w w w . s b f g n o s i a . o r g . b r / r e v i s t a
Original
Article
Biofilm
inhibition
activity
of
compounds
isolated
from
two
Eunicea
species
collected
at
the
Caribbean
Sea
Yenny
Martínez
Díaz
a,
Gina
Vanegas
Laverde
b,
Luis
Reina
Gamba
b,
Humberto
Mayorga
Wandurraga
c,∗,
Catalina
Arévalo-Ferro
d,
Freddy
Ramos
Rodríguez
b,
Carmenza
Duque
Beltrán
b,
Leonardo
Castellanos
Hernández
baUniversidadNacionaldeColombia,SedeBogotá,FacultaddeCiencias,PosgradoInterfacultadesdeMicrobiología,BogotáD.C.,Colombia
bUniversidadNacionaldeColombia,SedeBogotá,FacultaddeCiencias,DepartamentodeQuímica,GrupodeEstudioyAprovechamientodeProductosNaturalesMarinosyFrutasde
Colombia,BogotáD.C.,Colombia
cUniversidadNacionaldeColombia,SedeBogotá,FacultaddeCiencias,DepartamentodeQuímica,GrupodeProductosNaturalesVegetalesBioactivosyQuímicaEcológica,
BogotáD.C.,Colombia
dUniversidadNacionaldeColombia,SedeBogotá,FacultaddeCiencias,DepartamentodeBiología,GrupodeComunicaciónyComunidadesBacterianas,BogotáD.C.,Colombia
a
r
t
i
c
l
e
i
n
f
o
Articlehistory:
Received30April2015 Accepted9August2015
Availableonline26September2015
Keywords:
Anti-biofilm
Euniceafusca Euniceasp. Lipidcompounds Terpenoids
Marinenaturalproducts
a
b
s
t
r
a
c
t
Biofilmhasaprimaryroleinthepathogenesisofdiseasesandintheattachmentofmulticellularorganisms toafouledsurface.Becauseofthat,thecontrolofbacterialbiofilmshasbeenidentifiedasanimportant target.Inthepresentstudy,fivelipidcompoundsisolatedfromsoftcoralEuniceasp.andthreeterpenoids togetherwithamixtureofsterolsfromEuniceafuscacollectedattheColombianCaribbeanSeashowed differenteffectivenessagainstbiofilmformationbythreemarinebacteriaassociatedwithimmersed fouledsurfaces,Ochrobactrumpseudogringnonense,AlteromonamacleodiiandVibrioharveyi,andagainst twoknownbiofilmformingbacteria,PseudomonasaeruginosaATCC27853andStaphylococcusaureus
ATCC25923.ThepurecompoundswerecharacterizedbyNMR,HRESI-MS,HRGC-MSandopticalrotation. Themosteffectivecompoundswerebatylalcohol(1)andfuscosideEperacetate(6),actingagainstfour strainswithoutaffectingtheirmicrobialgrowth.Compound1showedbiofilminhibitiongreaterthan30% againstA.macleodii,andupto60%againstO.pseudogringnonense,V.harveyiandS.aureus.Compound 6inhibitedO.pseudogringnonenseandV.harveyibetween25and50%,andP.aeruginosaorS.aureusup to60%at0.5mg/ml.Theresultssuggestthatthesecompoundsexhibitspecificbiofilminhibitionwith lowerantimicrobialeffectagainstthebacterialspeciesassayed.
©2015SociedadeBrasileiradeFarmacognosia.PublishedbyElsevierEditoraLtda.Allrightsreserved.
Introduction
Allimmersedsurfacesaresubjecttocolonizationby microor-ganismsasbacteria,microalgaeandthenmacroorganismssuchas invertebrates,ina complexlayercalledbiofouling(Vianoetal., 2009).Bacteria arethefirst colonizersand theirbiofilm forma-tiontakesplaceinmultiplestages(Jadhavetal.,2013).Biofilms areabundantinnatureandaredevelopedwhenplanktonic bacte-riaadheretoasurfaceandinitiatetheformationofamicrocolony thatexistsasacommunityencasedinanextracellularmatrixthat confersahighdegreeofprotection(O’tooleetal.,2000;Huigens etal.,2007).
∗ Correspondingauthor.
E-mail:hmayorgaw@unal.edu.co(H.MayorgaWandurraga).
In marine environments, biofilms are formed by bacteria, diatomsandprotozoa(Vianoetal.,2009;Dobretsovetal.,2013). Bacterialbiofilmsareknownforbeingimplicatedinthe regula-tionofa subsequentsettlementbyinvertebratelarvae,mediate larval metamorphosis, provide suitable food source to newly metamorphosed juveniles,and serve asbiofilterstoabsorband biodegradeexcessivenutrients(Yang etal.,2014).Ontheother hand, biofilms are prevalent in medical, dental, industrial and marineenvironmentalsettings,wheretheyareundesirabledueto theirpathogenicity,andresistancetowardantimicrobialagentsor anti-biofoulingtechnologies(Dusaneetal.,2011;Rautetal.,2013). Inthissense,inhibitionofbiofilmsmayplayanimportantrolein biofoulingprevention(Quinnetal.,2012;Dobretsovetal.,2013).
Althoughadiversityofstrategieshasbeendevelopedtocontrol biofouling,thesearchfornovelandeffectivetechnologies contin-ues(Dusane etal.,2011).Oneofthemostecologicallyrelevant antifoulingstrategiesistoapplythechemicaldefensesofsessile
http://dx.doi.org/10.1016/j.bjp.2015.08.007
marineorganisms that keep theirbody surfacesfree of fouling (Jadhavetal.,2013;MajikandParvatkar,2014).Those antifoul-ingcompoundscouldbeisolatedfromsponges,algae,bryozoans, andcorals,whichproducesuchcompoundspresumablyasameans ofprotectionfrompredationorfoulingortoreducecompetition forspace(Mora-Cristanchoetal.,2011).Theuseofthesemarine naturalproductsismore favoredthancommonly usedbiocides becauseof theirbiocompatibility, stability, biodegradabilityand lowtoxicityunderdifferentenvironmentalconditions(Qietal., 2008;Dusaneetal.,2011;Telloetal.,2011).Therefore,continuous searchesforantifoulingcompoundsfrommarineinvertebratesof theColombianCaribbeanSeahavebeenreported(Mayorgaetal., 2011;Telloetal.,2011,2012;Cuadradoetal.,2013).However,an anti-biofilmpossibilityofthecompoundsassayedinthepresent investigationhasnotbeenstudiedbefore.
Theaimofthepresentstudywastoevaluatetheanti-biofilm activityoflipidsandterpenoidcompoundsisolatedfromEunicea sp.andEuniceafuscaDuchassaingandMichelotti(phylumCnidaria, class Anthozoa, subclass Octocorallia, order Alcyonacea, family Plexauridae),collectedatSantaMartaBay(Colombia),againstthree marinebacteriaandtwoknownbiofilmformingbacteriabythe microtiterplatemethod.
Materialsandmethods
Generalexperimentalprocedures
Forhigh-resolutionelectrospraymassspectrometry(HRESIMS), a LC-MS-IT-TOF was used on positive mode, and for ESIMS a LCMS-2010-ESI(Shimadzu,Tokyo,Japan)waslikewiseused. Opti-calrotation wasmeasuredona Polax-2L(Atago,Japan)and an ADP440(Bellingham+Stanley,USA).TheNMRspectraat400MHz for1Hand100MHzfor13CandDEPTinCDCl
3 wererecordedon aBrukerAvance400spectrometer,withTMS.Highperformance liquidchromatographyHPLCwasperformedonaMerck-Hitachi (L-4250UV/Visdetector,USA)at210nm,andonaLunaC18column 250mm×4.60mmi.d.×5m(Phenomenex).Forgas
chromatog-raphy mass spectrometry HRGCMS, Agilent equipment model 7890A-5975C with a DB-1 (30m×250m×0.25m) capillary
columnwasemployed.Allsolventsusedwereanalyticalgrade.
Coralmaterial
Euniceasp.andEuniceafuscaDuchassaingandMichelotti, sam-pleswerecollectedbyhandatSantaMartaBay(Latitude11.25N, Longitude74.2W)in2007,throughscubadivingat6–14mdeepby Prof.Dr.S.Zea,andvoucherspecimensweredepositedatthe Insti-tutodeCienciasNaturalesdelaUniversidadNacionaldeColombia codedasICN-MHN-PONo.250andNo.252respectively.The col-lectionof freshcoral colonies wassuperficiallyair-driedin the shade,immediatelyfrozenandwasthentransportedbyairplane tolaboratoryinBogota,wherewaskeptinfreezeruntilextraction.
Extractionandisolationofcompounds
ThesoftcoralEuniceasp.wascutintosmallpiecesandwas partiallyair-dried(wetweight850g,600gdryweight),extracted threetimeswithdichloromethane:methanol(1:1)atroom tem-perature.Thecombinedextractswereconcentrated byrotatory evaporation,andthecrudeextract(25g)waspartitionedbetween dichloromethane:water(1:1).Theorganicfraction(15g)was sub-jectedtosilicagel,columnchromatography(CC)withsolventsof increasingpolarityhexane,benzene,ethylacetateandmethanol, toobtainfifteenfractions(F1–F15)inabioguidedmannerusingan antimicrobialtestwithseveralmarinebacteriaisolatedfromheavy
colonizedsurfaces byMora-Cristancho etal. (2011).The bioac-tivefractionswerefurtherfractionatedoverSiO2CCasfollows.F4 (0.3g),elutedwithhexane-benzene(1:1),waschromatographed using an elution gradient with hexane:benzene (10:0–0:10) to givecompound 3 (60mg).Fraction F7(0.8g), eluted with ben-zene:ethylacetate(9:1),wassubjectedtoCCwithbenzene-ethyl acetate(10:0–2:8)andsubfractionF7.1waspurifiedbyHPLCusing methanoltoobtaincompounds 4(5mg)and 5(7mg).Fraction F10 (1.1g), eluted with benzene:ethylacetate (2:8),was sepa-ratedonCCusingbenzene-ethylacetate(5:5–0:10).Subsequently, subfractionF10.6,elutedwithbenzene:ethylacetate(4:6), con-tainscompound1(150mg)andsubfractionF10.9,waspurifiedby reverse-phaseCC(LichroprepRP-18,Merck)andfinallybyHPLCto affordcompound2(9mg).
FromE.fusca,thefollowingproductswerepreviouslyisolated: fuscosideE,fuscosideB(7),(+)-germacreneD(8),themixtureof sixsterols(9–14),andthecompoundfuscosideEperacetate(6)was semi-synthesized(Reinaetal.,2011).
Compoundsidentificationandfattyacidanalysis
To determinethe structure of compounds from Euniceasp., opticalrotation,NMR, ESIMSand HRESIMSdatawererecorded. Compounds2,4and5weresubjectedtomethanolysisaspreviously reported(Ramosetal.,2006),andthemixtureoffattyacidmethyl esterswasanalyzedbyHRGCMSasdescribedearlier(Castellanos etal.,2010).
Bacterialstrains
Two reference gram-positive strains, Staphylococcus aureus (ATCC 25923)and Staphylococcus epidermidis (ATCC 51400310) and two gram-negative Pseudomonas aeruginosa (ATCC 27853) andKlebsiellapneumoniae(206),commonlyreportedasbiofilms forming strains, were used (Quinn et al., 2012). They were suppliedbythemolecularepidemiologylaboratoryofthe Biotech-nologyInstitute(UniversidadNacionalde Colombia).Wildtype marinebacteriaassociated withfouledsurfaceswerealsoused; thesegram-positivestrains:Kokuriasp.(10-4DEP),Bacillus mega-terium(16-Ains5)andOceanobacillusiheyensis(31-C),andthese gram-negative strains: Vibrio campbellii (6-8PIN),Ochrobactrum pseudogringnonense(4-4DEP),Alteromonamacleodii(29-C)and Vib-rioharveyi(Phy-2A),aswasreportedforMora-Cristancho etal. (2011).
Microtiterplatebiofilmproductionassay
Inordertoselectthemostsuitablereferencebacteriaas con-trolfortheassaywithmarinestrains,thebiofilmformationwas testedbytheirabilitytoadheretowellsofpolystyrene(353072-BD FalconTMClear96-well)usingamodificationofprotocolbyO’toole
etal.(2000).Pre-inoculumsweregrownintrypticasesoy broth
(TSB)toanopticaldensityat600nm(OD600)of0.2–0.3.Fromeach culturedbroth,20lwasinoculatedtothewellsandincubatedfor
24hand48hat37◦C.Afterremovaloftheplanktoniccellsby
inordertoestablishwhetherthechangefrom37◦Cto25◦Chad
affectedtheirbiofilmformation,forthesubsequentassayunder thoseconditionswithmarinestrains.
Biofilminhibitionactivityassay
Threemarinestrains:O.pseudogringnonense,A.macleodiiand V.harveyi,andtworeferencestrains:P.aeruginosaandS.aureus, selectedbecauseoftheirstrongbiofilm-formingabilityconfirmed in this study,were usedin the assayof compounds (1–14), as previouslyreported(Telloetal.,2011),andfollowingthebiofilm productionassaydescribedabove.Pre-inoculumsweregrownto anOD600of0.1–0.2inmarinebroth(MB)andTSBrespectively. Twentylofinoculated cultureweremixed withtwodifferent
aliquot, 100g/wellor 5g/well ofpurecompound completed
withmediumtoconcentrationsat0.5mg/mlor0.025mg/ml,and incubatedfor48hat25◦C.Planktoniccellswerediscarded,and
finallythedifferentialstainingwasquantifiedusingaCVassay.The valuesareexpressedintermsofthepercentageofbiofilminhibited incomparisontotheuntreatedcontrolbiofilms(positivecontrol). Thepercentageofgrowthinhibitionwascalculatedforeachassay asacontrol.Additionally,toevaluatethecompounds’effectonthe remainingviablebacteria,aftertheassay,10lofthesuspension
fromeachwellwasinoculatedintoLuriaBertani(LB)agarplate solidmedium,andbacterialgrowthwastested.
Antimicrobialactivityassay
BioguidedseparationofcompoundsfromEuniceasp.was per-formedagainstthesevenmarinestrainssetupasdescribedby
Mora-Cristancho et al. (2011). Moreover, the concentration at
which compounds(1–14)couldbebactericidalwasestablished throughamodificationoftheKirby-Bauerdiskdiffusion suscep-tibilitytesting(Wilkinsetal.,1972),usingtworeferencestrains, K.pneumoniaeandS.aureus,andtwomarinestrains,A.macleodii andV.harveyi.LBsolidagarplateswereinoculatedwiththetested microorganisms.Thenfilterpaperdisksof5mmdiameter impreg-natedwithdifferentamountsofcompounds(3g/disk,7.5g/disk,
15g/diskand30g/disk)wereplacedontheinoculatedplates
andincubatedat37◦Cforreferencestrainsorat25◦Cformarine
strains.Thegrowthinhibitionhalosweremeasuredfor24and48h ofincubation.
Statisticalanalyses
Biofilminhibitionandgrowthinhibitiondatawerestatistically analyzedbyANOVA,andallexperimentswereconductedin trip-licateforeachstrain.Todetectdifferencesbetweenandamong thegroupsofdataorwhensignificantdifferencesweredetected, pairwisecomparisonsweremadeamongallthegroupsusingthe Turkey’smethodtoadjustformultiplecomparisons(Montgomery, 2007).
Results
IdentificationofcompoundsisolatedfromEuniceasp
CompoundsfromEuniceasp.wereidentifiedbycomparingthe spectroscopicdata withthose previously published. Compound
1 (176mg/kg yield from the coral) had the molecular formula C21H44O3,asdeducedfromHRESIMSwitha[M+Na]+peakatm/z 367.3194 (calcd. for C21H44O3Na+, 367.3183). Optical rotation [␣]D25+3.2(c1.5,CHCI3)andits1Hand13CNMRspectrashowed acloseresemblancetothosepublishedbyQuijanoetal.(1994)
and Sun et al. (2005) for (S)-(+)-3-octadecyloxy-l,2-propane
diol,analkylglycerol alsoknownasbatylalcohol. Compound2
(10.6mg/kg)hadthemolecularformulaC34H65NO3asestablished byHRESIMS.ItsmethanolysisandanalysisbyHRGCMSdisplayed a singlevolatilecompound identifiedas methylhexadecanoate. The negative optical rotation [␣]D25 −9.7 (c 0.3, CHCl3) and NMR spectra suggest that 2, (2S,3R,4E,8E)-N -hexadecanoyl-2-amino-4,8-octadecadiene-1,3-diol, was a ceramide as reported
by Shin and Seo (1995) and Han et al. (2005). Compound 3
(70mg/kg) was recognized as octadecyl (9Z)-9-octadecenoate (stearyl oleate), onthe basis of HRESIMS, and NMR data were coincident tothose of thewax previously synthetized (Vieville etal.,1995).Compound4(5.9mg/kg)wasopticallyinactiveandits methanolysisgaveamixtureofmethylhexadecanoateandmethyl (9Z)-9-octadecenoateinproportion2:1byHRGCMS.Accordingto ESIMS,theNMRdatawereidenticaltothoseofthesynthesized glycerolipid 1,3-dihexadecanoyl-2-(9Z-octadecenoyl)-glycerol as reportedbyStamatovandStawinski(2007).NMRspectroscopic featuresof5(8.2mg/kgyieldfromthecoral)weresimilartothose of 4exceptfor thelackof doublebond signalsand it wasalso optically inactive. It revealed the presence of methyl hexade-canoateandmethyloctadecanoateina1:2ratioasperHRGCMS. InagreementwithESIMSanalysis,compound5waspostulatedas 1,3-dioctadecanoyl-2-hexadecanoyl-glycerol.
1
2
3
4
5
6 7
0.00 0.20 0.40 0.60 0.80 1.00
48h 24h
OD
621
nm
P. aeruginosa
ATCC 27853
K. pneumoniae
206
S. aureus
ATCC 25923
S. epidermidis
ATCC 51400310
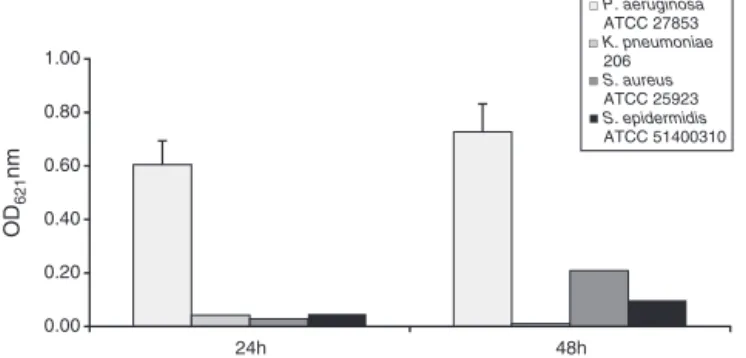
Fig.1.Biofilmformingabilityofreferencestrainsat37◦C.Microtiterplatemethod wasusedforquantifyingbiofilmformation,dyedwithcrystalviolet.Thebarson thegraphrepresentmean±SDofthebiofilmformationfromthreeindependent experiments.TheX-axisgivestheincubationtime(h).
0.0 0.4 0.8 1.2 1.6 2.0
O. pseudogringnonense 4-4DEP
A. macleodii 29-C
V. harveyi Phy-2A
P. aeruginosa ATCC 27853
OD
621
nm
Fig.2. Biofilmformingabilityofmarinestrainsduring48hat25◦C.Microtiterplate methodandCVstainingtoquantifywereused.Betweenthereferencestrains,P. aeruginosawasusedaspositivecontrolinthepresentstudyduetoitshigherbiofilm formationlevel.Thebarsonthegraphrepresentmean±SDofbiofilmformation fromthreeindependentexperiments.TheANOVAtestshowedastaticallysignificant differencerelativetocontrol(p<0.05).
Biofilmproductionassaybymicrotiterplatetest
The results showed that the biofilm was higher after 48h ofincubation for three referencestrains. From these,P. aerugi-nosaexhibitedthehighest biofilm formationlevel, significantly more than other strains (Fig. 1), followed by S. aureus being the selected reference strains. On the other hand, the assay withthose two strains at 25◦C determined that the S. aureus’
biofilmdecreasedwhileP.aeruginosashowedhigherlevelsinits biofilmformation.Thus, P.aeruginosawasusedaspositive con-trolin biofilmsformation assay of marine strains. Then, those resultsshowedthatthemarine strainsO. pseudogringnonese, A. macleodii and V. harveyi had higher biofilmformation, suchas shownbythecontrolstrainP.aeruginosa(Fig.2),whiletheother marine bacteria practically did not show any biofilm produc-tion.
Biofilminhibitionactivityassayofcompounds
Theanti-biofilm activityofcompounds1–8andsterols9–14
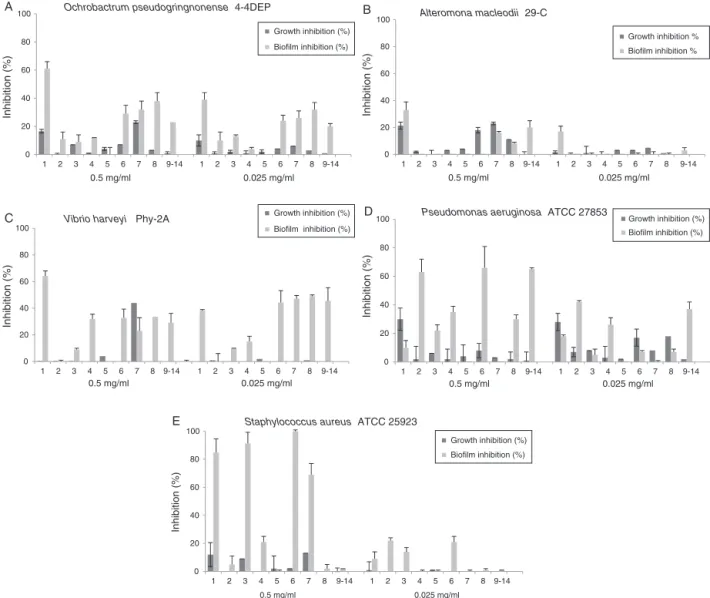
wasdeterminedtowardthreemarinestrains,andtworeference strains,allselectedinthepresentstudyduetotheirstrong biofilm-formingability.Asaresult,O.pseudogringnonense wasinhibited over50%onlybycompound1,upto61%at0.5mg/ml,whileat 0.025mg/ml,compounds 1,7,8 hadan inhibition closeto30% (Fig.3).Thestrainexhibitedlowresponse toothercompounds. Onlycompound1wasupto33%andthemixture9–14decreased thebiofilmformationofA.macleodiiat0.5mg/ml(Fig.3).Other compoundsslightlyreduced microbialgrowthwithouteffecton biofilms.InV.harveyi,biofilminhibitionwasshownby1upto64%
at0.5mg/ml; andat0.025mg/mlcompounds7and8 werethe mostactive.ResultswiththereferencestrainP.aeruginosa,shown ascompounds 6,(9–14) and2 at0.5mg/mlintheirorder, had thehighestlevelofeffectivenessreaching6upto66%,whereas at 0.025mg/ml, 2 and (9–14) showed effectiveness as well. Additionally,S.aureusastheonlygram-positive strainincluded wasthemostsusceptibleandcompounds1,3,6and7showed highpercentagesofinhibitionupto100%,at0.5mg/ml(Table1) andat0.025mg/mltheseexhibitedlowactivity.Inallcases,the compounds were less active against bacterial growth and the assessmentofbacterialviabilitybyplatingonagarshowedthat eachstraingrewagain.
9
12
R= R=
13 14
10 11
Biofilmsformationwassignificantlyreducedascomparedto untreated biofilmsof each of thesebacteria at thetested con-centrationsofcompounds.ThiswasvalidatedbyANOVA,witha significancelevelof95%andameancomparisonTukeytest,with an␣errorof0.05.
AntimicrobialactivityinextractsandcompoundsfromEunicea softcorals
Inthisstudy,thecrudeextractofEuniceasp.evidenced mod-erategrowthinhibitionagainstsixofthesevenmarinebacteria. Theorganicfractionandisolatedcompounds1to5showedmild antimicrobialactivitytowardO.pseudogringnonense,Kokuriasp., andV.harveyi.Thedeacetylatedof6,theterpene8andthesterols (9–14)fromEuniceafuscaalsohadlowactivityagainstBacillussp., O.pseudogringnonense andV. harbeyi asdetailedby Reinaetal. (2011).Finally,thediskdiffusionsusceptibilitytestdidnot evi-dence that compounds 1–14 couldbe bactericide, even at the highestassayedconcentration(30g/disk),confirmingtheirlow
antibacterialactivity.
Discussion
0 20 40 60 80 100 5 4 3 2 1 0.5 mg/ml 5 4 3 2 1 9-14 8 7 6 0.025 mg/ml
8 9-14 7 6
Inhibition (%)
B Alteromona macleodii 29-C
Growth inhibition % Biofilm inhibition %
0 20 40 60 80 100 5 4 3 2 1 0.5 mg/ml 5 4 3 2 1 9-14 8 7 6 0.025 mg/ml 9-14 8 7 6 Inhibition (%)
C Vibrio harveyi Phy-2A Growth inhibition (%)
Biofilm inhibition (%)
0 20 40 60 80 100 5 4 3 2 1 0.5 mg/ml 5 4 3 2 1 9-14 8 7 6 0.025 mg/ml 9-14 8 7 6 Inhibition (%)
D Pseudomonas aeruginosa ATCC 27853
Growth inhibition (%) Biofilm inhibition (%) 0 20 40 60 80 100 5 4 3 2 1
0.5 mg/ml
5 4 3 2 1 9-14 8 7 6
0.025 mg/ml
9-14 8 7 6
Inhibition (%)
A Ochrobactrum pseudogringnonense 4-4DEP
Growth inhibition (%) Biofilm inhibition (%)
0 20 40 60 80 100 5 4 3 2 1 0.5 mg/ml 5 4 3 2 1 9-14 8 7 6 0.025 mg/ml 9-14 8 7 6 Inhibition (%)
E Staphylococcus aureus ATCC 25923
Growth inhibition (%) Biofilm inhibition (%)
Fig.3.Biofilminhibitionandgrowthinhibitioneffectofisolatedcompounds1tomixture9–14fromEuniceasp.andEuniceafuscaagainstmarineandreferencebacteria. Biofilmwasquantifiedusingthemicrotiterplatetestandcrystalvioletassay.TheX-axisgivesthenumberoftheisolatedcompoundanditsconcentrationat0.5mg/ml or0.025mg/ml.Thebarsonthegraphrepresentmean±SDasapercentageofbiofilminhibitionoftriplicateexperiments.Biofilmsformationwassignificantlyreducedas comparedtountreatedbiofilms(control)ofeachofthesebacteriaatthetestedconcentrationsofthecompounds(ANOVAp<0.05)withasignificancelevelof95%anda meancomparisonTukeytestwithan␣errorof0.05.
acylglycerols: 1,3-dihexadecanoyl-2-(9Z-octadecenoyl)-glycerol (4), and 1,3-dioctadecanoyl-2-hexadecanoyl-glycerol (5). For decades,thefamilyof bioactivelipidshasgrowntremendously
(McAnoy et al., 2005; Deniau et al., 2010; Blunt et al., 2014).
Therefore, the possibilities of biofilm inhibition of lipids from Euniceasp.,togetherwithterpenoids,fuscosideEperacetate(6), fuscoside B(7), (+)-germacrene D (8), and sterols (9–14), from Eunicea fusca (Reina et al., 2011), were essayed by microtiter
Table1
Biofilminhibition(valuesfrom25%)ofcompoundsisolatedfromEuniceasp.andEuniceafuscaat0.5or0.025mg/ml,againstthreemarinestrainsandtworeferencebacteria.
Meanpercentageinhibitionofbiofilm*
Compound O.pseudogringnonense
4-4DEP A.macleodii 29-C V.harveyi Phy-2A P.aeruginosa ATCC27853 S.aureus ATCC25923
0.5 0.025 0.5 0.025 0.5mg/ml 0.025 0.5 0.025 0.5 0.025
1 61±6.2 39±5.8 33±6.0 64±4.2 38±1.3 85±10.3
2 63±9.2 42±1.8
3 91±9.0
4 32±3.7 35±4.6 26±4.8
5
6 29±6.5 33±6.4 44±9.0 66±14.3 100±0.0
7 32±6.1 26±5.3 47±2.9 69±8.4
8 38±6.8 32±5.0 34±0.0 49±1.6 30±3.4
9–14 29±7.0 45±10.0 65±1.4 37±5.4
Biofilmwasquantifiedbymicrotiterplatetestandcrystalviolet.Inthistable,theeffectivenessofmorethan25%ofinhibitionwasconsideredincomparisontotheuntreated controlbiofilms(positivecontrol).
*Datarepresentmean
platetests,a frequentlyused techniquefor quantifying biofilm formation(O’tooleetal.,2000;Al-SohaibaniandMurugan,2012).
Accordingtotheresultsobtainedinthisstudy,outofthefour referencestrains,P.aeruginosaevidencedthebestbiofilm forma-tion,followedbyS.aureusandtheywereusedforfurtheressays (Fig.1).P.aeruginosaandS.aureusaremodelorganisms,knownfor theircapacityofbiofilmformationandtheirinherentresistance toantimicrobialagents(O’tooleetal.,2000;Quinnetal.,2012). Ontheotherhand,theresultsaboutmarinestrainsshowedthat O.pseudogringnonense,A.macleodiiandV. harveyihad the high-estbiofilmformation(Fig.2),asthecontrolstrainP.aeruginosaat thesameculturecondition.AspublishedbyVikrametal.(2011), biofilmformationandbioluminescenceproductioninV.harveyiare aknownquorumsensing-controlledprocess,anditwasrecently studiedalsoinO.pseudogringnonense(Cuadradoetal.,2013),while A.macleodiihasbeencommonlyfoundinbiofilmsfromdifferent depthsofoceans(Ivars-Martinezetal.,2008).Thesemarine bacte-riatogetherwithreferencestrainsaremore efficient,andwere selectedaspositivecontrolforthefurtherbiofilminhibitiontests. Consequently,compounds1–8andsterols9–14weretestedfor assessingtheanti-biofilmability,andhaddifferenteffectiveness dependingonthebacteria,andsomewereactiveagainstvarious microorganisms(Fig.3).Compounds1and6werethemost effec-tiveastheyactedagainstfourstrains.Compound1inhibitedA. macleodii,O.pseudogringnonense,V.harveyiandS.aureustoa per-centagegreaterthan30%againstthefirst,andabove60%forthe lastthree.Compound6affectedO.pseudogringnonenseandV. har-veyiwitheffectivenessbetween25and50%,andP.aeruginosaand S.aureusabove60%ofbiofilminhibition(Table1).Compounds7
and8hadaneffectgreaterthan25%againstthreemicroorganisms. Compound4andmixtureofsterols9–14 wereeffectiveagainst twostrains,withapercentagehigherthan25%.Compounds2and
3showedeffectivenessonlytoonestrainbutabove50%.Incontrast, compound5wasinactivetobiofilminhibitionagainstallstrains.
Additionally, compounds 1–5, 8 and sterols 9–14 had low antibacterial activity by the disk-diffusion test against marine bacteria.Results,consistentwiththoseofthediskdiffusion suscep-tibilitytest,showedthatnoneofthecompounds1–8and(9–14) displayedabactericidaleffectevenatthehighestassayed concen-trationandthattheyonlyevidencedtheirlowantibacterialactivity. Interestingly,theresults confirmedthat thecompounds exhib-itedgrowthinhibitionmuchlowerthantheanti-biofilmactivity, andthataproportionofthebiofilmbacteriastillremainedviable, suggestingthatthereisbiofilminhibitionwithoutaffectingtheir growth,andthatthesecompoundsarelittletoxic.
Inthepresent study,thealkylglycerol– batylalcohol 1– is describedforthefirsttimeasananti-biofilmcompound. Alkyl-glycerolsarefoundinmarineinvertebratesinhigherlevelsthanin landvertebrates(WatschingerandWerner,2013).Theirbiological activitiescomprisestimulationofhematopoiesis,immunological defenses,antitumorandantimetastasis(Deniauetal.,2010;Iannitti andPalmieri,2010).Batylalcohol1waspreviouslyisolatedfrom sharkliver oil(Myers and Crews, 1983), from theinvertebrate spongeDesmapsamnaanchorata(Quijanoetal.,1994),fromthesoft coralPlexauraflexuosa(KindandBergmann,1942),fromCladiella species(Radhika,2006),andfromthegorgoniaScirpeariagracilis, showingmoderategrowthinhibitionformarinebacterialspecies asPseudoalteromonaspiscida,Ruegeriasp.,Vibrioalginolyticus,V. furnissiiandV.harveyi(Qietal.,2008).Onlydodecylglycerolwas foundasaneffectiveantibacterial againstStreptococcus faecium (Vedetal.,1984).Inpreviousstudies,otherstructurallyrelated naturallipidsshowedpotentialanti-biofilm,suchasaglycolipid composedofglucoseandpalmiticacid,whichdisruptedbiofilms ofCandidaalbicans,P.aeruginosaandBacilluspumilus(Dusaneetal., 2011),andthelinoleicacidthatreducedbiofilmaccumulationof Streptococcusmutans(Jungetal.,2014).
Inearlierworks,ceramide2wasobtainedfromanemone Para-condylactisindicus,gorgonianAcabariaunhlata(ShinandSeo,1995), softcoralDendronephthyagigantean(Hanetal.,2005),and zoan-thids, Palythoa caribaeorum and Protopalyhtoa variabilis. Other ceramideshavebeenrecognizedfor creatinganepidermal per-meability barrier, and showedcytotoxic or antitumoractivities (Almeidaetal.,2012;Bluntetal.,2014).Waxesandglycerolipids havebeencommonlydetectedbyanalyticaltechniques(Vieville
etal.,1995;McAnoyetal.,2005).Wax3waspreviously
biosyn-thetizedby Corynebacteriumsp.(Seoetal.,1982)and hasbeen detected in the eyelids of humans and mammalians (Butovich etal.,2007).Thepurificationofglycerolipids4and5hasnotbeen reported from natural sources. Nevertheless, another regioiso-merof4,called1,2-dihexadecanoyl-3-(9Z-octadecenoyl)-glycerol, whichisquiral,wasdetectedinbovineheart(Shinzawa-Itohetal., 2007).Inthesameway,theregioisomerof5, 1,2-dioctadecenoyl-3-hexadecanoyl-glycerol,wasdetectedinrapeseedoil(Beermann etal.,2007).
EuniceafuscaisthesourceoffuscolandfuscosidesA–E hav-inganti-inflammatoryactivity,aswellas(+)-germacreneD8and thesterolsmixture(9–14)(Shin andFenical,1991;Reinaetal., 2011).The(−)-germacreneDisa commoncompound ofplants (Reinaetal.,2011),andtheessentialoilthatcontainsgermacrene fromAchilleamillefoliuminhibitsbiofilmsofListeriamonocytogenes andListeriainnocua(Jadhavetal.,2013).Rautetal.(2013) demon-stratedthebiofilminhibitionofotherterpenoidsagainstCandida albicans.Moreover,bioactivesterolsmixtureorpurecompounds exhibitantibacterialorantifoulingactivities(Al-Lihaibietal.,2010;
Castellanos et al., 2010; Blunt et al., 2014). In the meanwhile,
otherditerpenoidssuchas xenicanes,dolabelanesand cembra-noids frommarinesources possess anti-biofilm activityagainst marine bacterialisolates (Viano et al., 2009; Tello et al., 2011, 2012).Notwithstanding,previousworkshavenotyetreportedthe possibilityof lipidsand terpenoidshereassayedasanti-biofilm compounds.
Inthepresentstudy,batylalcohol1andfuscosideEperacetate6
werethemosteffectivecompoundsagainstthebestbiofilm forma-tionbythebacteriatestedhere,andduetotheirlowantibacterial effect theycouldbe consideredas less toxic anti-biofilm com-poundstotheenvironment.Theresultsarestimulatingforfurther researchandbetterunderstandingthestructure-activity relation-shiportheactionmodeofthemostactivecompoundsfoundin thisstudy.In thissense,furtherstudiestodeterminethe mini-malinhibitoryconcentration(MIC)withsomeofthesesynthesized compoundsareunderdevelopmentinourlaboratory.
Conclusion
The present study showed the anti-biofilm capacity of five lipid compounds isolated from soft coral Eunicea sp., together with three terpenoids and a mixture of sterols from E. fusca collectedatCaribbeanSea,againstthreemarinebacteria,O. pseu-dogringnonense,A.macleodiiandV.harveyi,andtwoknownbiofilm formingbacteria, P.aeruginosaand S. aureus.They showed dif-ferenteffectiveness andthemostactivecompoundswerebatyl alcoholandfuscosideEperacetate.Thesecompoundsexhibit spe-cificbiofilminhibitionandcouldbeconsideredlesstoxictothe environmentbyhavinglowerantimicrobialactivity.
Author’scontributions
workandcontributedtothebiologicalstudies.FARRsupervised the laboratory work and contributed to critical reading of the manuscript. CDBcontributed withstudydesign and supervised theresearchproject.LCHsupervisedtheresearchprojectashead oftheresearch team,and contributedtocritical reading ofthe manuscript.Alltheauthorshave read thefinal manuscript and approveditssubmission.
Conflictsofinterest
Theauthorsdeclarenoconflictsofinterest.
Acknowledgment
ThisworkwassupportedbyUniversidadNacionalde Colom-bia[grantnumber5815/2012and 20406/2014],andColciencias [grantnumber1101-521-28468/2011].TheMinisteriode Ambi-ente, Vivienda y Desarrollo Territorial granted permission for researchonmarineorganisms[No.4of10/02/2010,andNo.0306 of22/02/2011].TheauthorsaregratefultoprofessorsSvenZea, MonicaPuyanaandEdissonTello.
References
Almeida,J.G.L.,Maia,A.I.V.,Wilke,D.V.,Silveira,E.R.,Braz-Filho,R.,LaClair,J.J., Costa-Lotufo,L.V.,Pessoa,O.D.,2012.PalyosulfonoceramidesAandB:unique sulfonylatedceramidesfromtheBrazilianzoanthidsPalythoacaribaeorumand
Protopalyhtoavariabilis.Mar.Drugs10,2846–2860.
Al-Lihaibi,S.S.,Ayyad,S.E.N.,Shaher,F.,Alarif,W.M.,2010.Antibacterialsphingolipid andsteroidsfromtheblackcoralAntipathesdichotoma.Chem.Pharm.Bull.58, 1635–1638.
Al-Sohaibani,S.,Murugan,K.,2012.Anti-biofilmactivityofSalvadorapersicaon car-iogenicisolatesofStreptococcusmutans:invitroandmoleculardockingstudies. Biofouling28,29–38.
Beermann,Ch.,Winterling,N.,Green,A.,Möbius,M.,Schmitt,J.J.,Boehm,G.,2007.
Comparisonofthestructuresoftriacylglycerolsfromnativetransgenic medium-chainfattyacid-enrichedrapeseedoilbyliquidchromatography-atmospheric pressure chemical ionization ion-trap mass spectrometry (LC–APCI-ITMS). Lipids42,383–394.
Blunt,J.W.,Copp,B.R.,Keyzers,R.A.,Munro,M.H.G.,Prinsep,M.R.,2014.Marine naturalproducts.Nat.Prod.Rep.31,160–258.
Butovich,I.A.,Uchiyama,E.,McCulley,J.P.,2007.Lipidsofhumanmeibum: mass-spectrometricanalysisandstructuralelucidation.J.LipidRes.48,2220–2235.
Castellanos,L.H.,Mayorga,H.W.,Duque,C.B.,2010.Studyofthechemical compo-sitionandantifoulingactivityofthemarinespongeClionadelitrixextract.Vitae 17,209–224.
Cuadrado,C.T.S.,Castellanos,L.,Arévalo-Ferro,C.,Osorno,O.E.,2013.Detectionof quorumsensingsystemsofbacteriaisolatedfromfouledmarineorganisms. Biochem.Syst.Ecol.46,101–107.
Deniau,A.L.,Mosset,P.,Pédrono,F.,Mitre,R.,LeBot,D.,Legrand,A.B.,2010.Multiple beneficialhealtheffectsofnaturalalkylglycerolsfromsharkliveroil.Mar.Drugs 8,2175–2184.
Dobretsov,S.,Abed,R.M.M.,Teplitski,M.,2013.Mini-review:Inhibitionofbiofouling bymarinemicroorganisms.Biofouling29,423–441.
Dusane,D.H.,Pawar,V.S.,Nancharaiah,Y.V.,Venugopalan,V.P.,Kumar,A.R.,Zinjarde, S.S.,2011.Anti-biofilmpotentialofaglycolipidsurfactantproducedbyatropical marinestrainofSerratiamarcescens.Biofouling27,645–654.
Fusetani, N., 2011. Antifouling marine natural products. Nat.Prod. Rep. 28, 400–410.
Han,A.R., Song,J.I., Jang, D.S., Min,H.Y.,Lee, S.K.,Seo, E.K., 2005. Cytotoxic constituentsoftheoctocoralDendronephthyagigantea.Arch.Pharm.Res.28, 290–293.
Huigens,R.W.,Richards,J.J.,Parise,G.,Ballard,T.E.,Zeng,W.,Deora,R.,Melander,C., 2007.InhibitionofPseudomonasaeruginosabiofilmformationwith bromoagelif-erinanalogues.J.Am.Chem.Soc.129,6966–6967.
Iannitti,T.,Palmieri,B.,2010.Anupdateonthetherapeuticroleofalkylglycerols. Mar.Drugs8,2267–2300.
Ivars-Martinez,E.,Martin-Cuadrado,A.B.,D’Auria,G.,Mira,A.,Ferriera,St., John-son,J.,Friedman,R.,Rodriguez-Valera,F.,2008.Comparativegenomicsoftwo ecotypesofthemarineplanktoniccopiotrophAlteromonasmacleodiisuggests alternativelifestylesassociatedwithdifferentkindsofparticulateorganic mat-ter.ISMEJ.2,1194–1212.
Jadhav,S.,Shah,R.,Bhave,Mr.,Palombo,E.A.,2013.Inhibitoryactivityofyarrow essential oil on Listeria planktonic cells and biofilms. Food Control 29, 125–130.
Jung,J.E.,Pandit,S.,Jeon,J.G.,2014.Identificationoflinoleicacid,amaincomponent ofthen-hexanefractionfromDryopteriscrassirhizoma,asananti-Streptococcus
mutansbiofilmagent.Biofouling30,789–798.
Kind,C.A.,Bergmann,W.,1942.Contributionstothestudyofmarineproducts. XI.Theoccurrenceofoctadecylalcohol,batylalcohol,andcetylpalmitatein gorgonias.J.Org.Chem.7,424–427.
Majik,M.S.,Parvatkar,P.T.,2014.NextgenerationbiofilminhibitorsforPseudomonas aeruginosa:synthesisandrationaldesignapproaches.Curr.Top.Med.Chem.14, 81–109.
Mayorga,H.W.,Urrego,N.F.,Castellanos,L.H.,Duque,C.B.,2011.Cembradienesfrom theCaribbeanSeawhipEuniceasp.TetrahedronLett.52,2515–2518.
McAnoy,A.M.,Wu,Ch.C.,Murphy,R.C.,2005.Directqualitativeanalysisof triacyl-glycerolsbyelectrospraymassspectrometryusingalineariontrap.J.Am.Soc. MassSpectrom.16,1498–1509.
Montgomery,D.C.,2007.DesignandAnalysisofExperiments.Limusa,Mexico.
Mora-Cristancho,J.A.,Arévalo-Ferro,C.,Ramos,F.A.,Tello,E.,Duque,C.,Lhullier, C.,Falkenberg,M.,Schenkel,E.P.,2011.Antifoulingactivitiesagainstcolonizer marinebacteriaofextractsfrommarineinvertebratescollectedinthe Colom-bianCaribbeanSeaandontheBraziliancoast(SantaCatarina).Z.Naturforsch. 66c,515–526.
Myers,B.L.,Crews,Ph.,1983.Chiraletherglyceridesfromamarinesponge.J.Org. Chem.48,3583–3585.
O’toole,G.,Kaplan,H.B.,Kolter,R.,2000.Biofilmformationasmicrobial develop-ment.Annu.Rev.Microbiol.54,49–79.
Qi,S.H.,Zhang,S.,Yang,L.H.,Qian,P.Y.,2008.Antifoulingandantibacterial com-poundsfromthegorgoniansSubergorgiasuberosaandScirpeariagracilis.Nat. Prod.Rep.22,154–166.
Quijano,L.,Cruz,F.,Navarrete,I.,Gómez,P.,Rios,T.,1994.Alkylglycerolmonoethers inthemarinespongeDesmapsammaanchorata.Lipids29,731–734.
Quinn,G.A.,Maloy,A.P.,McClean,St.,Carney,B.,Slater,J.W.,2012.Lipopeptide biosurfactantsfromPaenibacilluspolymyxainhibitsingleandmixedspecies biofilms.Biofouling28,1151–1166.
Radhika,P.,2006.Chemicalconstituentsandbiologicalactivitiesofthesoftcorals ofgenusCladiella:areview.Biochem.Syst.Ecol.34,781–789.
Ramos,F.,Takaishi,Y.,Kawazoe,K.,Osorio,C.,Duque,C.,Acu ˜na,R.,Fujimoto,Y., Sato,M.,Okamoto,M.,Oshikawa,T.,Ahmed,Sh.U.,2006.Immunosuppressive diacetylenes,ceramidesandcerebrosidesfromHydrocotyleleucocephala. Phy-tochemistry67,1143–1150.
Raut,J.S.,Shinde,R.B.,Chauhan,N.M.,Karuppayil,S.M.,2013.Terpenoidsofplant ori-gininhibitmorphogenesis,adhesion,andbiofilmformationbyCandidaalbicans. Biofouling29,87–96.
Reina,E.,Puentes,C.,Rojas,J.,García,J.,Ramos,F.A.,Castellanos,L.,Aragón,M., Ospina,L.F.,Fuscoside,E.,2011.Astronganti-inflammatoryditerpenefrom CaribbeanoctocoralEuniceafusca.Bioorg.Med.Chem.Lett.21,5888–5891.
Seo,Ch.W.,Yamada,Y.,Okada,H.,1982.Synthesisoffattyacidestersby Corynebac-teriumsp.S-401.Agric.Biol.Chem.46,405–409.
Shin,J.,Fenical,W.,1991.FuscosidesA–D:antiinflammatoryditerpenoidglycosides ofnewstructuralclassesfromtheCaribbeangorgonianEuniceafusca.J.Org. Chem.56,3153–3158.
Shin,J.,Seo,Y.W.,1995.IsolationofnewceramidesfromthegorgonianAcabaria undulata.J.Nat.Prod.58,948–953.
Shinzawa-Itoh,K.,Aoyama,H.,Muramoto,K.,Terada,H.,Kurauchi,Ts.,Tadehara,Y., Yamasaki,A.,Sugimura,T.,Kurono,S.,Tsujimoto,K.,Mizushima,Ts.,Yamashita, E.,Tsukihara,T.,Yoshikawa,Sh.,2007.Structuresandphysiologicalrolesof13 integrallipidsofbovineheartcytochromecoxidase.EMBOJ.26,1713–1725.
Stamatov,St.D.,Stawinski,J.,2007.Regioselectiveandstereospecificacylationacross oxirane-andsilyloxysystemsasanovelstrategytothesynthesisof enantiomer-icallypuremono-,di-andtriglycerides.Org.Biomol.Chem.5,3787–3800.
Sun,X.F.,Wu,Q.,Wang,N.,Cai,Y.,Lin,X.F.,2005.Novelmutualpro-drugsof 2´,3´-dideoxyinosinewith3-octadecyloxy-propane-1,2-diolbystraightforward enzymaticregioselectivesynthesisinacetone.Biotechnol.Lett.27,113–117.
Tello,E.,Castellanos,L.,Arevalo-Ferro,C.,Rodríguez,J.,Jimenez,C.,Duque,C.,2011.
AbsolutestereochemistryofantifoulingcembranoidepimersatC-8fromthe CaribbeanoctocoralPseudoplexauraflagellosa.Revisedstructuresof plexau-rolones.Tetrahedron67,9112–9121.
Tello,E.,Castellanos,L.,Arévalo-Ferro,C.,Duque,C.,2012.Disruptionin quorum-sensingsystemsandbacterialbiofilminhibitionbycembranoidditerpenes isolatedfromtheoctocoralEuniceaknighti.J.Nat.Prod.75,1637–1642.
Ved,H.S.,Gustow,E.,Mahadevans,V.,Pieringer,R.A.,1984.Dodecylglycerolanew typeofantibacterialagentwhichstimulatesautolysinactivityinStreptococcus
faeciumATCC9790.J.Biol.Chem.259,8115–8121.
Viano,Y.,Bonhomme,D.,Camps,M.,Briand,J.F.,Ortalo-Magné,A.,Blache,Y.,Piovetti, L.,Culioli,G.,2009.DiterpenoidsfromtheMediterraneanbrownalgaDictyota
sp.evaluatedasantifoulingsubstancesagainstamarinebacterialbiofilm.J.Nat. Prod.72,1299–1304.
Vieville,C.,Mouloungui,Z.,Gaset,A.,1995.SynthesisandanalysisoftheC1–C18 alkyloleates.Chem.Phys.Lipids75,101–108.
Vikram,A.,Jesudhasan,P.R.,Jayaprakasha,G.K.,Pillai,S.D.,Patil,Bh.S.,2011.Citrus limonoidsinterferewithVibrioharveyicell-cellsignallingandbiofilmformation bymodulatingtheresponseregulatorLuxO.Microbiology157,99–110.
Watschinger,K.,Werner,E.R.,2013.Orphanenzymesinetherlipidmetabolism. Biochimie95,59–65.
Wilkins,T.D.,Holdeman,L.V.,Abramson,I.J.,Moore,W.E.C.,1972.Standardized single-discmethodforantibioticsusceptibilitytestingofanaerobicbacteria. Antimicrob.AgentsChemother.1,451–459.