w ww . e l s e v i e r . c o m / l o c a t e / b j p
Original
Article
Anticonvulsant
mechanism
of
saponins
fraction
from
adventitious
roots
of
Ficus
religiosa
:
possible
modulation
of
GABAergic,
calcium
and
sodium
channel
functions
Damanpreet
Singh
1,
Rajesh
Kumar
Goel
∗DepartmentofPharmaceuticalSciencesandDrugResearch,PunjabiUniversity,Patiala,Punjab,India
a
r
t
i
c
l
e
i
n
f
o
Articlehistory:
Received8May2015 Accepted27October2015 Availableonline21July2016
Keywords:
BAYk-8644 Bicuculline Clonic
Convulsionsepilepsy
Ficusreligiosa
Veratridine
a
b
s
t
r
a
c
t
Inourpreviousstudies,quantifiedsaponins-richfractionfromadventitiousrootextractofFicusreligiosa L.,Moraceae,showedanticonvulsanteffectinacute,aswellaschronicmicemodelsofepilepsy.The presentstudywasdesignedtorevealputativeanticonvulsantmechanismofquantifiedsaponins-rich fractionusingtargetspecificanimalmodels.Theanticonvulsanteffectofquantifiedsaponins-rich frac-tionwasinitiallystudiedinmaximalelectroshockandpentylenetetrazoltestat1,2and4mg/kg;i.p.doses. Basedontheresultsofinitialanticonvulsanttesting,differentgroupsofmicewereinjectedwithvehicle orquantifiedsaponins-richfraction(4mg/kg;i.p.),30minpriortoaninjectionofN-methyl-d-aspartic acid(100mg/kg;s.c.),bicuculline(5mg/kg;i.p.),strychninehydrochloride(2mg/kg;i.p.),BAYk-8644 (37.5g;i.c.v.),veratridine(500g/kg;i.p.)andtheconvulsiveepisodeswerestudied.Treatmentwiththe extract(1,2and4mg/kg)showedsignificantprotectioninmaximalelectroshockand pentylenetetrazol-inducedconvulsiontests,inadose-dependentmanner.Moreover,quantifiedsaponins-richfractionat 4mg/kgdoseshowedsignificantincreaseinlatencytoclonicconvulsions,decreaseinseizureseverity andincreaseinaveragewaveamplitudeinbicuculline,BAYk-8644andveratridinetests,respectively, ascomparedtovehiclecontrol.However,SRFtreatmentfailedtoabolishN-methyl-d-asparticacidand strychnine-inducedconvulsions,indicatedbyinsignificantchangeintheappearanceofturningbehavior andonsetoftonicextension,respectively,ascomparedtovehiclecontrol.Fromtheresultsofpresent study,itisconcludedthatquantifiedsaponins-richfractionsuppressmaximalelectroshock, pentylenete-trazol,bicuculline,BAYk-8644andveratridine-inducedconvulsions,indicatingitsGABAergic,Na+and Ca2+channelmodulatoryeffects.Furtheritcanbecorrelatedthatquantifiedsaponins-richfractioncauses deactivationofvoltage-gatedNa+andCa2+channels,withouteffectingligand-gatedNa+andCa2+ chan-nels.Morestudiesarerequiredatmolecularlevelsusinginvitrotechniquestounderstandtheexact molecularinteractionsofquantifiedsaponins-richfractionwiththesepathways.
©2016SociedadeBrasileiradeFarmacognosia.PublishedbyElsevierEditoraLtda.Thisisanopen accessarticleundertheCCBY-NC-NDlicense(http://creativecommons.org/licenses/by-nc-nd/4.0/).
Introduction
Ficus religiosaL., Moraceae,is a medicinally importantplant ofthegenusFicus,and hasbeenextensivelyusedin traditional medicine for a wide range of ailments. Its different botanical partshavebeenusedfortheethnomedicaltreatmentofepilepsy. Many of its traditional uses have been validated in different experimentalstudiesthroughouttheglobe.Itsdifferentpartshave shown a variety of neurological effects including antiamnesic,
∗ Correspondingauthor.
E-mail:rkgoel@pbi.ac.in(R.K.Goel).
1Presentaddress:RegulatoryResearchCentre,CSIR–InstituteofHimalayan
BioresourceTechnology,Palampur,HimachalPradesh,India.
acetylcholinesterase inhibitory, parasympathetic modulatory, antianxietyand reversalofreserpine-inducedbehavioral effects (Singhetal.,2011a).Apartfromthesepharmacologicaleffects,it hasalsobeenstudiedforitsanticonvulsantpotentialindifferent experimentalstudies.
In ourprevious study, the crude fruit extract of F. religiosa
haveshownanticonvulsantactivity,whichwasfoundtobedue tomodulationofserotonergicfunctionsofthebrain(Singhand Goel, 2009; Goel and Singh, 2013).The flavonoid-rich fraction ofthefruitextract incombinationwithphenytoinalsoshowed protection in kindling mice model, along with attenuation of associated cognitive and behavioral impairments (Singh et al., 2014a). Its leaf extract wasstudied in acute animal models of convulsion,butwasfoundtobeineffective(Singhetal.,2011b). Inarecentstudythecrudebarkextractoftheplantalsoshowed
http://dx.doi.org/10.1016/j.bjp.2015.10.007
protectioninacuteanimalmodelsofconvulsions,whichwasfound tobeduetoGABAaminotransferaseinhibitoryactivityofits bioac-tivemetabolites(Singhetal.,2014b).Thecrudeadventitiousroot extractofF.religiosahasalsobeenstudiedforitsanticonvulsant activity(Patiletal.,2011).Whenpartitioned,onlythesaponins-rich fraction(SRF)oftheextractretainedanticonvulsantactivity,rest allotherfractionswerefoundtoineffective.Thestudyindicated saponinspresentintheadventitiousrootextracttoberesponsible foritsactivity(Singhetal.,2012).SRFpersealsoprevented behav-ioralimpairmentsassociatedwithkindlinginmice,butfailedto preventcognitivedeficit(Singhetal.,2013).
Thesaponinsareatypeofnaturallyoccurringsurface-active gly-cosides,whicharegenerallyproducedbyplantswithanexception ofsomelowermarineanimalsandbacteria(Francisetal.,2002). Thetherapeutic role ofsaponins hasbeensuggested inseveral central as wellas peripheral pathological conditions like, neu-rodegeneration, epilepsy, cognitive impairments, hypertension, atherosclerosis, inflammation, allergic reactions, cancer, hyper-glycemiaand manymore(Radadetal.,2004;Nah etal.,2007). Thesaponinsisolatedfromotherplantshavebeenfoundto inter-actwithallthepathologicalprocessesinvolvedinepilepsy.They showedGABAergicagonist(Kimuraetal.,1994;Kimetal.,2001; Choietal.,2003),glutamatergicantagonist(KimandRhim,2004; Pengetal.,2009),glycinergicagonist(Nohetal.,2003;Kimetal., 2004),Ca2+(Zhongetal.,1995;Kimetal.,2008)andNa+channel blockadeeffects(Liuetal.,2001;Kimetal.,2005;Chindoetal., 2009).Duetowidespectrumofneuronalpathwayinteractionby saponins,thepresentstudywasenvisagedtounderstandthe puta-tiveanticonvulsantmechanismofSRFofadventitiousrootextract ofF.religiosa,byusinganimalmodelsofepilepsyinvolving pri-marilymodulationofcalcium,sodium,glutamateandGABAergic pathways.
Methods
Plantmaterial,extraction,fractionationandquantification
SRFwaspreparedandquantifiedasdescribedinourprevious study(Singhetal.,2012).Briefly,theadventitious rootsofFicus religiosawerecollected,cleaned,shade-dried,powderedand sub-jectedtorepetitiveextractionwith50%ethanolin apercolator. Afterdryingthecombinedpercolate,theresultantextractwas dis-persedinwaterandfractionatedwithhexane,chloroform,ethyl acetateandbutanol.Thebutanolfractionwasprecipitatedandthe totalsaponinscontentwasdeterminedintheprecipitatescollected usingcolorimetricmethodwithvanillin-sulphuricacidsystem,as discussedinourpreviousstudy.Thedoseswerepreparedfreshly beforeuseandwasinjectedintraperitoneally(i.p.).Thedosesof SRFwereselectedbasedontheresultsofourpreviousstudy(Singh etal.,2012)andwasadministeredat1,2and4mg/kg.
Animals
MaleSwissalbinomice,weighing20–30gobtainedfromCCS Haryana Agricultural University, Hisar, were employed in the presentstudy. Theanimalswerehousedin standard cages and maintainedatroomtemperaturewithnaturaldayandnightcycles. Theanimalswereallowedfreeaccesstofood(standardlaboratory rodent’schow)andwaterduringthestudyperiod.Allthe exper-imentswereconductedbetween9amand4pm.Allprocedures werecarriedoutaccordingtotheguidelinesofCommitteeforthe PurposeofControlandSupervisiononExperiments onAnimals (CPCSEA),IndiaandapprovedbytheInstitutionalAnimalEthical Committee(no.:107/99/CPCSEA-2009-4.1).
Drugsandchemicals
Pentylenetetrazol(PTZ)(dissolvedinnormalsaline),strychnine hydrochloride(dissolvedinnormalsaline),bicuculline(dissolved inminimum0.1NHCl,volumewasmakeupwithnormalsaline, pHwasadjusted to6.65withNaOH), N-methyl-d-aspartic acid
(NMDA)(dissolved in normal saline)and veratridine (dissolved inminimum0.1NHCl,volumewasmakeupwithnormalsaline, pH was adjusted to 6.65 with NaOH) were purchased from Sigma–Aldrich (St. Louis, MO). Reference drugs, diazepam and phenytoinwereobtained locallyfrom Jackson LaboratoriesLtd. (Amritsar, India) and Cadila Laboratories (Ahmedabad, India), respectively.
Maximalelectroshock(MES)-inducedconvulsions
Miceindifferentgroupswereinjectedwiththevaryingdoses ofSRF(1,2and4mg/kg;i.p.),vehicle(10ml/kg;i.p.)andphenytoin (25mg/kg;i.p.).After30minofthesetreatments,allthegroups weredeliveredacalibrated(throughacurrentcalibrator[Rolex, Ambala,India])transauricularelectroshockof56mAfor0.2susing aconvulsiometer(Rolex,Ambala,India),viaapairofcrocodileear clips.Thedurationoftonichindlimbextension(seconds)wasnoted andwascomparedwiththatofvehiclecontrol(Swinyardetal., 1952).
PTZ-inducedconvulsions
PTZatadoseof75mg/kgwasgiventofivedifferentgroupsof micepretreated30minpriorwiththevaryingdosesofSRF(1,2and 4mg/kg;i.p.),vehicle(10ml/kg;i.p.)anddiazepam(5mg/kg;i.p.). Latencytoclonicconvulsions(min)wasnotedandwascompared withthatofvehiclecontrol.
NMDA-inducedconvulsions
Miceintwodifferentgroupsweretreatedwitheithervehicle (10ml/kg)orSRF(4mg/kg,i.p.)30minprior toasubcutaneous injectionofNMDA(100mg/kg).Thereafter,themicewereobserved fortheappearanceofturningbehaviorfornext30min.Turning behaviorwascharacterizedastwoconsecutive360cycles com-pletedby thesameanimal (Bhutada et al.,2010).Thetest was performedtodeterminetheroleofglutamatergicprocessesforthe anticonvulsanteffectofSRF.
Bicuculline-inducedconvulsions
Bicuculline wasadministered(5mg/kg;i.p.)in twodifferent groupsofmice,30minaftertreatmentwithvehicle(10ml/kg;i.p.) orSRF(4mg/kg;i.p.).Themicewereobservedfortheappearance ofclonic–tonicseizuresanddeathforaperiodof30minafter bicu-cullineinjection.Antagonismofbicucullineseizureswasdefinedas theabsence/delayofclonic–tonicseizuresfor30min(Irifuneetal., 2003).ThetestwasperformedtoexaminetheGABAergiceffectsof SRF.
Strychnine-inducedconvulsions
BAYk-8644-inducedconvulsions
BAYk-8644wasusedtoinduceconvulsionsasdescribedby
VonVoigtlanderetal.(1987)withslightmodifications.Afreehand
i.c.v.injection ofBAY k-8644 (37.5g/10l) wasmade in con-sciousmouse,30minaftervehicle(10ml/kg;i.p.)orSRF(4mg/kg;
i.p.)treatmentbyvisuallocationmethoddescribedbyHaleyand McCormick(1957).Briefly,eachanimalwasfirmlygripedbytautly pullingthelooseskinbehindthehead,amidlinewasdrawnusing amarkerthroughtheanteriorbaseoftheearsandtheinjection wasmade2mmoneithersidetheline.Acrossbetweentheleft eye–rightearandrighteye–leftearwasdrawntolocatethe mid-pointtodrawmidline.Theinjectionsweremadewithhypodermic needleof0.4mmexternaldiameterattachedtoa10lHamilton syringe(Hamilton,Bonaduz,Switzerland).Theneedlewascovered withapolypropylenetube,leaving3mmofthetipregion,soasto insertthisportionthroughtheskullintothebrainofmouse.The syringewasheldatanangleofaround45degreetotheskull,with thebevelofneedlefacingup,pointinginthedirectionofthetail. Inducedseizureswereratedaccordingtoascaledevisedasstage1, scratchingandtwistingoftheforelimbs(score1);stage2,rearing andwalking(score2);stage3,intermittentclonicjerksoflimbs withtonicflexionofforelimbsandtailflexion;stage4,head bob-bingwithcomplexgroomingactions(lickingoffurandscratching); stage5,jumping,squeakingandtonicextensionofhindlimbs;stage 6,barrelrolling(KaurandGoel,2011).Theoccurrenceofseizure signsandlatencywererecordedfor1h.Thetestwasperformedto investigatetheroleofCa2+channels.
Veratridine-inducedseizure
Veratridine seizures in mice were induced by the methods describedbyOtoometal.(2006)andOtoomandSequeira(2011), with slight modifications. Two different groups of mice were treatedwithvehicle(10ml/kg;i.p.)orSRF(4mg/kg;i.p.),30min prior to an intraperitoneal injection of veratridine (500g/kg). After15minofveratridineadministration,electroencephalogram (EEG)recordingwasperformedusingaDigitalPolygraph(PC-2004, MedicaidSystem,Chandigarh,India)bythemethoddescribedby
OtoomandSequeira(2011),butusingsurfaceelectrodes.Average waveamplitude(V/min)andwavepatterninvehiclecontrol ani-malswerecomparedwiththatofnormal(naïve)andSRFtreated animals.
Statisticalanalysis
Alltheresultswereexpressedasmean±SEM.Thesignificance ofdifferencebetweenmeanswasdeterminedusingone-way anal-ysisof variance (ANOVA)followed byTukey’s test in MES,PTZ and veratridine tests. Unpaired Student’s t-test was employed todeterminesignificanceofdifferencebetweenmeaninNMDA, bicuculline,strychnineandBAYk-8644-inducedconvulsiontests. Statisticalanalysisforthepercentagemortalityandpercentageof animalsshowingturningbehavior pergroupwasperformedby Chi-squaretest.Theresultswereregardedassignificantatp<0.05.
Results
EffectonMES-inducedconvulsions
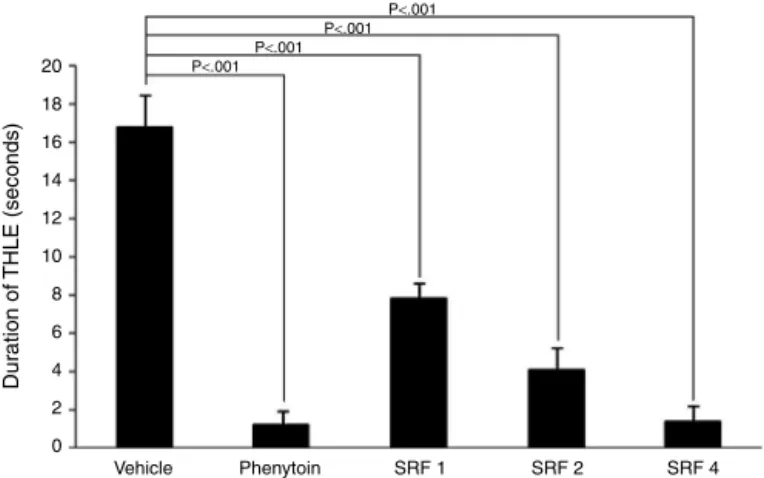
Treatment with SRF showed significant (p<0.001) dose-dependentdecreaseinthedurationoftonichindlimbextension at 1mg/kg (7.8±0.86s), 2mg/kg (4.1±1.2s), and 4mg/kg (1.4±0.8s),ascomparedtovehiclecontrol,withmaximum pro-tection observedat 4mg/kg. The anticonvulsantactivity of SRF
20
18
16
14
12
10
8
6
4
2
0
Vehicle
P<.001 P<.001
P<.001 P<.001
Phenytoin
Dur
ation of
THLE (seconds)
SRF 1 SRF 2 SRF 4
Fig.1. Effectdifferentdosesofsaponins-richfractionfromtheadventitiousroot extractofFicusreligiosaonthedurationofMES-inducedtonichindlimbextension. THLE,tonichindlimbextension;SRF1,2and4,saponins-richfraction1,2and 4mg/kg,respectively.
16
Vehicle Diazepam N/C
Con
vulsion latency (min)
SRF 1 SRF 2 SRF 4
14
12
10
8
6
4
2
0
P<.017 P<.001
P<.001
Fig.2. Effectdifferentdosesofsaponins-richfractionfromtheadventitiousroot extractofFicusreligiosaonthelatencytoPTZ-inducedconvulsions.N/C,no convul-sions;SRF1,2and4,saponins-richfraction1,2and4mg/kg,respectively.
at 4mg/kg was found to be comparable to phenytoin treated (1.2±0.8s)group(Fig.1).
EffectonPTZ-inducedconvulsions
InPTZ-inducedconvulsionstest,treatmentwithSRFat1mg/kg (p=0.017),2mg/kg(p<0.001)and4mg/kg(p<0.001)significantly increasedthelatencytoclonicconvulsions,ascomparedto vehi-clecontrolat5.58±0.14min,9.54±0.64minand12.28±1.12min, respectively.Similarly,asinMEStest,SRFshoweddose-dependent anticonvulsanteffect.SRFwasfoundtobelesseffectiveasthatof standarddiazepam,asitstreatmentonlydelayedtheclonic con-vulsions,wereasdiazepampretreatmentcompletelyabolishedthe inductionofPTZconvulsions(Fig.2).
EffectonNMDA-inducedconvulsions
TreatmentwithSRFshowednoprotectionagainstconvulsions inducedbyNMDA,indicatedbyinsignificantchangeintheonset andoccurrenceofturningbehaviorinmiceascomparedtovehicle control(Fig.3AandB).
Effectonbicuculline-inducedconvulsions
8 NS
Vehicle SRF 4 Vehicle SRF 4
NS
A
B
Onset of tur
ning beha
vior (min)
% animals sho
wing tur
ning beha
vior
120
100
80
60
40
20
0 7
6
5
4
3
2
1
0
Fig.3. Effectsofsaponins-richfractionfromtheadventitiousrootextractofFicusreligiosaonNMDA-inducedconvulsions.(A)Effectofsaponins-richfractionontheonset ofNMDA-inducedturningbehavior.(B)Effectofsaponins-richfractiononthepercentageofanimalsshowingNMDA-inducedturningbehavior;NS,notsignificant;SRF4, saponins-richfraction4mg/kg.
5
A
B
Vehicle SRF 4 Vehicle
% mor
tality
Con
vulsion latency (min)
SRF 4
P<.001 120 NS
100
80
60
40
20
0 4.5
4
3.5
3
2.5
2
1.5
1
0.5
0
Fig.4.Effectsofsaponins-richfractionfromtheadventitiousrootextractofFicusreligiosaonbicuculline-inducedconvulsions.(A)Effectofsaponins-richfractiononthe latencytoconvulsionsinducedbybicuculline;(B)Effectofsaponins-richfractiononthepercentagemortality;NS,notsignificant;SRF4,saponins-richfraction4mg/kg.
3.5 120
100
80
60
40
20
0 3
2.5
2
1.5
1
0.5
Vehicle SRF 4 Vehicle
NS NS
A
B
Con
vulsion latency (min) % mor
tality
SRF 4
Fig.5.Effectsofsaponins-richfractionfromtheadventitiousrootextractofFicusreligiosaonstrychnine-inducedconvulsions.(A)Effectofsaponins-richfractiononthe latencytoconvulsionsinducedbystrychnine.(B)Effectofsaponins-richfractiononthepercentagemortality;NS,notsignificant;SRF4,saponins-richfraction4mg/kg.
However,nosignificantprotectionagainstmortalitywasobserved aftertreatmentwithSRFasthatofvehiclecontrol(Fig.4AandB).
Effectonstrychnine-inducedconvulsions
TreatmentwithSRFshowednoprotectionagainstconvulsions inducedby strychnine,indicated by insignificantchangein the latencytoconvulsions,ascomparedvehiclecontrol.Moreover,no significantprotectionwasobservedonthepercentagemortalityas thatofcontrol(Fig.5AandB).
EffectonBAYk-8644-inducedconvulsions
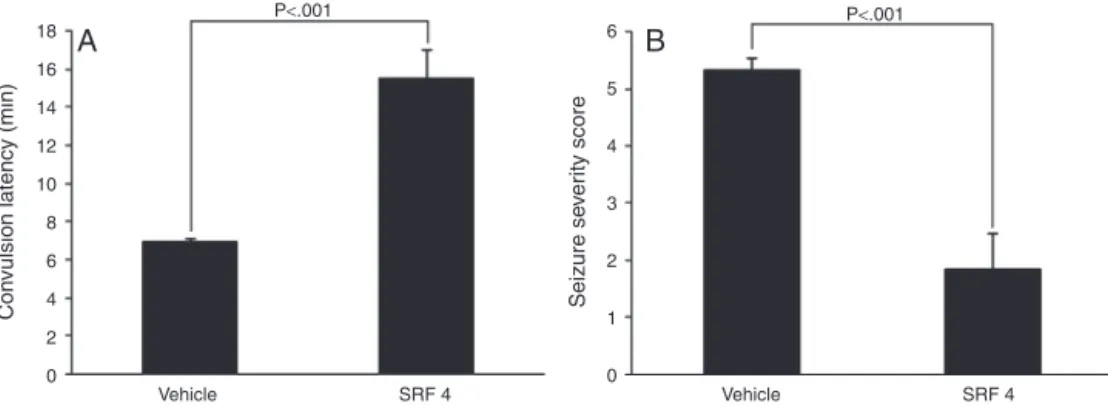
BAY k-8644, a Ca2+ channel agonistadministration in mice resultedinconvulsionswithalatencyofaround7.28±0.27min
in vehicle control group. Treatment with SRF, significantly (p<0.001) increased the latency to BAY k-8644-induced con-vulsions to 15.85±0.89min. SRF treatment also significantly (p<0.001)reducedtheseizureseverityscoreto1.83±0.6,as com-paredtovehiclecontrol(5.33±0.21)group(Fig.6AandB).
Effectonveratridine-inducedseizure
18
A
B
P<.001Vehicle
Con
vulsion latency (min)
Seizure se
ver
ity score
SRF 4 Vehicle SRF 4
P<.001
16
14
12
10
8
6
4
2
0
6
5
4
3
2
1
0
Fig.6.Effectsofsaponins-richfractionfromtheadventitiousrootextractofFicusreligiosaonBAYk-8644-inducedconvulsions.(A)Effectofsaponins-richfractiononthe latencytoconvulsionsinducedbyBAYk-8644;(B)Effectofsaponins-richfractionontheseizureseverityscore;SRF4,saponins-richfraction4mg/kg.
90
80
70
60
50
40
30
20
10
0
P<.001 P<.001
Naïve Vehicle
A
ver
age amplitude (
µ
V/min)
SRF 4
Fig.7.Effectsofsaponins-richfractionfromtheadventitiousrootextractofFicus
religiosaonveratridine-inducedchangesinwaveamplitude.SRF4,saponins-rich
fraction4mg/kg.
A
B
C
50 µV
2 sec. 0.2 sec.
Fig.8.Effectsofsaponins-richfractionfromtheadventitiousrootextractofFicus
religiosaonveratridine-inducedchangesinwavepatternduringseizuresphase.(A)
Naïvegroup;(B)vehiclecontrolgroup;(C)saponins-richfractiontreatedgroup (4mg/kg).
Discussion
It is important tostudy the mechanism of a novel anticon-vulsant component, as it might act on well-recognized targets innovelwaysand/orbynovelcombinationsofactionson well-recognizedtargets.In somecases,suchapracticehad revealed entirelynewantiepileptictargetsliketopiramateandlamotrigine (Rogawski,2006a).Hence,tostudytheanticonvulsantmechanism of SRF, thepresent study wascarried out. Treatmentwith SRF
resultedinsuppressionofMESandPTZ-inducedconvulsionsina dose-dependentmanner.Theresultsofthisinitialscreeningare inlinewiththeresultsofourpreviousstudy(Singhetal.,2012). SRFtreatmentresultedinmarkedsuppressionofBAYk-8644and veratridine-inducedconvulsions, and showed partialprotection againstbicuculline-inducedconvulsion.Howeveritwasfoundto betotallyineffectiveagainststrychnineandNMDA-induced con-vulsions.Since,MESandPTZtestsareconsideredtobethe“gold standards”inearlystagesofdrugtesting(Rogawski,2006b),hence initialconfirmationoftheanticonvulsanteffectofSRFwascarried inthesetestsatalldoses.Theothertests(NMDA,strychnine, bicu-culline,BAYk-8644andveratridine-inducedconvulsiontests)were performedtoinvestigateitsputativeanticonvulsantmechanism andeffectindifferentacuteseizuremodelsofvariedphenotype. Asinglemosteffectivedosewasusedtoreducethenumberof animalsrequiredinthestudy,duetoethicalconstraints.
Someofthepreviousinvitro studiesrevealedthat saponins isolatedfromginsengandotherFicusspeciescausesblockadeof voltagedependentNa+channels,hencedecreasesneuronal exci-tation (Liu et al., 2001; Kim et al., 2005; Chindo et al., 2009). Hence,tostudy theeffectof SRFonNa+ channels, veratridine-inducedseizuremodelwasused.Veratridineisacommonlyused experimentaltoolinvariouselectrochemicalstudies,itopensNa+ channels during sustained membrane depolarization by inhibi-tinginactivation,leadingtoNa+influx,secondarilyitcausesCa2+ influx,increasedpumpactivityandinturnexocytosis,leadingto experimentalseizures in rodents(Ulbricht,1998; Otoom etal., 2006).Inthepresentstudy,administrationofveratridinein vehi-clecontrolanimalsresultedinseizures,indicatedbycharacteristic EEG changes, these changes are in line with a previous study (Otoom and Sequeira,2011).Several clinically used antiepilep-ticdrugs actingthroughblockadeofNa+ channels,likevalproic acid,phenytoinandcarbamazepinehavebeenfoundtosuppress veratridine-inducedabnormalneuronalexcitationinexperimental studies(OtoomandAlkadhi,2000a,b).Sinceinourstudy, pretreat-mentwithSRF,abolishedtheveratridine-inducedseizureactivity indicated by normalization of EEGpattern, indicating itseffect mightbeduetoNa+channelblockadeactivity.
Panaxatriol saponins, the main constituents extracted from
Panax notoginseng have beenreported topossess aninhibitory
byBAYk-8644,whichinduceconvulsionsthroughactivationof voltage-gatedCa2+ channels.SRFpretreatmentinhibitedBAY k-8644-inducedconvulsion,therebysuggestingitseffecttobedueto inhibitionofCa2+channels.
Severalpreclinicalstudiesindicatingthemodulationof GABAer-gic functionsby plantisolated saponins have beenreported in literature.SaponinsisolatedfromPanaxginsengshowedGABAA receptorbindingintheratbrain(Kimuraetal.,1994;Kimetal., 2001).ThesesaponinsalsoincreasedtheGABA-mediatedinward peakcurrentinXenopusoocytes(Choietal.,2003).Effectiveness ofSRFininitialanticonvulsantscreeninginPTZ(GABAAreceptor antagonist)testindicateditsGABAergicmodulatoryeffect.Since severalotheranticonvulsantmoleculesactingthroughother mech-anismshavealsoshownactivityagainstPTZconvulsions,henceto furtherconfirmtheGABAergiceffectofSRF,itseffectwasstudiedin bicuculline(GABAAreceptorantagonist)test.TreatmentwithSRF resultedinsuppressionofbicuculline-inducedconvulsions, indi-catedbyincreaseinlatencytoclonicconvulsions,buthoweverit wasfoundtobeineffectiveinreducingmortality.Furtherstudies arerequiredtounderstandtheexactGABAergicmodulatoryeffects ofSRF.
InhibitionofNMDA-inducedexcitatoryresponsesbyplant iso-latedsaponinshasalsobeenwelldocumentedinseveralstudies. SaponinsisolatedfromginsenghaveshowninhibitionofNMDA receptor-mediatedepilepticdischargesinculturedhippocampal neurons(KimandRhim,2004).GinsenosideRb3haveshown neu-roprotectiveroleonhippocampalneurons,throughfacilitationof Ca2+-dependentdeactivationofNMDAreceptors(Pengetal.,2009). Clinicallyuseddrugslikefelbamateandlacosamideactthrough inhibitionofNMDAreceptor(Rogawski,2006).Therefore,tostudy theeffectofSRFonNMDA-mediatedexcitatoryresponses, NMDA-inducedconvulsionstestwasused.HoweverSRFfailedtosuppress NMDA-induced convulsions, indicating lack of NMDA receptor interactionsofSRF.
Theroleofglycinergicreceptormechanisminthepathogenesis ofclinicalandexperimentalepilepsieshasbeenwellestablished.In differentstudies,theglycinergicdrugshaveshownsuppressionof epilepticdischarge(Laubeetal.,2002;ChattipakornandMcMahon, 2003).Ginsenosideshaveshownenhancementofglycine-induced inwardpeakcurrentinhumanglycinereceptorchannelactivity expressedinXenopusoocytes(Nohetal.,2003).Theyhavealsobeen foundtoantagonizeNMDAreceptorsthroughtheglycine modu-latorysiteinratculturedhippocampalneurons(Kimetal.,2004). Theseliteraturefindingssuggesttheglycinergiceffectsofsaponins. HencetostudytheglycinergicmodulationofSRF,itseffectwas studiedagainstconvulsionsinducedbystrychnine(glycine recep-tor antagonist). Since, the SRF was found to be ineffective in suppressingstrychnine-induced convulsions, thereby indicating lackofeffectivenessasdirectinteractionwithglycinergicpathway. Theresultsof thepresentstudy showedthatSRFtreatment inhibitedMES-inducedconvulsionsandsuppressedBAYk-8644, veratridineandGABAAreceptorantagonists(PTZand bicuculline)-induced convulsions at tested doses, indicating it to be acting throughmultiplemechanismsasGABAagonistaswellasNa+and Ca2+ channelsinhibitor. Severalclinically used drugs have also beenfoundtobeactingthroughmultiplemechanisms(Köhling, 2002).Interestingly, SRFshowedprotectionin BAYk-8644 and veratridine tests,which actthrough activationof voltage-gated Ca2+andNa+channels,respectively,butwasfoundtobe ineffec-tiveagainstconvulsionsinducedbyNMDA(receptor-operatedion channels)receptoragonist,NMDA.Thesimilaranticonvulsant pro-fileisshownbyethosuximide,aclinicallyusedantiepileptic(Turski etal.,1990;OtoomandSequeira,2011).Theseresultsindicated theeffectivenessofSRFinblockadeofvoltage-gatedionchannels andineffectivenesstoward ligand-gatedNa+ andCa2+channels. Since,veratridineactbydepolarizationofexcitablecells,thereby
preventinginactivationofNa+channels,leadingtoanincreasein influxofNa+andCa2+(Jordánetal.,2000),thereforeitisnotexactly clearthattheanticonvulsanteffectofSRFisduetoblockadeofNa+ orCa2+channelsorboth,asithasalsoshownprotectionagainst seizuresinducedbyBAYk-8644.As,ethosuximidewhichshows anticonvulsanteffectthroughtheblockadeofCa2+channelsalso suppressveratridine-inducedseizures.Morestudiesatmolecular levelsarerequiredtounderstandtheseeffectsaccurately.
Conclusion
TheresultsofpresentstudyconcludedthatSRFexhibitits anti-convulsanteffectthroughmodulationofGABAergic,Na+andCa2+ channel functions.Effectiveness ofSRFin BAY k-8644and ver-atridinetestsindicateditsvoltage-gatedNa+ andCa2+channels inhibitoryeffectsbutnotligand-gatedNa+andCa2+channels.More studiesarerequiredatmolecularlevelstounderstandtheexact molecularinteractionsofSRFwiththesepathways.
Ethicaldisclosures
Protectionofhumanandanimalsubjects. Theauthorsdeclare thattheproceduresfollowedwereinaccordancewiththe regula-tionsoftherelevantclinicalresearchethicscommitteeandwith thoseoftheCodeofEthicsoftheWorldMedicalAssociation (Dec-larationofHelsinki).
Confidentialityofdata. Theauthorsdeclarethatnopatientdata appearinthisarticle.
Righttoprivacyandinformedconsent. Theauthorsdeclarethat nopatientdataappearinthisarticle.
Authors’contribution
Thefirstauthorconductedtheexperiments,andsecondauthor plannedand supervised the study.Both theauthors wrotethe manuscript.
Conflictsofinterest
Theauthorsdeclarenoconflictsofinterest.
Acknowledgements
WearethankfultotheUniversityGrantCommission,NewDelhi, India,forprovidingfinancialassistance[VideF.No.:34-130/2008 (SR)]for theprojectand projectfellowship toMr.Damanpreet Singh.
References
Bhutada,P.,Mundhada,Y.,Bansod,K.,Dixit,P.,Umathe,S.,Mundhada,D.,2010.
Anticonvulsantactivityofberberine,anisoquinolinealkaloidinmice.Epilepsy Behav.18,207–210.
Chattipakorn,S.C., McMahon,L.L.,2003. Strychnine-sensitiveglycinereceptors depresshyperexcitabilityinratdentategyrus.J.Neurophysiol.89,1339–1342.
Chindo,B.A.,Anuka,J.A.,McNeil,L.,Yaro,A.H.,Adamu,S.S.,Amos,S.,Connelly,W.K., Lees,G.,Gamaniel,K.S.,2009.AnticonvulsantpropertiesofsaponinsfromFicus platyphyllastembark.BrainRes.Bull.78,276–282.
Choi,S.E.,Choi,S.,Lee,J.H.,Whiting,P.J.,Lee,S.M.,Nah,S.Y.,2003.Effectsof ginseno-sidesonGABA(A)receptorchannelsexpressedinXenopusoocytes.Arch.Pharm. Res.26,28–33.
Czapi ´nski,P.,Blaszczyk,B.,Czuczwar,S.J.,2005.Mechanismsofactionof antiepilep-ticdrugs.Curr.Top.Med.Chem.5,3–14.
Francis,G.,Kerem,Z.,Makkar,H.P.,Becker,K.,2002.Thebiologicalactionofsaponins inanimalsystems:areview.Br.J.Nutr.88,587–605.
Haley,T.J.,McCormick,W.G.,1957.Pharmacologicaleffectsproducedby intracere-bralinjectionofdrugsintheconsciousmouse.Br.J.Pharmacol.Chemother.12, 12–15.
Irifune,M.,Takarada,T.,Shimizu,Y.,Endo,C.,Katayama,S.,Dohi,T.,Kawahara,M., 2003.Propofol-inducedanesthesiainmiceismediatedbygamma-aminobutyric acid-Aandexcitatoryaminoacidreceptors.Anesth.Analg.97,424–429.
Jordán.,J.,Galindo,M.F.,Calvo,S.,González-García,C.,Ce ˜na,V.,2000.Veratridine inducesapoptoticdeathinbovinechromaffincellsthroughsuperoxide produc-tion.Br.J.Pharmacol.130,1496–1504.
Kaur,M.,Goel,R.K.,2011.Anti-convulsantactivityofBoerhaaviadiffusa:plausible roleofcalciumchannelantagonism.Evid.BasedComplement.Alternat.Med. 2011,1–7.
Kim,H.S.,Hwang,S.L.,Nah,S.Y.,Oh,S.,2001.Changesof[3H]MK-801,[3H] mus-cimoland[3H]flunitrazepambindinginratbrainbytheprolongedventricular infusionofginsenosideRcandRg1.Pharmacol.Res.43,473–479.
Kim,J.H.,Hong,Y.H.,Lee,J.H.,Kim,D.H.,Nam,G.,Jeong,S.M.,Lee,B.H.,Lee,S.M.,Nah, S.Y.,2005.AroleforthecarbohydrateportionofginsenosideRg3inNa+channel
inhibition.Mol.Cells19,137–142.
Kim,S.,Kim,T.,Ahn,K.,Park,W.K.,Nah,S.Y.,Rhim,H.,2004.GinsenosideRg3 antagonizesNMDAreceptorsthroughaglycinemodulatorysiteinratcultured hippocampalneurons.Biochem.Biophys.Res.Commun.323,416–424.
Kim,S.,Nah,S.Y.,Rhim,H.,2008.Neuroprotectiveeffectsofginsengsaponinsagainst L-typeCa2+channel-mediatedcelldeathinratcorticalneurons.Biochem.
Bio-phys.Res.Commun.365,399–405.
Kim,S.,Rhim,H.,2004. GinsenosidesinhibitNMDAreceptor-mediated epilep-tic discharges in cultured hippocampal neurons. Arch. Pharm. Res. 27, 524–530.
Kimura,T.,Saunders,P.A.,Kim,H.S.,Rheu,H.M.,Oh,K.W.,Ho,I.K.,1994.Interactions ofginsenosideswithligand-bindingsofGABA(A)andGABA(B)receptors.Gen. Pharmacol.25,193–199.
Köhling, R., 2002. Voltage-gated sodium channels in epilepsy. Epilepsia 43, 1278–1295.
Laube,B.,Maksay,G.,Schemm,R.,Betz,H.,2002.Modulationofglycinereceptor function:anovelapproachfortherapeuticinterventionatinhibitorysynapses? TrendsPharmacol.Sci.23,519–527.
Liu,D.,Li,B.,Liu,Y.,Attele,A.S.,Kyle,J.W.,Yuan,C.S.,2001.Voltagedependent inhibitionofbrainNa(+)channelsbyAmericanginseng.Eur.J.Pharmacol.413, 47–54.
Löscher,W.,Schmidt,D.,1988.Whichanimalmodelsshouldbeusedinthesearch fornewantiepilepticdrugs?Aproposalbasedonexperimentalandclinical considerations.EpilepsyRes.2,145–181.
Nah,S.Y.,Kim,D.H.,Rhim,H.,2007.Ginsenosides:areanyofthemcandidatesfor drugsactingonthecentralnervoussystem?CNSDrugRev.13,381–404.
Noh,J.H.,Choi,S.,Lee,J.H.,Betz,H.,Kim,J.I.,Park,C.S.,Lee,S.M.,Nah,S.Y.,2003.
Effectsofginsenosidesonglycinereceptoralpha1channelsexpressedin Xeno-pusoocytes.Mol.Cells15,34–39.
Otoom,S.,Sequeira,R.P.,2011.Veratridineinducedabsencelike-seizureinthefreely movingrats:astudycorrelatingthebehaviouralfindingswiththe electrophys-iologicalactivities.Neuroendocrinol.Lett.32,487–490.
Otoom,S.A.,Alkadhi,K.A.,2000a.Actionofcarbamazepineonepileptiformactivity oftheverartidinemodelinCA1neurons.BrainRes.885,289–294.
Otoom,S.A.,Alkadhi,K.A.,2000b.Epileptiformactivityofveratridinemodelinrat brainslices:effectsofantiepilepticdrugs.EpilepsyRes.38,161–170.
Otoom,S.A.,Handu,S.S.,Wazir,J.F.,James,H.,Sharma,P.R.,Hasan,Z.A.,Sequeira, R.P.,2006.Veratridine-inducedwetdogshakebehaviourandapoptosisinrat hippocampus.BasicClin.Pharmacol.Toxicol.98,423–426.
Patil,M.S.,Patil,C.R.,Patil,S.W.,Jadhav,R.B.,2011.Anticonvulsantactivityofaqueous rootextractofFicusreligiosa.J.Ethnopharmacol.133,92–96.
Peng,L.L.,Shen,H.M.,Jiang,Z.L.,Li,X.,Wang,G.H.,Zhang,Y.F.,Ke,K.F.,2009. Inhi-bitionofNMDAreceptorsunderliestheneuroprotectiveeffectofginsenoside Rb3.Am.J.Chin.Med.37,759–770.
Radad,K.,Gille,G.,Rausch,W.,2004.Useofginsenginmedicine:perspectiveson CNSdisorders.Iran.J.Pharmacol.Ther.3,30–40.
Rogawski,M.A.,2006a.Moleculartargetsversusmodelsfornewantiepilepticdrug discovery.EpilepsyRes.68,22–28.
Rogawski,M.A.,2006b.Diversemechanismsofantiepilepticdrugsinthe develop-mentpipeline.EpilepsyRes.69,273–294.
Singh,D.,Goel,R.K.,2009.AnticonvulsanteffectofFicusreligiosa:roleofserotonergic pathways.J.Ethnopharmacol.123,330–334.
Singh,D.,Singh,B.,Goel,R.K.,2011a.Traditionaluses,phytochemistryand pharma-cologyofFicusreligiosa:areview.J.Ethnopharmacol.134,565–583.
Singh,D.,Singh,B.,Goel,R.K.,2011b.HydroethanolicleafextractofFicusreligiosa lacksanticonvulsantactivityinacuteelectroandchemoconvulsionmice mod-els.J.Pharm.NegativeResults2,58–61.
Singh,D.,Singh,B.,Goel,R.K.,2012.Roleofsaponinsfortheanticonvulsanteffectof adventitiousrootsofFicusreligiosa.Pharm.Biol.50,816–822.
Singh,P.,Singh,D.,Goel,R.K.,2014a.FicusreligiosaL.figs–apotentialherbaladjuvant tophenytoinforimprovedmanagementofepilepsyandassociatedbehavioral comorbidities.EpilepsyBehav.41,171–178.
Singh,D.,Gawande,D.,Singh,T.,Poroikov,V.,Goel,R.K.,2014b.Revealing pharma-codynamicsofmedicinalplantsusinginsilicoapproach:acasestudywithwet labvalidation.Comput.Biol.Med.47,1–6.
Singh,D.,Mishra,A.,Goel,R.K.,2013.EffectofsaponinsfractionfromFicusreligiosa onmemorydeficit,behavioralandbiochemicalimpairmentsin pentylenetetra-zolkindledmice.EpilepsyBehav.27,206–211.
Swinyard,E.A.,Brown,W.C.,Goodman,L.S.,1952.Comparativeassaysof antiepilep-ticdrugsinmiceandrats.J.Pharmacol.Exp.Ther.106,319–330.
Turski,L.,Niemann,W.,Stephens,D.N.,1990.Differentialeffectsofantiepileptic drugsandbeta-carbolinesonseizuresinducedbyexcitatoryaminoacids. Neu-roscience39,799–807.
Ulbricht,W.,1998.Effectsofveratridineonsodiumcurrentsandfluxes.Rev.Physiol. Biochem.Pharmacol.133,1–54.
VonVoigtlander,P.F.,Ochoa,M.C.,Lewis,R.A.,1987.Biochemicalandfunctional interactionsofaselectivekappaopioidagonistwithcalcium.Adv.Exp.Med. Biol.221,345–355.