses, measuring individual feed intake can be replaced by ventilation frequency, more easily assessed by means of technology, such as using SmartTags. These devices have been developed to measure ventilatory frequency and amplitude of free-swimming fish as an indicator of fish welfare (Damsgard, 2008).
1.5.1.2. Importance of assessing personality
Personality can be an important tool to better understand both ecological and biological dynamics of any organism and hence should be included as an explanatory variable to understand differences in individual survival, reproductive success, species diversity on behavioural interactions, population dynamics (i.e. growth, fecundity and survival), social assemblages patterns, and for improvement on conservation and management of natural resources (Mittelbach et al., 2014). In cultured fish, the advantages of characterizing proactive or reactive traits have now being extensively reviewed (Castanheira et al., 2015; Conrad et al., 2011b; Huntingford and Adams, 2005). As an example, reactive individuals have higher neural plasticity, higher life span and a more robust hormonal regulation (e.g. Castanheira et al., 2015). On the other hand, proactive fish often recover faster from stressful situations (Ward et al., 2004), display lower susceptibility to diseases (Mackenzie et al., 2009), higher growth rates (Basic et al., 2012) and often higher reproductive success (King et al., 2013; Wilson et al., 2010). By accepting the presence of such traits through scientific outputs, fish farmers are provided with tools to understand individual variation in fish for aquaculture practices, in terms of stress responsiveness, fish quality and performance, adaptation, growth, survival, reproduction success and disease resistance, having a positive impact on the productivity, health and welfare of the farmed fish. In this