w ww.e l s e v i e r . c o m / l o c a t e / b j p
Original
Article
Isolation
and
characterization
of
flavanols
from
Anthocephalus
cadamba
and
evaluation
of
their
antioxidant,
antigenotoxic,
cytotoxic
and
COX-2
inhibitory
activities
Madhu
Chandel
a,
Manish
Kumar
a,
Upendra
Sharma
b,
Neeraj
Kumar
b,
Bikram
Singh
b,∗,
Satwinderjeet
Kaur
a,∗aDepartmentofBotanicalandEnvironmentalSciences,GuruNanakDevUniversity,Amritsar,India
bNaturalProductChemistryandProcessDevelopmentDivision,CSIR-InstituteofHimalayanBioresourceTechnology,Palampur,India
a
r
t
i
c
l
e
i
n
f
o
Articlehistory:
Received4November2015 Accepted25February2016 Availableonline2May2016
Keywords:
Anthocephaluscadamba Antioxidant
Antigenotoxic Cytotoxic
Cyclooxygenase-2inhibitoryactivity
a
b
s
t
r
a
c
t
Insearchofleadmoleculesforuseindiseasepreventionandasfoodadditivefromnaturalsources, two flavanols wereisolated from leaves ofAnthocephalus cadamba (Roxb.)Miq., Rubiaceae.Their structureswereestablishedas6-hydroxycoumarin-(4′′→8)-(−)-epicatechinand
6-hydroxycoumarin-(4′′→8)-(−)-epicatechin-(4→6′′′)-(−)-epicatechinonthebasisofspectroscopicdata.Boththecompounds
exhibitedpotentantioxidantandantigenotoxicactivity.6-Hydroxycoumarin-(4′′→8)-(−)-epicatechin
scavenged DPPH, ABTS+.and superoxide anion radicalswith IC
50 values of 6.09g/ml, 5.95g/ml
and42.70g/mlrespectivelywhereastheIC50valuesfor6-hydroxycoumarin-(4′′→8)-(−
)-epicatechin-(4→6′′′)-(−)-epicatechinwere6.62g/mlforDPPHfreeradicals,6.93g/mlforABTSradicalcationsand
49.08g/mlforsuperoxideanionradicals.Boththecompoundsalsoexhibitedpotentreducing poten-tialinreducingpowerassayandprotectedtheplasmidDNA(pBR322)againsttheattackofhydroxyl radicalsgeneratedbyFenton’sreagentinDNAprotectionassay.InSOSchromotest, 6-hydroxycoumarin-(4′′→8)-(−)-epicatechindecreasedtheinductionfactorinducedby4NQO(20g/ml)andaflatoxinB1
(20g/ml)by31.78%and65.04%respectivelyataconcentrationof1000g/ml.Ontheotherhand, 6-hydroxycoumarin-(4′′→8)-(−)-epicatechin-(4→6′′′)-(−)-epicatechindecreasedthegenotoxicityofthese
mutagensby37.11%and47.05%respectively.ItalsoshowedcytotoxicityinCOLO-205cancercellline withGI50of435.71g/ml.Boththecompoundsshowedmoderatecyclooxygenase-2inhibitoryactivity.
©2016SociedadeBrasileiradeFarmacognosia.PublishedbyElsevierEditoraLtda.Thisisanopen accessarticleundertheCCBY-NC-NDlicense(http://creativecommons.org/licenses/by-nc-nd/4.0/).
Introduction
Cancerisamajorpublichealthprobleminallpartsoftheworld. Diversemolecular,biochemicalandcellularmechanismsateach stageof carcinogenesisareaccountable foraltering normal cell cycleprocess and converting a normal cell toa cancerous one (Seifriedetal.,2007).Ithasbeenobservedthatabout20%ormore ofcancercasescouldbepreventedbyincreasedintakeoffruitsand vegetablesindailydiet(RuhulAminetal.,2009).Natural prod-ucts/phytochemicalsarebeingexploredfortheirchemopreventive propertiesduetotheirnon-toxicnature,protectiveeffectsagainst oxidants and theirrecognition as dietary additives (Suh et al., 2009;Linetal.,2011;Liuetal.,2011;Wangetal.,2011;Kundu
∗ Correspondingauthors.
E-mails:bikramsingh@ihbt.res.in(B.Singh),satwinderjeet.botenv@gndu.ac.in (S.Kaur).
etal.,2014;Bohnetal.,2014).Oxidativestressisthemajor deter-minantinthedevelopmentofdiversediseases(Halliwell,1997; Flora,2007; Halliwelland Gutteridge, 2007; Ziech etal., 2010; Ayala-Pena,2013).Plantderivedconstituentsprovideprotection fromROS-inducedDNAdamageandconsequentlyfrom carcino-genesis(Lamsonetal.,2010).Numerousreportshaveshownthe capabilityofphytoconstituentstoprovideprotectionagainstfree radicalinducedailments(Sahinetal.,2010;Ungvarietal.,2010; Negietal.,2011;Scapagninietal.,2011;Xiongetal.,2012;Ziech etal.,2012;Carmona-Ramirezetal.,2013).Phytochemicalsisolated fromdifferentpartsoftheplantsbelongingtodiverseclassesof plantsecondarymetabolitesareaccountableforantioxidant prop-ertiesofmedicinalplants(Chungetal.,1998;Pietta,2000).Toxic effectsofantioxidantsofsyntheticoriginhavelimitedtheir uti-lizationinfoodproducts(Itoetal.,1986;Peters etal.,1996;Li etal.,2002).Naturalplantproductsarefrequentlyreportedas effi-cientchemopreventiveagents(SurhandFerguson,2003).Immense researchcarriedoutinplantscienceshasleadtotheidentification
http://dx.doi.org/10.1016/j.bjp.2016.02.007
of various plants used in ancient times for their medicinal potential.
Anthocephaluscadamba(Roxb.)Miq.,Rubiaceae,isanAyurvedic medicinalplant,usedintreatingvariousailments.Itisusedasa folkmedicineinthetreatmentoffeverandanemia,asantidiuretic andfor improvementof semenquality. Earlier,in astudyfrom thesamelaboratory,wehavereportedthatamongallfractions, EAAC fractionof A. cadamba leavesexhibited highest potential tocounteroxidativestressinvariousinvitroantioxidantassays (Chandeletal.,2012).Therefore,thepresentstudywasundertaken towardanefforttoisolatetheactivecompoundsresponsibleforthe potentialantioxidantactivityofEAACfractionandtoevaluatethe isolated molecules for theirantioxidant/antigenotoxic/cytotoxic properties.
Materialsandmethods
Bacterialstrain/celllinesandchemicals
EscherichiacoliPQ37 strainwaspurchasedfromInstitut Pas-teur,France.HeLaandCOLO-205cancercelllineswereobtained fromNationalCentreforCellSciences(NCCS),Pune. 2,2-Diphenyl-1-picrylhydrazyl (DPPH), ferric chloride, l-ascorbic acid, NADH (nicotinamideadeninedinucleotide),PMS(phenazine methosul-phate),NBT(nitrobluetetrazoliumchloride),ortho-nitrophenyl -d-galactopyranoside(ONPG),para-nitrophenylphosphate(PNPP), fetal bovineserum(FBS), DMEMculturemedium, RPMIculture medium, MTT, dimethyl sulfoxide (DMSO), penicillin, trypsin, antibiotic/antimycotic solution, HEPES, NaHCO3, streptomycin
wereobtainedfromHiMediaPvt.LimitedMumbai,India.Potassium persulfate, ABTS [2,2-azinobis(3-ethylbenzothiazoline-6-sulfonic acid)diammoniumsalt]andrutinfromSigma(St.Louis,MO,USA). PlasmidpBR322waspurchasedfromGeneiPvt.Ltd.,Banglore.All otherreagentsusedwereofanalyticalgrade(AR).
Collectionofplantmaterial
The plant material used (leaves of Anthocephalus cadamba (Roxb.) Miq., Rubiaceae) was procured during July 2010 from campusofGuruNanakDevUniversity(G.N.D.U.),Amritsar, Pun-jab(India).ThespecimenwasidentifiedandkeptatHerbarium, Department of Botanical and Environmental Sciences, G.N.D.U. withVoucherspecimenno.6557/2011.
ColumnchromatographyofEAACfraction
EAACfraction(15g)waspreparedasmentionedinthe fraction-ationprocedure(Fig.1)anddissolvedin10mlofMeOH,mixedwith silicagelandtheslurrywasmade.Thecolumnwaselutedusinga gradientofhexane/EtOAc(100/0),(98/2),(95/5),(85/15),(80/20), (75/25),(70/30),(60/40),(50/50),(40/60),(30/70),(20/80),(10/90), (0/100)andfinallythecolumnwaselutedwithMeOH.Three frac-tionswerecollectedwhenelutingwithhexane/EtOAc((10/90)viz. EALAC1,EALAC2andEALAC3.Agradientofhexane/EtOAc(100/0), (75/25),(70/30),(65/35),(60/40),(55/45),(50/50),(40/60),(20/80), (0/100)wasusedtofurtherfractionateEALAC2(500mg)andthe columnwaselutedwithMeOH.Compound1(20mg)wasobtained fromfractionselutingin(55:45)n-hexane/EtOAc.
EALAC3 (7.91g) was dissolved in EtOAc and washed with H2O (3×300ml). The EtOAc layerwas dried over sodium
sul-fate and concentrated to obtain EALAC3-S fraction. A gradient ofCHCl3/MeOH(100/0),(95/5),(92/8),(90/10),(85/15),(80/20),
(75/25),(70/30),(60/40),(50/50),(30/70),(0/100)wasusedto col-umnchromatograph EALAC3-Sfraction and finally elutionwas donewithMeOH.Thinlayerchromatographyoffractionscollected inCHCl3/MeOH(90/10)gave singlespotandthefractionswere
concentratedandlyophilizedtoobtaindarkbrowncolored com-pound2(50mg).Compound1wasagainobtainedfromprecipitates obtainedatgradientCHCl3/MeOH(95/5)asmentionedinFig.1.
Antioxidantactivity
DPPH-radicalscavengingassay
ScavengingofDPPHradicalswasassayedusingtheprotocolof Blois(1958)withminormodifications.
ABTSradicalscavengingassay
ThespectrophotometricanalysisofABTS+•scavengingactivity wasdeterminedaccordingtotheprotocolgivenbyReetal.(1999) withslightmodifications.
Reducingpowerassay
Reducing potential of both the compounds wasdetermined usingthemethodofOyaizu(1986).
Superoxideanionradicalscavengingassay
Themeasurementofsuperoxideanionscavengingactivityof theisolatedcompoundswasperformedaccordingtothemethod ofNishikimietal.(1972)withslightmodifications.
Rutinwasused asstandard antioxidantcompound inDPPH, ABTS.+,reducingpowerandsuperoxideanionradicalscavenging
assays.
DNAprotectionassay
Tomeasurethehydroxylradicalscavengingeffectoftheisolated compounds,DNAnickingexperimentwasperformedaccordingto theprotocolofLeeetal.(2002).
Antigenotoxicactivity
SOSchromotest
Anthocephalus cadamba (Leaves powder, 1.4 g)
Refluxed with 95% ethanol
Ethanol extract (ETAC) after solvent evaporation (325.4 g)
Dissolved in distilled water
Aqueous ethanol extract Ethyl acetate
EAAC (15 g) Marc
Subjected to column chromatography eluting with a gradient of Hex:EtoAc
Hex:EtoAc::10:90
EALAC1 EALAC2 (500 mg)
Hex:EtoAc::55:45
Compound 1
Upper EtoAc layer Lower aqueous layer
Filteration through sodium-sulfate
Concentrated and dried (EALAC3-S, 1.92 g)
Subjected to column chromatography eluting with a gradient of CHCI3:MeOH
CHCI3:MeOH::95:5 CHCI3:MeOH::90:10
Compound 2
Compound 1 Precipitates (200 mg)
CHCI3:MeOH::95:5 Subjected to column chromatography
eluting with a gradient of CHCI3:MeOH
Subjected to column chromatography eluting with a gradient of Hex:EtoAc
EALAC3 (7.91 g)
Dissolved in EtoAc
EtoAc soluble part
Fig.1. Isolationofcompounds1and2fromleavesofAnthocephaluscadamba.
(hydroxymethyl)amino-methane.Theabsorptionwasmeasuredat 420nmusingareferencesolutioninwhichcultureisreplacedbyL medium.
Theenzymeactivitieswerecalculatedaccordingtothe simpli-fiedmethod:
Enzymeunits (U)=A420×1000
t
A420: opticaldensityat 420nm; t: substrateconversiontime in
minutes.
Inductionfactor (IF)= Rc Ro
Rc:-galactosidase activity/alkalinephosphatase activity deter-minedforthetestcompoundatconcentrationc,
Ro:-galactosidaseactivity/alkalinephosphataseactivityinthe absenceofthetestcompound.
Anti-genotoxicitywas expressedas percentage inhibition of genotoxicityaccordingtotheformula:
Inhibition (%)=100−IF1−IF0 IF2−IF0
×100
where:
IF2istheinductionfactorofpositivecontrol(4NQOandaflatoxin
B1)
IF0 theinductionfactor oftheblank(without anytest
com-pound).
Cytotoxicity
MTTassay
The cytotoxicityof compounds 1 and 2 on Helaand COLO-205 cancer cell lines was assessed by MTT assay. Cell plating (1×104/well)wasdonein96-wellplates.After24hincubation,
cells were incubated with different concentrations of the test sampleinDMSO(0.1%)for24h.Additionof10lofMTT(5mg/ml, phosphate-buffered saline solution) was done followed by 2h incubation.The mediumwasdiscardedandDMSOwasusedto dissolvethecrystalsofreducedMTTformed.Opticaldensitywas noticedat570nm.The%ofcytotoxicitywasdeterminedusingthe formulagivebelow:
%cytotoxicity=A0−A1
A0 ×100 where,
A0=absorbanceofcontrol A1=absorbanceoftestsample
InvitroCOX-2inhibitoryactivity
InhibitionofCOX-2activityofisolatedcompoundsfromEAAC fractionfromleavesof A. cadamba wasassessed withthehelp of ‘COX (ovine/human)inhibitor screening assay’ kit (Item No. 560131,CaymanChemicalsCompany,USA).
Statisticalanalysis
The results were presented as the mean±standard error. Regressionanalysiswascarriedoutbybestfit methodandIC50
valueswerecalculatedusingregressionequation.Thedatawere analyzedforstatisticalsignificanceusinganalysisofvariance (one-wayANOVA).Thedifferenceamongaveragevalueswascompared byhonestlysignificantdifference(HSD)usingTukey’stest.The sig-nificancewascheckedat*p≤0.05.
Resultsanddiscussion
Analysisofcompounds1and2
Compound1
HRMSofcompound1displayedamolecularionpeakatm/z 452.7750correspondingtothemolecularformulaC24H20O9(see
supplementarydataassociatedwiththisarticle).Totaltwentyfour carbonsignalswereobservedin13CNMRspectraofcompound1
includingtenmethines,twomethylenesandtwelvequaternaryas evidentfromDEPTspectra.SignalformethyleneatıC28.2(C-4)
correlatingwithıH2.86(1H,m)andıH 2.94(1H,m),inHMQC
spectrumwasfoundtobeadjacenttotwooxygenatedmethines atıH 4.20 (1H,m), ıC66.0 (C-3)and ıH 4.82(1H,overlapped), ıC79.1(C-2)byHMBCanalysis,hence,suggestedthepresenceof
flavan-3-oltypeofunitincompound1.Threearomaticsignalat [ıH6.84(1H,s);ıC114.9(C-2′)],[ıH6.72(1H,m),ıC115.5(C-5′)]
and[ıH6.69(1H,m),ıC118.4(C-6′)]showedlongrangecorrelation
withquaternaryaromaticcarbonsatıC130.7(C-1′),ıC144.9(C-3′)
andıC145.3(C-4′)inHMBCspectraofcompound1.Other
oxy-genatedaromaticcarbonsC-5,C-7,C-9,C3′andC4′wereobserved atı156.2,152.5,151.0,144.9and145.3respectively.Onthebasis
oftheseNMRchemicalshiftsandcomparisonwithliterature val-ues(Agrawal andBansal,1989)partialskeleton ofcompound1 appearedtobe(−)-epicatechin.
In1HNMRspectrum,onlyonesinglet forH-6wasobserved
insteadofmeta-coupledprotonsforH-6andH-8incaseof(− )-epicatechinthatindicatedthepresenceofsubstitutionatC-8.13C
NMRspectrumofcompound1showedadditionalsignalsforester typecarbonylatıC169.8,methyleneat[ıH2.83–2.88(2H,m),ıC
37.2(C-3′′)],aliphaticmethineat[ı
H4.45(12H,m),ıC34.3(C-3′′)],
aromaticmethines[ıH6.78(1H,m),ıC114.3(C-5′′)],[ıH6.63(1H,
m),ıC114.0(C-7′′)],[ıH6.99(1H,m),ıC118.3(C-8′′)]andthree
qua-ternarycarbonsatıC152.5(C-6′′),144.1(C-9′′)and134.3(C-10′′).
TheanalysisoftheseNMRvaluessuggested6-hydroxycoumarin skeleton.ThesubstitutionatC-8wasconfirmedby13CNMR
spec-trumwhichrevealeddownfieldquaternarycarbonforC-8atıC
105.1.Thisquaternarycarbonatı105.1(C-8of(−)epicatechin) showedlongrangecorrelations(HMBC)withmethineatı34.3 (C-4′′of6-hydroxycoumarin)suggestedthatbothmoietieswereC-C linkedwhichwasfurtherconfirmedfromspectraldata.Thus,on thebasisofNMRspectraldata(seesupplementarydataassociated withthisarticle)thestructureofcompound1waselucidatedas 6-hydroxycoumarin-(4′′→8)-(−)-epicatechin.Thestructureis ten-tativelyassignedasforcoumarinunitlinkageatC-6insteadofC-8 similarNMRvaluesandcorrelationareexpected.
Compound2
HRMSofcompound2displayeda molecularionpeakatm/z 741.8964(seesupplementarydataassociatedwiththisarticle) cor-respondingtothemolecularformulaC39H32O15.1Hand13CNMR
signals arevery similartotheNMR of compound 1with addi-tionalsignalscorrespondingtoonemoreepicatechinmoiety.The linkagebetweentwoepicatechinsunitswereestablishedbylong rangecorrelationbetween[ıH2.97–3.07(1H,m),ıC36.7(C-4)]
C-4andıC107.3(C-6′′′)inHMBCspectrum(seesupplementarydata
associatedwiththisarticle).Thus,onthebasisofNMRspectraldata, ‘compound2’wascharacterizedas6-hydroxycoumarin-(4′′→ 8)-(−)epicatechin-(4→6′′′)-(−)-epicatechin.
O
O HO
O
HO
OH
OH OH
OH
1
O
O HO
O
HO
OH
OH OH
OH
HO OH
O HO
OH OH
2
6"
2"
4"
8
5 4
2 1' 3'
6"
2"
4"
8
5 4
2 1' 3'
6"' 5"'
3"'
2"' 1""
3""
4""
Antioxidantactivity
Compounds1and2exhibitedpotentradicalscavenging activ-ityinDPPHassay.Thecompounds1and2scavengedtheDPPH radicalsby77.08%and76.91%respectivelywithIC50of6.09g/ml
(compound 1) and 6.62g/ml (compound 2) which waslower thanthestandardantioxidantcompoundrutin(IC5054.05g/ml)
y=11.34ln(x)+29.51 r∗∗∗= 0.966
0 20 40 60 80 100
100 80 60 40 20 0
Concentration (µg/ml)
y=19.12ln(x)+15.91 r∗∗=0.943
0 20 40 60 80 100
100 80 60 40 20 0
Inhibition, %
Inhibition, %
Concentration (µg/ml)
DPPH assay ABTS+. assay
y=0.737x+6.489 r∗∗∗=0.992
0 20 40 60 80 100
100 80 60 40 20 0
Concentration (µg/ml)
y=0.618x+23.61 r∗∗∗=0.957
0 20 40 60 80 100
100 80 60 40 20 0
Inhibition, %
Inhibition, %
Concentration (µg/ml)
radical anion Superoxide
scavenging assay Reducing power assay
Fig.2.Antioxidantactivityofcompound1fromAnthocephaluscadambaleavesindifferentassays.
scavengingeffectexhibitedbycompounds1and2was28.35%and 24.99%whichdosedependentlyincreasedto99.76%and99.59% respectivelyatthehighesttestedconcentrationof100g/ml.The IC50valueforcompound1wascalculatedas5.95g/ml,whereas
compound2showedIC50 valueof6.93g/ml whichwaslower
thanthestandardantioxidantcompoundrutin(Figs.2and3).In reducingpowerassayboththecompoundsviz.1and2showed potentreductionpotentialof76.00%and55.57%athighesttested concentration(100g/ml)respectively.Standardantioxidant com-poundrutinshowedhigherIC50(IC50ofrutin160.77g/ml)than
theisolatedcompounds(IC50ofcompound1:70.27g/ml;IC50
ofcompound2:85.48g/mlwithrespecttorutin)(Figs.2and3). Bothcompoundsviz.compounds1and 2exhibitedpronounced superoxideanionradicalscavengingwithIC50of42.70g/mland
49.08g/mlrespectivelywhichwaslessthanthatofstandardrutin (IC5058.75g/ml)(Figs.2and3).Boththecompoundsshowedthe
abilitytoprotectthedamagedpBR322plasmidDNAfromtheattack ofhydroxylradicalsgeneratedbyFenton’sreaction(Fig.4).
Thehigher antioxidantactivityofcompounds1and 2might beattributedtothepresence ofmore hydroxyl groups present intheAandBringofthemolecules.Thenumberand configura-tionofhydroxylgroupsandthearrangementoffunctionalgroups aboutthenuclearstructuremayberesponsiblefortheantioxidant capacityofphenolics(Caoetal.,1997;ShekherPannalaetal.,2001). Theresultsofthepresentstudyareinaccordancewiththe sev-eralreportsshowingthepotentantioxidantactivityofflavonoids (Horvathovaetal.,2003;Gulcinetal.,2005;Rahmanetal.,2006; Kalpanaetal.,2009;Emametal.,2010;Hoetal.,2012;Jayasinghe et al.,2012; Tatsimo etal., 2012).Thereis a clearrelationship betweentheantioxidantpowerandthestructuralcharacteristics offlavonoids.Thepotentantioxidantactivityofcompounds1and 2mightbeduetothepresenceofO-dihydroxygroupsinthe B-ring,themeta5,7-dihydroxyarrangementsintheAringanda-OH
groupatposition3.Variousstudiesreportedthepresenceofabove mentionedstructuralcharacteristicsasthemajordeterminantsof antioxidantactivityofflavonoids(RattyandDas,1988;Borsetal., 1990;VanAckeretal.,1996;Rice-Evansetal.,1997;Pietta,2000; Lopez-Velezetal.,2003;Villanoetal.,2005;WolfeandLiu,2008). Moreover,flavonoidscanterminateradicalchainreactionsby servingaselectronandhydrogendonorsandtherebyconverting freeradicalstomorestableproducts(Kellyetal.,2002;Yenand Chen,1995).Thesuperoxideanionsscavengingactivityand antiox-idationof flavonols(quercetin,rutin,morin), flavones(acacetin, hispidulin)andflavanones(hesperidin,naringin)wasstudiedby Yuting et al. (1990). Rutin was found to be the most potent scavengerfollowedbyquercetinandnaringin, whilemorinand hispidulinwereveryweak.Caietal.(1997)attributedthe anti-carcinogeniceffectsofdifferentclassesofflavonoidsi.e.flavonol (quercetin),flavones(luteolin),andisoflavone(genistein)totheir antioxidantactivity.Quercetinandluteolinwerefoundtobepotent scavengerofH2O2andsuperoxideanionradicalsandalsoinhibited
thelipidperoxidationefficiently,whilegenisteinshoweda moder-ateeffectinradicalscavengingandexhibitedweakinhibitoryeffect inlipidperoxidationassay.
y=11.62ln(x)+28.02 r∗∗∗=0.955
0 20 40 60 80 100
100 80 60 40 20 0
Inhibition, %
Concentration (µg/ml)
y=20.21ln(x)+10.89 r∗∗=0.932
0 20 40 60 80 100
100 80 60 40 20 0
Inhibition, %
Concentration (µg/ml)
DPPH assay ABTS+. assay
y=0.533x+4.434 r∗∗∗=0.996
0 20 40 60 80 100
100 80 60 40 20 0
Inhibition, %
Concentration (µg/ml)
y=0.697x+15.79 r∗∗∗=0.950
0 20 40 60 80 100
100 80 60 40 20 0
Inhibition, %
Concentration (µg/ml)
radical anion Superoxide
scavenging assay Reducing power assay
Fig.3. Antioxidantactivityofcompound2fromAnthocephaluscadambaleavesindifferentassays.
wogonoside isolated from radixof Scutellaria baicalensis. Dose-dependentscavengingofhydroxylradicals,DPPHradicalsandalkyl radicalswasobservedbybaicaleinandbaicalinwhilewogoninand wogonosideshowedveryweakinhibitoryeffectsontheseradicals. Amongallthetestedcompoundsbaicaleinwasthemosteffective antioxidantanditspotentantioxidantactivitywasattributedto thepresenceofo-tri-hydroxylstructureintheAring.Hiranoetal. (2001)evaluatedtheDPPH•scavengingactivityofflavanols (cate-chin,epicatechin[EC],epigallocatechin[EGC],epicatechingallate [ECG], epigallocatechin gallate [EGCG]), flavonols (myricetin, quercetin,kaempferol)andflavones(apigeninandluteolin).EGCG wasthemostpotentDPPHradicalscavenger,whileluteolinwas theleastactive.Theyhavealsoevaluatedtheeffectofflavonoids onLDL oxidation and the inhibitory effectwas in theorder of
luteolin>ECG>EC>quercetin>catechin>EGCG>EGC>myricetin> kaempferol>apigenin.
Variousotherworkersgaveacomparativeaccountof antioxi-dantactivityofflavonoidsindifferentinvitroantioxidantassays andattributedthedifferenceintheiractivitytothepresenceor absence ofsubstituents onring A,ring Bor ring C (Gao etal., 1999;Pietta,2000;Miraetal.,2002;KhandujaandBhardwaj,2003; Emametal.,2010).
Antigenotoxicactivity
Identification of natural products with specific molecular and cellular targets can provide an effective approach to can-cer chemoprevention. Such an approach can be accomplished
1 2 3 4 5 6 1 2 3 4 5 6
100 70 50
10 10 50 70 100
Compound 2
Nicked circular form Linear form Supercoiled form
Compound 1
Figure4.Effectofcompounds1and2fromAnthocephaluscadambaleavesinplasmidDNAnickingassay. Lane1:NegativeControl(DW+pBR322plasmidDNA)
Table1
Antigenotoxiceffectofcompound1fromleavesofAnthocephaluscadambaagainst4NQOandAFB1inSOSchromotestusingE.coliPQ37testerstrain.
Treatment Dose(g/ml) -Galactosidaseunits Alkalinephosphataseunits R Inductionfactor(IF) Mean±SE Mean±SE
4NQO(direct-actingmutagen)
Control(nontreatedcells) 6.23±0.55 29.17±1.99 0.21 1.00 Positivecontrol(4NQO) 20g/ml 46.74±4.19 16.57±0.66 2.82 13.43 Negativecontrol(compound1only) 10 4.52±0.24 23.72±2.30 0.19 0.90a
30 4.38±0.29 24.34±2.08 0.18 0.86a
100 4.44±0.28 26.43±2.01 0.17 0.81a
300 4.92±0.19 24.06±1.22 0.20 0.95a
1000 5.10±0.18 23.76±1.67 0.21 1.00a
4NQO+compound1 10 30.58±3.83 12.65±0.45 2.42 11.52
30 34.34±2.95 12.60±0.49 2.73 13.00
100 33.86±2.92 14.32±1.62 2.36 11.24a
300 33.89±2.84 16.09±1.44 2.11 10.04a
1000 31.95±6.21 16.03±0.93 1.99 9.48a
AflatoxinB1(S9-dependentmutagen)
Control(nontreatedcells) 10.42±0.75 61.92±5.04 0.17 1.00
Positivecontrol(aflatoxinB1) 20g/ml 97.42±2.99 60.87±5.19 1.60 9.41
Negativecontrol(compound1only) 10 10.53±0.85 62.57±3.16 0.17 1.00a
30 11.42±0.19 61.13±3.98 0.19 1.12a
100 8.93±1.29 60.73±2.62 0.15 0.88a
300 9.03±1.03 61.00±5.48 0.15 0.88a
1000 9.48±0.51 67.18±5.52 0.14 0.82a
AflatoxinB1+compound1 10 61.10±9.04 59.18±6.71 1.04 6.12a
30 61.27±6.48 57.55±5.18 1.06 6.24a
100 57.13±8.15 56.98±5.02 1.00 5.88a
300 51.65±4.71 69.77±10.6 0.74 4.35a
1000 45.68±4.06 68.45±9.59 0.67 3.94a
R,-galactosidaseunits/alkalinephosphataseunits;IF,Rc/Ro.
aLevelofstatisticalsignificance:p≤0.05withrespectto4NQOandAflatoxinB1.
throughisolation,characterizationandpreclinicalevaluationfor their development as chemopreventive agents (Lippman and Hong,2002;Gupta,2007).Evaluationofantimutagenicactivities of natural products becomes necessary, as there is concor-dancebetweenantimutagenicityandanticarcinogenicity(Maron and Ames, 1983; El-Sayed et al., 2007). Antimutagenic stud-ies of botanical extracts form the foundation for selecting the leadextractsfor longtermand costlyinvivo chemoprevention investigationstogetherwiththeseparation and chemical struc-tural elucidation ofpossible active compounds(EI-Sayedet al., 2013).
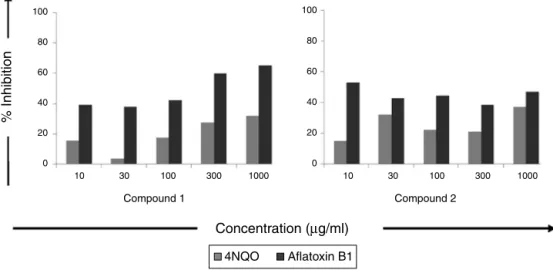
IntheSOSchromotest,itwasascertainedthatdifferent concen-trationsofcompounds1and2addedtotheindicatorbacteriawere notgenotoxicastheinductionfactorinducedbythetesteddoses wasbelow1.5.Table1andFig.5showedthatataconcentrationof 10g/ml,compound1inhibitedthegenotoxicityofAFB1(IF=9.41)
by39.12%whichdosedependentlyincreasedandata concentra-tionof1000g/ml,itshowedaninhibitionof65.04%.Similarly, compound1inhibitedtheinductionfactorof4NQO(IF=13.43)by 15.37%ataconcentrationof10g/mland31.78%at1000g/ml.As seenfromTable2andFig.5,compound2didnoteffectively mod-ulatedthegenotoxicityof4NQOandAFB1.Compound2reduced theinductionfactor of4NQO(IF=10.62)and AFB1(IF=8.80)by 37.11%and47.05%athighesttestedconcentrationof1000g/ml respectively.
Epicatechinsareoneofthecomponentsofteapolyphenolsand in a report by Itoet al. (1989),protective effects of hot water greenteaextractswereevaluatedagainsttheAFB1-induced chro-mosomal aberrations in bone marrow cells. Mutagenic effects mightbeinhibitedbycatechinsduetoflavanol–mutagenadduct formation(Stich,1991).Kuroda(1996)reportedthe bioantimu-tagenic activity of catechins against the direct-actingmutagen
100
80
60
40
20
0 100
80
60
40
20
0
10 30 100 300 1000 10 30 100
Compound 1 Compound 2
Concentration (
µ
g/ml)
% Inhibition
4NQO Aflatoxin B1 300 1000
Table2
Antigenotoxiceffectofcompound2fromleavesofAnthocephaluscadambaagainst4NQOandAFB1inSOSchromotestusingE.coliPQ37testerstrain.
Treatment (g/ml) -Galactosidaseunits Alkalinephosphataseunits R Inductionfactor(IF) Mean±SE Mean±SE
4NQO(direct-actingmutagen)
Control(nontreatedcells) 5.71±1.14 26.72±1.19 0.21 1.00 Positivecontrol(4NQO) 20g/ml 56.16±6.19 25.17±1.55 2.23 10.62 Negativecontrol(compound2only) 10 4.16±0.74 19.20±3.74 0.22 1.04a
30 3.92±0.72 19.62±3.54 0.20 0.95a
100 5.01±0.53 19.31±3.25 0.26 1.24a
300 5.38±0.39 20.05±3.59 0.27 1.29a
1000 6.20±0.62 19.96±3.67 0.31 1.48a
4NQO+compound2 10 43.24±5.13 22.59±2.35 1.91 9.10
30 41.01±6.15 22.67±2.17 1.81 8.62
100 35.26±4.24 22.32±2.21 1.58 7.52
300 40.67±5.80 23.15±2.34 1.78 8.48
1000 37.73±7.02 25.46±2.67 1.48 7.05
AflatoxinB1(S9-dependentmutagen)
Control(nontreatedcells) 11.92±0.21 78.13±3.18 0.15 1.00
Positivecontrol(aflatoxinB1) 20g/ml 95.80±1.34 72.63±2.69 1.32 8.80
Negativecontrol(compound2only) 10 11.08±0.99 74.63±2.13 0.15 1.00a
30 11.62±0.35 76.77±1.81 0.15 1.00a
100 12.92±0.43 76.10±4.41 0.17 1.13a
300 11.15±0.18 76.57±1.82 0.15 1.00a
1000 10.58±0.32 79.52±0.67 0.13 0.87a
AflatoxinB+compound2 10 56.12±2.51 79.83±3.26 0.70 4.67a
30 62.13±1.37 75.40±1.99 0.82 5.47a
100 60.30±6.34 75.40±1.98 0.80 5.33a
300 66.38±3.91 76.35±2.24 0.87 5.80a
1000 59.45±3.22 77.43±1.83 0.77 5.13a
R,-galactosidaseunits/alkalinephosphataseunits;IF,Rc/Ro.
aLevelofstatisticalsignificance:p≤0.05withrespectto4NQOandAflatoxinB1.
i.e.4NQO.Matsumotoetal.(1996)andWeisburgeretal.(1997) reportedthat epicatechins modulatethe activityof detoxifying enzymesi.e.reductionofcytochromeP450andincreaseinphase II enzymes. Bhouri et al. (2011) reported the antigenotoxicity of two flavonoids, kaempferol 3-O--isorhamninoside (K3O-ir) andrhamnocitrinand3-O--isorhamninoside(R3O-ir)againstthe genotoxicityinducedbynitrofurantoineandaflatoxinB1.K3O-ir andR3O-irreducedthegenotoxicityofaflatoxinB1significantly by96.64%and90.26%,respectivelyatthehighesttested concentra-tionof10g/ml.Flavonoidscanalsoactasdesmutagensbydirectly interactingwithmutagensandinactivatingthem(Heoetal.,1994). Antigenotoxicpotentialofapigenin(flavone)againstmitomycinC inducedgenotoxicdamageinmousebonemarrowcellswas stud-iedbySiddiqueandAfzal(2009).Resultsofthestudydemonstrated thatapigenineffectivelydiminishedthegenotoxictyofmitomycin Casreflectedfromdecreaseofsisterchromatidexchanges(SCEs) andchromosomalaberrationsinmousebonemarrowcells.Hayder etal.(2004)reportedtheantigenotoxicactivityofextractsfrom Myrtuscommunisandalltheextractswerefoundeffectiveagainst thegenotoxicityofAFB1andnifuroxazideandtheyattributedthe potentialofthetestedextractstowardantigenotoxicitytothe pres-enceofflavonoids,coumarinsandtannins.
AntiproliferativeandinvitroCOX-2inhibitoryactivity
Only compound 2 exhibited cytotoxicity against COLO 205 celllinewithpercentinhibitionof 52.06%at500g/ml(Fig.6). Howeverboththecompoundswerefoundtobeineffectiveagainst HeLa cellline.Procyanidins(flavonoids)-richgrape seedextract showedgrowthinhibitoryeffectand inductionofapoptoticcell deathina humanprostate carcinomaDU145cell line(Agarwal et al., 2002). Wang et al. (1999) investigated the mechanism of inductionof apoptosisby apigenin,myricetin, quercetinand kaempferol in HL-60 leukemiacells. Apigenin was foundtobe mostpotentinreducingthecellviabilitywithIC50of50M.Park
and Min(2011) reported that quercetininhibited invasion and
proliferationofgliomacellsbydownregulationofphospholipase D1,aregulatorofcellproliferationandtumorigenesis.Compound 1fromA.cadambaleavesinhibitedtheCOX-2by17.79%at concen-trationof1Mwhereas compound2showed25.64%inhibition atsameconcentration.Compound2wasfoundeffectiveagainst COLO-205 and this antiproliferative activity of the compound may partly be due to the inhibition of COX-2. Overexpression of COX-2hasbeen observedin colon tumors (Kawamori etal., 1998; Crofford, 1997; Roelofs et al., 2014). Therefore specific COX-2 inhibitors could potentially serve as chemopreventive agents. Ye et al. (2004) investigated the anticancer activity of genistein on human oral squamous carcinoma line (SCC-25). Genisteininhibitedthegrowthoforalsquamouscarcinomacells withIC50ofapproximately200MviaG2/Marrest.Genisteinat
aconcentrationof0.1Meffectivelydecreasedtheexpressionof COX-2.
100
80
60
40
20
0
25 50 125
Concentration (µg/ml)
% Inhibition
Colo-205 cell line
250 500
Conclusions
The isolated phytochemicals from A. cadamba have the potential to alleviate the genotoxicity of environmental muta-gens/carcinogens.Thesehavealsobeenfoundtopossesssignificant antioxidantactivitygreaterthanthatofstandardcompoundrutin. The results clearly show the chemopreventive potential of A. cadambaphytochemicalsand canbeapromisingnaturalsource inhealthandmedicine.
Ethicaldisclosures
Protectionofhumanandanimalsubjects. Theauthorsdeclare thattheproceduresfollowedwereinaccordancewiththe regula-tionsoftherelevantclinicalresearchethicscommitteeandwith thoseoftheCodeofEthicsoftheWorldMedicalAssociation (Dec-larationofHelsinki).
Confidentialityofdata. Theauthorsdeclarethattheyhave fol-lowed theprotocolsof theirworkcenter onthepublication of patientdata.
Right to privacy and informed consent. The authors have obtainedthewritteninformedconsentofthepatientsorsubjects mentionedinthearticle.Thecorrespondingauthorisinpossession ofthisdocument.
Authors’contributions
MCcarriedoutextraction,isolationandmajorbioactivitypart ofpresentwork.MKandMCcollectivelyconductedcytotoxicand COX-2inhibitoryactivitiesalongwithstatisticallyanalysisofthe data.US,NKandBScarriedoutstructuralelucidationand char-acterizationofisolatedcompounds.SKdesigned,supervisedand criticallycheckedthemanuscript.Allauthorsreadandapproved thefinalversionofmanuscript.
Conflictsofinterest
Theauthorsdeclarenoconflictsofinterest.
Acknowledgements
The authors are thankful to UGC (DRS-SAP), New Delhi for providingfinancialassistanceandDirector,IHBT,Palampurfor pro-vidingthenecessaryfacilitiestocarryoutisolationpart.
AppendixA. Supplementarydata
Supplementarydataassociatedwiththisarticlecanbefound,in theonlineversion,atdoi:10.1016/j.bjp.2016.02.007.
References
Agrawal,P.K.,Bansal,M.C.(Eds.),1989.Carbon-13NMRofFlavonoids.Elsevier, Amsterdam.
Agarwal,C.,Singh,R.P.,Agarwal,R.,2002.Grapeseedextractinducesapoptoticdeath ofhumanprostatecarcinomaDU145cellsviacaspasesactivationaccompanied bydissipationofmitochondrialmembranepotentialandcytochromecrelease. Carcinogenesis23,1869–1876.
Ayala-Pena,S.,2013.RoleofoxidativeDNAdamageinmitochondrialdysfunction andHuntington’sdiseasepathogenesis.FreeRadic.Biol.Med.62,102–110. Bhouri,W.,Sghaier, M.B., Kilani,S.,Bouhlel, I.,Dijoux-Franca,M.-G., Ghedira,
K.,Ghedira,L.C.,2011.Evaluationofantioxidantandantigenotoxicactivity oftwoflavonoidsfromRhamnusalaternusL.(Rhamnaceae):Kaempferol 3-O--isorhamninosideandrhamnocitrin3-O--isorhamninoside.FoodChem. Toxicol.49,1167–1173.
Blois,M.S.,1958.Antioxidantdeterminationsbytheuseofastablefreeradical. Nature29,1199–1200.
Bohn,S.K.,Blomhoff,R.,Paur,I.,2014.Coffeeandcancerrisk,epidemiological evi-dence,andmolecularmechanisms.Mol.Nutr.FoodRes.58,915–930. Bors,W.,Heller,W.,Michel,C.,Saran,M.,1990.Flavonoidsasantioxidants:
deter-minationofradicalscavengingefficiencies.MethodsEnzymol.186,343–354. Cai,Q.,Rahn,R.O.,Zhang,R.,1997.Dietaryflavonoids,quercetin,luteolinand
genis-tein,reduceoxidativeDNAdamageandlipidperoxidationandquenchfree radicals.CancerLett.119,99–107.
Cao,G.,Sofic,E.,Prior,R.L.,1997.Antioxidantandprooxidantbehaviorofflavonoids: structure–activityrelationships.FreeRadic.Biol.Med.22,749–760.
Carmona-Ramirez, I., Santamaria, A., Tobon-Velasco, J.C., Orozco-Ibarra, M., Gonzalez-Herrera,I.G.,Pedraza-Chaverri,J.,Maldonado,P.D.,2013.Curcumin restoresNrf2levelsandpreventsquinolinicacid-inducedneurotoxicity.J.Nutr. Biochem.24,14–24.
Chandel,M.,Sharma,U.,Kumar,N.,Singh,B.,Kaur,S.,2012.Antioxidantactivityand identificationofbioactivecompoundsfromleavesofAnthocephaluscadambaby ultra-performanceliquidchromatography/electrosprayionizationquadrupole timeofflightmassspectrometry.AsianPac.J.Trop.Med.5,977–985. Chung,K.-T.,Wong,T.-Y.,Huang,Y.-W.,Lin,Y.,1998.Tanninsandhumanhealth:a
review.Crit.Rev.FoodSci.Nutr.38,421–464.
Crofford,L.J.,1997.COX-1andCOX-2tissueexpression:implicationsand predic-tions.J.Rheumatol.49,15–19.
Devasagayam,T.P.,Subramanian,M.,Singh,B.B.,Ramanathan,R.,Das,N.P.,1995. Protection ofplasmid pBR322 DNA byflavonoids against single stranded breaksinducedbysingletmolecularoxygen.J.Photochem.Photobiol.B30, 97–103.
EI-Sayed, W.M., Hussin, W.A., Mahmoud, A.A., Alfredan, M.A., 2013. The Conyza triloba extracts with high chlorophyll and free radical scav-enging activity had anticancer activity in cell lines. Biomed. Res. Int., http://dx.doi.org/10.1155/2013/945638.
El-Sayed,W.M.,Hussin,W.A.,Franklin,M.R.,2007.Theantimutagenicityof 2-substitutedselenazolidine-4-(R)-carboxylicacids.Mutat.Res.627,136–145. Emam,A.M.,Mohamed,M.A.,Diab,Y.M.,Megally,N.Y.,2010.Isolationandstructure
elucidationofantioxidantcompoundsfromleavesofLaurusnobilisandEmex spinosus.DrugDiscov.Ther.4,202–207.
Flora,S.J.,2007.Roleoffreeradicalsandantioxidantsinhealthanddisease.CellMol. Biol.53,1–2.
Gao,Z.,Huang,K.,yang,X.,Xu,H.,1999.Freeradicalscavengingandantioxidant activitiesofflavonoidsextractedfromtheradixofScutellariabaicalensisGeorgi. Biochim.Biophys.Acta16,643–650.
Gulcin,I.,Berashvili,D.,Gepdiremen,A.,2005.Antiradicalandantioxidantactivity oftotalanthocyaninsfromPerillapankinensisdecne.J.Ethnopharmacol.101, 287–293.
Gupta, S., 2007. Prostatecancer chemoprevention: current status and future prospects.Toxicol.Appl.Pharmacol.224,369–376.
Halliwell,B.,1997.Antioxidantsandhumandiseases:ageneralintroduction.Nutr. Rev.55,44–52.
Halliwell,B.,Gutteridge,J.M.C.,2007.RadicalsinBiologyandMedicine.Oxford Uni-versityPress,NewYork.
Hayder,N.,Abdelwahed,A.,Kilani,S.,BenAmmar,R.,Mahmoud,A.,Ghedira,K., Chekir-Ghedira,L.,2004.Anti-genotoxicandfree-radicalscavengingactivities ofextractsfrom(Tunisian)Myrtuscommunis.Mutat.Res.564,89–95. Heo,H.Y.,Lee,S.J.,Kwon,C.H.,Kin,S.W.,Sohn,D.H.,Au,W.W.,1994.
Anticlasto-geniceffectsofgalanginagainstbleomycininducedchromosomalaberrations inmousespleenlymphocytes.Mutat.Res.311,225–229.
Hirano,R.,Sasamoto,W.,Matsumoto,A.,Itakura,H.,Igarashi,O.,Kondo,K.,2001. AntioxidantabilityofvariousflavonoidsagainstDPPHradicalsandLDL oxida-tion.J.Nutr.Sci.Vitaminol.47,357–362.
Ho,R.,Violette,A.,Cressend,D.,raharivelomanana,P.,Carrupt,P.A.,Hostettmann, K.,2012.Antioxidantpotentialandradical-scavengingeffectsofflavonoidsfrom theleavesofPsidiumcattleianumgrowninFrenchPolynesia.Nat.Prod.Res.26, 274–277.
Horvathova,K.,Novotny,L.,Vachalkova,A.,2003.Thefreeradicalscavengingactivity offourflavonoidsdeterminedbythecometassay.Neoplasma50,291–295. Ito,N.,Hirose,M.,Fukishima,S.,Tsuda,H.,Shirai,T.,Tatematsu,M.,1986.
Stud-iesonantioxidants:theiranticarcinogenicandmodifyingeffectsonchemical carcinogenesis.FoodChem.Toxicol.24,1099–1102.
Ito,Y.,Ohnishi,S.,Fujie,K.,1989.ChromosomeaberrationsinducedbyaflatoxinB1 inratbonemarrowcellsinvivoandtheirsuppressionbygreentea.Mutat.Res. 222,253–261.
Jayasinghe,L.,Amarasinghe,N.R.,Arundathie,B.G.S.,Rupasinghe,G.K.,Jayatilake, N.H.A.N.,Fujimoto,Y.,2012.AntioxidantflavonolglycosidesfromElaeocarpus serratusandFiliciumdecipiens.Nat.Prod.Res.26,717–721.
Kalpana,K.B.,Srinivasan,M.,Menon,V.P.,2009.Evaluationofantioxidantactivity ofhesperidinanditsprotectiveeffectonH2O2inducedoxidativedamageon pBR322DNAandRBCcellularmembrane.Mol.Cell.Biochem.323,21–29. Kawamori,T.,Rao,C.V.,Seibert,K.,Reddy,B.S.,1998.Chemopreventiveactivityof
celecoxib,aspecificcyclooxygenase-2inhibitor,againstcoloncarcinogenesis. CancerRes.58,409–412.
Kelly, E.H., Anthony, R.T., Dennis, J.B., 2002. Flavonoid antioxidants: chem-istry,metabolismandstructure–activityrelationships.J.Nutr.Biochem.13, 572–584.
Khanduja,K.L.,Bhardwaj,A.,2003.Stablefreeradicalscavengingand antiperoxida-tivepropertiesofresveratrolcomparedinvitrowithsomeotherbioflavonoids. IndianJ.Biochem.Biophys.40,416–422.
Kuroda,Y.,1996.Bio-antimutagenicactivityofgreenteacatechinsincultured Chi-nesehamsterV79cells.Mutat.Res.361,179–186.
Lamson,D.W.,Gu,Y.H.,Plaza,S.M.,Brignall,M.S.,Brinton,C.A.,Sadlon,A.E.,2010. ThevitaminC:vitaminK3system-enhancersandinhibitorsoftheanticancer effect.Altern.Med.Rev.15,345–351.
Lee,J.C.,Kim,H.R.,Kim,J.,Jang,Y.S.,2002.Antioxidantactivityofethanolextract ofthe stemof Opuntia ficus-indicavar. saboten. J. Agric. Food Chem.50, 6490–6496.
Li,Y.,Seacat,A.,Kuppusamy,P.,Zweier,J.L.,Yager,J.D.,Trush,M.A.,2002. Cop-perredox-dependentactivationof2-tert-butyl(1,4)hydroquinone:formationof reactiveoxygenspeciesandinductionofoxidativeDNAdamageinisolatedDNA andculturedrathepatocytes.Mutat.Res.518,123–133.
Lin,K.-W.,Yang,S.-C.,Lin,C.-N.,2011.Antioxidantconstituentsfromthestemsand fruitsofMomordicacharantia.FoodChem.127,609–614.
Lippman,S.M.,Hong,W.K.,2002.Cancerpreventionscienceandpractice.Cancer Res.62,5119–5125.
Liu,J.F.,Ma,Y.,Wang,Y.,Du,Z.Y.,Shen,J.K.,Peng,H.L.,2011.Reductionoflipid accumulationinHepG2cellsbyluteolinisassociatedwithactivationofAMPK andmitigationofoxidativestress.Phytother.Res.25,588–596.
Lopez-Velez,M.,Martinez-Martinez,F.,Valle-Ribes,C.D.,2003.Thestudyof phe-noliccompoundsasnaturalantioxidantsinwine.Crit.Rev.FoodSci.Nutr.43, 233–244.
Maron,D.M.,Ames,B.N.,1983.RevisedmethodsfortheSalmonellamutagenicity test.Mutat.Res.113,173–215.
Matsumoto,N.,Kohri,T.,Okushio,K.,Hara,Y.,1996.Inhibitoryeffectsoftea cat-echins,blackteaextractandoolongteaextractonhepatocarcinogensisinrat. Jpn.J.CancerRes.87,1034–1038.
Mira,L.,Fernandez,M.T.,Santos,M.,Rocha,R.,Florencio,M.H.,Jennings,K.R.,2002. Interactionsofflavonoidswithironandcopperions:amechanismfortheir antioxidantactivity.FreeRadic.Res.36,1199–1208.
Negi,G.,Kumar,A.,Sharma,S.S.,2011.Nrf2andNF-kappaBmodulationby sul-foraphanecounteractsmultiplemanifestationsofdiabeticneuropathyinrats andhighglucose-inducedchanges.Curr.Neurovasc.Res.8,294–304. Nishikimi,M.,Rao,N.A.,Yagi,K.,1972.Theoccurrenceofsuperoxideanioninthe
reactionofreducedphenazinemethosulphateandmolecularoxygen.Biochem. Biophys.Res.Commun.46,849–853.
Oyaizu,M.,1986.Studiesonproductofbrowningreactionpreparedfromglucose amine.Jpn.J.Nutr.44,307–315.
Park,M.H.,Min,D.S.,2011.Quercetin-induceddownregulationofphospholipaseD1 inhibitsproliferationandinvasioninU87gliomacells.Biochem.Biophys.Res. Commun.412,710–715.
Peters,M.M.C.G.,Rivera,M.I.,Jones,T.W.,Monks,T.J.,Lau,S.S.,1996.Glutathione conjugatesoftert-butyl-hydroquinone,ametaboliteoftheurinarytracttumor promoter3-tert-butyl-hyroxyanisole,aretoxictokidneyandbladder.Cancer Res.56,1006–1011.
Pietta,P.-G.,2000.Flavonoidsasantioxidants.J.Nat.Prod.63,1035–1042. Quillardet,P.,Hofnung,M.,1985.TheSOSchromotest,acolorimetricbacterialassay
forgenotoxins:procedures.Mutat.Res.147,65–78.
Rahman, M.M., Ichiyanagi, T., Komiyama, T., Hatano, Y., Konishi, T., 2006. Superoxide radical and peroxynitrite-scavenging activity of anthocyanins; structure–activity relationship and their synergism. Free Radic. Res. 40, 993–1002.
Ratty,A.K.,Das,N.P.,1988.Effectsofflavonoidsonnonenzymaticlipidperoxidation: Structure–activityrelationship.Oncology39,69–79.
Re,R.,Pellegrini,N.,Proreggente,A.,Pannala,A.,Yang,M.,Rice-Evans,C.,1999. AntioxidantactivityapplyinganimprovedABTSradicalcationdecolourization assay.FreeRadic.Biol.Med.26,1231–1237.
Rice-Evans,C.A.,Miller,N.J.,Paganga,G.,1997.Antioxidantpropertiesofphenolic compounds.TrendsPlantSci.2,152–159.
Roelofs,H.M.J.,Morsche,R.H.M.,VanHeumen,B.W.H.,Nagengast,F.M., Peters, W.H.M.,2014.Over-expressionofCOX-2mRNAincolorectalcancer.BMC Gas-troenterol.,http://dx.doi.org/10.1186/1471-230X-14-1.
RuhulAmin,A.R.M.,Kucuk,O.,Khuri,F.R.,Shin,D.M.,2009.Perspectivesforcancer preventionwithnaturalcompounds.J.Clin.Oncol.27,2712–2725.
Sahin,K.,Tuzcu,M.,Gencoglu,H.,Dogukan,A.,Timurkan,M.,Sahin,N.,Aslan,A., Kucuk,O.,2010.Epigallocatechin-3-gallateactivatesNrf2/HO-1signaling path-wayincisplatin-inducednephrotoxicityinrats.LifeSci.87,240–245.
Scapagnini,G.,Vasto,S.,Abraham,N.G.,Caruso,C.,Zella,D.,Fabio,G.,2011. Modula-tionofNrf2/AREpathwaybyfoodpolyphenols:anutritionalneuroprotective strategyforcognitiveandneurodegenerativedisorders.Mol.Neurobiol.44, 192–201.
Seifried,H.E.,Anderson,D.E.,Fisher,E.I.,Milner,J.A.,2007.Areviewoftheinteraction amongdietaryantioxidantsandreactiveoxygenspecies.J.Nutr.Biochem.18, 567–579.
ShekherPannala,A.,Chan,T.S.,O’Brien,P.J.,Rice-Evans,C.A.,2001.FlavonoidsB-ring chemistryandantioxidantactivity:fast-reactionkinetics.Biochem.Biophys. Res.Commun.282,1161–1168.
Siddique,Y.H.,Afzal,M.,2009.AntigenotoxiceffectofapigeninagainstmitomycinC inducedgenotoxicdamageinmicebonemarrowcells.FoodChem.Toxicol.47, 536–539.
Stich,H.F.,1991.Thebeneficialandhazardouseffectsofsimplephenoliccompounds. Mutat.Res.259,307–324.
Suh,Y.,Afaq,F.,Johnson,J.J.,Mukhtar,H.,2009.Aplantflavonoidfisetininduces apoptosisincoloncancercellsbyinhibitionofCOX-2and Wnt/EGFR/NFkappaB-signalingpathways.Carcinogenesis30,300–307.
Surh,Y.,Ferguson,L.R.,2003.Dietaryandmedicinalantimutagensand anticarcino-gens:Molecularmechanismsandchemopreventivepotentialhighlightsofa symposium.Mutat.Res.9485,1–8.
Tatsimo,S.J.N.,Tamokou,J.D.D.,Havyarimana,L.,Csupor,D.,Forgo,P.,Hohmann, J., Kuiate, J.-R., Tane, P., 2012. Antimicrobial and antioxidant activity of kaempferol rhamnosidederivativesfrom Bryophyllum pinnatum.BMC Res. Notes,http://dx.doi.org/10.1186/1756-0500-5-158.
Ungvari,Z.,Bagi,Z.,Feher,A.,Recchia,F.A.,Sonntag,W.E.,Pearson,K.,deCabo,R., Csiszar,A.,2010.Resveratrolconfersendothelialprotectionviaactivationofthe antioxidanttranscriptionfactorNrf2.Am.J.Physiol.HeartCirc.Physiol.299, 18–24.
VanAcker,S.A.B.E.,Berg,D-J.V.D.,Tromp,M.N.J.L.,Griffioen,D.H.,Bennekom,W.P.V., Vijgh,W.J.F.V.D.,Bast,A.,1996.FreeRadic.Biol.Med.20,331–342.
Villano,D.,Fernandez-Pachon,S.F.,Troncoso,A.M.,Garcia-Parrilla,C.,2005. Com-parisonofantioxidantactivityofwinephenoliccompoundsandmetabolites invitro.Anal.Chim.Acta538,391–398.
Vinson,J.A.,Dabbagh,Y.A.,Serry,M.M.,Jang,J.,1995.Plantflavonoids,especiallytea flavonols,arepowerfulantioxidantsusinganinvitrooxidationmodelforheart disease.J.Agric.FoodChem.43,2800–2802.
Wang,I.-K.,Lin-Shiau,S.-Y.,Lin,J.-K.,1999.Inductionofapoptosisbyapigeninand relatedflavonoidsthroughcytochromecreleaseandactivationofcaspase-9and caspase-3inleukaemiaHL-60cells.Eur.J.Cancer35,1517–1525.
Wang,S.,Meckling,K.A.,Marcone,M.F.,Kakuda,Y.,Tsao,R.,2011.Can phytochem-icalantioxidantrichfoodsactasanti-canceragents?FoodRes.Int.44,2545– 2554.
Weisburger,J.H.,Rivenson,A.,Garr,K.,Aliaga,C.,1997. Tea,orteaandmilk, inhibitmammarygland andcoloncarcinogenesisinrats.CancerLett.114, 1–5.
Wolfe,K.L.,Liu,R.H.,2008.Structure–activityrelationshipsofflavonoidsinthe cel-lularantioxidantactivityassay.J.Agric.FoodChem.56,8404–8411.
Xiong,N.,Huang,J.,Chen,C.,Zhao,Y.,Zhang,Z.,Jia,M.,Zhang,Z.,Hou,L.,Yang,H.,Cao, X.,Liang,Z.,Zhang,Y.,Sun,S.,Lin,Z.,Wang,T.,2012.Dl-3-n-butylphthalide,a nat-uralantioxidant,protectsdopamineneuronsinrotenonemodelsforParkinson’s disease.Neurobiol.Aging33,1777–1791.
Ye,F.,Wu,J.,Dunn,T.,Yi,J.,Tong,X.,Zhang,D.,2004.Inhibitionofcyclooxygenase-2 activityinheadandneckcancercellsbygenistein.CancerLett.28,39–46. Yen,G.-C.,Chen,H.-Y.,1995.Antioxidantactivityofvariousteaextractsinrelation
totheirantimutagenicity.J.Agric.FoodChem.43,27–32.
Yuting,C.,Rongliang,Z.,Zhongjian,J.,Yong,J.,1990.Flavonoidsassuperoxide sca-vengersandantioxidants.FreeRadic.Biol.Med.9,19–21.
Ziech,D.,Anestopoulos,I.,Hanafi,R.,Voulgaridou,G.P.,Franco,R.,Georgakilas,A.G., Pappa,A.,Panayiotidis,M.I.,2012.Pleiotrophiceffectsofnaturalproductsin ROS-inducedcarcinogenesis:theroleofplantderivednaturalproductsinoral cancerchemoprevention.CancerLett.327,16–25.