*Correspondence: Amelia Avachat. Sinhgad college of Pharmacy. Vadgaon (Bk.) - Pune 411041 - India. E-mail: prof_avachat@yahoo.com
A
vol. 51, n. 3, jul./sep., 2015 http://dx.doi.org/10.1590/S1984-82502015000300018
Formulation and characterization of self emulsifing pellets of
carvedilol
Vikas Bhandari, Amelia Avachat
*Sinhgad College of Pharmacy, Vadgaon (Bk.), India
The purpose of present study was aimed at developing self emulsifying drug delivery system in liquid and then in pellet form that would result in improved solubility, dissolution and permeability of the poorly water soluble drug carvedilol. Pellets were prepared using extrusion-spheronization technique incorporating liquid SEDDS (carvedilol, capmul MCM EP, cremophore EL, tween 20, propylene glycol), adsorbents ( and crospovidone), microcrystalline cellulose and binder (povidone K-30). Ternary
phase diagram was constructed to identify diferent oil-surfactant-cosurfactant mixtures according to
the proportion of each point in it. The optimal CAR-SEDDS pellets showed a quicker redispersion with a droplet size of the reconstituted microemulsion being 160.47 nm, which was almost unchanged
after solidiication. SEM analysis conirmed good spherical appearance of solid pellets; DSC and XRD analysis conirmed that there was no crystalline carvedilol in the pellets. Pellets were then capable of transferring lipophilic compounds into the aqueous phase and signiicantly enhancing its release with
respect to pure drug.
Uniterms: Drugs/self emulsifying/delivery system. Pellets/self emulsifying/preparation. Pellets/self emulsifying/delivery system. Carvedilol/pharmacokinetics.
O propósito do presente estudo foi desenvolver um sistema líquido de liberação de fármacos auto
emulsiicante e, então, na forma de pélete, que poderia resultar em aprimoramento da solubilidade, da dissolução e permeabilidade do fármaco carvedilol, pouco solúvel em água. Os péletes foram preparados utilizando-se a técnica de extrusão-esferonização, incorporando líquido SEDDS (carvedilol,
capmul MCM EP, Cremofor EL, Tween 20, propileno glicol), adsorventes (e crospovidona), celulose
microcristallina e ligante (povidona K-30). O diagrama de fase ternário foi construído para identiicar as
misturas diferentes de óleo-tensoativo-co-tensoativo, de acordo com a proporção em cada ponto delas.
Os péletes CAR-SEDDS mostraram redispersão mais rápida, com tamanho de gota da microemulsão reconstituída de 160,47 nm, que se mostrou quase inalterada após a solidiicação. A análise por SEM conirmou a aparência esférica dos péletes sólidos. Análise por DSC e XRD conirmou que não havia carvedilol cristalino nos péletes. Estes foram, então capazes de transferir os compostos lipofílicos para a fase aquosa, aumentando, signiicativamente, sua liberação em relação ao fármaco puro.
Unitermos: Fármacos/sistema líquido de liberação auto emulsiicante. Péletes/auto emulsiicante/ preparação. Péletes/auto emulsiicante/sistema líquido de liberação. Carvedilol/farmacocinética.
INTRODUCTION
In recent years, an increasing number of new chemical entities and many already existing drugs show low aqueous solubility, which are prone to poor oral absorption, high intra-inter-subject variability and lack of dose availability. Thus, to increase solubility of drugs
which are mainly BCS II type, various solubilization techniques or formulation strategies have been developed like the use of cyclodextrins, nanoparticles, solid dispersions, permeation enhancers, liposomes, etc. (Wang et al., 2010).
that are capable of forming oil-in-water emulsions upon gentle agitation provided by the GIT motion (Abdalla, Klein, Mader, 2008).
However, SEDDS are present in the liquid state, which can be packed in hard or soft gelatin capsule directly but suffer from the drawbacks related to stability and compatibility. Incorporation of liquid SEDDS into solid dosage form is therefore compatible and desirable where spray drying, self emulsifying granules, tablet or pellets can be a preferred and viable option (Iosio et al., 2008).
Pellets have many advantages, over conventional
solid dosage forms viz; flexibility in designing and
developing the dosage form and improving the safety and efficacy. Because the pellets disperse freely in the gastrointestinal tract, drug absorption is maximized with a subsequent reduction in peak plasma fluctuations and
hence minimizing potential side efects without lowering
drug bioavailability. The pellets also reduce variations in gastric emptying rates and overall transit time and therefore a reduction of intra and inter-subject variability of plasma
proiles is achieved. In addition, pellets reduce the problem
of high local concentration of drugs and thus avoiding irritation that may be caused by certain active constituents. The most widely used techniques for pellet production in the pharmaceutical industry are extrusion/spheronization (ES), solution/suspension layering, and powder layering. The process of ES has become the method of choice in the preparation of pellet-based dosage forms since it offers many advantages over the other methods, including the
spherical shape with a narrow size distribution, good low
properties, low friability, uniform packing characteristics and reproducible scalability (Abdalla, Mader, 2007).
Carvedilol is a non-selective beta-blocker indicated in the treatment of mild to moderate congestive heart failure (CHF). It blocks beta-1 and beta-2 adrenergic receptors as well as the alpha-1 adrenergic receptors and is classified according to the Biopharmaceutical
Classiication System as a drug with low solubility and
is presented as an immediate-release dosage form in the WHO essential drug list. Carvedilol is available in the market in 3.125, 6.25, 12.5, and 25mg tablets. Its serum concentration is not only affected by its low solubility
but also by P-glycoprotein (P-gp) activity and irst pass
metabolism (http://www.drugbank.ca/drugs/DB01136). In this study, we developed a novel CAR-loaded SEDDS pellets using extrusion-spheronization technique. An efficient CAR-SEDDS formulation was evaluated by solubility study, self-emulsifying grading test, phase diagrams, and redispersibility study. Finally, the optimum pellets were characterized by scanning electron microscopy (SEM), differential scanning calorimetry
(DSC), X-ray difraction (XRD), and reconstitution test
(Hua et al., 2012).
MATERIAL AND METHODS
Material
C a r v e d i l o l w a s k i n d l y g i f t e d b y M e d l e y Pharmaceuticals Ltd. Capmul MCM EP, capmul PG12, captex 500, captex 300 were gifted by Abitec Corp.,
USA. Capryol 90, labrail M 2125 CS, labrail M 2130
CS, labrafac CC, labrasol, lauroglycol FCC, transcutol P were supplied by Gattefosse, France. Oleic acid, tween 80, tween 20, span 20, span 80, propylene glycol, PEG 400, PEG 200 was purchased from Loba Chemie Pvt. Ltd., Mumbai. Cremophore EL, cremophore RH 40 were obtained from Signet Chemicals, Mumbai. Polaxamer 407, solutol HS 15 was supplied by BASF. MCC PH 101 was obtained from RanQ Pharmaceuticals Nashik. Crospovidone, povidone K-30 and were obtained from and used in R & D Medley Pharmaceuticals Ltd., Mumbai.
Solubility of carvedilol
The solubility of carvedilol in various oils, surfactants
and co surfactants was determined by using the shake lask
method. An excess amount (50 mg) of carvedilol was added to each vial containing 1ml of the selected vehicle i.e. oil, surfactant or cosurfactant. Oils used were capmul MCM
C8, capryol 90, labrail M 2125, labrail M 2130, captex
500, captex 300, oleic acid, capmul PG 12, capmul MCM EP. Surfactants was selected from labrafac, cremophore EL, cremophore RH 40, labrasol, tween 20, tween 80, solutol
HS 15, polaxamer 407; while propylene glycol, PEG 400,
transcutol P, span 20, PEG 200, lauroglycol FCC, span 80 were used as cosurfactants. After sealing of each vial proper mixing of carvedilol with the vehicles was done in order to facilitate solubilization. Mixtures were shaken for 48 h in a mechanical shaker maintained at room temperature. After 48h each vial was centrifuged at 5,000 rpm for 10min.
Undissolved carvedilol was removed by iltering through 0.44 µ Whatman ilter paper. Aliquots of iltrate were diluted
with methanol, and the concentration of dissolved carvedilol
was quantiied by U.V. Spectrophotometer at 285 nm (Hua
et al., 2012).
Selection of surfactant and cosurfactant
The self - emulsiication ability of surfactants was
assessed to select the best surfactant from a large pool of
mixed in 1:3 (v/v) and vortexed to form a homogenous mixture (Borhade, Nair, Hedge, 2008). The ratio of oil to surfactant was decided on the basis of the requirements stated by Pouton for spontaneously emulsifying systems and represents a Type III system. Oil-surfactant mixture, 1 mL dispersed in 100 mL of double distilled water in a glass beaker was mixed with gentle stirring. Visual test
was used to assess self-emulsiication of surfactants in terms of dispersibility, ease of emulsiication and inal
appearance using a grading system as mentioned below. Various cosurfactants were screened by mixing them with the selected surfactants in 1:2 v/v ratio. An oily phase was added to each of this (S+Co-S) mixture in 1:3 (v/v) and vortexed gently to form a homogenous mixture. They were evaluated using a visual test as explained below. The emulsions were allowed to stand for 2 h and their transmittance was measured at 638.2 nm by UV-1800 double beam spectrophotometer (Shimadzu, Japan) using double distilled water as blank (Date, Nagarsenker, 2007).
Self emulsifying grading test
The selected oil, surfactants, and cosurfactants were
homogenized at diferent ratios (4:4:2, 3:4:3, 2:4:4 etc.
v/v/v) in eppendrof tubes by vortexing for 5 min. Then 1 mL of the mixture that has a transparent appearance was added to 100 mL water with magnetic stirring (50 rpm) to determine the emulsion forming process and final appearance, which has been divided into four grades using a visual grading system.
Grade A: Rapid forming micro emulsion, which is
clear or slightly bluish in appearance, self-emulsiication
time- less than 1 min.
Grade B: Rapid forming, slightly less clear emulsion,
which has a bluish white appearance, self-emulsiication
time-less than 2 min.
Grade C: Bright white emulsion (similar to milk
in appearance), self-emulsiication time-less than 2 min.
Grade D: Dull, grayish white emulsion with a slightly oily appearance that is slow to emulsify,
self-emulsiication time-more than 2 min.
Construction of ternary phase diagram
Ternary diagrams of surfactant, cosurfactant and
oil were plotted using ProSim software; each of them,
representing an apex of the triangle. Ternary mixtures with varying compositions of oil, surfactant, and cosurfactant were prepared. For any mixture, the total of surfactant, cosurfactant and oil concentrations always added to 100%. The large numbers of such mixtures with varying surfactant, cosurfactant and oil concentrations were prepared in this investigation as mentioned in Table I and Table II. The percentage of surfactant, cosurfactant and oil used herein was decided based on the requirements stated by Pouton for the spontaneously emulsifying systems. Compositions were evaluated for SEDDS formation by diluting 100µl of each of the mixtures to 10ml double distilled water. The globule size of the resulting dispersion
was determined by Nanophox (NX0088). Dispersions,
having globule size 250nm or below were considered
desirable. The area of SEDDS formation was identiied in
the ternary system based on the desired globule size (Date, Nagarsenker, 2007).
Formulation optimization of SEDDS
SEDDS were optimized for following parameters:
• Drug loading
• Amount of oily phase • Droplet size
Diferent batches of SEDDS were prepared as shown
in Table I and Table II. Oil, surfactant and cosurfactant were accurately measured and mixed using stirrer followed by vortex mixing until a solution (CAR-SEDDS) was obtained (Setthacheewakul et al., 2010).
Development of CAR-SEDDS pellets
Preparation of CAR-SEDDS pellets
SEDDS 20ml containing carvedilol 5% was
adsorbed on (speciic surface area 110 m2/g). This was
TABLE I - Diferent batches of SEDDS (primary batches)
Ingredients (µL) X1 X2 X3 X4 Y1 Y2 Y3 Y4
Capmul MCM EP 100 200 300 400 100 200 300 400
Cremophore EL 600 533 467 400 -- -- --
--Tween 20 -- -- -- -- 600 533 467 400
S/CoS ratio 2:1 2:1 2:1 2:1 2:1 2:1 2:1 2:1
further passed through mesh size 30 (595 microns) and mixed with microcrystalline cellulose PH 101, crospovidone, povidone K-30 in planetary mixture for
10min. Suicient quantity of water was added to form a
wet mass. This wet mass was then extruded through the extruder (Umang Pharmatech Pvt. Ltd. Mumbai, India) at 10 rpm which was then spheronised on spheroniser at 500 rpm for 2-5 min. Pellets were dried in hot air oven at 60 °C for 40 min. Dried pellets were passed through mesh size 16 (1190 microns) to get uniform sized pellets. Pellets were filled into size 0 hard gelatin capsules (Iosio et al., 2011).
Characterization of CAR-SEDDS and CAR-SEDDS pellets
Scanning electron microscopy (SEM)
The outer macroscopic structure of the pellets was examined by scanning electron microscopy (JEOL JSM-6360 A). Prior to microscopy, samples were coated with
carbon by sputtering for 2 min by autoine coater (JEOL
JFC-1600).The samples were scanned at a voltage of 10 kV (Zhang et al., 2012).
Differential scanning calorimetry (DSC)
The physical state of carvedilol in solid SEDDS
was characterized by the diferential scanning calorimetry
analysis by DSC-SIO6300, Japan. The sample (about 2.5 mg) was placed on standard aluminum pans, and dry
nitrogen was used as eluent gas. The sample was scanned
at a scanning rate of 10°C/ min between 40-260 °C and 40
ml/min nitrogen low (Prabagar et al., 2009).
X-ray powder diffraction (XRPD)
To verify the physical state of carvedilol in solid
SEDDS, X-ray powder scattering measurement were carried out with an X-ray difractometer (Bruker AXS,
DH Advanced, Germany) at room temperature using
monochromatic CuKα-radiation (k = 1.5406 Å) at 30 mA
and at 40 kV over a range of 2θ angles from 0 ° to 40 °
with an angular increment of 0.02 °per s (Yi et al., 2008).
Reconstitution study
Solid SEDDS 100 mg prepared as described above were dispersed in 10 mL distilled water, by vortex mixing (30 s), and then incubated for 30 min at 25 °C (Borhade,
Nair, Hedge, 2008). The particle size of reconstituted solid SEDDS emulsion was determined by Nanophox
(NX0088) (Yi et al., 2008).
In vitro dissolution Test
The quantitative in vitro release test was performed
in 500 mL of 0.1 N HCl using USP XXIV dissolution
apparatus 1. The basket was rotated at 50 rpm. The liquid and solid SEDDS formulations (pellets in hard gelatin capsules) were analysed to dissolution studies. Results were compared with those of plain drug and marketed formulation. During the release studies, 5 mL sample of the medium was taken out and subjected to drug analysis using UV spectrophotometrically at 285 nm. The removed volume was replaced each time with 5ml of fresh medium. Similarly, dissolution studies were also done in pH 4.6
acetate bufer and pH 6.8 phosphate bufer to study the efect of pH on carvedilol release.
Permeability studies
The methods employed were modiied with respect
to the experimental procedures described in the literature. To check the intraduodenal permeability, the duodenal part of the small intestine of the chick was isolated and taken for in vitro diffusion study. Then this tissue was thoroughly washed with Ringer’s solution to remove the mucous and lumen contents. The liquid SEDDS sample was diluted with 1 mL of distilled water while for the tablet and pellet sample a suspension was made in distilled water. The resultant sample (6.25 mg/mL) was injected into the lumen of the duodenum using a syringe, and two ends of intestine were tightly closed. Then the tissue was placed in a chamber of organ bath with continuous aeration. The
receiver compartment was illed with 30 ml of phosphate-bufer (pH 6.8). The absorbance was measured using a
UV-VIS spectrophotometer at a wavelength of 285 nm.
TABLE II - Diferent batches of SEDDS (inal batches)
Ingredients (µL) F1 F2 F3 F4 F5 F6
Capmul MCM EP 350 400 450 350 400 450
Cremophore EL 433 400 367 -- --
--Tween 20 -- -- -- 433 400 367
Propylene Glycol 217 200 183 217 200 183
Droplet Size (nm) 136.22±1.32 142.89±1.17 246.29±1.38 213.63±1.27 244.95±1.24 291.83±1.15
The percent difusion of the drug was calculated against
time and plotted on a graph (Ghosh et al., 2006).
RESULTS AND DISCUSSION
Solubility of carvedilol
Among the various oils higher solubility of carvedilol was found in capmul MCM EP, so it was selected as the oil phase. Data suggested that drug has more solubility in MCT rather than LCT because medium chain triglycerides (MCT) possess higher ester content per gram than long chain triglycerides (LCT), so drug has higher solubility in MCT than LCT Among the various surfactants, solubility of carvedilol was found highest in labrasol, cremophore EL and tween 20 while among the cosurfactants higher solubility was found in transcutol P, propylene glycol,
polyethylene glycol 200. According to self emulsiication
ability and % transmittance study cremophore EL and tween 20 were selected as sufactants and propylene glycol was selected as cosurfactant for further study. Solubility of carvedilol in various vehicles (oil, surfactant, cosurfactant) is shown in Figure 1.
Self-emulsifying grading test
It was seen that as the oil component increases in the formulation beyond a certain limit there was generation
of non-clear dispersion. Among the X1-X4 and Y1-Y4 the X3, X4, Y3, Y4 show grade B while X1, X2, Y1, Y2
exhibited grade A type emulsion. Formulation F1-F6 shows grade B type of emulsion.
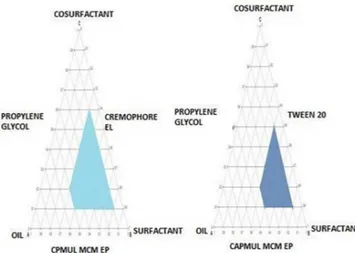
Ternary phase diagrams
Ternary phase diagrams of oil (capmul MCM EP), Surfactants (cremophor EL, tween 20) and cosurfactant (Propylene glycol) were constructed (Figure 2).The largest microemulsion region was observed when cremophore EL was used as surfactant. The coloured region indicates better
self-emulsiication region. Self-emulsion formation area
was increased with an increase in surfactant concentration.
Formulation optimization of SEDDS
Based on the ternary phase diagram, eight diferent
preliminary batches of SEDDS were formulated using capmul MCM as oil, cremophore EL and tween 20 as a surfactant and propylene glycol as cosurfactant (Table I). For further characterization through particle size analysis formulation, which contained cremophore EL as a surfactant showed lower particle size than other formulations. Different batches F1-F6 (Table II) were prepared using capmul MCM EP, cremophore EL, tween 20 and propylene glycol.
Though the batch containing 10% oil had the least particle size the required amount of carvedilol could not be solubilized into it. The same was the case with SEDDS containing 20 or 30% oil. Beyond 40% the globule size
FIGURE 1 - Solubility of carvedilol in various vehicles (oil, surfactant, cosurfactant).
was get increased which was not acceptable. So, according to % of oil content, drug loading and droplet size analysis formulation F2 and F5 were taken for further study.
Development of CAR-SEDDS pellets
Preparation of CAR-SEDDS pellets
Solid SEDDS were successfully prepared using colloidal silicon dioxide and microcrystalline cellulose PH 101 as a solid carrier, crospovidone as a disintegrant and povidone K-30 is as a binder. Solid pellets were prepared using an extrusion-spheronization technique and then
illed into 0 size gelatin capsule.
Characterization of CAR-SEDDS pellets
Scanning electron microscopy (SEM)
Pellet surfaces and cross-sections were studied using scanning electron microscope. As shown in Figure 3 SEM micrographs of carvedilol-SEDDS pellets reveal that the pellets had a spherical shape. Carvedilol-SEDDS appear to be entrapped in the matrices. The images show that the surface of the pellets is full of pinholes, which probably allow the ingress of the aqueous phase into the matrix, and
allow difusion of the entrapped carvedilol from SEDDS
out of the surface and the core of the matrix. Due to this, the liquid carvedilol-SEDDS is released through the mini-channels and then forms oil-in-water microemulsion thus making carvedilol ready for absorption as an oily droplet solution.
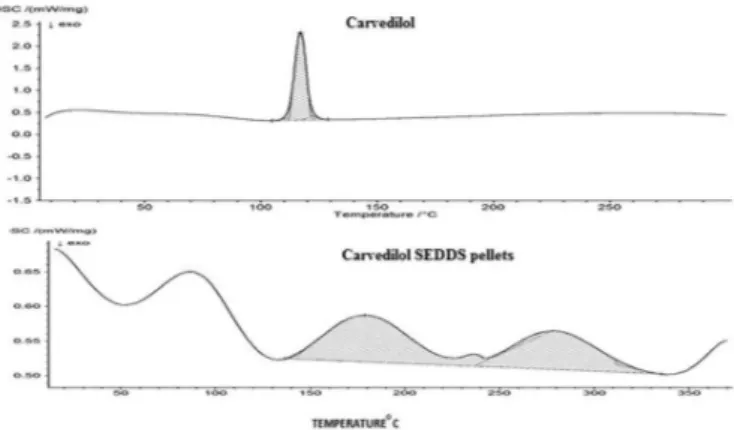
Differential scanning calorimetry (DSC)
The physical state of carvedilol in the solid SEDDS was investigated since it would have an important
inluence on the in vitro and in vivo release characteristics. DSC curves of pure carvedilol and the solid SEDDS of carvedilol are shown in Figure 4. Pure carvedilol showed a sharp endothermic peak at a temperature of 116 °C corresponding to its melting point. No obvious peak of the drug was found in the solid SEDDS of carvedilol, indicating that the drug must be present in the molecularly dissolved state in solid SEDDS.
X-ray powder diffraction (XRPD)
From X-ray powder diffractograms shown in
Figure 5, the internal physical state of carvedilol in the solid SEDDS was further verified. No obvious peaks representing crystals of carvedilol were seen for the solid SEDDS, indicating that the drug is present in the molecularly dissolved state in solid SEDDS (Dixit, Nagarsenker, 2008).
Reconstitution properties of CAR-SEDDS pellets
The average droplet size of emulsion from both liquid and solid SEDDS was less than 250 nm. The droplet size of the emulsion from the solid SEDDS was
no signiicantly diferent from the droplet size of the liquid
SEDDS. From these results, incorporating the liquid SEDDS in colloidal silicon dioxide and MCC PH 101 by extrusion and spheronization did not have a remarkable effect on droplet size. The solid SEDDS preserved the self-emulsification performance of the liquid SEDDS. Droplet size of liquid SEDDS is 142.89 nm and that of solid SEDDS is 160.47 nm.
The mean droplet size of the diluted SEDDS preconcentrates was low, and all were found to be in the
FIGURE 3 - SEM images of pellets and pellet surface.
FIGURE 4 - DSC of carvedilol and CAR SEDDS pellets.
nanometric range (20-250nm). Droplets size results of SEDDS formulation are reported in Table III. In all eight formulations, which were tested, the droplet size increased upon decreasing ammount of S+Co-Smix. SEDDS shows good uniformity in the droplet size distribution even after diluted with water.
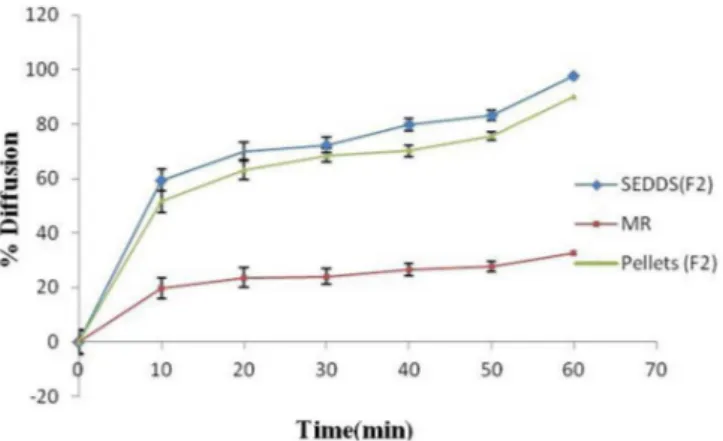
In vitro dissolution Test
As shown in Figure 6, the release performance of CAR from solid and liquid SEDDS was significantly improved compared to conventional tablets and plain drug. From the in vitro dissolution studies, it was observed that, plain drug showed very less release (46%) after 60 min, 6.25 mg immediate release tablet showed only 68% release after 60 min, whereas, liquid SEDDS showed a rapid release of 75% (F2) and 65% (F5) in 0.1 N HCl in just 10 min and 86%(F2) and 80%(F5) in 20 min. Solid SEDDS showed a release of 90% (F2) and 80% (F5) within 30 min only.
Figure 7 illustrates that, % drug release from CAR SEDDS pellets was 100% and surprisingly same at
diferent pH conditions viz; 0.1 N HCl, pH 4.6 and pH 6.8 bufer while plain drug does not show it. This indicates that
SEDDS has rendered the release of carvedilol independent of the pH which is not so with the plain drug nor with the marketed product.
Carvedilol as such is reported to have pH dependant
solubility, maximum solubility being in the acidic pH. One of the objectives of the present study was also to
nullify the efect of pH on the release of carvedilol so as to
achieve uniform drug release through GIT. So, the release of carvedilol, the prepared CAR-SEDDS and the marketed
product was seen in diferent pH conditions, which mimic
the in-vivo conditions.
Permeability studies
In vitro intestinal permeability data are shown in
Figure 8. The drug difused at a faster rate from the SEDDS
than from the tablet dosage form. The total percentage
difusion was much higher for the SEDDS than for the tablet dosage form. After 1 hour of difusion, 97.8% of the drug was difused from the liquid SEDDS, as compared
to 91% from the CAR-SEDDS pellets and 32% from marketed tablet.
TABLE III - Droplet size of SEDDS
Batch X1 X2 X3 X4 Y1 Y2 Y3 Y4
Droplet
size(nm) 23.41±0.64 33.41±0.55 130.89±0.68 144.84±1.27 37.62±0.58 69.25±0.67 196.33±0.64 249.58±1.38
FIGURE 6 - In vitro Dissolution release study of marketed drug, plain drug and CAR-SEDDS pellet formulations F2 & F5 in 0.1 N HCl, where, L F2-SEDDS F2,L F5-SEDDS F5,S F2-solid SEDDS F2,S F5-solid SEDDS F5, MR-marketed formulation, Plain-plain drug tablet.
FIGURE 7 - Efect of pH on carvedilol release.
CONCLUSION
Carvedilol has only 25-35% absolute bioavailability
due to high irst pass metabolism and insolubility in water.
The present research work was directed towards the development of SEDDS which were incorporated into pellets, which would increase solubility and permeability of carvedilol.
Type of oil, surfactant and cosurfactant concentration play a vital role in SEDDS formation. Based on the solubility studies capmul MCM EP was selected as oil phase, cremophore EL, tween 20 was selected as the surfactant and propylene glycol was selected as cosurfactant based on its emulsification properties and the resulting droplet size.
Fastest reconstitution and smallest particle size was achieved with formulation F2, in which the liquid SEDDS had a droplet size of 142.89nm and solid SEDDS had a droplet size of 160.47nm.
Conclusively, a SEDDS consisting of capmul MCM EP (40% v/v) emulsified with cremophore EL (40% v/v) and propylene glycol (20%v/v) was successfully developed with an increased dissolution rate, increased solubility and ultimately, increased permeability of a poorly water soluble drug, carvedilol. The liquid CAR SEDDS were converted into solid pellets by using the extrusion spheronization technique. The SEM characterization of SEDDS pellets revealed spherical shape of pellets. The developed formulation exhibited significantly higher dissolution property compared to 6.25mg immediate release marketed tablet.
Thus, the SEDDS pellets could be an effective formulation strategy for other oil-soluble drugs with low oral absorption.
LIST OF ABBREVIATIONS
CAR-Carvedilol
SEDDS-Self emulsifying drug delivery system SEM-Scanning electron microscopy
XRD-X ray difraction
ES-Extrusion-spheronization
DSC-Diferential scanning calorimetry
rpm-Revolutions per minute nm- Nanometer
GIT- Gastro intestinal tract
ACKNOWLEDGEMENT
Authors are thankful to Medley Pharmaceuticals,
Mumbai; Abitec Corp., USA, Gattefosse, France, Signet
chemicals, India, for providing gift samples.
REFERENCES
ABDALLA, A.; KLEIN, S.; MADER, K. A new
self-emulsifying drug delivery system (SEDDS) for poorly soluble drugs: Characterization, dissolution, in vitro
digestion and incorporation into solid pellets. Eur. J. Pharm. Sci., v.35, n.5, p.457-464, 2008.
ABDALLA, A.; MADER, K. Preparation and characterization
of a self-emulsifying pellet formulation. Eur. J. Pharm. Biopharm., v.66, n.2, p. 220-226, 2007.
BORHADE, V.; NAIR, H.; HEGDE, D. Design and Evaluation of
Self-microemulsifying Drug Delivery System (SMEDDS) of tacrolimus. AAPS PharmSciTech., v.9, n.1, p.13-21, 2008.
DATE, A.A.; NAGARSENKER, M.S. Design and evaluation
of self-nanoemulsifying drug delivery systems (SNEDDS) for cefpodoxime proxetil. Int. J. Pharm., v.329, n.1-2, p.166-172, 2007.
DIXIT, R.; NAGARSENKER, M. Self-nanoemulsifying
granules of ezetimibe: Design, optimization and evaluation.
Eur. J. Pharm. Sci., v.35, n.3, p.183-192, 2008.
G H O S H , P. K . ; M A J I T H I YA , R . J . ; U M R E T H I A , M.L.;MURTHY, S.R. Design and development of
microemulsion drug delivery system of acyclovir for improvement of oral bioavailability. AAPS PharmSciTech., v.7, 3, p.E1-E6, 2006.
HUA, X.;LIN, C.;CHEN, D.; ZHANG, J.; LIU, Z.; WUB, W.;SONG, H. Sirolimus solid self-microemulsifying
pellets: Formulation development, characterization and bioavailability evaluation. Int. J. Pharm., v.438, n.1-2, p.123-33, 2012.
IOSIO, T.; VOINOVICH, D.; GRASSI, M.; PINTO, J.F.; PERISSUTTI, B.; ZACCHIGNAA, M.; QUINTAVALLE, U.; SERDOZ, F. Bi-layered self-emulsifying pellets prepared by co-extrusion and spheronization: Inluence of formulation
variables and preliminary study on the in vivo absorption.
Eur. J. Pharm. Biopharm., v.69, n.2, p.686-697, 2008.
IOSIO, T.;VOINOVICH, D.; PERISSUTTI, B.; SERDOZ, F.; HASA, D.; GRABNAR, I.; ACQUA, S.D.; ZARAD, G.P.; MUNTONI, E.; PINTO, J.F. Oral bioavailability of
PRABAGAR, B.; LEE, B.J.; OH, D.H.; KIM, J.O.; HONG, M.J.; JEE, J.P.;KIM, J.A.; YOO, B.K.; WOO, J.S.; YONG, C.S.; CHOI, H.G. Enhanced oral bioavailability
of dexibuprofen by a novel solid self-emulsifying drug delivery system (SEDDS). Eur. J. Pharm. Biopharm., v.72, n.3, p.539-545, 2009.
SETTHACHEEWAKUL, S.; MAHATTANADUL, S.; PHADOONGSOMBUT , N.; PICHAYAKORN, W.;
WIWATTANAPATAPEE, R. Development and evaluation of self-microemulsifying liquid and pellet formulations of curcumin, and absorption studies in rats. Eur. J. Pharm. Biopharm., v.76, n.3, p.475-485, 2010.
WANG, Z.; SUN, J.; WANG, Y.; LIU, X.; LIU, Y.; FU, Q.; MENG, P.; HE, Z. Solid self-emulsifying nitrendipine
pellets: Preparation and in vitro/in vivo evaluation. Int. J. Pharm., v.383, n.1-2, p.1-6, 2010.
YI, T.; WAN, J.; XU, H.; YANG, X. A new solid
self-microemulsifying formulation prepared by spray-drying to improve the oral bioavailability of poorly water soluble drugs. Eur. J. Pharm. Biopharm., v.70, n.2, p.439-444, 2008.
ZHANG, Y.; WANGA, R.; WUA, J.; SHEN, Q. Characterization
and evaluation of self-microemulsifying sustained-release pellet formulation of puerarin for oral delivery. Int. J. Pharm., v.427, n.2, p.337-344, 2012.
Received for publication on 25th September 2013