DESALINATION
E L S E V I E R Desalination 128 (2000) 81-90
www.elsevier.com/locate/desal
The rejection of anionic dyes and salt from water solutions using
a polypropylene microfilter
John J. Porter a*, Arlindo C. Gomes b
aSchool of Textile Fiber and Polymer Science, College of Engineering and Science, Clemson University, Clemson, SC 29634, USA
Tel. +1 (864) 656-5960; Tax +1 (864) 656-5973, email: porter@mail.clemson.edu bDepartment of Chemistry, University of Da Beira Interior, Covilha, Portugal
Received 1 September 1999; accepted l December 1999
Abstract
Previous work reported by this laboratory showed that inorganic membranes such as stainless steel and ceramic microfilters were capable of rejecting anionic dyes and sodium nitrate from water solutions. It was of interest to see if this were possible with organic membranes such as propylene microfilters. Experimental data are presented showing that a polypropylene microfilter will reject both salt and Direct Red 2 from aqueous solutions when the conductivity of the solution is below 500/~Siemens. The use of microfiltration to remove color is an important phenomenon considering that microfiltration comprises the largest fraction of the total membrane production in the world and is now used commercially for tertiary biological wastewater treatment. The effect ofpH and salt concentration on the filtration rate and color rejection is also presented.
Kegwords: Organic membranes; Microfiltration; Tertiary treatment; Dye filtration
1. I n t r o d u c t i o n but they also have been recently shown to be The increase in the use o f microfilters in capable o f removing soluble anionic dyes from recent years has been so significant that they now aqueous solutions [2,3]. Data reported by this comprise the largest faction o f total membrane laboratory [2,3] have shown that titania-coated stainless steel and ceramic microfilters are production [ 1 ]. Not only are these filters able to capable o f rejecting Direct Red 2 and Acid Red 1 remove suspended solids from aqueous solutions, from deionized water. Since these experiments were performed with deionized and microfiltered water having a conductivity o f less than
*Corresponding author. 1/~Siemens, the ionic rejections could not be
0011-9164/00/$- See front matter © 2000 Elsevier Science B.V. All rights reserved PII: S0011-9164(00)00025-4
82 di.Z Porter, A.C. Gomes / Desalination 128 (2000) 81-90
attributed to the presence of dynamically formed
membranes produced during the course of the [I ] Heat Exchanger L] I Filtrate or
experiment. To see if the rejection mechanism nov, ~ Permeate
were a characteristic of inorganic titania mem- M~er P .. . . Flow
~
" Gage Meterbranes, an organic polypropylene microfilter was
selected for evaluation. The dyes used for this ~ ]~ [ Filtration Module ] work are representative o f the types of soluble p ...
Control Pressure Temp. Pressure
dyes which would be present in many industrial va.,e c~on ceton Gage waste streams and resistant to normal biological
treatment processes. ~ , l ~ 1 ~ N
The data describing the filtration character- ~ ] ] [ - ~
istics of ceramic [2] and stainless steel tubular microfilters [3] were obtained on tubes coated
with a fused asymmetric titanium dioxide layer Feed Supply
having a nominal pore diameter of 0.2 microns. =:~ 0a Concentrate Return
Microfilters such as these are generally described L [4] as being able to separate suspended particu-
late with a particle diameter of greater than Fig. 1. Laboratory filtration system. 0.1 microns from aqueous solutions. When they
are used to remove suspended material from
water, it is generally assumed that the filter will three channels, 5.5 mm in diameter. The module was supplied by Microdyne Technologies, Inc., not remove salts. Because salt rejection was
observed in the previous study [2,3], it was the (Raleigh, NC, US) and had a total surface area of
2 . .
objective o f this research to investigate the 362.9cm and a membrane nominal pore dla- filtration properties of a commercial organic meter of 0.2 microns. It could be operated at polypropylene microfilter to see if similar reject- temperatures below 65°C and could withstand ions would occur with this organic membrane, pHs of 1-14. The pore size was obtained from the module manufacturer, Akzo Nobel Faser A.G. (Wuppertal, Germany). The tubes were cleaned using a laboratory detergent solution in deionized
2. Laboratory set-up
water before use. Analytical measurements for All the laboratory tests were conducted on the conductivity were made with a Myron L DS pilot unit shown in Fig. 1. The system is conductance meter standardized against gravi- constructed of 316 stainless steel tubes and metrically prepared sodium nitrate solutions), a fittings, except forthemulti-stagepump impeller, Milton Roy Spectronic 20D colorimeter, and an which is fabricated from Dupont Delrin plastic Orion Model 250A laboratory pH meter. The pHs with a temperature limit of 70°C. The unit was of all solutions were adjusted with reagent grade fitted with temperature and pressure cut-off 10% nitric acid and 10% sodium hydroxide. switches to protect the pump impeller and allow Direct Red 2 was chosen for the study because of for more independent operation. A 115-L stain- its use in previous work [2], its stability to hot less steel, steam jacketed tank was used to supply water and its extensive use in the textile industry.
feed water to the pilot unit. The dye was crystallized from water and acetone
T h e p o l y p r o p y l e n e m o d u l e u s e d w a s 3 0 m m i n using a published procedure [5] to obtain diameter and 700mm in length and contained relatively pure dye, free of commercial additives.
J.J. Porter, A.C. Gomes / Desalination 128 (2000) 81-90 83 The laboratory detergent used for cleaning the with the inorganic membranes, it was desirable to module and filtration system was obtained from conduct studies with an organic microfilter to see Baxter Scientific and labeled as micro concen- how it would perform under similar conditions. trated cleaning solution. Chemical constituents in The organic microfilter selected for evaluation the cleaner were glycine N , N ' - 1,2-ethanediylbis was a polypropylene tubular module supplied by (N-carboxymethyl)-tetrasodium salt; benzene MicrodyneTechnologies, Inc. (Raleigh, NC, US) sulfonic acid, dimethylammonium salt; benzene and should be the least polar of any membrane sulfonic acid, dodecyl-, cpd. with 2,2',2"- material otherthanfluorocarbonmembranes. nitrilotris-ethanol; poly(oxy-l, 2-ethaanediyl), An experiment was performed in the a l p h a - ( 4 - n o n y l ( p h e n y l ) - o m e g a - h y d r o x y , laboratory to evaluate the effect of temperature
branched, on the filtration rate and conductivity rejection o f
Flow rates in the tube were maintained at the polypropylene membrane. The results are 4.3 m/s to provide turbulence (Reynold's shown in Fig. 2 withthe data previously obtained numbers>30,000)andtominimizeconcentration with the stainless steel and ceramic tubes. The polarization and surface fouling. Conductivity curve obtained with the polypropylenemicrofitler rejections were determined using Eq. (1). is linear and does not have the same curvature as
the data shown for the two inorganic membranes.
% r e j e c t i o n - The decrease in viscosity o f the aqueous solution
with increasing temperature accounts for the Conductivity of concentration -conductivity &permeate
c o n d u c t i v i t y o f c o n c e n t r a t e shape of the curves for the two inorganic
• 100 (1) membranes. Evidently the cylindrical shape o f
the tubular polypropylene membrane restricts the thermoplastic organic membrane surface from It is possible to get a reasonable estimate of expanding and the pores actually decrease in the total electrolyte concentration (for a simple diameter as the polypropylene expands with monovalent salt) in PPM by multiplying the increasing temperature. The two inorganic conductance by 0.6 [2]. In this study the membranes are much more rigid than the poly- additional electrolyte, other than that contributed propylene and should show no significant by the dyes, would be sodium nitrate, nitric acid, expansion over the narrow temperature range
sodium hydroxide, used. Since the fluid in all cases was deionized
water, the relative shape of the curves should be similar if the polypropylene pores do not change
3. Results and discussion with increasing temperature. It is most interesting
that the conductivity rejections obtained with the The rejection o f simple salts and ionic dyes by three membranes is similar considering that they 0.2micron ceramic and stainless steel micro- represent two entirely different chemical filters was recently reported [2,3] by this compositions, ln the cases presented the rejection laboratory. Both inorganic microfilters were is more a function of the solution and the pore coatedwith afused layeroftitanium dioxide and size than the m e m b r a n e c o m p o s i t i o n . are generally used to remove small particulate A second experiment, shown in Fig. 3, was from aqueous solutions. Neither of the micro- conducted where the pH was changed during the filters is supposed to be able to reject soluble filtration to see the effect o f p H on the filtration salts and ionic dyes from aqueous solutions, characteristics of the membrane. The filtration Because of the unusual rejection properties found rate remained relatively constant or increased
8 4 JJ. Porter, A.C. Gomes / Desalination 128 (2000) 81 90
1750 -~-Stainless Steel Filter, 0.66 M Pa j j 100 - = - Ceramic Filter, 0.66 M Pa % Conductivity Rejections /
--e- Polypmpylene Filter, 0.21 M Pa / I
A 1400 - ~ - % Cond. Rej. Stainless Steel Filter 80 ~.J~ --~-% Cond. Rej. Ceramic Filter
.~.
. j 1050 ~ - 60
~,
~00 40~- ~i Itrati ~fn R a ~ o-~
350 ~ 20
Deionized Water, pH = 5 - 8, 10 - 30 I~Siemens
0 ~ , , 0
25 35 45 55 65 75
Temperature, °C
Fig. 2. Filtration rate for deionized water using stainless steel, ceramic and polypropylene microfilters at a flow velocity of 4.3 m/s.
leo,
= IPressur 0' 'MPa
1 1 ,
~3 80 ! 9 X ,-- u N • ~ / p N o f S o l u t i o n - - - - ~ O -- ._~ 60 - ~ > _ 7 =.~ " .> ~ ~ "6 , - ~ ,
40
' J
~
5
,- .o C) o. ° ~ ~ 20 3 Filtration L / m2 / % C o n d u c t i v i t y R e j e c t i o n , = 6 - 1 0 0 gSiemens 0 4 ~ 1 0 1 2 3 4 5 6 7 T i m e of Filtration in HoursFig. 3. Effect of pH on filtration rate for deionized water using a polypropylene microfilter at a flow velocity of 4.3 m/s at 30°C.
J.J. Porter, A.C. Gomes / Desalination 128 (2000) 81 90 85
C H 3 - - CH 3
slightly o v e r the pH range o f 4 - 1 0 used for the ~H2 ~ ) - - ~ ~ .NH2
experiment. As was found with the previous work ~ N = N ~ N = N , @
[2,3], the c o n d u c t i v i t y rejection averaged 35% and decreased as the pH was lowered to four. At
the end o f the e x p e r i m e n t the conductivity SO3Na SO3Na
rejection increased when the pH was adjusted to
7.5. T h e filtration rates for the p o l y p r o p y l e n e Fig. 4. Structure of Direct Red 2 dye, C.I. 23500. m o d u l e were significantly higher than the
inorganic m e m b r a n e s considering the fact that
the p o l y p r o p y l e n e microfilter was operated at a w i d e l y used by the textile and paper industry. pressure o f two atmospheres and the inorganic The structure for the dye is shown in Fig. 4. It is m e m b r a n e s [2,3] were operated at 6.5atm resistant to degradation at the pHs and tempera-
pressure, tures used ( 3 0 - 6 0 ° C ) for the study. Data were
taken a pH from 10 to 4 to show the effect o f p H 3.1. Results w i t h D i r e c t R e d 2 dye on the filtration properties o f the p o l y p r o p y l e n e
microfilter (shown in Figs. 5-8). The a v e r a g e To investigate the m e m b r a n e filtration o f conductivity and dye rejection for each experi- larger ions Direct Red 2 was selected because it ment is shown in Table 1. The average rejections was used for the previous studies [2,3] and is are lower than those obtained with the inorganic
Table 1
Performance ofpolypropylene, ceramic and stainless steel microfilters with deionized water containing Direct Red 2 dye at flow velocities of 4.3 m/s
Membrane Temp., °C Permeate rate a, L/m2/h Conductivity a, #Siemens Color a, % rejection Concentrate % rejection
TiO 2 + stainless steel 30 129 102 61 88
• Surface: 294 c m 2 45 156 266 43 84
• Cross-sec: 1.85 cm 2 60 180 351 41 83
• Avg. press in tube, 0.66 MPa
TiO 2 + ceramic 30 435 150 54 99
• Surface: 396 cm 2 45 423 178 65 96
• Cross-see: 1.1 l cm 2 60 605 498 42 95
• Avg. press in tube, 0.66 MPa
Polypropylene 30 331 58 71 56
• Surface: 363 cm 2 45 407 217 7 44
• Cross-sec: 0.713 cm 2 45 399 4420 2 18
• Avg. press in tube, 0.21 MPa 60 498 330 5 43
86 J . I Porter, A.C. Gomes / Desalination 128 (2000) 81 90 100 - • 11 Pressure = 0.21 M Pa I , l ~ ~ pH of Solution -- /
1
X 80 ~ 9~" _L~ % Rejection Direct Red
' - 0 ~ i )~ c , o "0 ~ 60 ~ ~" ~ 7 . ' - - "-" 0 0 ¢ j . - ~ 0 IZ ~ .~-, Filtration Rate, ~ / ~- ~" - / 5 "1" .O ~tY' 40 m 2 / h r x 0.1 :~ 20 ~ l , 3 0 1 2 3 4 5 6 7 8
Time of Filtration in Hours
Fig. 5. Filtration rate for deionized water and Direct Red 2 using a polypropylene microfilter at a flow velocity o f 4.3 m/s at 30°C. Pressure = 0.21 M Pa p
trl
'
80 ~:~--o-~ ~ ~ 9 X~--" : % Rejection Direct Red 2
~ E ' ~ ~ (conc. = 18 ppm ) / s j c c ~ ~ ..x ~ ' ~ 0 0 i a: .~, ~ - ~ . . . . _ _ _ o ~ Filtration Rate,
_d-
~= o L / m 2 / hr x 0.1 % Conductivity Rejection, u. J I Range = 120- 300 pSiemens20
J /I
1
r
,
3
0 l ~ ' ~ ~ ~ ~ ~ ~ ~ 1 0 1 2 3 4 5 6 7Time of Filtration in Hours
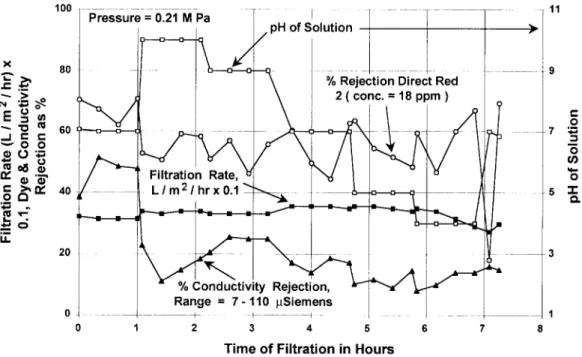
Fig. 6. Filtration rate for deionized water and Direct Red 2 using a polypropylene microfilter at a flow velocity o f 4.3 m/s at 45 °C.
J.J. Porter, A.C. Gomes / Desalination 128 (2000) 8I 90 87 . 11 100 Pressure = 0.21 M Pa [
~
pH of Solution I • X 80 9 Filtration Rate, ° ~ 0 "~ n, . ~ , ~ 0 c- ~,n,' 40 t ~ 5 -I-,o.~ = % Rejection Direct Red 2 o. _~ ¢~ i conc. = 18 ppm ) u-. % Conductivity Rejection, Range = 280- 380 laSiemens 0 1 0 1 2 3 5 6 T i m e o f F i l t r a t i o n in H o u r s
Fig. 7. Filtration rate for deionized water and Direct Red 2 using a polypropylene microfilter at a flow velocity of 4.3 m/s at 60°C.
oo
~= ' Pressure =~0.21 M Pat
11 x 80 9 .~ ._~ ~ - - - p H of Solution ~ ' O ~ 60 ~ 7 ".~ . . , I C e " "~ ~ 0 0 ~ Filtration Rate, "~ ~ o ~ L / m 2 hr x 0.1• ect,on Direct Red
E ' - o ppm ) 20 ' 3 I % Conductivity Rejection, j e t - R a n g e = 4300-4500 gSiemens 0 ~ '1 0 1 2 3 4 5 6 7 T i m e o f F i l t r a t i o n in H o u r s
Fig. 8. Filtration rate for deionized water and Direct Red 2 at high conductivity using a polypropylene microfilter at a flow velocity of 4.3 m/s at 45°C.
88 J.J. Porter, A.C. Gomes / Desalination 128 (2000) 81-90
membranes [2,3], also shown in Table 1. At the the solution from the surface of the membrane to three temperatures approximately 50% of the dye a distance of many angstroms and influence to was rejected, which is very significant for a passage of ions. In previous work with a ceramic microfilter having pores 0.2micron in diameter, membrane [2], it was possible to show that the The polypropylene membrane evidently has a relation between the percent conductivity slightly larger average pore diameter than the rejection vs. the conductivity of the filtering inorganic membranes. This would account for the solution was very similar to the Debye length and larger filtration rates at the lower pressure. This conductivity that exist between the ions in would also account for the lower rejections aqueous solutions. The correlation that was obtained with the organic membrane. The fact developed from the work with the ceramic that salt rejection did occur indicates that similar membrane is shown in Eq. (2):
electrostatic forces were effecting the filtration of
the polypropylene membrane that was found with % conductivity rejection = 980
the two inorganic microfiiters. (2)
It would be interesting to investigate the ionic * (cond. of concentrate, ~tSiemens) -°5 dye rejection ofmicrofilters having nominal pore
diameters of 0.1 microns and smaller. It is logical Israelachvili [6] developed an equation using to expect microfilters with smaller pores than Grahame's equation [7] to determine charge those used for this study to reject dyes and salts effects that reach up to 300 A into very dilute better. Microfilters are now commercially avail- solutions. The equation is shown below as Eq. (3) able with a wide range o f rejection properties. In
many cases it may not be necessary to use a Debye length, namometers _- _1 _-3.04
nanofilter to reject soluble ionic dyes. A micro- ~: (3)
filter may be used and give filtration rates that
are much higher than those available with * (sodium nitrate, equiv./L)o.5 nanofiltration. The limitation of using micro-
filters for ionic dye removal is that less than where 1/~:isthe Debye length. This equation can 100% o f the dye is rejected. In those applications be applied to solutions of 1" 1 electrolytes where where >95% of the dye must be rejected, it will the surface potentials are below 25 mV. The be necessary to use a membrane with smaller surface potential was not measured, but sodium pores or nanofiltration. The limitation with using nitrate was a 1 : 1 electrolyte and accounted for at nanofiltration is that a much lower filtration rates least 98% o f the ions in solution for the data will be obtained requiring more membrane shown in Fig. 9. Since the conductivity o f a surface and higher filtration pressures. Additional sodium nitrate solution is essentially linear with the salt concentration, it is possible to substitute work using microfilters having nominal pore
the conductivity into Eq. (3) in place of the sizes smaller than 0.2 microns may provide a
beneficial solution to this problem, sodium nitrate concentration to give Eq. (4): Debye length, A = 1150 *
3.2. Correlation with the Debye length (4)
One plausible explanation for the rejection of (sodium nitrate conductivity,laSiemens) -°5 sodium and nitrate ions by a microfilter having a
nominal pore diameter of 2000 A is the presence The only difference in Eq. (2) and Eq. (4) is the o f an electrostatic field. The field can reach into value of the constants 980 and 1150. As sodium
~ Porter, A.C. Gomes / Desalination 128 (2000) 81 90 89 1 0 0 ~ : ~ - s ~ :: : : • _ _ ~ : - 1 0 0 Data taken at o • • pH -- 4 - 10 • '/a C o n d . Rej. C e r a m t c M e m . , 0 . 6 6 M Pa ~. / I o % C o n d . Rej. P o l y p r o p y l e n e M e m . , 0.21 M Pa / ~ / % R e 80 j. C e r a m i c M e m . = 9 8 0 x ( C o n d . ) -05 80 ~ ~ - 0 . 3 2 E ~== ! ~ .... % Rej. P o l y p r o p y l e n e M e m . = 35 x ( C o n d . ) .~° O - 0.5 ¢n ~ so A\~v - - D e b y e L e n g t h , A = 1150. x ( C o n d . ) _ 60 .~"
"
i_
i112 I- 2 "
4 0 0 4 0 e - • • ~ O • • • r ~ - - a k . • 2 0 • - - - " - - - - " ~ r ~ .. . . -- . . . . 2 0°°
-
1
'
o - - ' ~ - - , _ , , . . . . - ; - ~ : - - ; - - " - - - - _ . . . . ~ - - o 0 1 0 0 0 2 0 0 0 3 0 0 0 4 0 0 0 5 0 0 0 C o n c e n t r a t e C o n d u c t i v i t y , H m h o s / c mFig. 9. Correlation of conductivity rejection with conductivity for polypropylene and ceramic microfilters at a flow velocity of 4.3 m/s at 45°C.
nitrate was used in our experiments [2] and When the salt or electrolyte concentration is Israelachvili's [7], the similar correlation of the raised to give conductivity's o f greater than data with the conductivity of the solution 40001aSiemens, the rejection approaches zero but indicates that the same type of electrostatic forces is still measurable. This was also found with the are controlling both processes, stainless steel and ceramic microfilter which both While the polypropylene membrane gave a showed higher rejections at high conductivity much weaker field effect, as the curve in Fig. 9 concentrations. The degree of fouling in all cases shows, the electrostatic forces appearto influence no doubt influences the results obtained. the rejection in a similar manner. Another However, in all cases microfiltered and deionized explanation for the smaller rejection of ions may water was used and the system cleaned frequently be the presence of larger pores in the polypro- to eliminate and minimize this problem. The use pylene membrane than the ceramic membrane, as of stainless steel for the fabrication of the labora- proposed earlier to account for the higher tory system should minimize fouling. Rejections filtration rates obtained with the organic that can be expected in commercial applications membrane. This would explain the lack of that use normal tap water should be higher than correlation with Israelachvili's [7] equation, those reported here, and for this reason it is Considering that no rejection was expected with important to collect data carefully before assum- the organic polypropylene membrane, the results ing that ionic dyes and salts are not rejected in
90 ~ Porter, A.C. Gomes / Desalination 128 (2000) 81-90
4. Conclusions inert characteristics of the polypropylene mem-
branes discussed in this paper are a decided Many times it is assumed that low molecular
advantage in most industrial applications. weight ions or even larger molecules will pass
freely through microfilters similar to the ones used in the experiments reported in this paper.
The data presented show that caution should be Acknowledgments
taken with the use o f any microfilter. Careful The authors are indebted to Walter Pupa of analysis of the filtration data must be made MicrodyneTechnologies, Inc. (Raleigh, NC, US) before it is assumed that ions or molecules in the for supplying the polypropylene module (Akzo stream will freely pass through the microfilter. Nobel Faser A.G.) used in this work and for Trace contaminants would be sufficient to providing helpful discussions during the course influence the passage of ions o f low molecular of the study. Funding for this work was received weight. Good quality tap water and deionized from the School of Textiles, Fiber and Polymer water can produce similar results causing the Science, Clemson University, and the Fundagfio microfilter to reject electrolytes after a few hours para a Ci4ncia e Tecnologia Praxis XXI, ref. of operation. The time necessary to cause the 3/3.2/Papel/2328/95, University of Da Beira f l t e r to reject ions will depend on the Interior, Covilha, Portugal.
construction materials used for the filtration system and the intrusion of trace particulate from
any source. The filtering characteristics of References relatively inert membranes, such as the
polypropylene membrane used in this study, no [1] The Membrane Business-- 10 Years Back,10 Years doubt can change as they are used and repeatedly Forward, Membrane Technology Separations Plan-
cleaned over time. ning Conference, 1997, Newton, MA, USA.
The important advantage of using micro- [2] J.J. Porter and S. Zhuang, J. Membr. Sci.,l10(1996)
119. filtration is that the membranes should have a
much higher filtration rate per unit area and [3] J.J. Porter and R.S. Porter, J. Membr. Sci., 101 (1995)
67.
require much less membrane surface. Because of [4] H. Winston, W.S. Sirkar and K.K. Sirkar, Membrane this the membrane cost may be much less in Handbook, VanNostrandReinhold, NewYork, 1992, many applications. More work is needed with p. 455.
microfiltershavinglowernominalporediameters [5] M.H. Hall and W.S. Perkins, Textile Res. J., 41 than the microfilters used in this study, i.e., <0.2 (1971) 923.
microns. It should be possible to take advantage [6] D.C. Grahame, J. Chem. Physics, 21 (1953) 1054. o f the electrostatic properties of the membranes [7] J.N. Israelachvili, Intermolecular and Surface Forces,