w ww . e l s e v i e r . c o m / l o c a t e / b j p
Original
Article
Flax
lignan
concentrate
attenuate
hypertension
and
abnormal
left
ventricular
contractility
via
modulation
of
endogenous
biomarkers
in
two-kidney-one-clip
(2K1C)
hypertensive
rats
Sameer
Hanmantrao
Sawant,
Subhash
Laxmanrao
Bodhankar
∗DepartmentofPharmacology,PoonaCollegeofPharmacy,BharatiVidyapeethDeemedUniversity,Erandwane,Pune,India
a
r
t
i
c
l
e
i
n
f
o
Articlehistory:
Received2December2015 Accepted10May2016 Availableonline14June2016
Keywords:
Flaxlignanconcentrate Antihypertensiveactivity 2K1Chypertension Endothelin-1 Renalangiotensin-II
a
b
s
t
r
a
c
t
ThepresentinvestigationwasdesignedtostudytheeffectofflaxlignanconcentrateobtainedfromLinum
usitatissimumL.,Linaceae,intwo-kidney,oneclip(2K1C)hypertensionmodelinWistarrats.2K1C
Gold-blattmodelratsweredividedrandomlyintosixgroups:sham,2K1Ccontrol,captopril(30mg/kg),flax
lignanconcentrate(200,400and800mg/kg).Flaxlignanconcentrateandcaptoprilwereadministered
dailyforeightconsecutiveweeks.Sham-operated,and2K1Ccontrolratsreceivedthevehicle.
Treat-mentwithflaxlignanconcentrate(400and800mg/kg)significantlyanddose-dependentlyrestoredthe
hemodynamicparameterssystolicbloodpressure,diastolicbloodpressure,meanarterialbloodpressure
andleftventricularfunctions.Theflaxlignanconcentratesignificantlyrestoredtheelevatedhepatic,
renalandcardiacmarkerenzymesintheserum.Italsorestoredtheorgansweights(kidneyandheart),
serumelectrolytelevelandhistologicalabnormalities.Furthermore,flaxlignanconcentratesignificantly
elevatedthelevelofbiochemicalmarkersthatisenzymaticantioxidantssuperoxidedismutase,
glu-tathioneanddecreasedmalondialdehydeintheheartandkidneytissues.Meanwhile,wefoundthat
plasmanitricoxideandplasmanitricoxidesynthasecontentsweresignificantlyincreasedintheflax
lignanconcentrate-treatedgroup,andplasmaendothelin-1andrenalangiotensin-IIlevelswere
signif-icantlylowerthan2K1Chypertensivegroup.Inconclusion,theantihypertensiveandantioxidanteffect
offlaxlignanconcentrateweredose-dependentandatthehighestdose(i.e.800mg/kg)similartothose
ofcaptopril(30mg/kg).Itissuggestedthatflaxlignanconcentratereducedbloodpressurebyreduction
ofrenalangiotensin-IIlevel,inhibitionofplasmaendothelin-1production,inductionofthenitricoxide,
nitricoxidesynthaseandinvivoantioxidantdefensesystem.
©2016SociedadeBrasileiradeFarmacognosia.PublishedbyElsevierEditoraLtda.Thisisanopen
accessarticleundertheCCBY-NC-NDlicense(http://creativecommons.org/licenses/by-nc-nd/4.0/).
Introduction
Theone-thirdadultpopulationoftheworldisaffectedby
hyper-tension, and it can beconsidered as one of themost common
chronicdiseasenowadays(Leeetal., 2009;Gosaviet al.,2011;
Ghoshetal.,2012;Shivakumaretal.,2014).Theworld’sadult
pop-ulationwithhypertensionislikelytoincreasefromonebillionin
2000to1.56billionby2025(Kearneyetal.,2005).
Renin–angiotensin–aldosteronesystem(RAAS)playsan
impor-tantroleinhypertension.Renalischemialeadstosecretereninby
kidneytissue whichis responsiblefor catalyzingthehydrolysis
of angiotensin-I (Ang-I) from the N-terminus of
angiotensino-gen.Ang-IisconvertedintoAng-IIbytheangiotensin-converting
enzyme (ACE). Ang-II is the primary product of RAAS, which
∗ Correspondingauthor.
E-mail:subhash.bodhankar@bharatividyapeeth.edu(S.L.Bodhankar).
leadstovasoconstrictionandhypertensionthroughbindingtothe
Ang-IIreceptorandstimulatingsynthesisofaldosterone(Kamble
et al., 2013; Badole et al., 2015). It is well known that Ang-II
alsostimulatethegenerationofsuperoxideanionradical(O2−•)
(Griendling et al., 1994; Rajagopalan et al., 1996), which
con-tributetodecreasednitricoxide(NO)bioavailabilityandimpaired
endothelium-dependentvasorelaxation(Gryglewskietal.,1986).
SyntheticACEinhibitorstherapyiscommonlyusedtodaytotreat
hypertension.However,this advanced antihypertensivetherapy
hasserioussideeffectssuchasangioedemaanddrycough(Coulter
andEdwards,1987).Therefore,theuseofanantioxidantmaybe
thepossibletherapyforthepreventionandtreatmentof
hyper-tensionwiththeestablishedantihypertensivedrug(Gosavietal.,
2011,2014;Visnagrietal.,2013).
Flaxseedorlinseed(LinumusitatissimumL.,Linaceae)isbrown
or yellowcoloredseed harvested fromtheblue flowersofflax
crop. It has been used as food in India and around theworld
for a long time. Flaxseed mainly contains omega-3 fatty acid,
http://dx.doi.org/10.1016/j.bjp.2016.05.005
␣-linolenicacid,dietaryfiberandsecoisolariciresinoldiglucoside
(SDG)lignan(Bassettetal.,2009).Flaxseedcontainstento
hun-dredtimesmorelignanthanmostotheredibleplantsseeds.Itis
reportedthatflaxseedalsocontainsotherlignanslikematairesinol,
lariciresinol, hinokinin, arctigenin, pinoresinol and demethoxy
secoisolariciresinolin smallquantitywithseveralphenolicacid
compounds(Prasadetal.,1998;Johnssonetal.,2000).Antidiabetic
(Prasadetal.,2000), antihyperlipidemic(Raygudeetal.,2012a),
cardioprotective(Zanwaretal.,2011,2013),renoprotective(Ghule
etal., 2011,2012,2015), antiatherogenic(Prasad,1997; Prasad
etal.,1998),antioxidant,anticancer,antiviral,bactericidaland
anti-inflammatory(Chenetal.,2002;Collinsetal.,2003;Kinniryetal.,
2006;Rajeshaetal.,2006;Zanwaretal.,2010)potentialsofflaxseed
havebeenalreadyreported.
Clinicalstudiesinvolvingpatientswithperipheralartery
dis-easeandhighbloodpressure(Rodriguez-Leyvaetal.,2013;Khalesi
etal.,2015)reportedthatconsumptionofflaxseedsinthedietfor
thedurationofmorethanthreemonthsloweredbloodpressure.
Theantihypertensivepotentialofflaxlignaninchronic
hyperten-siveconditionhasnotbeenwellexplainedinanimals.Theobjective
ofthepresentworkwastostudytheeffectofflaxlignan
concen-trate(FLC)intwo-kidney,one-clip(2K1C)inducedhypertension
inWistarrats.
Materialsandmethods
Experimentalprotocol
MaleWistarratsweighing(200–250g)werepurchasedfrom
NationalToxicology Centre,Pune, India.They weremaintained
at 25±1◦C temperature and 45–55% relative humidity under
12h light/dark cycle. The animals had access to food pellets
(manufacturedbyPranavAgroIndustriesLtd.,Sangli,India)and
wateradlibitum.Theexperimentalprotocolwasapprovedbythe
InstitutionalAnimalEthics Committee(IAEC)constitutedasper
guidelinesofCommitteeforthePurposeofControland
Supervi-sionofExperimentsonAnimal(CPCSEA),India.TheIAECapproval
numberisCPCSEA/PCL/08/2014-15.
Drugsandchemicals
Captopril,sulfanilicacid,N-(1-naphthyl)ethylenediaminewas
purchasedfromSigma–AldrichCorporation,USA.Absolute
alco-hol(manufacturedbyChangshuYangyuanChemicals,China)was
purchasedfromtherespectivevendor.Analyticalgrade hexane,
hydrochloricacid, and sodium hydroxide werepurchased from
QualigenesFineChemicalsPvt.Ltd.,Mumbai,India.
Collectionandauthenticationofplantseeds
Seeds of Linum usitatissimum L., Linaceae (flaxseeds) were
obtainedfromPunjabraoDeshmukhKrishiVidyapeeth,Collegeof
Agriculture,Nagpur,India.Aftertheauthenticationoftheseeds,
avoucherspecimenwasdepositedatourInstitute,PoonaCollege
ofPharmacy,Pune,India.Theflaxseedswerestoredinacoldroom
beforeprocessingforoilextractionatourRealWorldNutritionLab,
BharatiVidyapeethDeemedUniversity,Pune,India.
Preparationofflaxlignanconcentrate(FLC)
Preparation of FLC was carried out as described previously
(Zanwaretal.,2013).Theflaxseedcakewasdefattedbyhexane
toremove residual oil. Thedefatted cakewas thenhydrolyzed
withaqueoussodiumhydroxidefor1hatroomtemperaturewith
intermittentshakingfollowedbyextractionwith50%ethanol.The
filtratewasacidifiedtopH3using1Mhydrochloricacid.The
fil-tratewasdriedusingrotavacapparatusat50◦C.Thedrypowderof
hydroalcoholicextractwaslabeledasFLC.
PreparationofdrugsolutionandselectionofFLCdose
CaptoprilandFLCweredissolvedindistilledwater.Thisstudy
was carried out using three doses of FLC (i.e. 200, 400 and
800mg/kg,p.o.)andonedoseofcaptopril(i.e.30mg/kg,p.o.).
Experimentalinductionofhypertension
Wistar rats weighing 200–250g were anesthetized with
50mg/kgintraperitonealadministrationofthiopentalsodium.The
furonthebackofeach ratwasshaved,andtheskinwas
disin-fected.Aflankincisionwasmadeintheleftlumbarareaparallel
tothelongaxisoftherat.Therenal pedicelwasexposed with
thekidneyretractedtotheabdomen.Leftrenalarterywas
con-strictedtoinducetwo-kidney,one-clip hypertension(2K1C),as
previouslydescribedbyKharinandKrandycheva(2004).Briefly,
a loop of the left renal artery was pulled into a segment of
polyurethane tube [MRE 040-S20, Braintree Scientific; internal
diameter(ID)=0.50mm,length2mm].Themuscleandskinlayer
(incisionsite)weresuturedwithahighlysterilesutureneedle.After
oneweekoftherecoveryperiod,theanimalswereusedforthe
furtherexperiment.Ratsinsham-operatedgroupunderwentthe
exposureoftheleftrenalartery,butthearterywasnotconstricted.
Themuscleandskinlayer(incisionsite)weresuturedwithasterile
sutureneedle.Afteroneweekoftherecoveryperiod,theanimals
wereusedforthefurtherexperiment.
Experimentaldesign
Theratswererandomlydividedintosixgroups,eachcontaining
sixrats:
GroupI:Sham-operated(vehicledistilledwaterp.o.)
GroupII:2K1Ccontrol(vehicledistilledwaterp.o.)
GroupIII:2K1C+captopril(30mg/kgp.o.)
GroupIV:2K1C+FLC(200mg/kgp.o.)
GroupV:2K1C+FLC(400mg/kgp.o.)
GroupVI:2K1C+FLC(800mg/kgp.o.)
FLCandcaptoprilwereandadministeredtotheratsorallyusing
anoralfeedingneedledailyforeightconsecutiveweeks.The
sham-operatedand2K1Ccontrolratsreceivedvehicledistilledwater.At
theendofthestudyperiod,bloodwascollectedbyaretro-orbital
punctureforthemeasurementofbiochemicalparameters.
Assessmentofhemodynamicchanges
Each rat was anesthetized with intraperitoneal injection of
urethane(1.25g/kg).Thetracheawascannulatedtoassist
respi-ration.Thesystolicbloodpressure(SBP),diastolicbloodpressure
(DBP)and meanarterialbloodpressure (MABP)weremeasured
byinvasivetechniqueattheendoftheeighthweek.A
polyethyl-enecannula(PE50)filledwithheparinizedsaline(100IU/ml)was
insertedintotherightcarotidartery.Thecannulawasconnected
toatransducerandthesignalwasamplified.Theleft
ventricu-larhemodynamicchangesweremeasuredusingaMillarmikro-tip
transducercatheter(ModelSRP-320;MillarInstrument,Inc.
320-7051,Houston, TX 77023-5417) inserted intothe left ventricle
viatherightcarotidarteryandconnectedtoabioamplifier(Adil
etal.,2015,2016a;Visnagrietal.,2015).Maximumfirst
deriva-tiveofventricularpressure(dP/dtmax),minimumfirstderivativeof
pressure(EDP)signals wereobtainedfromprimarysignals (left
ventricularsystolicpressureandbloodpressure)bymeansof
Pow-erlab8-channeldataacquisitionsystem(ADInstrumentsPvt.Ltd.,
withLabChart7.3Prosoftware,Australia).
Samplecollectionanddeterminationofbiomarkers
Serumandplasmasamplecollection
Attheendofthestudyperiodand1hafterthetestsubstance
administration,thebloodwascollectedbyretro-orbitalpuncture
underanesthesia.Serumsampleswerecollectedwithoutadded
anticoagulant.Serumsampleswerecollectedaftercentrifugation
for10minat845×gand4◦C.Thebloodwascollectedinto
anti-coagulantcontainingtubesandimmediatelycentrifuged(10min
at845×g and4◦C temperatures)for plasmasamplecollection.
Theserumandplasmasampleswerestoredat−80◦Cuntilbeing
analyzed.
Heartandrenaltissuesamples
At the end of the experimental period, all the rats were
humanely euthanized. The heart and kidneys were removed
for furtherexperiments. The portions of the heart and clipped
renal tissues were individually homogenized in 10% cold
Tris–hydrochloridebuffer(10mmol/l,pH7.4)usingtissue
homog-enizer(Remi,India)andcentrifugedat5283×gfor15minat0◦C.
Theclearsupernatantcollectedafterthecentrifugationwasused
forbiochemicalandmolecularestimations.
Measurementofbiologicalserummarkers
TheserumelectrolytessuchasNa+,K+andCl−wereestimated
usingcommerciallyavailablemeasurementkits(CoralClinical
Sys-tem,Goa,India).Creatinekinase(CK-MB),lactatedehydrogenase
(LDH),aspartateaminotransferase(AST),alanineaminotransferase
(ALT),alkalinephosphatase(ALP),totalprotein,bloodureanitrogen
(BUN),uricacidandcreatininewerealsomeasuredbyusing
com-merciallyavailablemeasurementkits(AccurexPvt.Ltd.,Mumbai,
India).
Estimationofendogenousantioxidantenzyme
Thesuperoxidedismutase(SOD)concentrationwasdetermined
bythemethodpreviouslydescribedelsewhere(Kambleetal.,2013;
Adiletal.,2014;Aswaretal.,2015;Honmoreetal.,2015).The
SODactivitywasexpressedasU/mgofprotein.Theglutathione
(GSH)assaywasperformedaccordingtothemethodpreviously
describeelsewhere(Moronet al.,1979;Kandhareet al.,2011a,
2015a;Kumaret al.,2014; Ketkaretal., 2015;Goswami etal.,
2016;Adiletal.,2016b).Theamountofreducedglutathionewas
expressedasg/mgofprotein.Malondialdehyde(MDA)levelinthe
kidneyandhearttissuesweremeasuredbythemethodpreviously
describedelsewhere(SlaterandSawyer,1971;Patiletal.,2011,
2015;Raygudeetal.,2012a,b;Saraswathietal.,2014;Kandhare
et al., 2016a), and thevalues wereexpressed in nanomoles of
MDA/mgofprotein.
Determinationofnitricoxide(NO),nitricoxidesynthase(NOS),
endothelin-1(ET-1),Ang-IIlevel
NOishighlyunstablefreeradical,whichisconvertedinto
sta-blemetabolitesnitrateandnitriteintheequimolarratio(Schlaich
etal.,2007;Visnagrietal.,2014;Kandhareetal.,2016a,2015b;
Sarkaretal.,2015).TheplasmaNOlevelwasdeterminedasnitrite
bytheacidicGriessreaction.Theassaywasperformedbyarapid,
simplespectrophotometricmethoddescribedelsewhere(Miranda
etal.,2001;Gosavietal.,2012a,b;Kandhareetal.,2013a,2014a).
Theprincipleofthisassayisareductionofnitratebyvanadium.
ThenitritereactswithsulfonamideandN-(1-naphthyl)
ethylenedi-aminetoproduceapinkazo-productwithmaximumabsorbanceat
543nm.Theconcentrationswerecalculatedusingastandardcurve
ofsodiumnitrateandtheresultswereexpressedinmol/l.ET-1,
NOS[GenxbioHealthSciencesLtd.,India]intheplasmandAng-II
level[RayBiotech,Inc.,USA]intherenaltissuehomogenatewere
measuredusingElisakitsaspertheinstructionsaregivenbythe
manufacturer.
Histopathologicalexamination
The excised heart and kidney samples were cleaned and
immediatelyfixedinneutralbuffered10%formalinsolution.The
specimenswereroutinelyprocessedandembeddedinparaffin.The
specimenswerecutinsectionsof5mthicknessbymicrotome
andstainedwithMasson’strichromeformicroscopicexamination.
Thesectionswereobservedunderthemicroscopeand
photomicro-graphsofthetissuesectionweretakenusingamicroscopecamera
(NikonCoolpix).Theparametersofhistopathologicalassessment
ofthekidneysectionsweremainlyperivascularedema,fibrosis,
glomerular necrosisand collagendeposition.The parametersof
histopathologicalassessmentoftheheartsectionsweremyocardial
degeneration,collagendeposition,andfibrosis.
Statisticalanalysis
The data wereexpressed as mean±standard errorof mean
(SEM)andstatisticalanalysiswascarriedoutbyone-wayANOVA
followedbyposthocDunnett’stestusingGraphPadPrism5.0
soft-ware(GraphPadSoftware,SanDiego,CA,USA).Differenceswitha
valueofp<0.05wereconsideredstatisticallysignificant.
Results
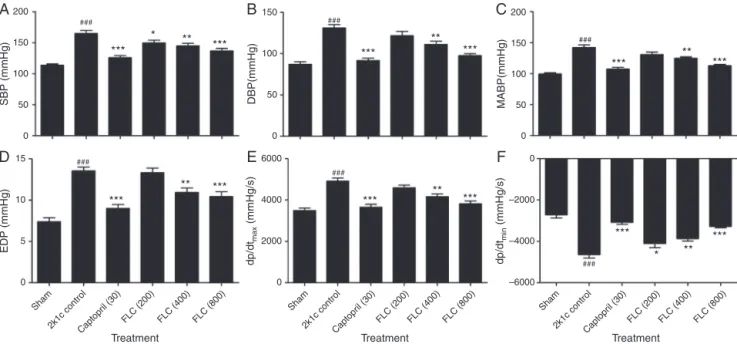
EffectofFLConhemodynamicparametersandleftventricular
contractilefunctionofheart
Fig.1presentstheeffectofthreedifferentconcentrations(200,
400and800mg/kg)onhemodynamicparameters andleft
ven-tricularcontractilefunctioninalltheI–VIgroupsafter8weeks.
Comparedtoshamoperatedgroup,theratsin2K1Ccontrolgroup
showedsignificant(p<0.001each)increase inSBP, DBP,MABP,
EDP,dP/dtmax and dP/dtmin after 8weeks. Captopril (30mg/kg)
and FLC (800mg/kg) treatment showed a significant (p<0.001
each)decreaseintheSBP,DBP,MABP,EDP,dP/dtmaxanddP/dtmin.
FLC(400mg/kg)treatmentalsoshowedsignificant(p<0.01each)
decreaseinSBP,DBP,MABP,EDP,dP/dtmaxanddP/dtmincompared
to2K1C-controlgroup.TreatmentwithFLC(200mg/kg)showed
significant(p<0.05)decreaseinSBP,anddP/dtmin;butdidnotshow
anysignificantdecreaseinDBP,MABP,EDPanddP/dtmaxvalues
(Fig.1).
EffectofFLConorgansweightandelectrolyte
The2K1C-controlhypertensive(group-II)ratsshowed
signifi-cant(p<0.001)increaseintheweightsofkidneyandheart.The
serumsodiumion(Na+)andchlorideion(Cl−)levelssignificantly
increasedwhilethatofserumpotassiumion(K+)leveldecreased
compared to sham. The treatments with captopril (30mg/kg)
and FLC (400, 800mg/kg) reduced organs weight and restored
thoseions(sodium,chloride,andpotassium)leveltonearnormal
(Table1).
EffectofFLConserumcardiac,hepaticandrenalmarkersand
serumtotalproteinlevel
The activities of CK-MB, LDH, AST, ALT, ALP, total protein,
0
15
10
EDP (mmHg)
dp/dt
max
(mmHg/s)
dp/dt
min
(mmHg/s)
DBP(mmHg) MABP(mmHg)
SBP (mmHg)
5
Treatment
0 0 –6000
–4000 –2000 0 0 50 100 150 200
2000 4000 6000 0 50 100 150
Sham
2k1c controlCaptopr il (30)
FLC (200) FLC (400) FLC (800)
Treatment Sham
2k1c controlCaptopr il (30)
FLC (200) FLC (400) FLC (800)
Treatment Sham
2k1c controlCaptopr il (30)
FLC (200) FLC (400) FLC (800) 50
100 150
###
###
###
###
###
### ***
***
***
***
*** ***
*** ***
*** ***
*** ***
* **
** **
**
* **
** 200
A
B
C
F
E
D
Fig.1. EffectofadministrationofFLCandcaptoprilonhemodynamicparameters(A)SBP,(B)DBP,(C)MABP,(D)EDP,(E)dP/dtmaxand(F)dP/dtmininmodified2K1C
hypertensiveWistarrats(n=6).Dataareexpressedasmean±S.E.M.andstatisticalanalysiswascarriedoutbyone-wayANOVAfollowedbyposthocDunnett’stest; ns=non-significant,*p<0.05,**p<0.01,***p<0.001ascomparedwith2K1Ccontrolgroup-II,#p<0.05,##p<0.01,###p<0.001comparedtoshamgroup-I.
Table1
EffectofFLC(200,400and800mg/kg)andcaptopril(30mg/kg)onorgans(heartandkidney)weightsandserumelectrolytesin2K1ChypertensiveWistarrats.
Parameter Treatment
Sham 2K1Ccontrol 2K1C+captopril
(30mg/kg)
2K1C+FLC (200mg/kg)
2K1C+FLC (400mg/kg)
2K1C+FLC (800mg/kg)
Organweight(g)
Heartweight 1.18±0.03 1.54±0.04d 1.20±0.02c 1.44±0.04ns 1.38±0.05b 1.27±0.02c
Kidneyweight 1.41±0.03 2.18±0.05d 1.53
±0.04c 2.02
±0.05ns 1.94
±0.05b 1.62
±0.04c
Electrolyte(mEq/l)
Sodium 144±1.57 183±6.29d 152±3.39c 179±5.45ns 161±5.03b 155±3.75c
Potassium 6.22±0.22 3.67±0.19d 5.73
±0.27c 3.81
±0.19ns 4.65
±0.20b 5.4
±0.15c
Chloride 104±2.12 131±4.21d 115
±1.66c 126
±2.5ns 120
±2.1a 116
±1.79b
Valuesareexpressedasmean±SEMforn=6rats.Dataareexpressedasmean±S.E.M.andstatisticalanalysiswascarriedoutbyone-wayANOVAfollowedbyposthoc
Dunnett’stest;ns=non-significant.
ap<0.05ascomparedwith2K1Ccontrol(group-II). b p<0.01ascomparedwith2K1Ccontrol(group-II). c p<0.001ascomparedwith2K1Ccontrol(group-II). d p<0.001comparedtoshamgroup(I).
Table2
EffectofFLC(200,400and800mg/kg)andcaptopril(30mg/kg)onserumcardiac,hepatic,renalmarkersandserumtotalproteinin2K1ChypertensiveWistarrats.
Parameter Treatment
Sham 2K1Ccontrol 2K1C+captopril (30mg/kg)
2K1C+FLC (200mg/kg)
2K1C+FLC (400mg/kg)
2K1C+FLC (800mg/kg)
Aspartateaminotransferase(AST)(IU/l) 90.6±3.21 162±5.69d 108
±5.53c 144
±4.09ns 136
±4.96b 121
±3.97c
Alanineaminotransferase(ALT)(IU/l) 37.5±2.13 75.3±1.82d 50.8
±2.75c 68.6
±2.37ns 62.5
±1.38b 59.9 ±3.05c
Alkalinephosphatase(ALP)(IU/l) 73.1±4.54 186±6.98d 129
±13.6c 166
±8.22ns 140
±9.48b 140
±10b
Totalprotein(mg/dl) 5.31±0.22 8.50±0.33d 6.01±0.27c 7.54±0.24ns 7.00±0.26b 5.98±0.29c
Bloodureanitrogen(BUN)(mg/dl) 17.80±0.70 36.10±1.81d 23.80±1.38c 31.10±1.05a 29.40±1.27b 27.20±1.05c
Uricacid(mg/dl) 1.93±0.08 3.90±0.15d 2.22±0.07c 3.52±0.10a 3.38±0.10b 2.98±0.09c
Creatinine(mg/dl) 0.77±0.07 1.83±0.04d 0.90
±0.05c 1.49
±0.12ns 1.36
±0.14b 1.12 ±0.09c
Creatininekinase(CK-MB)(IU/l) 345±13.9 563±16.6d 464
±14.1c 531
±11.4ns 500
±10.6b 484
±11.5c
Lactatedehydrogenase(LDH)(IU/l) 655±10.6 770±10.7d 672
±8.1c 732
±12.6a 712
±9.72b 680
±5.71c
Valuesareexpressedasmean±SEMforn=6rats.Dataareexpressedasmean±S.E.M.andstatisticalanalysiswascarriedoutbyone-wayANOVAfollowedbyposthoc
Dunnett’stest;ns=non-significant.
Table3
EffectofFLC(200,400and800mg/kg)andcaptopril(30mg/kg)onendogenousantioxidantenzymesin2K1ChypertensiveWistarrats.
Parameter Treatment
Sham 2K1Ccontrol 2K1C+captopril
(30mg/kg)
2K1C+FLC (200mg/kg)
2K1C+FLC (400mg/kg)
2K1C+FLC (800mg/kg)
SOD(Unit/mgprotein)
Kidney 12.47±0.27 5.58±0.21d 9.16±0.34c 6.61±0.34ns 7.21±0.44b 8.62±0.32c
Heart 4.6d7±0.39 3.04±0.08d 4.25±0.11c 3.32±0.17ns 4.13±0.11b 4.22±0.08c
MDA(nmolofMDA/mgprotein)
Kidney 3.42±0.23 7.93±0.31d 4.33
±0.28c 6.95
±0.17a 6.44
±0.20b 5.45
±0.29c
Heart 4.35±0.24 8.06±0.23d 5.86±0.09c 7.80±0.16ns 6.91±0.13b 6.05±0.25c
GSH(g/mgprotein)
Kidney 8.11±0.26 4.58±0.26d 6.41
±0.21c 4.94
±0.28ns 5.66
±0.18b 5.99
±0.15c
Heart 7.76±0.36 3.91±0.24d 6.74±0.20c 3.86±0.23ns 5.14±0.15b 5.34±0.18c
Valuesareexpressedasmean±SEMforn=6rats.Dataareexpressedasmean±S.E.M.andstatisticalanalysiswascarriedoutbyone-wayANOVAfollowedbyposthoc
Dunnett’stest;ns=non-significant.
ap<0.05ascomparedwith2K1Ccontrol(group-II). bp<0.01ascomparedwith2K1Ccontrol(group-II). c p<0.001ascomparedwith2K1Ccontrol(group-II). d p<0.001comparedtoshamgroup(I).
hypertensiverats(2K1C-controlgroup-II).Thetreatmentwith
cap-topril(30mg/kg)andFLC(200,400,800mg/kg)showedareduction
intheactivitiesofthesecardiac,hepaticandrenalmarkerstoward
near normal. Captopril (30mg/kg) showed the highest activity
than the test drug FLC (200, 400, 800mg/kg). FLC (800mg/kg)
showedsignificant(p<0.001)reductioninserumlevelofall
mark-ers,exceptinALP(p<0.01).TreatmentwithFLC(400mg/kg)also
showedsignificant(p<0.01)reductioninserumlevelofallmarkers.
However,FLCinlowdose(200mg/kg)didnotshowanysignificant
inhibitioninthelevelofserumCK-MB,LDH,AST,ALT,ALP,total
pro-tein,andcreatinine;butshowedasignificantreductioninserum
levelofBUNanduricacidcomparedtothe2K1Ccontrolanimals
(Table2).
Endogenousantioxidantenzymes
TheSOD and GSHactivityin thetissues (kidneyand heart)
of2K1Chypertensiveratsweredecreasedsignificantly(p<0.001)
after8weeks.Captopril(30mg/kg),aswellasFLC(400,800mg/kg),
restoredtheSODandGSHactivityinthetissues(kidneyandheart)
aftereight weeks. On the other hand,FLC (200mg/kg) didnot
showanysignificantrestorationoftheSOD andGSHactivityin
thetissues.MDAlevelinthetissues(kidneyandheart)of2K1C
hypertensiveratsincreasedsignificantlyafter8weeks.Captopril
(30mg/kg)andFLC(800mg/kg)treatedgroupshadsignificantly
decreasethe levelof MDAthan in theFLC(400mg/kg) treated
group.However,FLC(200mg/kg)treatedgroupdidnotshowany
significantrestorationofMDAlevel(Table3).
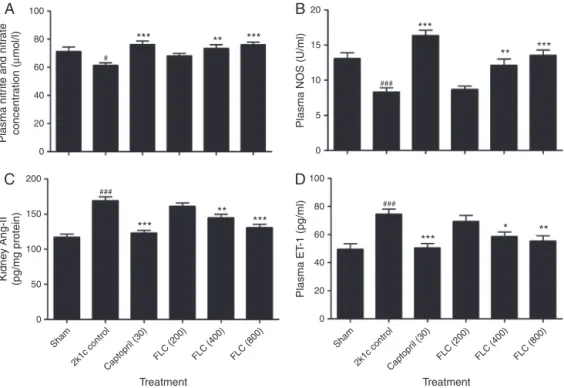
EffectofFLConNO,NOS,Ang-IIandET-1level
The2K1C-controlratsshowedasignificantdecreaseinplasma
NOandNOSlevelascomparedtotheshamoperatedgrouprats.The
ratstreatedwithcaptopril(30mg/kg)andFLC(400and800mg/kg)
significantlyelevatedplasmaNOandplasmaNOSlevels.However,
2K1ChypertensiveratstreatedwithFLC(200mg/kg)didnotshow
anysignificanteffect(Fig.2AandB).
Theincreased blood pressure in the2K1C hypertensiverats
wasalsolinkedwithsignificantlyincreasedkidneyAng-IIlevels
compared withsham-operated group.Captopril(30mg/kg) and
FLC (400 and 800mg/kg) treatment in 2K1C hypertensive rats
showeddecreasesinAng-IIlevelsofthekidney(Fig.2C).Plasma
ET-1level waselevatedsignificantlyin the2K1C-controlgroup
compared withshamgroup.Captopril (30mg/kg)and FLC(400
and 800mg/kg) significantly decreased the plasma ET-1 level
dose-dependentlytowardnearnormallevel(Fig.2D).
EffectofFLConhistopathologyofkidney
The histopathological examination of kidney tissues in the
sham-operatedratsshowednormalglomeruluscellandtubuliwith
theabsenceofperivascularedema,fibrosis,andcollagen
deposi-tion.Ontheotherhand,thehypertensive2K1Cgroupratsshowed
a significantincrease inperivascular edema,fibrosis, and
colla-gendeposition.Captopril(30mg/kg)andFLC(400and800mg/kg)
showedadecreaseinperivascularedema,fibrosis,collagen
depo-sition, and necrosis. However, FLC (200mg/kg) did not show
significantprotectionfromhypertensivedamage(Fig.3).
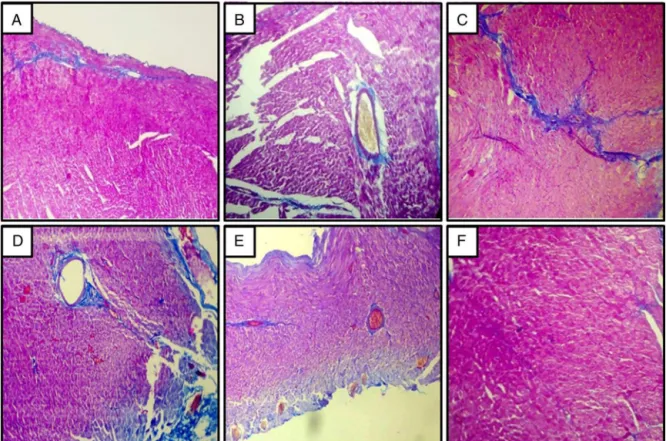
EffectofFLConhistopathologyofheart
The 2K1C hypertensive group rats showed severe
myocar-dialdegeneration,hypertrophy,andfibrosis.Captopril(30mg/kg)
treatedgroupshowedminimalmyocardialdegenerationand
col-lagendepositionandfibrosis.FLC(400and800mg/kg)treatment
alsoshowedadecreaseinmyocardialdegenerationandcollagen
depositionand fibrosis.However,FLC(200mg/kg)didnotshow
anysignificantprotection(Fig.4).
Discussion
The2K1Cisaclassicalmethodtoinducehypertensioninrats
similartohuman,whichisprimarilybasedonRAAS(Thurstonetal.,
1980;PonchonandElghozi,1996;Kandhareetal.,2011b).Themain
mechanismbehindthe2K1ChypertensionisRAASratherthana
disturbanceinkidneyfunction(Nogueiraetal.,2012).Unilateral
renalarteryocclusiondecreasesperfusionpressureinsidethe
kid-neyandstimulatesreninsynthesis,whichthenproduceAng-IIand
increasestheperipheralresistanceandbloodpressure(Pickering,
1989).
Our studydemonstrated that higher doses of FLC (400 and
800mg/kg) significantly decreased systolic, diastolic and mean
arterial blood pressures in the 2K1C hypertensive Wistar rats.
Secoisolariciresinoldiglucoside(SDG),amainconstituentofFLC,
isreportedtohaveasimilartypeofresultsinnormotensiveand
100
A
B
D
C
Plasma nitr
ite and nitr
ate
concentr
ation (
µ
mol/l)
Kidne
y Ang-II
(pg/mg protein)
Plasma ET
-1 (pg/ml)
Plasma NOS (U/ml)
80
60
40
20
0
200
150
###
### ### #
***
*** ***
***
***
*** ***
** **
* **
**
100
50
Treatment 0
Sham
2k1c controlCaptopr il (30)
FLC (200) FLC (400) FLC (800)
Treatment 0
20 40 60 80 100 0 5 10 15 20
Sham
2k1c controlCaptopr il (30)
FLC (200) FLC (400) FLC (800)
Fig.2.EffectofadministrationofFLCandcaptoprilonplasmaNOlevels(A),plasmaNOSactivity(B),Ang-IIofclippedkidney(C)andplasmaET-1(D)in2K1Chypertensive Wistarrats(n=6).Dataareexpressedasmean±S.E.M.andstatisticalanalysiswascarriedoutbyone-wayANOVAfollowedbyposthocDunnett’stest;ns=non-significant, *p<0.05,**p<0.01,***p<0.001ascomparedwith2K1Ccontrolgroup-II,#p<0.05,##p<0.01,###p<0.001comparedtoshamgroup-I.
Fig.4. EffectofadministrationofFLCandcaptoprilonhearthistologyin2K1ChypertensiveWistarrats.Photomicrographofsectionsoflungsof(A)sham-operatedrats (group-I)showednormalcardiacmusclefiberswiththeabsenceofmyocardialfibrosis.(B)2K1Ccontrol(group-II)showedseveremyocardialdegeneration,hypertrophy,andfibrosis. (C)2K1C+Captopril(30mg/kg)(group-III)showedminimalmyocardialdegenerationandcollagendepositionandmyocardialfibrosis.(D)2K1C+FLC(200mg/kg)(group-IV) showednosignificantdecreaseincollagendepositionandmyocardialfibrosis.(E)2K1C+FLC(400mg/kg)(GroupV)showedadecreaseinmyocardialdegenerationand collagendepositionandfibrosis.(F)2K1C+FLC(800mg/kg)(GroupVI)showedasignificantreductioninmyocardialdegeneration,collagendeposition,andfibrosis(Masson’s trichrome20×).
theactivityofSDGmaybedue tothestimulationof guanylate
cyclase-nitric oxide pathway and by inhibition of angiotensin
converting enzyme (ACE) (Prasad, 2004; Kamble et al., 2013).
Earlier,we havedeterminedSDGcontentinFLCbyusing
high-performanceliquidchromatography(HPLC)analysisandreported
bloodpressureloweringeffectofFLCinDOCA-salt-inducedrenal
hypertensionmodelinrats(SawantandBodhankar,2016).Inthe
presentstudy,theeffectofFLContherenin–angiotensinsystem
dependent2K1Chypertensiverat, similartothose of captopril,
leadus toconsiderthatSDG a mainconstituent fromFLCmay
possess ACE-inhibitor-like properties. However, the possibility
of a potentiating effect of SDG by other flavonoids and minor
constituentspresentinFLCcannotberuledout.
Thehemodynamicdatashowedthatleftventricleenddiastolic
pressure,maxdP/dtandmindP/dtincreasedin2K1Chypertensive
rats,whichareaclearsignofincreasedpreloadandafterloadinthe
heart.Thealteredleftventricularparametersin2K1Chypertensive
ratsalsoshoweddecreasecontractility,diastoliccomplianceand
dysfunctionintheheart(Wangetal.,2007;Junhongetal.,2008).
FLCandcaptoprilarerestoredEDP,maxdP/dtandmindP/dt
sig-nificantlyindicatedthatFLCandcaptoprildecreasedtheburdenon
theheart,increasedtheconformityofmyocardiumandimproved
cardiacfunction.ThesefindingsthussupportthatFLCcontaining
SDGasmainconstituenthasantihypertensivepotentialin2K1C
hypertensiverats.
Chronic hypertension leads to continuous accumulation of
interstitialcollagenfibersandanincreaseinheartweight(Rossi
andPeres,1992).ItisprovedthatAng-IIofRAASisalsoinvolved
inthetissue hypertrophyorhyperplasia.Therefore,RAASplays
animportantrole intheweightincreaseofheartandkidneyin
the2K1Chypertensivemodel(Kobayashietal.,1999).Thecurrent
results showed thecaptopril and FLC significantly prevent the
increaseinkidneyandheartweightassociatedwithhypertrophy,
whichmaybeduetoantihypertensiveeffectsofcaptoprilandFLC.
It is wellknownthatintracellular sodiumion concentration
increasesandpotassiumionconcentrationdecreasessignificantly
in hypertension (Adrogue and Madias, 2007). Our results are
thus,inaccordance withthepreviousstudyandsuggestedthat
restoration ofserum sodiumandpotassium ion maybedue to
antihypertensiveeffectsofFLC.
Theliverplaysanimportantroleinmetabolism,toxicityand
eliminationofendogenousandexogenouselements.Liverdamage
leadstoincreasedactivityofAST,ALT,ALPandtotalproteininthe
plasma(Navarroetal.,1993;Bhattacharjeeetal.,2009;Visnagri
etal.,2012;Kandhareetal.,2013b,c,2015d;Sarkateetal.,2015;
Devkaretal.,2016).ThereasonbehindtheelevationofAST,ALT,
ALPandtotalproteininthe2K1Chypertensionmaybeoxidative
stressthatcausedleakageoftheseenzymesfromlivertissuesdueto
membranedamage.AftertheadministrationofFLCandcaptopril,
therewasasignificantdecreaseintheserumactivitiesofAST,ALT,
ALPandtotalproteinthatclearlysignifiesthatcaptoprilandFLC
protectedthefunctionalcapacityofliverandpreventedoxidative
damageduetohypertension.
Inhypertension,volumeandpressureloadsonthekidneyslead
tothedysfunctionanddamagetotherenaltissues(Möhringetal.,
1975;Kandhareetal.,2015d).Bloodureanitrogen(BUN),serum
creatinine,anduricacidareconsideredasmarkersoftherenal
func-tion.Theyareproducedduetodisturbanceofproteinandnucleic
acidmetabolisminthehypertensivestress.Severalanimal
stud-iesof2K1Chypertensionhavereportedthathypertensionelevates
thelevelsofbloodureanitrogen,serumcreatinineanduricacid
duetohighblood pressuremaybethereason fortheelevated
levelsofBUN,creatinine,and uricacidintheserum(Kandhare
etal.,2012a,b,c,d,2015c).Thecurrentstudyshowedareduction
intheelevatedserumlevelofrenalmarkersbyFLCandexplainits
protectiveeffectin2K1Chypertensiverats.Hypertensioninduces
myocardialdamage,andtheserumlevelsofCK-MBandLDHare
consideredasstandardmarkers fortheidentificationofcardiac
damage(Mairetal., 1994).The decreased serumlevel ofthese
enzymesintheFLC-treatedgroupsofferedprotectiontoheartin
the2K1Chypertension.
ThepresentstudyshowedthattheactivityofSODandGSHin
theheartandkidneytissuesof2K1Ccontrolgroupwaslowerthan
thatinthesham-operatedgroup.Ontheotherhand,MDAactivity
inthe2K1Ccontrolgroupwassignificantlyhigherthaninthe
sham-operatedgroup.Ourresultsthussuggestedthathypertensionledto
increasedoxidativestress,whichisinagreementwiththeprevious
study(Caoetal.,2013).ThecurrentstudyshowedthatFLCand
captopriladministrationscavengingtheoxygenfreeradicalinthe
bloodleadingtotheiranti-oxidanteffects.
Bloodflow-inducedshearstressonendothelialcellsplayskey
roleintheproductionofNObytheendothelialNOS(Pohletal.,
1986;Kandhareetal.,2012d,2014b,2016b).NOisvasorelaxant
substanceandphysiologicalantagonist oftheAng-IIofRAASat
vascular levels.Ang-II reduces NObioavailability by promoting
superoxideanion, whichis responsibleforvasoconstriction and
increaseinbloodpressure(DeNicolaetal.,1992;Cosentinoetal.,
1994;Zhouetal.,2014).
TheantagonisticeffectsofNOandAng-IIcomeoutalsoin
inter-actionswithothervasoactivesubstanceET-1,whichisthepotent
vasoconstrictorreleased fromtheendothelium (Rubanyi, 1994;
Zhouetal.,2014).ET-1isoverexpressedinthevasculatureand
bloodinvariousmodelsofhypertension,includingthe2K1Cmodel,
whichincreasessystemicbloodpressure(IglarzandSchiffrin,2003;
Cao et al., 2013).In the present study, these biomarkers were
altered in the 2K1C control hypertensive rats, where
simulta-neouslythebloodpressureincreasedwithAng-IIandET-1,asa
synthesisofNOandNOSdecreased.Allthesechangeswereopposed
significantlybytheACEinhibitordrugcaptopriland FLC.
Dose-dependenteffectsofFLContherenin–angiotensinsystemmarkers
weresimilartothoseofcaptoprilandsuggestACEinhibitorlike
propertyofFLC.
Histologicalstudyoftheratheart andkidneys revealedthat
theFLCtreatmentinthe2K1Chypertensiveratsreducedcardiac
andrenaldamagecorrelatewiththevarioushemodynamic,
bio-chemicalobservations.Theseresultssupporttheantihypertensive
activityofFLCinthe2K1Chypertensiverats.
Conclusion
AntihypertensiveandantioxidanteffectsofFLCin2K1C
hyper-tensionweredose-dependentandatthehighestdose(800mg/kg)
similartothoseofcaptopril,whichismainlycharacterizedbythe
reductioninbloodpressure,restorationofalteredleftventricular
functionsandendogenousbiomarkers.ItisconcludedthatFLCmay
reducebloodpressurebyreductionofkidneyAng-IIlevel,
inhibi-tionofET-1productionandinductionoftheNO,NOSandinvivo
antioxidantdefensesystem.
Ethicalstatement
Theanimalexperimentsinthepresentworkwerecarriedout
strictlyfollowingtheguidelinesgivenbyCPCSEA,India.The
pro-tocolwasapprovedbytheInstitutionalAnimalEthicalCommittee
(IAEC)constitutedasperguidelinesofCPCSEA.
Ethicaldisclosures
Protectionofhumanandanimalsubjects. Theauthorsdeclare
thattheproceduresfollowedwereinaccordancewiththe
regula-tionsoftherelevantclinicalresearchethicscommitteeandwith
thoseoftheCodeofEthicsoftheWorldMedicalAssociation
(Dec-larationofHelsinki).
Confidentialityofdata. Theauthorsdeclarethatnopatientdata
appearinthisarticle.
Righttoprivacyandinformedconsent. Theauthorsdeclarethat
nopatientdataappearinthisarticle.
Authors’contributions
SScontributedincollectingplantsampleandidentification,a
confectionoftheherbarium,runningthelaboratorywork,analysis
ofthedataanddraftedthepaper.SLBsupervisedthelaboratory
workandcontributedtocriticalreadingofthemanuscript.Allthe
authorshavereadthefinalmanuscriptandapprovedthe
submis-sion.
Conflictsofinterest
Theauthorsdeclarenoconflictsofinterest.
References
Adil, M., Kandhare, A., Dalvi, G., Ghosh, P., Venkata, S., Raygude, K., Bod-hankar,S.,2016a.Ameliorativeeffectofberberineagainstgentamicin-induced nephrotoxicityinratsviaattenuationofoxidativestress,inflammation, apo-ptosis andmitochondrial dysfunction.Ren.Fail., http://dx.doi.org/10.3109/ 0886022X.2016.1165120.
Adil,M.,Kandhare,A.,Ghosh,P.,Venkata,S.,Raygude,K.,Bodhankar,S.,2016b. Ameliorativeeffectofnaringininacetaminophen-inducedhepaticandrenal toxicityinlaboratoryrats:roleofFXRandKIM-1.Ren.Fail.,http://dx.doi.org/ 10.3109/0886022X.2016.1163998.
Adil,M.,Kandhare,A.D.,Visnagri,A.,Bodhankar,S.L.,2015.Naringinameliorates sodiumarsenite-inducedrenalandhepatictoxicityinrats:decisiveroleof KIM-1,Caspase-3,TGF-beta,andTNF-alpha.Ren.Fail.37,1396–1407.
Adil,M.,Visnagri,A.,Kumar,V.S.,Kandhare,A.D.,Ghosh,P.,2014.Protectiveeffect ofnaringinonsodiumarseniteinducedtesticulartoxicityviamodulationof biochemicalperturbationsinexperimentalrats.Pharmacologia5,222–234. Adrogue,H.J.,Madias,N.E.,2007.Sodiumandpotassiuminthepathogenesisof
hypertension.N.Engl.J.Med.356,1966–1978.
Amat, N., Amat, R., Abdureyim,S., Hoxur, P., Osman, Z., Mamut,D., Kijjoa, A., 2014. Aqueous extract of Dioscorea opposita thunb. normalizes the hypertension in2K1C hypertensive rats. BMC Complement.Altern. Med., http://dx.doi.org/10.1186/1472-6882-14-36.
Aswar,U.M.,Kandhare,A.D.,Mohan,V.,Thakurdesai,P.A.,2015.Anti-allergiceffect ofintranasaladministrationoftype-Aprocyanidinpolyphenolsbased standard-izedextractofcinnamonbarkinovalbuminsensitizedBALB/cmice.Phytother. Res.29,423–433.
Badole,S.L.,Chaudhari,S.M.,Jangam,G.B.,Kandhare,A.D.,Bodhankar,S.L.,2015. CardioprotectiveactivityofPongamiapinnatainstreptozotocin–nicotinamide induceddiabeticrats.Biomed.Res.Int.2015,403291.
Bassett,C.M.,Rodriguez-Leyva,D.,Pierce,G.N.,2009.Experimentalandclinical researchfindingsonthecardiovascularbenefitsofconsumingflaxseed.Appl. Physiol.Nutr.Metab.34,965–974.
Bhattacharjee,N.,Pathak,S.,Khuda-Bukhsh,A.R.,2009.Ameliorationof carcinogen-inducedtoxicityinmicebyadministrationofapotentizedhomeopathicdrug, natrumsulphuricum200.Evid.BasedComplement.Alternat.Med.6,65–75. Cao,Y.J.,He,X.,Wang,N.,He,L.C.,2013.Effectsofimperatorin,theactivecomponent
fromRadixAngelicae(Baizhi),onthebloodpressureandoxidativestressin2K,1C
hypertensiverats.Phytomedicine20,1048–1054.
Chen,J.,Stavro,P.M.,Thompson,L.U.,2002.Dietaryflaxseedinhibitshumanbreast cancergrowthandmetastasisanddownregulatesexpressionofinsulin-like growthfactorandepidermalgrowthfactorreceptor.Nutr.Cancer43,187–192. Collins,T.F.,Sprando,R.L.,Black,T.N.,Olejnik,N.,Wiesenfeld,P.W.,Babu,U.S.,Bryant, M.,Flynn,T.J.,Ruggles,D.I.,2003.Effectsofflaxseedanddefattedflaxseedmeal onreproductionanddevelopmentinrats.FoodChem.Toxicol.41,819–834. Cosentino,F.,Sill,J.C.,Katusic,Z.S.,1994.Roleofsuperoxideanionsinthemediation
ofendothelium-dependentcontractions.Hypertension23,229–235. Coulter,D.M.,Edwards,I.R.,1987.Coughassociatedwithcaptoprilandenalapril.
DeNicola,L.,Blantz,R.C.,Gabbai,F.B.,1992.NitricoxideandangiotensinII. Glomeru-larandtubularinteractionintherat.J.Clin.Invest.89,1248–1256.
Devkar,S.T.,Kandhare,A.D.,Zanwar,A.A.,Jagtap,S.D.,Katyare,S.S.,Bodhankar, S.L.,Hegde,M.V.,2016.Hepatoprotectiveeffectofwithanolide-richfractionin acetaminophen-intoxicatedrat:decisiveroleofTNF-alpha,IL-1beta,COX-IIand iNO.Pharm.Biol.,1–10,http://dx.doi.org/10.3109/13880209.2016.1157193. Ghosh, P.,Kandhare, A.D.,Raygude,K.S.,Kumar, V.S.,Rajmane, A.R.,Adil,M.,
Bodhankar,S.L.,2012.Determinationofthelongtermdiabetesrelated compli-cationsandcardiovasculareventsusingUKPDSriskengineandUKPDSoutcomes modelinarepresentativewesternIndianpopulation.AsianPac.J.Trop.Dis.2, S642–S650.
Ghule,A.E.,Jadhav,S.S.,Bodhankar,S.L.,2011.RenoprotectiveeffectofLinum
usitatis-simumseedsthroughhaemodynamicchangesandconservationofantioxidant
enzymes in renal ischaemia-reperfusion injury in rats. Arab. J. Urol. 9, 215–221.
Ghule,A.E.,Jadhav,S.S.,Bodhankar,S.L.,2012.Effectofethanolicextractofseedsof
Linumusitatissimum(Linn.)onhemodynamicchangesandleftventricular
func-tioninrenalarteryoccludedrenovascularhypertensioninrats.Pharmacologia 3,283–290.
Ghule,A.E.,Kandhare,A.D.,Jadhav,S.S.,Zanwar,A.A.,Bodhankar,S.L.,2015. Omega-3-fattyacidaddstotheprotectiveeffectofflaxlignanconcentrateinpressure overload-inducedmyocardialhypertrophyinratsviamodulationofoxidative stressandapoptosis.Int.Immunopharmacol.28,751–763.
Gosavi,T.,Kandhare,A.,Raygude,K.,Ghosh,P.,Bodhankar,S.,2011.Acomparative studyontheefficacy,safetyandcosteffectivenessofViscumalbumand
Rau-wolfiaserpentinamothertinctureinhypertensivepatients.Deccan.J.Nat.Prod.
2,29–35.
Gosavi,T.P.,Ghosh,P.,Kandhare,A.D.,Kumar,V.S.,Adil,M.,Rajmane,A.R., Bod-hankar,S.L., 2012a.TherapeuticeffectofH. pylorinosode,a homeopathic preparationinhealingofchronicH.pyloriinfectedulcersinlaboratoryanimals. AsianPac.J.Trop.Dis.2,S603–S611.
Gosavi,T.P.,Kandhare,A.D.,Ghosh,P.,Bodhankar,S.L.,2012b.Anticonvulsant
activ-ityofArgentummetallicum,ahomeopathicpreparation.DerPharm.Lett.4,
626–637.
Gosavi,T.P.,Kumar,V.S.,Kandhare,A.D.,Zanwar,A.A.,Hegde,M.V.,Bodhankar,S.L., 2014.Acomprehensivemetaanalysisandsystematicreviewoneffectof genis-teinonmetabolicsyndrome.Pharmacologia5,120–126.
Goswami,S.,Kandhare,A.,Zanwar,A.A.,Hegde,M.V.,Bodhankar,S.L.,Shinde,S., Deshmukh,S.,Kharat,R.,2016.Orall-glutamineadministrationattenuated cutaneouswoundhealinginWistarrats.Int.WoundJ.13,116–124.
Griendling,K.K.,Minieri,C.A.,Ollerenshaw,J.D.,Alexander,R.W.,1994.Angiotensin IIstimulatesNADHandNADPHoxidaseactivityinculturedvascularsmooth musclecells.Circ.Res.74,1141–1148.
Gryglewski,R.J.,Palmer,R.M.J.,Moncada,S.,1986.Superoxideanionisinvolvedin thebreakdownofendothelium-derivedvascularrelaxingfactor.Nature320, 454–456.
Honmore,V.,Kandhare,A.,Zanwar,A.A.,Rojatkar,S.,Bodhankar,S.,Natu,A.,2015.
Artemisiapallensalleviatesacetaminopheninducedtoxicityviamodulationof
endogenousbiomarkers.Pharm.Biol.53,571–581.
Iglarz,M.,Schiffrin,E.L.,2003.Roleofendothelin-1inhypertension.Curr.Sci.Inc.5, 144–148.
Johnsson,P.,Kamal-Eldin,A.,Lundgren,L.N.,Aman,P.,2000.HPLCmethodfor anal-ysisofsecoisolariciresinoldiglucosideinflaxseeds.J.Agric.FoodChem.48, 5216–5219.
Junhong,W.,Jing,Y.,Jizheng,M.,Shushu,Z.,Xiangjian,C.,Hengfang,W.,Di,Y.,Jinan, Z.,2008.Proteomicanalysisofleftventriculardiastolicdysfunctionheartsin renovascularhypertensiverats.Int.J.Cardiol.127,198–207.
Kamble,H.,Kandhare,A.D.,Bodhankar,S.,Mohan,V.,Thakurdesai,P.,2013.Effectof lowmolecularweightgalactomannansfromfenugreekseedsonanimalmodels ofdiabetesmellitus.Biomed.AgingPathol.3,145–151.
Kandhare,A.,Raygude,K.,Ghosh,P.,Bodhankar,S.,2011a.Theameliorativeeffectof fisetin,abioflavonoid,onethanol-inducedandpylorusligation-inducedgastric ulcerinrats.Int.J.GreenPharm.5,236–243.
Kandhare,A.D.,Alam,J.,Patil,M.V.,Sinha,A.,Bodhankar,S.L.,2016a.Wound heal-ingpotentialofnaringinointmentformulationviaregulatingtheexpression ofinflammatory,apoptoticandgrowthmediatorsinexperimentalrats.Pharm. Biol.54(3),419–432.
Kandhare,A.D.,Bodhankar,S.L.,Mohan,V.,Thakurdesai,P.A.,2015a.Acuteand repeateddoses(28days)oraltoxicitystudy ofglycosidesbased standard-izedfenugreekseedextractinlaboratorymice.Regul.Toxicol.Pharmacol.72, 323–334.
Kandhare,A.D.,Bodhankar,S.L., Mohan,V., Thakurdesai,P.A., 2015b.Effectof glycosidesbasedstandardizedfenugreekseedextractinbleomycin-induced pulmonaryfibrosisinrats:decisiveroleofBax,Nrf2,NF-kappaB,Muc5ac, TNF-alphaandIL-1beta.Chem.Biol.Interact.237,151–165.
Kandhare,A.D.,Bodhankar,S.L.,Mohan,V.,Thakurdesai,P.A.,2015c. Pharmacoki-netics,tissuedistributionandexcretionstudyofafurostanolglycoside-based standardizedfenugreekseedextractinrats.Ren.Fail.37,1208–1218. Kandhare,A.D.,Bodhankar,S.L.,Singh,V.,Mohan,V.,Thakurdesai,P.A.,2013a.
Anti-asthmaticeffectsoftype-Aprocyanidinepolyphenolsfromcinnamonbarkin ovalbumin-inducedairwayhyperresponsivenessinlaboratoryanimals.Biomed. AgingPathol.3,23–30.
Kandhare,A.D.,Ghosh,P.,Bodhankar,S.L.,2014a.Naringin,aflavanoneglycoside, promotesangiogenesisandinhibitsendothelialapoptosisthroughmodulation ofinflammatoryandgrowthfactorexpressionindiabeticfootulcerinrats. Chem.Biol.Interact.219,101–112.
Kandhare, A.D., Ghosh, P., Ghule, A.E., Bodhankar, S.L., 2013b. Elucidationof molecularmechanisminvolvedinneuroprotectiveeffectofcoenzymeQ10in alcohol-inducedneuropathicpain.Fundam.Clin.Pharmacol.27,603–622. Kandhare,A.D.,Ghosh,P.,Ghule,A.E.,Zambare,G.N.,Bodhankar,S.L.,2013c.
Protec-tiveeffectofPhyllanthusamarusbymodulationofendogenousbiomarkersand DNAdamageinaceticacidinducedulcerativecolitis:roleofphyllanthinand hypophyllanthin.ApolloMed.10,87–97.
Kandhare,A.D.,Kumar,V.S.,Adil,M.,Rajmane,A.R.,Ghosh,P.,Bodhankar,S.L.,2012a. InvestigationofgastroprotectiveactivityofXanthiumstrumariumL.by modula-tionofcellularandbiochemicalmarker.OrientalPharm.Exp.Med.12,287–299. Kandhare,A.D.,Patil,A.,Guru,A.,Mukhrjee,A.,Sarkar,A.,Sengupta,A.,Parmar, H.M.,Muthal,A.P.,Wangikar,P.,Bodhankar,S.L.,2016b.Ameliorativeeffectof ferulicacidagainstaceticacidinducedulcerativecolitis:RoleofHO-1andNrf2. Pharmacologia7,114–124.
Kandhare,A.D.,Patil,M.V.,Bodhankar,S.L.,2015d.l-Arginineattenuatesthe ethyl-eneglycolinducedurolithiasisinininephrectomizedhypertensiverats:roleof KIM-1,NGAL,andNOs.Ren.Fail.37,709–721.
Kandhare,A.D.,Raygude,K.S.,Ghosh,P.,Ghule,A.E.,Bodhankar,S.L.,2012b. Neu-roprotectiveeffectofnaringinbymodulationofendogenousbiomarkersin streptozotocininducedpainfuldiabeticneuropathy.Fitoterapia83,650–659. Kandhare,A.D.,Raygude,K.S.,Ghosh,P.,Ghule,A.E.,Gosavi,T.P.,Badole,S.L.,
Bod-hankar,S.L.,2012c.EffectofhydroalcoholicextractofHibiscusrosasinensisLinn. leavesinexperimentalcolitisinrats.AsianPac.J.Trop.Biomed.2,337–344. Kandhare, A.D., Raygude,K.S.,Ghosh, P., Gosavi, T.P., Bodhankar,S.L., 2011b.
Patentabilityofanimalmodels:Indiaandtheglobe.Int.J.Pharm.Biol.Arc.2, 1024–1032.
Kandhare,A.D.,Raygude,K.S.,ShivaKumar,V.,Rajmane,A.R.,Visnagri,A.,Ghule,A.E., Ghosh,P.,Badole,S.L.,Bodhankar,S.L.,2012d.Ameliorativeeffectsquercetin againstimpairedmotornervefunction,inflammatorymediatorsandapoptosis inneonatalstreptozotocin-induceddiabeticneuropathyinrats.Biomed.Aging Pathol.2,173–186.
Kandhare,A.D.,Shivakumar,V.,Rajmane,A.,Ghosh,P.,Bodhankar,S.L.,2014b. Eval-uationoftheneuroprotectiveeffectofchrysinviamodulationofendogenous biomarkersinaratmodelofspinalcordinjury.J.Nat.Med.68,586–603. Kang,D.H.,Nakagawa,T.,Feng,L.,Watanabe,S.,Han,L.,Mazzali,M.,Truong,L.,Harris,
R.,Johnson,R.J.,2002.Aroleforuricacidintheprogressionofrenaldisease.J. Am.Soc.Nephrol.13,2888–2897.
Kearney,P.,Whelton,M.,Reynolds,K.,Muntner,P.,Whelton,P.,He,J.,2005.Global burdenofhypertension:analysisofworldwidedata.Lancet365,217–223. Ketkar,S.,Rathore,A.,Kandhare, A.,Lohidasan,S.,Bodhankar,S.,Paradkar,A.,
Mahadik,K.,2015.Alleviatingexercise-inducedmuscularstressusingneatand processedbeepollen:oxidativemarkers,mitochondrialenzymes,and myo-statinexpressioninrats.Integr.Med.Res.4,147–160.
Khalesi,S.,Irwin,C.,Schubert,M.,2015.Flaxseedconsumptionmayreduceblood pressure:asystematicreviewandmeta-analysisofcontrolledtrials.J.Nutr.145, 758–765.
Kharin,S.N.,Krandycheva,V.V.,2004.Methodofexperimentalconstrictionofrenal arteryformodelingofrenovascularhypertensioninrats.Bull.Exp.Biol.Med. 138,103–105.
Kinniry,P.,Amrani,Y.,Vachani,A.,Solomides,C.C.,Arguiri,E.,Workman,A.,Carter, J.,Christofidou-Solomidou,M.,2006.Dietaryflaxseedsupplementation ame-lioratesinflammationandoxidativetissuedamageinexperimentalmodelsof acutelunginjuryinmice.J.Nutr.136,1545–1551.
Kobayashi, S.,Ishida,A., Moriya,H.,Mori, N.,Fukuda, T.,Takamura, T.,1999. AngiotensinIIreceptorblockadelimitskidneyinjuryintwo-kidney,one-clip Goldblatthypertensiveratswithspecialreferencetophenotypicchanges.J.Lab. Clin.Med.133,134–143.
Kumar,V.S.,Rajmane,A.R.,Adil,M.,Kandhare,A.D.,Ghosh,P.,Bodhankar,S.L.,2014. Naringinamelioratesaceticacidinducedcolitisthroughmodulationof endoge-nousoxido-nitrosativebalanceandDNAdamageinrats.J.Biomed.Res.28, 132–145.
Lee,J.C.,Krochak,R.,Blouin,A.,Kanterakis,S.,Chatterjee,S.,Arguiri,E.,Vachani,A., Solomides,C.C.,Cengel,K.A.,Christofidou-Solomidou,M.,2009.Dietaryflaxseed preventsradiation-inducedoxidativelungdamage,inflammationandfibrosis inamousemodelofthoracicradiationinjury.CancerBiol.Ther.8,47–53. Mair,J.,Wagner,I.,Jakob,G.,Lechleitner,P.,Dienstl,F.,Puschendorf,B.,Michel,G.,
1994.Differenttimecoursesofcardiaccontractileproteinsafteracute myocar-dialinfarction.Clin.Chim.Acta231,47–60.
Miranda,K.M.,Espey,M.G.,Wink,D.A.,2001.Arapid,simplespectrophotometric methodfor simultaneous detection ofnitrate and nitrite.Nitric Oxide5, 62–71.
Möhring,J.,Möhring,B.,Haack,D.,Lazar,J.,Oster,P.,Schömig,A.,Gross,F.,1975. ThirstandSaltAppetiteinExperimentalRenalHypertensionofRats.In:Control MechanismsofDrinking.SpringerScience+BusinessMedia,pp.155–164. Moron,M.S.,Depierre,J.W.,Mannervik,B.,1979.Levelsofglutathione,glutathione
reductaseandglutathioneS-transferaseactivitiesinratlungandliver.Biochim. Biophys.Acta582,67–78.
Navarro,M.,Montilla,M.,Martín,A.,Jiménez,J.,Utrilla,M.,1993.Freeradical scav-engerandantihepatotoxicactivityofRosmarinustomentosus.PlantaMed.59, 312–314.
Nogueira,B.V.,Palomino,Z.,Porto,M.L.,Balarini,C.M.,Pereira,T.M.,Baldo,M.P., Casarini,D.E.,Meyrelles,S.S.,Vasquez,E.C.,2012.Granulocytecolony stimulat-ingfactorpreventskidneyinfarctionandattenuatesrenovascularhypertension. CellPhysiol.Biochem.29,143–152.
Morininpylorusligationinducedgastriculcerviamodulationofoxidative stress.DerPharm.Lett.7,131–139.
Patil,M.,Kandhare,A.,Bhise,S.,2011.Pharmacologicalevaluationofameliorative effectofaqueousextractofCucumissativusL.fruitformulationonwoundhealing inWistarrats.ChroniclesYoungSci.2,207–213.
Pickering,T.G.,1989.Renovascularhypertension:etiologyandpathophysiology. Semin.Nucl.Med.19,79–88.
Pohl,U.,Holtz,J.,Busse,R.,Bassenge,E.,1986.Crucialroleofendotheliuminthe vasodilatorresponsetoincreasedflowinvivo.Hypertension8,37–44. Ponchon, P., Elghozi, J.L., 1996. Contribution of the renin–angiotensin and
kallikrein–kininsystemstoshort-termvariabilityofbloodpressurein two-kidney,one-cliphypertensiverats.Eur.J.Pharmacol.297,61–70.
Prasad,K.,1997.Dietaryflaxseedinpreventionofhypercholesterolemic atheroscle-rosis.Atherosclerosis132,69–76.
Prasad,K.,2004.Antihypertensiveactivityofsecoisolariciresinoldiglucoside(SDG) isolatedfromflaxseed:roleofguanylatecyclase.Int.J.Angiol.13,7–14. Prasad,K.,Mantha,S.V.,Muir,A.D.,Westcott,N.D.,1998.Reductionof
hypercholes-terolemicatherosclerosisbyCDC-flaxseedwithverylowalpha-linolenicacid. Atherosclerosis136,367–375.
Prasad,K.,Mantha,S.V.,Muir,A.D.,Westcott,N.D.,2000.Protectiveeffectof sec-oisolariciresinoldiglucosideagainststreptozotocin-induceddiabetesandits mechanism.Mol.CellBiochem.206,141–149.
Rajagopalan,S.,Kurz,S.,Munzel,T.,Tarpey,M.,Freeman,B.A.,Griendling,K.K., Har-rison,D.G.,1996.AngiotensinII-mediatedhypertensionintheratincreases vascularsuperoxideproductionviamembraneNADH/NADPHoxidase acti-vation. Contributionto alterations of vasomotor tone. J. Clin. Invest. 97, 1916–1923.
Rajesha,J.,Murthy,K.N.,Kumar,M.K.,Madhusudhan,B.,Ravishankar,G.A.,2006. Antioxidantpotentialsofflaxseedbyinvivomodel.J.Agric.FoodChem.54, 3794–3799.
Raygude,K.S.,Kandhare,A.D.,Ghosh,P.,Bodhankar,S.L.,2012a.Anticonvulsant effectoffisetinbymodulationofendogenousbiomarkers.Biomed.Prev.Nutr. 2,215–222.
Raygude,K.S.,Kandhare,A.D.,Ghosh,P.,Ghule,A.E.,Bodhankar,S.L.,2012b. Eval-uationofameliorativeeffectofquercetininexperimentalmodelofalcoholic neuropathyinrats.Inflammopharmacology20,331–341.
Rodriguez-Leyva,D.,Weighell,W.,Edel,A.L.,LaVallee,R.,Dibrov,E.,Pinneker,R., Maddaford,T.G.,Ramjiawan,B.,Aliani,M.,Guzman,R.,Pierce,G.N.,2013.Potent antihypertensiveactionofdietaryflaxseedinhypertensivepatients. Hyperten-sion62,1081–1089.
Rossi,M.A.,Peres,L.C.,1992.Effectofcaptoprilonthepreventionandregressionof myocardialcellhypertrophyandinterstitialfibrosisinpressureoverloadcardiac hypertrophy.Am.HeartJ.124,700–709.
Rubanyi,G.,1994.Endothelins:molecularbiology,biochemistry,pharmacology, physiology,andpathophysiology.Pharmacol.Rev.46,325–415.
Saraswathi,K.Y.,Muthal,A.,Kandhare,A.,Rojatkar,S.,Bodhankar,S.,2014.Studyof methanolicextractofArtemisiapallensWallonenduranceoflaboratoryanimals. Pharmacologia5,298–309.
Sawant,S.H.,Bodhankar,S.L.,2016.Flaxlignanconcentratereversealterationsin bloodpressure,leftventricularfunctions,lipidprofileandantioxidantstatusin DOCA-saltinducedrenalhypertensioninrats.Ren.Fail.38,411–423.
Sarkar,A.,Sengupta,A.,Mukhrjee,A.,Guru,A.,Patil,A.,Kandhare,A.D.,Bodhankar, S.L.,2015.Antiulcerpotentialofmorininaceticacid-inducedgastriculcervia modulationofendogenousbiomarkersinlaboratoryanimals.Pharmacologia6, 273–281.
Sarkate,A.P.,Murumkar,P.R.,Lokwani,D.K.,Kandhare,A.D.,Bodhankar,S.L.,Shinde, D.B.,Bothara,K.G.,2015.DesignofselectiveTACEinhibitorsusingmolecular dockingstudies:synthesisandpreliminaryevaluationofanti-inflammatoryand TACEinhibitoryactivity.SARQSAREnviron.Res.26,905–923.
Schlaich,M.P.,Delles,C.,Schmieder,R.E.,2007.Involvementofendothelial mecha-nismsinl-arginine-inducedalterationsofrenalhaemodynamicsinhumans.J. Hypertens.25,1515–1516(authorreply1516–1517).
Shivakumar,V.,Kandhare,A.,Rajmane,A.,Adil,M.,Ghosh,P.,Badgujar,L.,Saraf,M., Bodhankar,S.,2014.Estimationofthelong-termcardiovasculareventsusing UKPDSriskengineinmetabolicsyndromepatients.IndianJ.Pharm.Sci.76, 174–178.
Slater,T.F.,Sawyer,B.C.,1971.Thestimulatoryeffectsofcarbontetrachlorideand otherhalogenoalkanesonperoxidativereactionsinratliverfractionsinvitro. Generalfeaturesofthesystemsused.Biochem.J.123,805–814.
Thurston,H.,Bing,R.F.,Swales,J.D.,1980.Reversaloftwo-kidneyoneclip renovas-cularhypertensionintherat.Hypertension2,256–265.
Visnagri,A.,Kandhare,A.D.,Bodhankar,S.L.,2015.Renoprotectiveeffectof berber-ineviaintonationonapoptosisandmitochondrial-dependentpathwayinrenal ischemiareperfusion-inducedmutilation.Ren.Fail.37,482–493.
Visnagri,A.,Kandhare,A.D.,Chakravarty,S.,Ghosh,P.,Bodhankar,S.L.,2014. Hes-peridin, aflavanoglyconeattenuates experimentaldiabeticneuropathy via modulationofcellularandbiochemicalmarkertoimprovenervefunctions. Pharm.Biol.52,814–828.
Visnagri,A.,Kandhare,A.D.,Ghosh,P.,Bodhankar,S.L.,2013.Endothelinreceptor blockerbosentaninhibitshypertensivecardiacfibrosisinpressure overload-inducedcardiachypertrophyinrats.Cardiovasc.Endocrinol.2,85–97. Visnagri,A.,Kandhare,A.D.,ShivaKumar,V.,Rajmane,A.R.,Mohammad,A.,Ghosh,
P.,Ghule,A.E., Bodhankar,S.L.,2012. Elucidationofameliorativeeffectof co-enzymeQ10instreptozotocin-induceddiabeticneuropathicperturbation bymodulationofelectrophysiological,biochemicalandbehavioralmarkers. Biomed.AgingPathol.2,157–172.
Wang,P.,Tang,F.,Li,R.,Zhang,H.,Chen,S.,Liu,P.,Huang,H.,2007.Contributionof differentNoxhomologuestocardiacremodelingintwo-kidneytwo-clip reno-vascularhypertensiverats:effectofvalsartan.Pharmacol.Res.55,408–417. Zanwar,A., Hegde,M.V., Bodhankar, S.L.,2011. Ethanolic extract ofseeds of
Linumussitattimum(Flaxlignanconcentrate)preventsdoxorubicin-induced
car-diotoxicityinrats.AtherosclerosisSuppl.12,146.
Zanwar,A.A.,Hegde,M.,Bodhankar,S.,2010.Invitroantioxidantactivityofethanolic extractofLinumusitatissimum.Pharmacol.Online1,683–696.
Zanwar,A.A.,Hegde,M.V.,Bodhankar,S.L.,2013.Protectiveroleofconcomitant administrationofflaxlignanconcentrateandomega-3-fattyacidon myocar-dialdamageindoxorubicin-inducedcardiotoxicity.FoodSci.Hum.Wellness2, 29–38.