w ww . e l s e v i e r . c o m / l o c a t e / b j p
Review
Folk
medicine,
phytochemistry
and
pharmacological
application
of
Piper
marginatum
Jennifer
Brú,
Juan
David
Guzman
∗DepartamentodeQuímicayBiología,DivisióndeCienciasBásicas,UniversidaddelNorte,Barranquilla,Colombia
a
r
t
i
c
l
e
i
n
f
o
Articlehistory:
Received16February2016 Accepted31March2016
Keywords: Pipermarginatum Piperaceae Folkmedicine Phytochemistry Pharmacology Biologicalapplications
a
b
s
t
r
a
c
t
PipermarginatumJacq.,Piperaceae,isawidelydistributedNeotropicalspeciesabundantintheCaribbean, exhibitingacharacteristicwingedpetioleandaheart-shapedleaf,itstwovegetativelandmarksforrapid identification.Thespecieshasbeenemployedbytraditionalindigenousculturesforitsreputedmedicinal properties.TheplantismostfrequentlyemployedbylocalhealersinCentralAmerica,theAntillesand SouthAmerica,foralleviatinggastrointestinalailments,administeredasadecoctionorinfusionforits tonic,diureticandcarminativeeffects.Thesebeneficialpropertiesmaybeattributedtothepresenceof variousphytochemicalswithinP.marginatum,withmostofthestudiesfocusingontheessentialoilof theplant.Monoterpenoids,sesquiterpenoidsandphenylpropanoidsofavariedchemicalstructurehave beenidentifiedintheessentialoil,whilephenylalkanoids,aristolactams,amidesandflavonoidshavebeen purifiedbychromatographictechniquesfromtheextracts.Thebiologicalandpharmacological exami-nationofP.marginatumshowedthattheplantmaybeavaluablesourceofmosquitocidal,antifungal, antitumoralandhemostaticagents.Futurebioguidedresearchmayyieldbiologicallyrelevantmolecules usefulinmedicineoragriculture.
©2016SociedadeBrasileiradeFarmacognosia.PublishedbyElsevierEditoraLtda.Thisisanopen accessarticleundertheCCBY-NC-NDlicense(http://creativecommons.org/licenses/by-nc-nd/4.0/).
Introduction
The species Piper marginatum Jacq., Piperaceae, was first describedin1781byDutchbotanistNikolausJosephvonJacquin. ThespecieshadbeencollectedduringexcursionstoCentraland SouthAmerica,andthemorphologicalcharacterswererecorded asheart-shaped,acuminated,multi-veinedandreticulatedleaves, withamarginal,groovedandwingedpetiole,andsolitary flow-ers(Jacquin,1786).TheSwissbotanistAnneCasimirPyramede Candollewasthefirsttoobservesmallmorphologicaldifferences betweendifferent collections of P.marginatum,and recognized threesubspecies:P.marginatumJacq,P.marginatumvar.anisatum
(Kunth)C.DC.andP.marginatumvar.catalpifolium(Kunth)C.DC. (Candolle,1902).Inthecomprehensivework“ThePiperaceaeof NorthernSouthAmerica”,WilliamTreleaseandTrumanG.Yuncker, differentiatedthesubspeciesbasedonthepuberulent(var. anisa-tum)orpillose(var.catalpifolium)natureofthenervesontheupper surfaceoftheleaves(TreleaseandYuncker,1950).Howeverthe difficultytocharacterize specimensatthesubgenuslevel given itsextensivehomoplasy(the developmentofsimilarcharacters byparallelor convergentevolution) wasrecognizedbyRicardo
∗ Correspondingauthor.
E-mail:jguzmand@uninorte.edu.co(J.D.Guzman).
Callejas(Callejas,1986),andthusthemodernclassifications con-siderthesubspeciesassynonymsofP.marginatumJacq.(Andrade etal.,2008).Inthiswork,thesensulatoofP.marginatumwas con-sidered, followingthe criteriaof theMissouriBotanical Garden (Tropicos.org,2015),whichcollatesunderP.marginatumseveral speciesandsubspecies,includingPipersan-joseanumC.DC.,Piper patulumBertol.,PiperuncatumTrel.,PiperquiriguanumTrel.,among others.
ThephylogeneticanalysisofthegenusPiperusingthesequence alignmentoftheinternaltranscribedspacer(ITS)ofthe18S-26S nuclearribosomalDNAandthechloroplastintronregionpsbJ-petA, indicatedthatthespeciesP.marginatumiscloselyrelatedtoP. mul-tiplinerviumandP.schwakei,andtogetherthesethreespeciesbuild theP.marginatumcomplex(Jaramilloetal.,2008).Moreoverthe Pothomorphegroupspecies(whichincludesP.auritum,P. pelta-tumandP.umbellatum)showedtobephylogeneticallyrelatedto theP.marginatumcomplex.
ThespeciesP.marginatumhasbeenwidelyrecognizedforits medicinalpurposeswithinanumberofindigenouscultureslocated intheCaribbeanandAmazonregions,fromCentralAmericaand theAntillestoBrazil(deNú ˜nezandJohnson,1943;Braga,1960; D’Angeloetal.,1997;DiStasiandHiruma-Lima,2002).Inaddition thedriedleaveshavebeenusedasanaturalsweetener(Hussain et al., 1990; Surana et al., 2006). Its major secondary metabo-litesare terpenoidsand phenylalkanoids(Andradeetal.,2008).
http://dx.doi.org/10.1016/j.bjp.2016.03.014
Inadditionanumberofbiologicalandpharmacologicalpublished data(Sequeda-Casta ˜nedaetal.,2015)seemstosupportitsusein traditionalmedicine.Thisreviewcoverstheethnomedicinal, phy-tochemicalandbiologicalliteraturepublishedonP.marginatum
withtheaimtoidentifytheresearchvoidsforfuture investiga-tionandcriticallyassessthepotentialapplicationofthespeciesin medicineandagriculture.
Folkmedicineandtraditionaluses
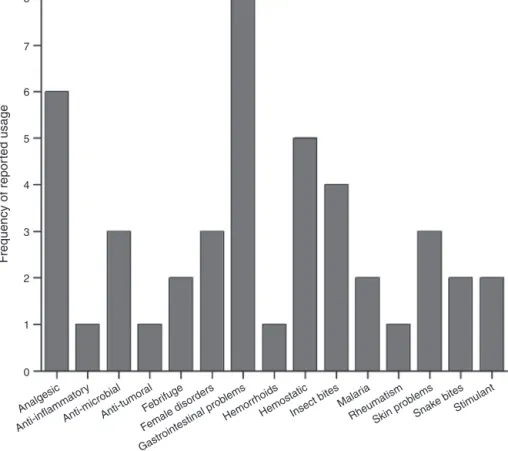
Theindigenous communitiesinCentralAmerica, theAntilles andSouthAmericarepeatedlyreportedtouseP.marginatumfor treatingavariedarrayofdiseasesandailments.Gastrointestinal problemsarethemostcommontherapeuticuseinthetraditional medicine,spanning differentlocationsand cultures (Fig.1,Box 1).The plant is recurrently employed either as a decoction or infusionforits tonic,diureticand carminative effects(Foungbe
etal.,1976;Johnson,1998;DiStasiandHiruma-Lima,2002;de
Albuquerque et al., 2007). It is also used to treat gallbladder, liver,stomach,spleen,urinaryandgastrointestinalailments(van denBerg,1982;Pereiraetal.,2011;YukesandBalick,2011),but alsodysentery(deNú ˜nezand Johnson,1943).In Central Amer-ica,thespeciesisknownas“Aniseto”and itis employedasan infusionfortreatingflatulencedisorders,inasimilarwaytostar anise.
PainreliefisthesecondmostfrequentuseofP.marginatumin thetraditionalmedicine(Fig.1,Box1).Theplantisusedtorelieve toothache,headachesandpaincausedbyitching,andasageneral analgesic(Hazlett,1986;GiraldoTafur,1996;DiStasiand Hiruma-Lima,2002;Pereiraetal.,2011).Theplantisemployedtopically asacataplasmtoalleviatethepainofthelimbsorabdomen,oras adecoctionorinfusionforteeth,headandstomachaches(García, 1974;GiraldoTafur,1996;YukesandBalick,2011).
ThespeciesP.marginatumisalsocommonlyusedasa hemo-static(Fig.1,Box1).Theplanthasbeenreportedtostopbleeding particularly in the case of ophidian accidents (de Nú ˜nez and Johnson,1943;Braga,1960;D’Angeloetal.,1997;Sánchezetal., 2011;Ortega-Galvan,2014).Reportsofthetopicalapplicationof theplanttoalleviateitchingandscratchingfrominsectbites includ-ingants(ElisabetskyandGely,1987)hasbeenrecurrentlyreported (Box1),anditremainstobedeterminedifthispropertyisduesolely totheanalgesiceffect.InadditionP.marginatumdisplays antimi-crobialpropertieswithrecordedapplicationsinBrazil(Corrêaand Pena,1984;D’Angeloetal.,1997),Colombia(DukeandVasquez, 1994),Cuba(Sánchezetal.,2011)andPuertoRico(deNú ˜nezand Johnson,1943).Thespeciesisalsoemployedtotreatfemale disor-ders,skinproblemsandinsectbites(vanAndeletal.,2008;Lans andGeorges,2011;Pereiraetal.,2011).InSuriname,Trinidadand PuertoRico,theplantiswidelyusedtotreatfemaledisorderssuch astocleanfemalesexualorgans,tohelpparturition,andtoreduce themenstruationflow,respectively(Morton,1977;vanAndeletal., 2008;LansandGeorges,2011).IntheFrenchGuiana,P.marginatum
isusedtotreatcutaneouseruptionsandskinrashes(Foungbeetal., 1976;Morton,1977;D’Angeloetal.,1997),andinBrazilitisused toalleviatehitchingcausedbyinsectbites(Pereiraetal.,2011).
Phytochemistry
The species P. marginatum shows a distinct phytochemistry withthepresenceofspecificsecondarymetabolites,notfoundin otherPiperspecies.Forinstance,P.marginatumistheonlyPiper
speciescontaininganethole,estragole, isoeugenolmethylether, thephenylalkanoids3-farnesyl-4-hydroxybenzoicand 3-farnesyl-4-methoxybenzoic acids and the glycosides marginatoside and vitexin(Parmaretal.,1997).NootherPiperspecieshaveshown thepresenceofthesechemotaxonomicmarkers.
8
7
6
5
4
3
2
F
requency of repor
ted usage
1
0
Analgesic
Anti-inflammator y
Anti-microbialAnti-tumor al Febr
ifuge
Female disorders
Gastrointestinal prob lems
HemorrhoidsHemostaticInsect bites Malar
ia
RheumatismSkin prob lems
Snak e bites
Stim ulant
Box1
TraditionalmedicineapplicationsofPipermarginatumbylocalcommunities.
Location Organoftheplant Medicinalproperties,or afflictionstreated
Modeofapplication References
Amazonregion Roots Carminative,diuretic,tonic, usefulagainsttoothacheand cobravenom
Baths DiStasiandHiruma-Lima(2002)
Roots Toalleviatepainanditchingof insectbites
Cataplasm
Fruitsandleaves Stimulant nd
Stem,leaves Againstitchingfromantbites Cataplasm ElisabetskyandGely(1987) nd Asantispasmodicandagainst
liverandgallbladderdiseases
Decoction vandenBerg(1982)
Brazil Leaves Rheumatism,bleedingskin wounds,toothacheandtumors
Decoction CorrêaandPena(1984),D’Angeloetal.(1997)
Leaves Toreduceswellings Rubbedwithfatas cataplasm,poultice
BranchandSilva(1983),DukeandVasquez(1994)
Roots Toalleviatehitchingcausedby insectbites,andalsoasateeth painreliever
Maceration Pereiraetal.(2011)
Leavesandfruits Asantispasmodic,totreat cough,andaffectionsofthe spleen,liverandintestinal problems
Usedtopically Pereiraetal.(2011)
Stem,leaves,roots Againsthighbloodpressure, asthma,erysipelas,problems withurinarysystemandasa diuretic
nd deAlbuquerqueetal.(2007)
nd Totreathemorrhoids Infusion RodriguesandAndrade(2014)
nd Hemostatic,snake-bite medicine
nd Braga(1960),D’Angeloetal.(1997)
Colombia Leaves,stem Forprotectingteethagainst cavities
Chewed DukeandVasquez(1994),García(1974)
Leaves Analgesic Infusionandcataplasm GiraldoTafur(1996) Roots Usedagainstmalariaandas
stimulant
Juice García(1974)
Entireplant Toreducefevers Decoction García(1974)
CostaRica Leaves Totreatheadaches Decoction Hazlett(1986)
Cuba nd Antiseptic,astringent,
antihemorrhagicand hemostatic
nd Sánchezetal.(2011)
DominicanRepublic Leaves Indigestionandflatulence disordersbutalsoagainst stomachpain
Infusion YukesandBalick(2011)
FrenchGuiana Leaves Usedincombinationwith Quassiaamaratotreatmalaria
Decoction Vigneronetal.(2005)
Leaves Totreatcutaneouseruptions andinsectbites
Decoction Foungbeetal.(1976)
Roots Diureticandsudorific Infusion Foungbeetal.(1976)
nd Usedtotreatskinrashes nd D’Angeloetal.(1997),Morton(1977)
Panama nd Carminative,diuretic,
emmenagogue,hemostatic
nd Johnson(1998)
nd Reducefeverandlung secretions
nd D’Angeloetal.(1997),Morton(1977)
PuertoRico Leaves Hemostatic Cataplasm deNú ˜nezandJohnson(1943)
Leaves Astreatmenttodysentery Infusion deNú ˜nezandJohnson(1943)
nd Reducesmenstruationflow nd Morton(1977)
Suriname Leaves Tocleansethevagina,cleanse theuterus,disguisebadsmell, enhancesexualpleasure amongstotherapplications
Steambath vanAndeletal.(2008)
TrinidadandTobago nd Tohelpparturition Infusionordecoctiondrank LansandGeorges(2011)
ndreferstonodata.
Accordingtotheessentialoil(EO)components,seven chemo-typeswererecognizedbyAndradeetal.intheircomprehensive studywith22samplesofP.marginatumcollectedthroughoutthe BrazilianAmazon(Andradeetal.,2008).Theexistenceofseven chemotypesmayinduce toconsiderthe assumptionof ancient characterofP.marginatum,allowingpotentialspeciationeventsto occurduringrecentevolution.Thishypothesismaybetestedinthe futurebymolecularphylogeneticanalysis.Thecompositionofthe EOoftheleaf,stemandinflorescencefromaP.marginatumspecies collectedinnearRecife,Brazil,showedthatthemajorcomponent
anEOrichingermacreneD(36.6%)(Jaramillo-Coloradoetal.,2015). Thesestudiessuggestthattherearemorethansevenchemotypes ontheEOofP.marginatumsensulato.Theseresultspointoutto adramaticvariationinchemicalcompositionforasetofrelated marginatum-phenotypespeciesbutinadditionitisnecessaryto considerthevariabilityassociatedtochronobiologicalphenomena (monthly,weeklyanddailyvariation)ashypothesizedbyMoraes etal.(2014).
MostofthephytochemicalstudiesonP.marginatumhavebeen carriedoutontheEOof theplant.Twentyfive monoterpen(e)-oids(1–25,Box 3), andfifty sevensesquiterpen(e)-oids(26–82,
The sole sesquiterpenoid which has been purified using chromatographic techniques is caryophyllene oxide (40) (de
Oliveira Chaves and de Oliveira Santos, 2002). Among the
thirty-nine phenylalkanoids (83–122, Box 2) reported for P.
marginatum, most of them were identified by CG-MS
anal-ysis of the EO of the plant, while some of them were isolatedtopuritybychromatographictechniques(apiol(87),(E )-asarone(89),croweacin(92), 2,6-dimethoxy-3,4-methylenedioxy-1-(2-propenyl)-benzene (95), 3-farnesyl-4-hydroxybenzoic acid
(99) and 3-farnesyl-4-methoxybenzoic acid (100), 2-hydroxy-3,4-methylenedioxypropiophenone (101), marginatine (106), 3,4-methylenedioxypropiophenone (108), 2-methoxy-4,5-methylenedioxypropiophenone (109), pipermargine (118) piperonal (119), 1-(1-(Z)-propenyl)-2,4,6-trimethoxybenzene (120), safrole (121), 2,4,5-trimethoxypropiophenone (122)) (de
Diaz and Gottlieb, 1979; Maxwell and Rampersad, 1988; de
ThealkaloidsoramidessofaridentifiedfromP.marginatumare thearistolactamscepharanoneB(123)andpiperolactamA(124), (E,E)-N-isobutyl-2,4-octadienamide(125)andcinnamoyl pirroli-dide (126) (de Oliveira Santos and de Oliveira Chaves, 1999a; deOliveiraChavesetal.,2003;deOliveiraChaveset al.,2006). Two flavanones 5,4′-dihydroxy-7-methoxyflavanone (127) and
5,7-dihydroxy-4′-methoxyflavanone(128),andtwoflavonoid
gly-cosidesmarginatoside(129)andvitexin(130)havebeenisolated fromtheleavesofP.marginatum(Tillequinetal.,1978;Reigada etal.,2007).Thecatecholaminenoradrenaline(131)was identi-fiedbyhighperformanceliquidchromatography(D’Angeloetal., 1997),andthefattyacidstearicacid(132)hasbeenpurifiedfrom theleaves(deDiazandGottlieb,1979).
Biologicalandpharmacologicalapplications
Thesweeteningeffectoftheplantrecordedinthetraditional applicationwasattributedtothepresenceof(E)-anethole(83),a sweetphenylpropanoidandmajorcompound incertain chemo-types of the EO of P. marginatum but also present in fennel (Foeniculum vulgare), staranise (Illicium verum), cicely(Myrrhis odorata) and anise root (Osmorhiza longistylis) (Hussain et al., 1990).(E)-Anetholehasbeenreportedtoprovideprotectionagainst chemically-inducedapoptosisandgenotoxicity(Abraham,2001; Galickaetal.,2014),andtobenon-carcinogenicinmice(Miller etal.,1983)posingnorisktohumanhealth(Newberneetal.,1999).
Most studies evaluating the biological and pharmacological properties of P. marginatum have focused on the essential oil (Box3).TheleafEOdemonstratedgrowthinhibitionofEscherichia coli bacteria with a minimum inhibitory concentration (MIC) valuebetween700and900g/mlagainsttwopathogenicstrains STEC0157 and EPEC0312 respectively (Duarte et al., 2007). A muchlowerMICvalueof120g/mlwasfoundagainstthe phy-topathogenicbacteriumXanthomonasalbilineans (Sánchezetal., 2012).TheleafEOwasalsoscreenedforfungalinhibitionagainst bothAlternariasolaniiandFusariumoxysporum,displaying moder-ateactivityindiskdiffusionassays(dosSantosetal.,2011;Duarte
etal.,2013).HoweveranotherstudyreportedthattheEOfromP. marginatumwasinactiveagainstF.oxysporumwithanMICvalue higherthan500g/ml,whileitwasfoundtobeslightlymoreactive againstTrichophytonrubrumandTrichophytonmentagrophyteswith respectiveMICvalues of500and 250g/ml (Tangarife-Casta ˜no etal.,2014).MoreoverboththeEOandtheethanolicextractofP. marginatumwerereportedtobeinactiveagainstCandidaalbicans
Box2
ReportedphytochemicalsidentifiedinPipermarginatum.
Class Compound Presentinorgan Reference
Monoterpeneand monoterpenoids
Allocymene(1) Leaf,stem Ramosetal.(1986)
Borneol(2) Leaf Andradeetal.(2008)
Camphene(3) Leaf Andradeetal.(2008)
Camphor(4) Leaf Andradeetal.(2008)
␦-3-Carene(5) Leaf,stem Andradeetal.(2008),Autranetal.(2009),Moraesetal. (2014),Ramosetal.(1986),Vogleretal.(2006) p-Cymene(6) Leaf,stem Andradeetal.(2008),Ramosetal.(1986),Vogleretal.
(2006)
8-p-Cymenol(7) Leaf,stem Vogleretal.(2006)
Isoborneol(8) Leaf Andradeetal.(2008)
Isopentylisovalerate(9) Leaf Autranetal.(2009),Moraesetal.(2014) Isosylvestrene(10) Leaf Andradeetal.(2008)
Limonene(11) Leaf,stem Andradeetal.(2008),Ramosetal.(1986),Vogleretal. (2006)
Linalool(12) Leaf,stem Andradeetal.(2008),Autranetal.(2009),Moraesetal. (2014),Ramosetal.(1986),Vogleretal.(2006)
p-Mentha-1-(7),8-diene(13) Leaf Andradeetal.(2008)
Myrcene(14) Leaf,stem Andradeetal.(2008),Ramosetal.(1986) (E)--ocimene(15) Leaf,stem Andradeetal.(2008),Ramosetal.(1986)
(Z)--ocimene(16) Leaf,stem Andradeetal.(2008),Autranetal.(2009),Moraesetal. (2014),Ramosetal.(1986)
␣-Phellandrene(17) Leaf,stem Andradeetal.(2008),Ramosetal.(1986) ␣-Pinene(18) Leaf,stem Andradeetal.(2008),Ramosetal.(1986)
-Pinene(19) Leaf,stem Andradeetal.(2008),Autranetal.(2009),Moraesetal. (2014),Ramosetal.(1986)
Sabinene(20) Leaf Andradeetal.(2008)
Sylvestrene(21) Leaf Autranetal.(2009),Moraesetal.(2014) ␣-Terpinene(22) Leaf,stem Andradeetal.(2008),Ramosetal.(1986) ␥-Terpinene(23) Leaf,stem Andradeetal.(2008),Ramosetal.(1986) ␣-Terpineol(24) Leaf Autranetal.(2009),Moraesetal.(2014) ␣-Terpinolene(25) Leaf,stem Andradeetal.(2008),Ramosetal.(1986)
Sesquiterpeneand sesquiterpenoids
␣-Acoradiene(26) Leaf,inflorescence Autranetal.(2009),Moraesetal.(2014) -Acoradiene(27) Leaf Autranetal.(2009),Moraesetal.(2014) Alloaromadendrene(28) Leaf Andradeetal.(2008)
Alloaromadendreneepoxide(29) Leaf Andradeetal.(2008)
Aromadendrene(30) Leaf Andradeetal.(2008)
Bicycloelemene(31) Leaf Sánchezetal.(2011)
Bicyclogermacrene(32) Leaf Andradeetal.(2008),Autranetal.(2009),Moraesetal. (2014),Sánchezetal.(2011)
-Bourbonene(33) Leaf,stem Andradeetal.(2008),Ramosetal.(1986)
␣-Cadinene(34) Leaf Moraesetal.(2014)
␦-Cadinene(35) Leaf,stem Andradeetal.(2008),Ramosetal.(1986)
␥-Cadinene(36) Leaf,stem,
inflorescence
Autranetal.(2009),Moraesetal.(2014)
␣-Cadinol(37) Leaf Andradeetal.(2008)
␦-Cadinol(38) Stem Ramosetal.(1986)
-Caryophyllene(39) Leaf,stem, inflorescence
Andradeetal.(2008),Autranetal.(2009),Moraesetal. (2014),Ramosetal.(1986)
Caryophylleneoxide(40) Leaf,stem Andradeetal.(2008),Autranetal.(2009),deOliveira ChavesanddeOliveiraSantos(2002)
␣-Copaene(41) Leaf,stem,
inflorescence
Andradeetal.(2008),Ramosetal.(1986),Autranetal. (2009),Moraesetal.(2014),Sánchezetal.(2011)
␣-Cubebene(42) Leaf,stem,
inflorescence
Andradeetal.(2008),Autranetal.(2009)
-Cubebene(43) Leaf Andradeetal.(2008)
Cyclosativene(44) Leaf Andradeetal.(2008)
-Dihydroagarofuran(45) Leaf Andradeetal.(2008) ar-Dihydroturmerone(46) Stem Autranetal.(2009)
-Elemene(47) Leaf,stem,
inflorescence
Andradeetal.(2008),Autranetal.(2009),Moraesetal. (2014),Ramosetal.(1986),Sánchezetal.(2011) ␦-Elemene(48) Leaf,stem Autranetal.(2009),Moraesetal.(2014),Ramosetal.
(1986)
␥-Elemene(49) Leaf, Ramosetal.(1986)
Elemol(50) Leaf,stem Andradeetal.(2008),Autranetal.(2009),Moraesetal. (2014),Ramosetal.(1986),Sánchezetal.(2011) ␣-Eudesmol(51) Leaf Andradeetal.(2008),Sánchezetal.(2011)
-Eudesmol(52) Leaf,stem Bernaletal.(2011),Ramosetal.(1986),Andradeetal. (2008),Sánchezetal.(2011)
10-epi-␥-Eudesmol(53) Leaf Andradeetal.(2008)
␥-Eudesmol(54) Leaf Andradeetal.(2008)
GermacreneA(55) Leaf Andradeetal.(2008)
GermacreneD(56) Leaf,inflorescence Andradeetal.(2008),Autranetal.(2009) GermacreneD-4ol(57) Leaf Andradeetal.(2008)
Globulol(58) Leaf Andradeetal.(2008)
Box2(Continued)
Class Compound Presentinorgan Reference
(E)--guaiene(60) Leaf Autranetal.(2009),Moraesetal.(2014) (Z)--guaiene(61) Leaf,inflorescence Autranetal.(2009),Moraesetal.(2014)
-Gurjunene(62) Leaf Andradeetal.(2008)
␥-Gurjunene(63) Inflorescence Autranetal.(2009)
␥-Himachalene(64) Leaf Autranetal.(2009),Moraesetal.(2014)
␣-Humulene(65) Leaf,stem,
inflorescence
Andradeetal.(2008),Autranetal.(2009),Moraesetal. (2014),Ramosetal.(1986)
HumuleneepoxideII(66) Leaf Andradeetal.(2008)
Isoledene(67) Leaf Autranetal.(2009),Moraesetal.(2014) (Z)-Isolongifolanone(68) Stem Autranetal.(2009)
Ledol(69) Leaf Autranetal.(2009),Moraesetal.(2014)
␣-Muurolene(70) Leaf Andradeetal.(2008)
␥-Muurolene(71) Leaf,stem Ramosetal.(1986)
epi-␣-Muurolol(72) Leaf Andradeetal.(2008)
(E)-Nerolidol(73) Leaf Andradeetal.(2008),Sánchezetal.(2011)
(Z)-Nerolidol(74) Stem Autranetal.(2009)
Patchoulol(75) Leaf,stem,
inflorescence
Autranetal.(2009),Moraesetal.(2014)
␣-Selinene(76) Leaf Andradeetal.(2008)
-Selinene(77) Leaf,stem Andradeetal.(2008),Autranetal.(2009)
7-epi-␣-Selinene(78) Leaf Andradeetal.(2008)
Selin-11-en-4␣-ol(79) Leaf Andradeetal.(2008)
Seychellene(80) Stem Autranetal.(2009)
Spathulenol(81) Leaf Andradeetal.(2008)
Valencene(82) Inflorescence Autranetal.(2009)
Phenylalkanoids (E)-anethole(83) Leaf,stem Andradeetal.(2008),Sánchezetal.(2011),Vogleretal. (2006)
(Z)-anethole(84) Leaf Andradeetal.(2008)
p-Anisaldehyde(85) Leaf,stem Vogleretal.(2006)
Anisylketone(86) Leaf,stem Vogleretal.(2006)
Apiol(87) Root Santosetal.(1998)
Asaricin(88) Leaf Andradeetal.(2008)
(E)-asarone(89) Leaf,stem,root, inflorescence
Andradeetal.(2008),Autranetal.(2009),Moraesetal. (2014),Sánchezetal.(2011),Sánchezetal.(2011),Santos etal.(1998)
(Z)-asarone(90) Leaf,stem,
inflorescence
Andradeetal.(2008),Autranetal.(2009),Moraesetal. (2014)
␥-Asarone(91) Leaf Andradeetal.(2008)
Croweacin(92) Root Andradeetal.(2008),deOliveiraSantosetal.(1997) Dillapiole(93) Leaf,stem Andradeetal.(2008),Ramosetal.(1986),Sánchezetal.
(2011)
3,4-Dimethoxycinnamicacid(94) Leaf Sánchezetal.(2011)
2,6-Dimethoxy-3,4-methylenedioxy-1-(2-propenyl)-benzene (95)
Root Santosetal.(1998)
Elemicin(96) Leaf,stem Autranetal.(2009),Moraesetal.(2014),Ramosetal. (1986),Sánchezetal.(2011)
Estragole(97) Leaf,stem Andradeetal.(2008),Ramosetal.(1986),Sánchezetal. (2011),Vogleretal.(2006)
Exalatacin(98) Leaf Andradeetal.(2008)
3-Farnesyl-4-hydroxybenzoicacid(99) Leaf,stem deOliveiraChavesanddeOliveiraSantos(2002),Maxwell andRampersad(1988)
3-Farnesyl-4-methoxybenzoicacid(100) Leaf,stem MaxwellandRampersad(1988)
2-Hydroxy-3,4-methylenedioxypropiophenone (101)
Leaf,stem Andradeetal.(2008),deDiazandGottlieb(1979),Ramos etal.(1986)
Isocroweacin(102) Leaf Sánchezetal.(2011)
Isolelemicin(103) Leaf,stem Andradeetal.(2008),Ramosetal.(1986) (E)-Isoosmorhizole(104) Leaf Andradeetal.(2008)
Isosafrole(105) Leaf Sánchezetal.(2011)
3,4-Methylenedioxy-1-(2E-octenyl)-benzene (marginatine,106)
Root Santosetal.(1998)
1-(3,4-Methylenedioxyphenyl)-propan-1-ol (marginatumol,107)
Leaf Reigadaetal.(2007)
3,4-Methylenedioxypropiophenone(108) Leaf,stem Andradeetal.(2008),deDiazandGottlieb(1979),Ramos etal.(1986),Reigadaetal.(2007)
2-Methoxy-4,5-methylenedioxypropiophenone (109)
Root,leaf Andradeetal.(2008),deDiazandGottlieb(1979),Oliveira SantosandOliveiraChaves(2000),Reigadaetal.(2007)
Methyleugenol(110) Leaf,stem Andradeetal.(2008),Ramosetal.(1986),Sánchezetal. (2011)
Methyl3-farnesyl-4-hydroxybenzoate(111) Leaf,stem MaxwellandRampersad(1988) Methyl3-farnesyl-4-methoxybenzoate(112) Leaf,stem MaxwellandRampersad(1988) (E)-methylisoeugenol(113) Leaf,stem,
inflorescence
Autranetal.(2009),Moraesetal.(2014)
Box2(Continued)
Class Compound Presentinorgan Reference
Myristicin(115) Leaf,stem Andradeetal.(2008),Ramosetal.(1986),Sánchezetal. (2011)
Norepinephrine(116) Leaf D’Angeloetal.(1997)
Nothosmyrnol(117) Leaf Sánchezetal.(2011)
1-(1E-propenyl)-2,4,6-trimethoxybenzene (pipermargine,118)
Root Santosetal.(1998)
Piperonal(119) Leaf,stem deDiazandGottlieb(1979) 1-(1-(Z)-propenyl)-2,4,6-trimethoxybenzene
(120)
Fruit deOliveiraChavesanddeOliveiraSantos(2002)
Safrole(121) Leaf,stem Andradeetal.(2008),deDiazandGottlieb(1979),Ramos etal.(1986),Sánchezetal.(2011)
2,4,5-Trimethoxypropiophenone(122) Root deOliveiraSantosanddeOliveiraChaves(1999b)
Alkaloidsandamides CepharanoneB(123) Wholeplant deOliveiraChavesetal.(2006) Cinnamoylpirrolidide(124) Stem deOliveiraChavesetal.(2003)
(E,E)-N-isobutyl-2,4-octadienamide(125) Root deOliveiraSantosanddeOliveiraChaves(1999a) PiperolactamA(126) Wholeplant deOliveiraChavesetal.(2006)
Flavonoids 5,4′-Dihydroxy-7-methoxyflavanone(127) Leaf Reigadaetal.(2007)
5,7-Dihydroxy-4′-methoxyflavanone(128) Leaf Reigadaetal.(2007)
Marginatoside(129) Leaf Tillequinetal.(1978)
Vitexin(130) Leaf Tillequinetal.(1978)
Others Noradrenaline(131) Leaf D’Angeloetal.(1997)
Stearicacid(132) Leaf,stem deDiazandGottlieb(1979)
fromstemandflowerbeingslightlymoreactivethantheEOfrom theleaf(Autranetal.,2009).AlowerLC50valueof8.3g/mlwas
reportedforaleafEOobtainedfromParaiba,Brazil(Costaetal., 2010)whereasaLC50valueof34g/mlwasfoundforaleafEO
fromaplantcollectedintheRondoniastateinBrazil(Santanaetal., 2015).MoreoverinthepresenceoftheEOat100g/ml,thegravid
A.aegyptifemalesreducedthenumberofeggslaidbyone-third comparedtothenegativecontrol(Autranetal.,2009).Theeffectof theP.marginatumEOonbrineshrimp(Artemiafranciscana) letha-lityandVerocellscytotoxicitywasfoundtobecomparableinthe concentration-responsewitha LC50 valueof22.4g/ml against
theA.franciscananaupliiafter24hofexposition(Olivero-Verbel etal.,2009),andanIC50valueof30.3g/mlagainsttheVerocells
(Tangarife-Casta ˜noetal.,2014).InadditiontheEOalso demon-stratedantiparasiticandinsecticidalproperties,with90%ofthe populationof Schistosomamansonibeinginhibitedwith5mgof theEO(Frischkornetal.,1978),and90%ofthepopulationofthe fireantSolenopsissaevissimabeinginhibitedwithaconcentration of427–480g/ml (Soutoetal.,2012).TheEOofP.marginatum
showedsignificant antioxidantactivity witha DPPH IC50 value
between1.2and1.5g/mlwhilethecontrolascorbicacidshoweda DPPHIC50valueof1.0g/ml(Jaramillo-Coloradoetal.,2015).
Fur-thermoretheEOdemonstratedrepellentactivityoftheredflour beetleTriboliumcastaneumfromaconcentrationof0.01l/ml,and alsoaconsiderableanti-alimentaryeffectagainstthecottonmoth
Spodopteralittoralis,beingnon-phytotoxicagainstLatuccasativa, and thussuggesting apotential applicationasan naturalagent tocontrolbeetlesand mothsin agriculturalsettings( Jaramillo-Coloradoetal.,2015).
Both leaf and root from P. marginatum collected in Yutaje, Venezuelawereextractedwithethanolandtheethanolicextracts weretestedforcytotoxicityagainstacollectionofcancercelllines (Villasmil et al., 2006).The leaf extract wasactive against the humancoloncarcinomalineHT-29witha GI50 of55g/mlbut
inactiveagainsttheothercancerlines,whereastherootextract wasfoundactiveagainstthehumanpancreaticcarcinomaPANC-1 (GI5065g/ml),andmoderatelyactiveagainstcolonHT-29(GI50
298g/ml)andlungA549(GI50240g/ml)carcinomacelllines
(Villasmiletal.,2006).Amurineinvivoexperimentwasperformed andtheleafextractshowedamarkedantitumoraleffectdecreasing byhalfthesizeofthetumorscomparedtothenegativecontrol.
Interestinglytherootextractwasinactivesuggestingthat poten-tialantitumoralcompoundsarepresentintheleafbutabsentin theroot,andthusthesecompoundscouldbeeasilydifferentiated byHPLCanalysisoftheextracts.Themethanolicextractobtained
from P. marginatum leaf collected in Pernambuco, Brazil, was
examinedforantifungalactivityagainstthephytopathogenicfungi
Colletotrichumscovillei,whichcausesanthracnoseonbellpeppers (Araújo et al.,2014).The methanolic extract displayed a dose-dependentinhibitionofmycelialgrowth,achieving50%inhibition withaconcentrationof750g/ml.Althoughthemethanolicextract was fractioned by column chromatography and a significantly active fractionwasseparated, theactiveantifungal compounds remainstobeidentified.Moreoverthehydroalcoholicextractwas screenedforactivityagainstLeishmaniainfantumamastigotesand showedanIC50valueof25g/ml,whilethepositivecontrol
pen-tamidineshowedanIC50valueof2.43g/ml(Iwanagaetal.,2014).
Box3
BiologicalandpharmacologicalactivitiesofPipermarginatum.
Extractorcompound Biologicalactivity Assay Potency References
LeafEO Antibacterial Invitroagainsttwopathogenic strainsofEscherichiacoli
MIC(STEC0157)=700g/ml MIC(EPEC0312)=900g/ml
Duarteetal.(2007)
InvitroagainstXanthomonas albilineans
MIC=120g/ml Sánchezetal.(2012)
Antifungal InvitroagainstFusarium oxysporum
Inhibitiondiameter=22.5mm, Controldiameter=69.9mm
dosSantosetal.(2011)
InvitroagainstAlternaria solanii
Inhibition%=57with10lof EO
Duarteetal.(2013)
Larvicidal InvitroagainstAedes aegyptilarvae
LC50=8.3g/ml
Controltemefos LC50=0.3g/ml
Costaetal.(2010)
LC50=34g/ml
LC90=85g/ml
Santanaetal.(2015)
EO Antifungal InvitroagainstCandidaalbicans MIC>2.0mg/ml Duarteetal.(2005) Antifungalandcytotoxicity Invitroactivityagainst
Fusariumoxysporum, Trichophytonrubrumand Trichophytonmentagrophytes andcytotoxicityagainstVero cellline
F.oxysporumMIC>500g/ml T.rubrumMIC=500g/ml T.mentagrophytes MIC=250g/ml.
IC50=30.3g/mlagainstVero
cellline
Tangarife-Casta ˜noetal. (2014)
Antioxidant,repellent, anti-alimentaryandphytotoxic
InvitroDPPHantioxidant, Triboliumcastaneumrepellent, Spodopteralittoralis, antialimentary,andLatucca sativaandLoliumperenne phytotoxicityactivities
DPPHinhibitionwith IC50=1.2–1.5g/ml,whilethe
controlascorbicacidshowed IC50=1.0g/ml.
EOrepellentof>50%T. castaneumfrom0.01l/ml EOanti-alimentarytoS. littoraliswith64–80%effect with100g/cm2
EO(atunknownconcentration) inhibitedrootgrowthofL. perenneby54–62%,but increasedrootgrowthofL. sativaby119–170%
Jaramillo-Coloradoetal. (2015)
Brineshrimplethality InvitroagainstArtemia franciscanacysts
LC50=22.38g/ml(at24h)
LC50=12.64g/ml(at48h)
Olivero-Verbeletal.(2009)
Cercaricidal InvitroagainstSchistosoma mansoni
At10mg,96%ofcercariaedied At5mg,90%ofcercariaedied At1mg,24%ofcercariaedied
Frischkornetal.(1978)
Larvicidal InvitroagainstAedesaegypti larvae
LeafEOLC50=23.8g/ml
StemEOLC50=19.9g/ml
FlowerEOLC50=19.9g/ml
Autranetal.(2009)
Insecticidal Invitroagainsttheworkersof thefireantSolenopsis saevissima(Smith)
LC50=122–167g/ml
LC90=427–480g/ml
Soutoetal.(2012)
Oviposition InvitroongravidAedesaegypti femalesandcountingthe numberofeggslaid
%eggslaid(leaf)=33% %eggslaid(stem)=32% %eggslaid(flower)=35% At100g/mlwithrespectto thenegativecontrol
Autranetal.(2009)
Ethanolicextract Cytotoxicity Invitroagainstmurine melanomaB16/BL6,human coloncarcinomaHT-29,human lungcarcinomaA549,human cervicalcarcinomaHeLa,and humanpancreaticcarcinoma PANC-1
Leaf:
B16/BL6GI50>300g/ml
HT-29GI50=55g/ml
A549GI50>300g/ml
HeLaGI50>300g/ml
PANC-1GI50>300g/ml
Root:
B16/BL6GI50>300g/ml
HT-29GI50=298g/ml
A549GI50=240g/ml
HeLaGI50>300g/ml
PANC-1GI50=65g/ml
Villasmiletal.(2006)
Antitumoral Invivomurinetumorinduction assay
Theleafextract(andnotthe rootextract)ofP.marginatum showedantitumoralactivity
Villasmiletal.(2006)
Methanolicextract Antifungal InvitroagainstColletotrichum scovillei
Thepercentageofinhibitionof mycelialgrowth(PIC)was50% withaconcentrationof 750g/ml,andreached70%at 1.5mg/ml
Araújoetal.(2014)
Hydro-alcoholicextract Anti-leishmanial InvitroagainstLeishmania infantumamastigotes
IC50=25g/ml
Thepositivecontrol pentamidineshowed IC50=2.43g/ml
Box3(Continued)
Extractorcompound Biologicalactivity Assay Potency References
Waterextract Toxicity InvivoonWistaradultratand albinomice
LD100=1g/kg(intraperitoneal)
LD>2g/kg(orally)
D’Angeloetal.(1997)
Bloodpressure Invivoonrat Dose-dependenthypertension wasobservedwithintravenous andoraladministrations
D’Angeloetal.(1997)
Vasdeferenscontractility Invivoonrat Dose-dependentcontractions withEC=38.02g/ml
D’Angeloetal.(1997)
Atriacontractility Invivoonguineapig Dose-dependentcontractions withdosesbetween2.5and 10g/ml
D’Angeloetal.(1997)
Perfusedmesentericbed pressure
Invivoonrat Dose-dependentincreaseof theperfusionpressureof mesentericarteriawith EC=159.6g
D’Angeloetal.(1997)
Analgesia Invivoonmice Areductionoftwist
movementswasobservedin treatedanimalswith0.5and 1.0mg/kgoraldoses
D’Angeloetal.(1997)
Anti-inflammatoryofpaw edema
Invivoonrat Dose-dependentreductionof swellingwith0.5and 1.0mg/kgoraldoses
D’Angeloetal.(1997)
Pleuralleukocytecount Invivoonrat Noeffectonexudatevolume andleukocytecountwith0.5 and1.0mg/kgoraldoses
D’Angeloetal.(1997)
Purifiedflavonoids Antifungal Invitroautobiographyagainst Cladosporuscladosporioidesand Cladosporussphaerospernum
Amountrequiredtoinhibit bothC.cladosporioidesandC. sphaerospernum:
(107)–10.0g (108)–5.0g (109)–5.0g (127)–1.0g (128)–1.0g
Reigadaetal.(2007)
countsinthepleurisyinducedbycarrageenin,suggestingthatthe anti-inflammatory effect is related to a vasoconstriction action of thewater extract of P.marginatum,and not a specific anti-inflammatoryaction(D’Angeloetal.,1997).
Among all the identified secondary metabolites present
in P. marginatum, only five have been tested for a
biological activity and these are three arylpropanoids 1-(3,4-methylenedioxyphenyl)-propan-1-ol (marginatumol, 107), 3,4-methylenedioxypropiophenone (108), 2-methoxy-4,5-methylenedioxypropiophenone (109), and the two flavanones 5,4′-dihydroxy-7-methoxyflavanone(127)and5,7-dihydroxy-4′
-methoxyflavanone(128).Abioautographicmethodwasemployed toevaluatetheirantifungal effect againstCladosporus
cladospo-rioides and Cladosporus sphaerospernum and the most active
compounds were the two flavanones (127) and (128), which inhibitedbothmicroorganismswithanamountaslittleas1g (Reigadaetal.,2007).Thetwo propiophenones(108)and(109) inhibitedthegrowthofthefungiwith5g,whereasthealcohol marginatumol,(107)required10gtoinhibitthefungi.
Conclusion
The species P. marginatum, widely used in the traditional medicine of the Caribbean region, primarily to treat gastroin-testinalproblemsbutalsoemployedasanalgesic,hemostaticand to cure insectbites, contains a variable mixture of terpenoids, phenylalkanoids, amides and flavonoids. Some of these com-pounds, includinganethole, estragole, isoeugenolmethyl ether, 3-farnesyl-4-hydroxybenzoic and 3-farnesyl-4-methoxybenzoic acids,marginatoside and vitexin,arenot presentin otherPiper
species,andarethuschemotaxonomicmarkersforP.marginatum. ThehighvariabilityofchemotypesobservedwithinP. margina-tumsensulatomayreflectuponspeciationevents,orotherfactors whichremainstobefullyinvestigated.Thedistinctivenessofthe
chemicalcompositiontranslateintoarangeofbiologicaland phar-macologicalapplications,whichaccordingtothereportedpotency, thelarvicidaleffectagainstA.aegypti,theantifungalactivityagainst phytopathogenicfungiandthehemostaticandantitumoral poten-tial,areworthhighlightingforfutureresearch.
Authors’contributions
JBandJDGsearchedtheliterature.JBcollecteddataintables. JDGorganizedtheinformation,preparedthefiguresandwrotethe paper.
Conflictsofinterest
Theauthorsdeclarenoconflictsofinterest.
Acknowledgments
The authors acknowledge financial support from the Uni-versidad delNorte (Agenda Interna2014-0014) for the project “Búsquedadenuevosagentesanti-tuberculosiseninflorescencias dePiperdelCaribeColombiano”.TheauthorsaregratefultoDr. MariaCristinaMartinez-Habibe(Uninorte)forusefuldiscussions.
References
Abraham,S.K.,2001.Anti-genotoxicityoftrans-anetholeandeugenolinmice.Food Chem.Toxicol.39,493–498.
Andrade,E.H.A.,Carreira,L.M.M.,daSilva,M.H.L.,daSilva,J.D.,Bastos,C.N.,Sousa, P.J.C.,Guimarães,E.F.,Maia,J.G.S.,2008.Variabilityinessential-oilcomposition ofPipermarginatumsensulato.Chem.Biodivers.5,197–208.
Araújo,E.R.,Harand,W.,Lima,I.C.,Dias,F.C.R.,Santana,A.A.D.d.,Carvalho,R.R.d.C., Laranjeira,D.,2014.ExtractsofPipermarginatumandAzadirachtaindicaforthe controlofColletotrichumscovilleiinbellpepper.Pesq.Agrop.Bras.49,88–94. Autran,E.S.,Neves,I.A.,daSilva,C.S.B.,Santos,G.K.N.,CâmaraC.A.G.d.,Navarro,
activitiesagainstAedesaegyptiofessentialoilsfromPipermarginatumJacq. (Piperaceae).Bioresour.Technol.100,2284–2288.
Bernal,H.Y.,Martínez,H.G.,Sánchez,G.F.Q.,2011.Pautasparaelconocimiento, conservaciónyusosostenibledelasplantasmedicinalesnativasenColombia: Estrategianacionalparalaconservacióndeplantas,Bogotá,Colombia.,pp.167. Braga,R.,1960.PlantasdoNordeste,especialmentedoCeará.ImprensaOficial,
For-taleza,Brasil,540pp.
Branch,L.C.,Silva,M.d.,1983.FolkmedicineofAlterdoChão,Pará,Brazil.Acta Amazon.13,737–797.
Callejas,R.,(Ph.D.thesis)1986.TaxonomicrevisionofPipersubgenusOttonia (Piper-aceae).CityUniversityofNewYork,NewYork,150pp.
Candolle,C.D.,1902.Piperaceae.vol3.Lipsiae,Borntraeger.GenevaBotanical Gar-den,124pp.
Corrêa,M.P.,Pena,L.d.A.,1984.DicionáriodasplantasúteisdoBrasiledasexóticas cultivadas.MinistériodaAgricultura,InstitutoBrasileirodeDesenvolvimento Florestal,RiodeJaneiro,747pp.
Costa,J.,Santos,P.F.d.,Brito,S.A.,Rodrigues,F.F.,Coutinho,H.D.,Botelho,M.A.,Lima, S.G.d.,2010.Composic¸ãoquímicaetoxicidadedeóleosessenciaisdeespécies dePiperfrentealarvasdeAedesaegyptiL.(Diptera:Culicidae).Lat.Am.J.Pharm. 29,463–467.
D’Angelo,L.C.A.,Xavier,H.S.,Torres,L.M.B.,Lapa,A.J.,Souccar,C.,1997. Pharma-cologyofPipermarginatumJacq.afolkmedicinalplantusedasananalgesic, antiinflammatoryandhemostatic.Phytomedicine4,33–40.
deAlbuquerque,U.P.,Monteiro,J.M.,Ramos,M.A.,deAmorim,E.L.C.,2007.Medicinal andmagicplantsfromapublicmarketinnortheasternBrazil.J.Ethnopharmacol. 110,76–91.
deDiaz,A.M.,Gottlieb,O.R.,1979.PropiophenonesfromPipermarginatum.Planta Med.35,190–191.
deNú ˜nez,E.H.,Johnson,C.H.,1943.ApreliminarystudyofPipermarginatumJacq.J. Am.Pharm.Assoc.32,234–236.
deOliveiraChaves,M.,deOliveiraSantos,B.,deOliveira,A.,2003.1-Cinnamoyl pyrrolididefromPipermarginatum.Biochem.Syst.Ecol.31,1213–1214. deOliveiraChaves,M.C.,deOliveira,A.H.,deOliveiraSantos,B.V.,2006.
Aristolac-tamsfromPipermarginatumJacq.(Piperaceae).Biochem.Syst.Ecol.34,75–77. deOliveiraChaves,M.C.,deOliveiraSantos,B.V.,2002.ConstituentsfromPiper
marginatumfruits.Fitoterapia73,547–549.
deOliveiraSantos,B.R.V.,deOliveiraChaves,M.C.,1999a. (E,E)-N-Isobutyl-2,4-octadienamidefromPipermarginatum.Biochem.Syst.Ecol.27,113–114. deOliveiraSantos,B.V.,Da-Cunha,E.V.,deOliveiraChaves,M.C.,Gray,A.I.,1997.
CroweacinfromPipermarginatum.Biochem.Syst.Ecol.25,471–472. de Oliveira Santos, B.V., de Oliveira Chaves, M.C., 1999b.
2,4,5-Trimethoxypropiophenone from Piper marginatum. Biochem. Syst. Ecol. 27,539–541.
DiStasi,L.C.,Hiruma-Lima,C.A.,2002.PlantasmedicinaisnaAmazôniaenaMata Atlântica.EditorUNESP,SãoPaulo,pp.125–126.
dosSantos,M.R.A.,Lima,R.A.,deFreitasFernandes,C.,Silva,A.G.,Facundo,V.A., 2011.AtividadefungicidadoóleoessencialdePipermarginatumL.(Piperaceae) sobreFusariumoxysporum(Schlecht)invitro.SaudePesq.4.
Duarte,M.C.T.,Figueira,G.M.,Sartoratto,A.,Rehder,V.L.G.,Delarmelina,C.,2005. Anti-Candidaactivity ofBrazilian medicinal plants.J.Ethnopharmacol. 97, 305–311.
Duarte,M.C.T.,Leme,E.E.,Delarmelina,C.,Soares,A.A.,Figueira,G.M.,Sartoratto, A.,2007.ActivityofessentialoilsfromBrazilianmedicinalplantsonEscherichia coli.J.Ethnopharmacol.111,197–201.
Duarte,Y.,Pino,O.,Infante,D.,Sánchez,Y.,Travieso,M.d.C.,Martínez,B.,2013.Efecto invitrodeaceitesesencialessobreAlternariasolaniSorauer.Rev.Protec.Veg.28, 54–59.
Duke,J.A.,Vasquez,R.,1994.AmazonianEthnobotanicalDictionary.Taylor&Francis, CRCPress,BocaRaton,pp.138–139.
Elisabetsky,E.,Gely,A.,1987.PlantesmédicinalesutiliséesenAmazoniecommefond potentieldenouveauxagentsthérapeutiquesdanslescasd’allergie,thrombose etinflammation.J.Agric.Tradit.Bot.Appl.34,143–151.
Foungbe,S.,Tillequin,F.,Paris,M.,Jacquemin,H.,Paris,R.-R.,1976.Surunepipéracée deGuyane,lePipermarginatumJacq.Ann.Pharm.Fr.34,339–343.
Frischkorn,C.,Frischkorn,H.,Carrazzoni,E.,1978.Cercaricidalactivityofsome essentialoilsofplantsfromBrazil.Naturwissenschaften65,480–483. Galicka,A.,Kr ˛etowski,R.,Nazaruk,J.,Cechowska-Pasko,M.,2014.Anetholeprevents
hydrogenperoxide-inducedapoptosisandcollagenmetabolismalterationsin humanskinfibroblasts.Mol.Cell.Biochem.394,217–224.
García,H.,1974.FloramedicinaldeColombia:botánicamédica,Segundaedicióned. TercerMundoEditores.
GiraldoTafur,C.,1996.MedicinatradicionaldelasmujeresSionadelresguardode BuenavistaenelríoPutumayo.Caldasia18,227–238.
Hazlett,D.,1986.Ethnobotanicalobservationsfromcabecarandguaymísettlements inCentralAmerica.Econ.Bot.40,339–352.
Hussain,R.,Poveda,L.,Pezzuto,J.,Soejarto,D.,Kinghorn,A.,1990.Sweeteningagents ofplantorigin:phenylpropanoidconstituentsofsevensweet-tastingplants. Econ.Bot.44,174–182.
Iwanaga,C.C.,Bruschi,F.L.,Cerquetani,J.A.,Cortez,D.A.G.,2014.Antileishmnaial evaluationactivityofcrudehydroalcoholicextractofPipermarginatumJacq.VI SimpósioInternacionaldePós-GraduacaoePesquisa,SaoPaulo,Brasil,pp.9. Jacquin,N.J.,1786.Iconesplantarumrariorum.LugduniBatavorum,Argentorati
Wappler,Whiteetfilium,LuchtmansandKönig,London.,pp.2.
Jaramillo-Colorado,B.,Julio-Torres,J.,Duarte-Restrepo,E.,Gonzalez-Coloma,A., Julio-Torres,L.F.,2015.Estudiocomparativodelacomposiciónvolátilylas
actividadesbiológicasdelaceiteesencialdePipermarginatumJacq.Colombiano. Bol.Latinoam.Caribe14,343–354.
Jaramillo,M.A.,Callejas,R.,Davidson,C.,Smith,J.F.,Stevens,A.C.,Tepe,E.J.,2008. AphylogenyofthetropicalgenusPiperusingITSandthechloroplastintron psbJ-petA.Syst.Bot.33,647–660.
Johnson,T.,1998.CRCEthnobotanyDeskReference.Taylor&Francis,CRCPress, BocaRaton,pp.631.
Lans,C.,Georges,K.,2011.Women’sknowledgeofherbsusedinreproductionin TrinidadandTobago.In:Rai,M.,Acharya,D.,Rios,J.L.(Eds.),Ethnomedicinal Plants:RevitalizingofTraditionalKnowledgeofHerbs.CRCPress,pp.115–134. Maxwell,A.,Rampersad,D.,1988.Prenylated4-hydroxybenzoicacidderivatives
fromPipermarginatum.J.Nat.Prod.51,370–373.
Miller,E.C.,Swanson,A.B.,Phillips,D.H.,Fletcher,T.L.,Liem,A.,Miller,J.A.,1983. Structure-activitystudiesofthecarcinogenicitiesinthemouseandratofsome naturallyoccurringandsyntheticalkenylbenzenederivativesrelatedtosafrole andestragole.CancerRes.43,1124–1134.
Moraes,M.M.,daSilva,T.M.,Silva,R.R.d.,Ramos,C.S.,daCamara,C.A.,2014.Circadian variationofessentialoilfromPipermarginatumJacq.Bol.Latinoam.Caribe13, 270–277.
Morton,J.F.,1977.Somefolk-medicineplantsofcentralAmericanmarkets.Pharm. Biol.15,165–192.
Newberne,P.,Smith,R.L.,Doull,J.,Goodman,J.I.,Munro,I.C.,Portoghese,P.S., Wag-ner,B.M.,Weil,C.S.,Woods,L.A.,Adams,T.B.,Lucas,C.D.,Ford,R.A.,1999.The FEMAGRASAssessmentoftrans-anetholeusedasaflavouringsubstance.Food Chem.Toxicol.37,789–811.
OliveiraSantos, B.V.d.,Oliveira Chaves,M.C.d.,2000. Assignments of1H and 13Cresonantesignalsin2-methoxy-4,5-methylenedioxypropiophenonewith the assistance of 1D and 2D NMR experiments. Acta Farm. Bonaer. 19, 45–48.
Olivero-Verbel,J.,Gueette-Fernandez,J.,Stashenko,E.,2009.Acutetoxicityagainst ArtemiafranciscanaofessentialoilsisolatedfromplantsofthegenusLippiaand PipercollectedinColombia.Bol.Latinoam.Caribe8,419–427.
Ortega-Galvan,J.,2014.MifamiliausaPipermarginatumparacurarlamordedura deserpiente,Megua,Baranoa,Atlántico,Colombia(Personalcommunication). Parmar,V.S.,Jain,S.C.,Bisht,K.S.,Jain,R.,Taneja,P.,Jha,A.,Tyagi,O.D.,Prasad,
A.K.,Wengel,J.,Olsen,C.E.,Boll,P.M.,1997.PhytochemistryofthegenusPiper. Phytochemistry46,597–673.
Pereira,L.A.,Barboza,G.E.,Bovini,M.G.,DeAlmeida,M.Z.,Guimarães,E.F.,2011. Car-acterizaciónyusodepimientasenunacomunidadQuilomboladelaAmazonía Oriental(Brasil).J.Bot.Res.Inst.Texas5,255–272.
Ramos,L.S.,daSilva,M.L.,Luz,A.I.R.,Zoghbi,M.G.B.,Maia,J.G.S.,1986.Essentialoil ofPipermarginatum.J.Nat.Prod.49,712–713.
Reigada,J.B.,Tcacenco,C.M.,Andrade,L.H.,Kato,M.J.,Porto,A.L.M.,Lago,J.H.G., 2007.ChemicalconstituentsfromPipermarginatumJacq.(Piperaceae)– antifun-galactivitiesandkineticresolutionof(RS)-marginatumolbyCandidaantarctica lipase(Novozym435).Tetrahedron-Asymmetry18,1054–1058.
Rodrigues,A.P.,Andrade, L.H.C.,2014.Levantamento etnobotânicodasplantas medicinaisutilizadaspelacomunidadedeInhamã,Pernambuco,Nordestedo Brasil.Rev.Bras.PlantasMed.16,721–730.
Sánchez,Y.,Correa,T.M.,Abreu,Y.,Martínez,B.,Duarte,Y.,Pino,O.,2011. Car-acterizaciónquímicayactividadantimicrobianadelaceiteesencialdePiper marginatumJacq.Rev.Protec.Veg.26,170–176.
Sánchez,Y.,Correa,T.M.,Abreu,Y.,Pino,O.,2012.EfectodelaceiteesencialdePiper marginatumJacq.ysuscomponentessobreXanthomonasalbilineans(Ashby) Dawson.Rev.Protec.Veg.27,39–44.
Santana,H.,Trindade,F.,RG,S.,Silva,A.,Militao,J.,Facundo,V.,2015.Essentialoils ofleavesofPiperspeciesdisplaylarvicidalactivityagainstthedenguevector, Aedesaegypti(Diptera:Culicidae).Rev.Bras.PlantasMed.17,105–111. Santos,B.V.d.O.,Chaves,E.M.C.d.O.,Gray,A.I.,1998.PhenylalkanoidsfromPiper
marginatum.Phytochemistry49,1381–1384.
Sequeda-Casta ˜neda,L.,Celis,C.,Gutiérrez,S.,Gamboa,F.,2015.Pipermarginatum Jacq.(Piperaceae):phytochemical:therapeutic,botanicalinsecticidaland phy-tosanitaryuses.Pharmacol.Online3,136–145.
Souto,R.,Harada,A.,Andrade,E.,Maia,J.,2012.InsecticidalactivityofPiper essen-tialoilsfromtheAmazonagainstthefireantSolenopsissaevissima(Smith) (Hymenoptera:Formicidae).Neotrop.Entomol.41,510–517.
Surana,S.,Gokhale,S.,Rajmane,R.,Jadhav,R.,Jadhav,R.,2006.Non-saccharide naturalintensesweeteners.Anoverviewofcurrentstatus.Nat.Prod.Rad.5, 270–278.
Tangarife-Casta ˜no, V., Correa-Royero, J.B., Roa-Linares, V.C., Pino-Benitez, N., Betancur-Galvis,L.A.,Durán,D.C.,Stashenko,E.E.,Mesa-Arango,A.C.,2014. Anti-dermatophyte,anti-Fusariumandcytotoxicactivityofessentialoilsandplant extractsofPipergenus.J.Essent.OilRes.26,221–227.
Tillequin,F.,Paris,M.,Jacquemin,H.,Paris,R.,1978.FlavonoidesdePipermarginatum. PlantaMed.33,46–52.
Trelease,W.,Yuncker,T.G.,1950.ThePiperaceaeofNorthernSouthAmerica,vol.1. UniversityofIllinoisPress,Urbana,pp.73–78.
Tropicos.org,2015.!PipermarginatumJacq.MissouriBotanicalGardenhttp://www. tropicos.org/Name/25001136(visited10.09.15).
vanAndel,T.,deKorte,S.,Koopmans,D.,Behari-Ramdas,J.,Ruysschaert,S.,2008. DrysexinSuriname.J.Ethnopharmacol.116,84–88.
Vigneron,M.,Deparis,X.,Deharo,E.,Bourdy,G.,2005.Antimalarialremediesin FrenchGuiana:aknowledgeattitudesandpracticesstudy.J.Ethnopharmacol. 98,351–360.
Villasmil,J.,Abad,M.J.,Arsenak,M.,Fernández,A.,Ruiz,M.-C.,Williams,B., Michelan-geli,F.,Herrera,F.,Taylor, P., 2006.Cytotoxic and antitumoractivities of Venezuelanplantextractsinvitroandinvivo.Pharmacol.Online3,808–816.
Vogler,B.,Noletto,J.A.,Haber,W.A.,Setzer,W.N.,2006.Chemicalconstituentsofthe essentialoilsofthreePiperspeciesfromMonteverde,CostaRica.J.Essent.Oil Bear.Plants9,230–238.