w ww . e l s e v i e r . c o m / l o c a t e / b j p
Original
Article
In
vitro
antitubercular
activity
of
extract
and
constituents
from
the
stem
bark
of
Disthemonanthus
benthamianus
Jean
Noel
Evina
a,
Dominique
Serge
Ngono
Bikobo
a,e,∗,
Auguste
Abouem
A.
Zintchem
a,b,
Norbert
Mbabi
Nyemeck
II
a,c,
Esther
Del
Florence
Moni
Ndedi
d,
Patrick
Hervé
Betote
Diboué
d,
Maximilienne
Ascension
Nyegue
d,
Alex
de
Théodore
Atchadé
a,
Dieudonné
Emmanuel
Pegnyemb
a,
Ulrich
Koert
c,
Christian
G.
Bochet
eaDepartmentofOrganicChemistry,FacultyofScience,UniversityofYaoundéI,Yaoundé,Cameroon
bDepartmentofChemistry,HigherTrainingCollege,UniversityofYaoundéI,Yaoundé,Cameroon
cFacultyofChemistry,Philipps-UniversitätMarburg,Marburg,Germany
dDepartmentofMicrobiology,FacultyofScience,UniversityofYaoundéI,Yaoundé,Cameroon
eDepartmentChemie,UniversitätFribourg,Fribourg,Switzerland
a
r
t
i
c
l
e
i
n
f
o
Articlehistory:
Received10May2017 Accepted12September2017 Availableonline4November2017
Keywords:
Fabaceae
Antitubercularactivity Distemonanthoside Flavonoids Phenolicacids
a
b
s
t
r
a
c
t
A new C-glycosylflavone, apigenin7-methylether 6-C-[-xylopyranosyl-(1→3)--glucopyranoside]
named distemonanthosidewas isolatedfromthestem barkofDistemonanthusbenthamianus Baill.,
Fabaceae,alongwithsixknowncompounds,sitosterol3-O--d-glucopyranoside,4-methoxygallicacid,
syringicacid,quercetin,6′′-O-acetylvitexin,quercetin3-O--d-glucopyranoside.Thestructuresofthose
compoundsandothersweredeterminedthroughspectralanalyses.Compoundsdistemonanthoside,
sitosterol3-O--d-glucopyranoside,4-methoxygallicacidandquercetinweretestedagainstaclinical
iso-latestrainofMycobacteriumtuberculosisAC45;theyexhibitedgoodtomoderateantitubercularactivities
withMICvaluesrangedfrom31.25to125g/ml.
©2017SociedadeBrasileiradeFarmacognosia.PublishedbyElsevierEditoraLtda.Thisisanopen
accessarticleundertheCCBY-NC-NDlicense(http://creativecommons.org/licenses/by-nc-nd/4.0/).
Introduction
Tuberculosis (TB) is a chronic contagious disease caused by severalspeciesofMycobacterium.Duetothefactthatthereisa doubtnowadaysontheefficiency ofcurrent antibiotics forthe treatmentoftuberculosis,micro-organismsdevelopedresistance inducinganincreaseofthenumberofpatientswiththedisease inworldwide(WHO,2016).ThisincreasingofMDR-TBincidence hasledtoanurgentneedforthediscoveryofnewplantnatural productsthatmaypotentiallyeradicateTB.Severalinvitrogrowth inhibitionofdifferentstrainsofM.tuberculosisbyplantextracts havebeenreported(Okunadeetal.,2004;CoppandNorriePearce, 2007;Gautametal.,2007;McGawetal.,2008).The Cameroo-nianmedicinalplantDistemonanthusbenthamianusBaill.,Fabaceae, isa largerainforesttreewidely distributedin Africa, especially inequatorialregion.Thisessenceishighlyappreciated industri-allyforheavyconstructionandsomecountriesusetoexportitas
∗ Correspondingauthor.
E-mail:ngonos@uy1.uninet.com(D.S.Bikobo).
“Movingi”.InMayombéregion(Congo),traditionalhealersemploy thestembarkinthetreatmentofseveraldiseasesas:parasitic, der-matitis,furuncles,acesandchancres.IntheChailluregion(Congo), thatplantisusedtocurebronchitisaffectionsandchildrenfever (Bouquet,1969).In previousworkscarried onD. benthamianus, mainlymethoxylatedflavonolsandflavoneswereisolated(King etal.,1952;Kingetal.,1954;MalanandRoux,1979;Happiand Mpondo,1994);this paperdescribestheisolation andstructure elucidationofconstituentsfromstembarkofD.benthamianus.The evaluationofantitubercularactivitiesofcompounds distemonan-thoside(1),sitosterol3-O--d-glucopyranoside,4-methoxygallic acid(2)andquercetinagainstresistantstrainofM.tuberculosiswas alsoexamined.
Materialandmethods
Generalprocedures
Meltingpointswereuncorrectedandweremeasuredona Met-tlerToledoinstrument.IRspectrawererecordedonanAlphaFT-IR SpectrometerfromBruker,while1D and2DNMR spectrawere
https://doi.org/10.1016/j.bjp.2017.09.006
obtainedonaBrukerDRX500(500MHzfor1Hand125MHzfor 13C spectra)spectrometer(Bruker,Rheinstetten,Germany)with
chemicalshiftsreportedinı(ppm)usingTMS(ıH)asan inter-nalstandard.TheHR-ESI-MSwasobtainedonLTQ-FTinstrument (ThermoScientific).UPLC–MSwasmeasuredbyaShimadzu UPLC-MSsystem.OpticalrotationsweremeasuredonaPerkin-Elmer 341polarimeter.Silica gel 60 (230–400 meshE. Merck, Darm-stadt,Germany)wasemployedforcolumnchromatography,the solventmixingsystemsforelutionweremainlyCH2Cl2/MeOHwith increasingpolarityeach.
Plantmaterial
Stem bark of Distemonanthus benthamianus Baill., Fabaceae, werecollectedatEséka(Koumoul)nearYaoundé(3◦38′60.00′′N,
10◦46′0.00′′E)intheCentreRegionofCamerooninMarch2014
andidentifiedbyVictorNana.Avoucherspecimen(No.45488HCN) wasdepositedattheNationalHerbariuminYaoundé,Cameroon.
Extractionandisolation
DriedandpowderedstembarkofD.benthamianus(254g)were extractedfor48hwithMeOH(3×1l)atroomtemperature.After
filtrationandevaporationofsolvent,thecrudeMeOHextract(16g) wassubjectedtoCC(150×3cm)[(SiO2),elutingwithagradient solventsystem(CH2Cl2/MeOH)]givingfourmainfractions:I(1.9g), II(3.8g),III(3.6g)andIV(6.7g).Fractions(100ml)werecollected andgroupedonthebasisofTLCanalysis.FractionII(3.8g)was sub-mittedtoCC(SiO2,100×1cm)usingsolventsystemCH2Cl2/MeOH
(50/1)togivesitosterol3-O--d-glucopyranoside(65mg).Fraction III(3.6g)wassubmittedtoCC(SiO2,100×1cm)usingsolvent
sys-temCH2Cl2/MeOH(60/1to5/1)togivefoursub-fractions(IIIa,IIIb, IIIcand IIId).Sub-fractionIIIc(1g) waschromatographed(SiO2, 50×1cm)usingCH2Cl2/MeOH(40/1–15/1)toafford compound
2(480mg)andcompound3(3mg).Sub-fractionIIId(0.65g)was subjectedtoasilicagelcolumningradientelutionmixturesolvent composedofCH2Cl2/MeOH(25/1–5/1)toaffordquercetin(8mg) andquercetin3-O--d-glucopyranoside(11mg).Usingthesame process,fractionIV(6.7g)gavethreesub-fractions(IVa,IVbandVc). Sub-fractionIVa(0.98g)wasfurtherchromatographedonasilica gelcolumn(100×1cm)usingCH2Cl2/MeOH(10/1)toafford com-pound4(4mg).Sub-fractionIVb(2.8g)waspurifiedbyrepeated CConsilicagel(100×1cm)withthesolventsystemCH2Cl2/MeOH (10/1–1/1)toprovidecompound1(28mg).
Structuralcharacterizationofdistemonanthoside(1)
Yellowsolid;[˛]25D =−54◦ (c0.05, MeOH);m.p.285–287◦C;
IRmaxKBrcm−1:3267,2923,2853,1595,1512,1226,1159;TLC,R f: 0.28(CH2Cl2/MeOH:90/10);ESI-MSm/z:ESI-MS:577.4[M−H]−•,
LC–MS:m/z579[M+H]+andESI-MS:m/z601.5[M+Na]+(Calcdfor C27H30O14Na=601.5);1HNMR(500MHz,DMSO-d
6),ıH:8.09(2H,
d,J=8.8Hz,H-2′andH-6′),6.96(2H,d,J=8.8Hz,H-3′and5′),6.82
(1H,s,H-3),6.44(1H,s,H-8),4.81(1H,d,J=10.0Hz,H-1′′),4.01b
(1H,H-3′′),3.86(3H,s,–OCH3),3.82(1H,d,J=7.0Hz,H-1′′′),3.72
(1H,dd,J=11.3;2.4Hz,H-6′′),3.42b (1H,H-6′′),3.39b(1H,H-2′′),
3.36b(1H,H-4′′),3.21b(1H,H-5′′),2.83(1H,dd,J=11.5;4.0Hz,
H-5′′′),2.81b(1H,H-3′′′),2.78b(1H,H-2′′′),2.39b(1H,H-5′′′);13CNMR
(125MHz,DMSO-d6):ıC:182.1(C-4),164.1(C-2),163.4(C-7),161.4 (C-9),161.2(C-4′),155.5(C-5),128.9(C-2′andC-6′)121.3(C-1′),
115.8(C-3′andC-5′),105.3(C-1′′′),104.7(C-6),104.1(C-10),102.3
(C-3),94.8(C-8),81.7(C-5′′),80.4(C-3′′),78.1(C-2′′),76.4(C-3′′′),
75.6(C-2′′′),73.5(C-1′′),71.3(C-4′′),69.2(C-4′′′),65.3(C-5′′′),60.8
(C-6′′),56.5(–OCH3).
bSignalpatternsareunclearduetooverlap.
Antitubercularactivity
MICvaluesweredeterminedfortheextractagainstM. tuberculo-sisstrainAC45(clinicalisolateobtainedfromSangmelimadistrict’s HospitalinSouthRegionofCameroon)employingthemicroplate AlamarBlueassay,usingRifampicinasreference.The96wellsplate received100lofMiddlebrook7H9mediumsupplementedwith 10%OADC(oleicacid,albumin,dextrose,catalase)2%glyceroland 0.05%v/voftween80.Brothandserialdilutionofcompoundswere madedirectlyontheplatewithdrugconcentrationsof0.244to 250g/ml.Plateswerecoveredandsealedwithparafilmand incu-batedat37◦Cfor14days.Then,40lAlamarBluesolutionwas
addedtotheplateandincubatedfor24h.Abluecolourinthewell wasinterpretedasnobacterialgrowthandpinkcolourwasscored asgrowth.TheMICwasdefinedasthelowestdrugconcentration, whichpreventedcolour changefrombluetopink.Theresultof antitubercularactivitydepictedinTable1.TheMICandMBCwere determinedaccordingtotheguidelinesofCLSI(2011).Each exper-imentwasperformedatleasttwiceaccordingtotheguidelinesof theClinicalandLaboratoryStandardsInstitute(CLSI,2011).
Acidhydrolysisof1
Compound 1 (8mg) wasdissolved in7% H2SO4 (0.5ml) and heatedonanaqueousbathat100◦Cfor4h.Thereactionmixture
wasdilutedwithH2OandextractedwithCH2Cl2.TheCH2Cl2layer wasevaporatedtodrynessandpurifiedbypreparativeTLCover silicagelwithCH2Cl2–MeOH(5/1)aseluent.Apigenin-7-methyl ether 6-C-glucoside (3mg) was isolated and identified through directcomparisonwithauthenticsamples(TLC,MP,andIR).The neutralizedand lyophilizedaqueuoushydrosylatesof the aque-oussolutiongaveonlyxylose.GC-MS(Column:5%phenyland95% methylsiliconeonultra2,0.2×46m,columntemp.:250◦C,
car-riergas:He0.8ml/min,sample:trimethylsilylderivatives:tR(min) xylose(19.29for1).
Table1
MICandMBCvaluesofthemethanolextractandtheisolatedcompoundsagainstclinicalisolatestrainofMycobacteriumtuberculosis(AC45).
Plantspecies/compounds MICa(g/ml) MICa(M) MBCb(g/ml) MBC/MIC
D.benthamianus 1250 ndc 2500 2
1 125 216.3 125 1
Sitosterol3-O--d-glucopyranoside 62.5 108.5 125 2
2 31.25 169.8 125 4
Quercetin 62.5 207.0 125 2
RMP 0.976 ndc 7.8125 8
RMP,Rifampicin.
Resultsanddiscussion
The detailed investigation of methanol extract of the stem barkofD.benthamianusledtotheisolationofsevencompounds. Six of them were identified as the known sitosterol 3-O--d -glucopyranoside (Ngono Bikobo et al., 2014), 4-methoxygallic acid(2)(Ouyangetal.,2007),syringicacid(3)(BayihaBaNjock et al., 2011), quercetin (Güvenalp and Demirezer, 2005), 6′′
-O-acetylvitexin (4) (Bayiha Ba Njock et al.,2011), quercetin 3-O--d-glucopyranoside(Muraietal.,2014).Thestructuresofthese compoundswereelucidatedbyNMRspectroscopyanalysis, includ-ing1Dand2Dtechniquesandalsobycomparingexperimentaldata withrespectiveliteraturedata
Compound 1 was obtained as yellow amorphous powder, [˛]25D =−54◦ (c=0.05,MeOH).Its molecularformula, C27H30O14 wasestablishedbynegative-ionHR-ESI-MS(Fig.S4).Thespectrum displayedthedeprotonatedmoleculepeak[M−H]−atm/z=577.4
inagreementwiththeaboveformula(calcd,577.44).TheIR spec-trum of 1 showed absorption bands characteristic of hydroxyl groups(3219cm−1),conjugatedcarbonylgroups(1652cm−1)and aromaticrings(1603and1572cm−1).UVspectralpropertiesof1
showedabsorptionmaximaatmax340nmand268nminMeOH, characteristic for a substituted flavone (Mabry et al., 1970). In addition,acidhydrolysisof1gaveapigenin7-methylether 6-C-glucosideand-xylosewhichwereidentifiedbyTLCanalysisand comparisonwithauthenticsamples(GC;tR19.29min).Inthe1H NMRspectrum(Table2)thesetofortho-coupledAA′BB′type
pro-tonsatıH8.04(2H,d,J=8.8Hz)and6.96(2H,d,J=8.8Hz),was respectivelyassignedtoH-2′/6′andH-3′/5′protonsoftheB-ringof
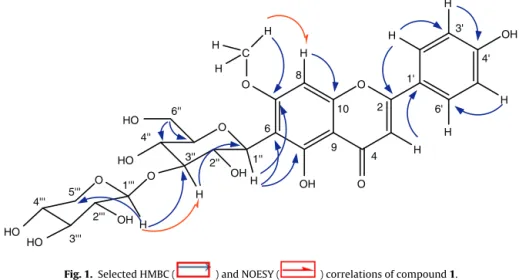
themolecule, whileanisolatedaromaticprotonappearedatıH 6.44(s, H-8)fromAring.Thespectrum alsorevealedthe pres-enceofamethoxylgroupatıH3.86and twosignalsassignable toanomericsugarprotons,whichwereidentifiedtobeaninner -glucopyranoseandaterminal-pyranosestructure ofxylose. Thiswasstrengthenedbytheobservationin13CNMRandDEPT spectraofelevencarbonsignals(Table2)amongwhichtwoare anomericcarbonsignalsatıC73.5and105.3,sevenmethinecarbon signals,twooxymethylenecarbonsatıC60.8and65.3.Sincethe anomericprotonsofglucoseandxyloseatıH4.81and3.82 exhib-itedlargecouplingconstants(J=10.0and7.0Hz),thesugarswere consideredofthe-pyranosetype.TheHMBCspectrumof com-pound1revealedcorrelationsoftheanomericprotonatıH4.81 (H-1′′)andcarbonsatıC161.3(C-7),155.6(C-5)104.7(C-6)and
81.7(C-5′′)(Fig.1),indicatingtheC-Cbondbetweentheinner
-glucopyranosylmoietyandtheaglyconeat6-position.Inaddition, H-1′′′atıH3.82correlatestobothC-3′′(ıC80.4)andC-5′′′(ıC65.3)
Table2
1Hand13CNMRspectroscopicdataofcompound1(500and125MHzinDMSO-d6) ıinppm.
Position ıC DEPT ıH(JinHz) HMBC(C→H)
Apignenin
2 164.1 C H-C(3);H-C(2′)
3 102.3 CH 6.82(s)
4 182.1 C H-C(3)
5 155.5 C H-C(1′′)
6 104.7 C H-C(1′′)
7 163.4 C H-C(1′′);CH3
O-8 94.8 CH 6.44(s)
9 161.4 C H-C(8)
10 104.1 C H-C(3)
1′ 121.3 C H-C(3);H-C(2′,
6′)
2′ 128.9 CH 8.04(d,8.8) H-C(3′,5′)
3′ 115.8 CH 6.96(d,8.8) H-C(2′,6′)
4′ 161.2 C H-C(2′);
H-C(5′)
5′ 115.8 CH 6.96(d,8.6) H-C(2′,6′)
6′ 128.9 CH 8.04(d,8.6) H-C(3′,5′)
7-OCH3 56.5 CH3 3.86(s)
Innerglucose
1′′ 73.5 CH 4.81(d,10) H-C(2′′);
H-C(5′′)
2′′ 78.1 CH 3.39a H-C(1′′)
3′′ 80.4 CH 4.01a H-C(1′′)
4′′ 71.3 CH 3.36a H-C(2′′);
H-C(5′′)
5′′ 81.7 CH 3.21a H-C(1′′);
H-C(6′′) 6′′ 60.8 CH2 3.72(dd,11.3;2.4)
3.42a
H-C(5′′)
Terminalxylose
1′′′ 105.3 CH 3.82(d,7.0) H-C(2′′′); H-C(5′′′)
2′′′ 75.6 CH 2.78a H-C(1′′′)
3′′′ 76.4 CH 2.81a H-C(1′′′);
H-C(5′′′)
4′′′ 69.2 CH 2.89a H-C(5′′′)
5′′′ 65.3 CH2 2.83(dd,11.5;4.0) 2.39a
H-C(1′′′); H-C(4′′′) aSignalpatternsareunclearduetooverlap.
revealingthatthe-xylopyranosylmoietywaslinkedtoC-3′′at ıC80.4,showingthatglucoseandxylosearelinkedthrougha1→3
type.ThiswasstrengthenedbytheNOESYcrosspeaksoftheprotons H-3′′(ıH4.01)withH-1′′′(ıH3.82)confirmingtheaforementioned
bonding.Theattachmentofamethoxylgrouptothe7-positionwas shownbytheobservationofthecrosspeaksatıH3.86(3H,s,OMe) andıC164.1(C-7)inthelong-rangeHMBCspectrum.Moreover theNOESY(Fig.1)experimentconfirmedthispositionthroughthe correlationbetweenH-8(ıH6.44)andthemethoxylproton sig-nalsatıH3.86.Thecompleteassignmentofallprotonandcarbon resonanceswasachievedaftercarefulanalysisofCOSY,HSQCand HMBCtechniques.
SomesignificantHMBCcorrelationsareshowninFig.1andin Table2.Compound1iscloselyrelatedtothepreviouslyreported swertisin 2′′-O-arabinoside from thetall beardediris (Takayuki
etal.,2012);meanwhile,differencesoccurinthesequenceofsugar moietiesandthisisexemplifiedbythevaluesoftheretentiontimes ofxylose[whichisclosetoreporteddata(Liuetal.,2009)].This assertionisalsostrengthenedbytheupperchemicalshiftvaluesof protonsofthexylosemoietiescomparedtothoseofarabinose(Gu etal.,2011).
Accordingly,1wasdefinedasapigenin7-methylether6-C-[ -xylopyranosyl-(1→3)--glucopyranoside]named
O O
OH O
OH
O
O
HO HO
O HO
HO
H H
H H
H H
H
C H
H H
2
4 6
8
10
9
1' 3'
4'
6'
1'' 3''
6''
5''' 1'''
3'''
H
H OH
OH 4'''
2'' 4''
2'''
Fig.1. SelectedHMBC( )andNOESY( )correlationsofcompound1.
Accordingto Cantrell et al. (2001) isolated compounds that exhibitaMICof64g/mlorlowerareconsideredpromising.For crudeextracts,theMICshouldbeequaltoorlowerthan125g/ml (Guetal.,2004).Thus,thevaluesof125,62.5,31.25and62.5g/ml for 1, sitosterol 3-O--d-glucopyranoside, 4-methoxygallicacid andquercetin,respectivelyobtainedhere,areasgoodasa promis-ingisolatedcompoundsexceptforcompound1(Table1).According toGuetal.themethanolextractofD.benthamianusshowedpoor inhibitoryactivityagainstM.tuberculosis,exhibitingaMICandMBC of1250and2500g/mlrespectively,suggestingthelow lipophilic-ity of its constituents (more polar compounds) when they act mutuallyinsynergy.AccordingtoPetersonandShanholtzer(1992) bacteriostaticactivityhasbeendefinedasaratioofMBCtoMICof >4.Thus,alltestedcompoundsexhibitedbactericidalactivity.The resultsofthepresentstudyareinaccordancewithpreviousreport regardingthevaluesofMICofisolatedcompounds(Guetal.,2004; Jiménez-Alleranesetal.,2007).
Conclusion
Thespecies D. benthamianus,is known asabundant sources offlavonoids.Compounds1,sitosterol3-O--d-glucopyranoside and 4-methoxygallicacid wereisolated for the first time from thisspecies.Thebioactivitystudyoftheisolatedcompounds indi-catedthatthreecompounds(sitosterol3-O--d-glucopyranoside, 4-methoxygallicacidandquercetin)exhibitedinteresting antitu-bercularactivity.
Authors’contributions
JNE(PhDstudent)contributedrunningthelaboratorywork,and draftedthepaper;EFMN,PHBDandMANcontributedto biologi-calstudies,runningthelaboratorywork,analysisofthedataand draftedthepaper;NMNcontributedtoanalysisofthedataand draftedthepaper;UK,ATA,DEPandCBcontributedtocritical read-ingofthemanuscript;AAZandDSNBcontributedincollectingplant samples,supervisedthelaboratorywork,didtheNMR investiga-tionsand revisedthepaper.Alltheauthorshaveread thefinal manuscriptandapprovedthesubmission.
Conflictsofinterest
Theauthorsdeclarenoconflictsofinterest.
Acknowledgements
Theauthorsgratefullyacknowledgefinancialsupportfromthe SwissNationalScience Foundation(SNSF)(No: IZK0Z2-157272) forresearchfellowshipsinSwitzerlandtoD.S.NgonoBikobo.We thank MrV. Nanafor the collectionand identification ofplant material.WethankMrFelixFehrofDepartmentofChemistryof UniversityofFribourgandKoert’steam,particularlyMrOliverBorn ofPhilipps-UniversitätMarburgforspectralanalysis.
AppendixA. Supplementarydata
Supplementarydataassociatedwiththisarticlecanbefound,in theonlineversion,atdoi:10.1016/j.bjp.2017.09.006.
References
BayihaBaNjock,G.,Bartholomeusz,T.A.,Foroozandeh,M.,Pegnyemb,D.E., Chris-tens,P.,Jeannerat,D.,2011.NASCA-HMBC,anewNMRmethodologyforthe resolutionofseverelyoverlappingsignals:applicationtothestudyof agathis-flavone.Phytochem.Anal.23,126–130.
Bouquet, A., 1969. Fetish and traditional medicine of Congo (Brazzaville). O.R.S.T.O.M.Paris36,177–178.
Cantrell, C.L.,Franzblau,S.G., Fischer,N.H.,2001. Antimycobacterialplant ter-penoids.PlantaMed.67,685–694.
CLSI,2011.SusceptibilityTestingofMycobacteria,Nocardiae,andOtherAerobic Acti-nomycetes,2nded.ClinicalandLaboratoryStandardsInstitute(CLSI),Wayne, PA,USA,ApprovedStandardM24-A2.
Copp,B.R.,NorriePearce,A.,2007.Naturalproductgrowthinhibitorsof
Mycobac-teriumtuberculosis.Nat.Prod.Rep.24,278–297.
Gautam,R.,saklani,A.,Jachak,S.M.,2007.Indianmedicinalplantsasasourceof antimycobacterialagents.J.Ethnopharmacol.110,200–234.
Gu,J.Q.,Wang,Y.,Franzblau,S.G.,Montenegro,G.,Yang,D.,Timmermann,B.N.,2004. AntitubercularconstituentsofValerianalaxiflora.PlantaMed.70,509–514. Gu,X.,Lee,S.G.,Bar-Peled,M.,2011.BiosynthesisofUDP-xyloseandUDP-arabinose
inSinorhizobiummeliloti1021:firstcharacterizationofabacterialUDP-xylose
synthase,andUDP-xylose4-epimerase.Microbiology157,260–269. Güvenalp,Z.,Demirezer,O.,2005.FlavonolglycosidesfromAsperulaarvensisL.Turk.
J.Chem.29,163–169.
Happi,E.N.,Mpondo,T.N.,1994.Twopolymethoxylatedflavonesfrom
Distemonan-thusbenthamianus.J.Nat.Prod.57,291–293.
Jiménez-Alleranes,A.,Meckes,M.,Torres,J.,Herrera-Luna,J.,2007. Antimycobacte-rialtriterpenoidsfromLantanahispida(verbenaceae).J.Ethnopharmacol.111, 202–205.
King,F.E.,King,T.J.,Sellars,K.,1952.Thechemistryofextractivesfromhardwoods. PartV.Theisolationof3,7,4′-trimethylquercetin(ayanin)fromtheheartwood
ofDistemonanthusbenthamianus.J.Chem.Soc.0,92–95.
King,F.E.,King,T.J.,Stokes,P.J.,1954.Thechemistryofextractivesfromhardwoods. PartsXIX.Thestructuresoffurthernewflavonesoccurringinayan(
Distemo-nanthusbenthamianus).J.Chem.Soc.0,4587–4594.
Liu,R.,Ma,S.,YU,S.,Pei,Y.,Zhang,S.,Chen,X.,Zhang,J.,2009.Cytotoxicoleanane triterpenesaponinsfromAlbiziachinensis.J.Nat.Prod.72,632–639.
Malan, E., Roux, D.G., 1979. Flavonoids from Distemonanthus benthamianus
Baillon.Methoxylatedflavonesandinter-relationshipsofbenthamianin,a [2]-benzopyrano-[4,3-b][I]-benzopyran.J.Chem.Soc.1,2696–2703.
McGaw,L.J.,Lall,N.,Meyer,J.J.M.,Eloff,J.N.,2008.ThepotentialofSouthAfrican plantsagainstMycobacteriuminfections.J.Ethnopharmacol.119,482–500. Murai,Y.,Kitajima,J.,Iwashina,T.,2014.FlavonoidsfromtwoalpineCampanula
speciesinJapan.Bull.Natl.Mus.Nat.Sci.40,113–118.
NgonoBikobo,D.S.,Mosset,P.,Abouem,A.,Zintchem,A.,Atchadé,A.T.,Balemaken Missi,M.,MbabiNyemeckII,N.,Pegnyemb,D.E.,2014.Campylospermine,anN -hydroxyalkaloidfromtheleavesofCampylospermumdensiflorum(Ochnaceae). Int.J.Pharm.Phytopharmacol.Res.6,719–728.
Okunade, L., Elvin-Lewis, M.P., Lewis, W.H., 2004. Natural antimycobacterial metabolites:currentstatus.Phytochemistry65,1017–1032.
Ouyang,M.A.,Wein,Y.S.,Su,R.K.,Kuo,Y.H.,2007.RhusemialinsA-C,newcyclolignan estersfromtherootsofRhusjavanicavar.roxburghiana.Chem.Pharm.Bull.55, 804–807.
Peterson,L.R.,Shanholtzer,C.J.,1992.Testsforbactericidaleffectsofantibacterial agents:technicalperformanceandclinicalrelevance.Clin.Microbiol.Rev.5, 420–432.
Takayuki,M.,Tsutomu,Y.,Nobuhiro,S.,Tsukasa,I.,2012.Phenoliccompounds, includingnovelC-glycosylflavone,fromtheflowersofthetallbeardedIris cul-tivar‘VictoriaFalls’.Nat.Prod.Commun.7,1591–1594.