145
The Effects of Trimetazidine on Heart Rate Variability in Patients with
Heart Failure
Yilmaz Gunes, Unal Guntekin, Mustafa Tuncer, Musa Sahin
Yuzuncu Yil University, Faculty of Medicine, Cardiology Department, Van, Turkey.Summary
Background: Reduced measures of heart rate variability (HRV) have been shown to be related with prognosis in heart failure. Chronic administration of trimetazidine in addition to the conventional therapy has been shown to improve functional class and left ventricular functions of heart failure patients.
Objective: To assess the effects of trimetazidine on HRV in optimally treated patients with heart failure of ischemic origin.
Methods: Trimetazidine 20 mg three times/day was added to therapy of 30 patients with heart failure being treated with angiotensinogen converting enzyme inhibitors or angiotensin receptor blockers, carvedilol, spironolactone, digitalis and furosemide. The etiology of heart failure was coronary artery disease in all patients. Patients were evaluated with echocardiography and 24-hour heart rate variability analysis before and 3 months after addition of trimetazidine.
Results: Mean left ventricular ejection fraction (LVEF) significantly increased after the addition of trimetazidine (33.5±5.1% to 42.5±5.8%, p<0.001). Of the HRV parameters, SDNN (97.3±40,1 to 110.5±29,2 msecs, p=0.049) and SDANN (80.5±29,0 to 98.3±30,5 msecs) were significantly increased after trimetazidine treatment. Baseline SDNN was significantly correlated with baseline LVEF (r=0.445, p=0.023, p=0.008) and the increment in SDNN was correlated with increase in LVEF (r=0.518, p=0.007).
Conclusions: Adding trimetazidine to optimal medical therapy in patients with heart failure of ischemic origin may improve heart rate variability in association with improved left ventricular ejection fraction. (Arq Bras Cardiol 2009; 93(2):145-148)
Key Words: Trimetazidine / administration and dosage; heart rate; heart failure.
Mailing Address: Yilmaz Gunes •
Yuzuncu Yil University, Faculty of Medicine, Cardiology Department, Van, Turkey E-mail: yilmazleman@yahoo.com
Manuscript received on 21/04/2008; reviewed manuscript received on 17/07/2008; accepted on 15/10/2008.
Introduction
Patients with chronic heart failure (CHF) present autonomic dysfunction with activation of the sympathetic nervous system and reduction in parasympathetic activity1,2. Analysis of the
heart rate variability (HRV) is a reliable and reproducible technique for assessing autonomic activity in patients with cardiovascular diseases3-6. Reduced measures of heart rate
variability have been shown to be related with prognosis in heart failure7,8. Several investigators have demonstrated that
measurements of HRV can be used to evaluate the effect of angiotensin-converting enzyme inhibitors and β-adrenergic receptor antagonists on cardiac autonomic activity in patients with heart failure9,10. Trimetazidine is a metabolic agent with
anti-ischemic properties operating independently of any hemodynamic changes. Previous studies have also suggested that trimetazidine modulates the postmyocardial infarction autonomic control of HRV, that is, that it reduces sympathetic overactivity and augments vagal influence11,12. Trimetazidine
acts as a partial inhibitor of fatty acid oxidation and in turn,
trimetazidine stimulates glucose oxidation. In heart failure, similar to what happens during acute myocardial ischemia, glucose and lactate oxidation are decreased and fatty acid oxidation is increased, increasing the oxygen requirement13.
In this study we aimed at assessing the effects of trimetazidine, added to conventional heart failure treatment, on HRV in patients with heart failure.
Methods
Thirty patients with heart failure and New York Heart Association (NYHA) functional class II or III who were already receiving optimal heart failure treatment consisting of angiotensinogen-converting enzyme inhibitors or angiotensin receptor blockers, carvedilol, spironolactone, digitalis and furosemide were included in the study. The etiology of the heart failure was coronary artery disease documented by coronary angiography in all patients. Following baseline echocardiographic examination, trimetazidine, at a dose of 20 mg three times/day, was added to the therapy and patients were reevaluated 3 months later. Exclusion criteria were; NYHA class IV, acute myocardial infarction in the previous 3 months, atrial fibrillation, LV ejection fraction (LVEF)> 40%, severe valvular heart disease, alcoholic cardiomyopathy,
Arq Bras Cardiol 2009; 93(2):145-148
Gunes et al
Trimetazidine and HRV in heart failure
pacemakers, renal failure (serum creatinine>2.0 mg/dL), chronic lung disease or any systemic disorder and use of additional drugs such as antiarrhythmics. The study was approved by hospital Ethics Committee and patients gave their written informed consent.
The echocardiographic examination was performed at rest, with the patient on the left lateral decubitus position, using a commercially available echocardiographic device (Vivid 3, General Electric) with a 3 MHz transducer, by two experienced echocardiographists who were blinded to the clinical data. Using M-mode echocardiography, long-axis measurements were obtained at a level distal to the mitral valve leaflets, according to current recommendations14. Left
ventricular ejection fraction was calculated via modified biplane Simpson’s method from apical four and two chamber views. Measurements were made off-line on three separate beats and then averaged for all parameters. During the entire echocardiographic study, a single-lead electrocardiogram was continuously recorded.
Heart Rate Variability Analysis
Twenty-four-hour ambulatory electrocardiogram Holter monitoring was performed using Aria digital Holter recorder (Spacelabs healthcare). The recordings were analyzed with specialcomputer software (Impresario Solo package). All recordings were visually examined and manuallyoverread to verify beat classification by two experienced examiners who were blinded to the clinical data. Only recordings with at least 20 hours of data and 80% or more of quantified sinus beats were included in the analysis. Abnormal beats and areasof artifact were automatically and manually identified
and excludedfrom the analysis. Moreover, the longest and
shortest true normal-to-normal intervals were identified for each recording in order to exclude all beats outside this range from the heart rate variability (HRV) analysis. The following
parameters were used in evaluation of HRV in timedomain:
(1) Standard deviation of all normal-to-normal intervals (SDNN [milliseconds]);(2) Standard deviation of mean of all normal-to-normal intervals in all consecutive5-min segments of the entire recording (SDANN [milliseconds]);(3) The root mean square of differences between adjacent normal-to-normal intervals (rMSSD [milliseconds]); (4) The percentage of R-R intervals with more than 50 milliseconds variation (pNN50); (5) Standard deviation of differences between successive normal-to-normal intervals (SDSD [milliseconds]).
Statistics
Data were presented as mean ± standard deviation (SD). Using an SPSS package 10.0 (SPSS Inc., Chicago, Illionis, USA) the changes in parameters after treatment were compared with paired t-tests. Pearson’s correlation analysis was used to assess the correlation between variables. A two-tailed p value < 0.05 was consideredsignificant.
Results
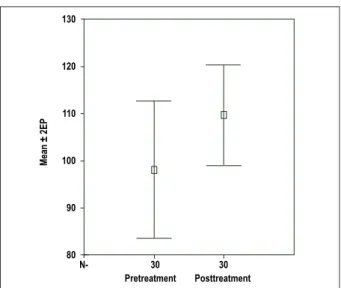
The mean age of the patients was 59.6±10.2 (38-75) years and 24 of them (80.0%) were males. Seven (23.3%) patients had diabetes and 16 (53.3%) had hypertension (Table 1). Mean systolic blood pressure was 122.3±16.7 mmHg and mean diastolic pressure was 78.5±11.4 mmHg. No significant changes occurred regarding blood pressures. A significant increase in mean LVEF was observed after the addition of trimetazidine (33.5±5.1% to 42.5±5.8%, p<0.001) (Table 2). Of the HRV parameters, SDNN and SDANN were significantly increased after trimetazidine treatment (Table 3, Figure 1, Figure 2). Baseline SDNN was significantly correlated with baseline LVEF (r=0.445, p=0.023). Also, the increment in SDNN was correlated with the increase in LVEF (r=0.518, p=0.007).
Discussion
In the present study, we observed that trimetazidine improved LVEF and HRV in patients receiving optimal treatment for heart failure of ischemic origin. Heart rate variability is a simple and noninvasive method used to identify vagal or sympathetic control of heart rate15,16. Changes in
the vagal or sympathetic tone of heart rate assessed by HRV analysis have been associated with mortality, arrhythmias and sudden cardiac death, particularly after acute myocardial infarction and heart failure7,8,17-19. The chronic administration
of trimetazidine in addition to the conventional therapy, in overall terms, has been shown to improve functional class and LV functions of heart failure patients20-22. Previous studies have
also suggested that trimetazidine modulates the autonomic control of HRV, i.e., that it reduces sympathetic overactivity and increases vagal influences11,12, 23.
Heart failure is associated with autonomic dysfunction, which can be quantified by measuring HRV. A reduction in SDNN identified patients at high risk of death7,8. In the
Table 1 - Baseline characteristics of the study patients (n=30).
Age years 59.6±10.2
Male sex 24 (80.0%)
Coronary artery disease 30 (100.0%)
Diabetes mellitus 7 (23.3%)
Hypertension 16 (53.3%)
Smoking history 20 (66.7%)
BP: blood pressure, LVEF: left ventricular ejection fraction
Pretreatment Post-treatment P value
Systolic BP (mmHg) 122.3±16.7 120.4±15.3 0.686
Diastolic BP (mmHg) 78.5±11.4 77.7±12.1 0.785
NYHA class I class II class III
19 (63.3%) 9 (30.0%) 2 (6.7%)
24 (80%) 6 (20.0%)
0
0.252
LVEF (%) 33.5±5.1 42.5±5.8 <0.001
Table 2 - Blood pressures, functional class and LVEF of the patients.
Original Article
Arq Bras Cardiol 2009; 93(2):145-148
Gunes et al Trimetazidine and HRV in heart failure
Pretreatment Posttreatment
M
ean ±
2E
P
130
120
110
100
90
80
N- 30 30
Figure 1 - Error bars for SDNN; before and after treatment with trimetazidine.
SE: Standard error, SDNN: Standard deviation of all normal-to-normal intervals
Pretreatment Posttreatment
M
ean ±
2E
P
120
110
100
90
80
70
60
N- 30 30
SE: Standard error, SDANN: Standard deviation of mean of all normal-to-normal intervals
Figure 2 - Error bars for SDANN; before and after treatment with trimetazidine.
Table 3 - Twenty-four hours heart rate variability parameters.
Pretreatment Post-treatment P value
Mean heart rate (bpm) 74.3±10.3 73.9±10.3 0.819
SDNN (msec) 97.3±40.1 110.5±29.2 0.049
rMSSD (msec) 42.2±20.1 46.5±22.0 0.323
PNN50 (%) 9.1±8.1 7.7±5.9 0.256
SDANN (msec) 80.5±29.0 98.3±30.5 0.008
SDSD (msec) 40.2±31.9 38.9±19.2 0.776
SDNN: Standard deviation of all normal-to-normal intervals, rMSSD: root mean square of differences between adjacent normal-to-normal intervals, PNN50: percentage of R-R intervals with more than 50 milliseconds variation, SDANN: Standard deviation of mean of all normal-to-normal intervals, SDSD: Standard deviation of differences between successive normal-to-normal intervals.
prospective analysis of the UK-Heart study , the evaluation of 433 outpatients with congestive heart failure (NYHA functional class I to III; mean LVEF 0.41±0.17), showed that the reduction in SDNN was a more powerful predictor of death risk, due to progressive heart failure, than the other conventional clinical measurements7. Aronson et al24 found
SDNN and SDANN to be useful predictors of survival after discharge in patients with decompensated heart failure. Therefore, our findings of increased SDNN and SDANN after the addition of trimetazidine to optimal medical therapy may have important clinical implications.
Trimetazidine has been reported to have a favorable effect on HRV parameters, explained by decreases in the sympathetic and increases in the parasympathetic activity in acute ischemic conditions11,23. Trimetazidine exerts
its anti-ischemic properties without affecting myocardial oxygen consumption and blood supply25-27. Trimetazidine
affects myocardial substrate use by inhibiting oxidative phosphorylation and by shifting energy production from free fatty acids (FFA) to glucose oxidation27. The beneficial
effect of this agent has been attributed to preservation of phosphocreatine and adenosine triphosphate (ATP) intracellular levels and reduction of cell acidosis, calcium overload and free radical-induced injury caused by ischemia26-32. In heart failure, similar to what happens during
acute myocardial ischemia, glucose and lactate oxidation are decreased and fatty acid oxidation is increased, increasing the oxygen requirement per ATP molecule produced. A major factor in the development and progression of heart failure is an already reduced availability of ATP, determining a metabolic state that has been defined as energy starvation13.
Limitations
The small number of study patients and the lack of a control group are the major limitations of the study. Since heart failure is a continually progressive process of autonomic imbalance, it is difficult to find the reference
point to define the timing of HRV measurement in heart failure patients. Comparisons of HRV determined in heart failure patients with similar clinical conditions in all populations are also difficult. It is possible that these limitations may be the cause of the variation and disparity in HRV predictive values. Therefore, instead of a placebo controlled study, progressive changes in measurements of HRV would be more important.
Conclusions
Trimetazidine, when added to optimal medical therapy in patients with heart failure of ischemic origin, may improve heart rate variability in association with improved
Original Article
Arq Bras Cardiol 2009; 93(2):145-148
Gunes et al
Trimetazidine and HRV in heart failure
References
1. Cohn JN, Levine TB, Olivari MT. Plasma norepinephrine as a guide to prognosis in patients with chronic congestive heart failure. N Engl J Med. 1984; 311 (13): 819-23.
2. Nolan J, Flapan AD, Capewell S, MacDonald T, Neilson JMM, Ewing DJ. Decreased cardiac parasympathetic activity in chronic heart failure and its relation to left ventricular function. Br Heart J. 1992; 67: 482-6.
3. Nolan J, Flapan AD, Goodefield NE, Prescott RJ, Bloomfield P, Neilson JMM, et al. Measurement of parasympathetic activity from 24-hour ambulatory electrocardiograms and its reproducibility and sensitivity in normal subjects, patients with symptomatic myocardial ischemia, and patients with diabetes mellitus. Am J Cardiol. 1996; 77: 154-8.
4. Chattipakorn N, Incharoen T, Kanlop N, Chattipakorn S. Heart rate variability in myocardial infarction and heart failure: review. Int J Cardiol. 2007; 120: 289-96.
5. Paschoal MA, Polessi EA, Simioni FC. Evaluation of heart rate variability in trained and sedentary climacteric women. Arq Bras Cardiol. 2008; 90: 74-9.
6. Pinheiro CH, Medeiros RA, Pinheiro DG, Marinho Mde J. Spontaneous respiratory modulation improves cardiovascular control in essential hypertension. Arq Bras Cardiol. 2007; 88: 651-9.
7. Nolan J, Batin PD, Andrews R, Lindsay SJ, Brooksby P, Mullen M, et al. Prospective study of heart rate variability and mortality in chronic heart failure: results of the United Kingdom Heart Failure Evaluation and Assessment of Risk Trial (UK-Heart). Circulation. 1998; 98: 1510-6.
8. Yi G, Goldman JH, Keeling PJ, Reardon M, McKenna WJ, Malik M. Heart rate variability in idiopathic dilated cardiomyopathy: relation to disease severity and prognosis. Heart. 1997; 77: 108-14.
9. Binkley PF, Haas GJ, Starling RC, Nunziata E, Hatton PA, Leier CV, et al. Sustained augmentation of parasympathetic tone with angiotensin-converting enzyme inhibition in patients with congestive heart failure. J Am Coll Cardiol. 1993; 21: 655-61.
10. Bullinga JR, Alharethi R, Schram MS, Bristow MR, Gilbert E. Changes in heart rate variability are correlated to hemodynamic improvement with chronic carvedilol therapy in heart failure. J Card Fail. 2005; 11: 693-9.
11. Ulgen MS, Akdemir O, Toprak N. The effects of trimetazidine on heart rate variability and signal-averaged electrocardiography in early period of acute myocardial infarction. Int J Cardiol. 2001; 77: 255-62.
12. Guler N, Eryonucu B, Gunes A, Guntekin U, Tuncer M, Ozbek H. Effects of trimetazidine on submaximal exercise test in patients with acute myocardial infarction. Cardiovasc Drugs Ther. 2003; 17: 371-4.
13. Katz AM. Is the failing heart energy depleted? Cardiol Clin. 1998; 16: 633-44.
14. Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, et al. Recommendations for chamber quantification: a report from the American Society of Echocardiography’s Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr. 2005; 18: 1440-63.
15. Bittencourt MI, Benchimol Barbosa PR, Drumond Neto C, Bedirian R, Barbosa EC, Brasil F, et al. Assessing autonomic function in hypertrophic cardiomyopathy. Arq Bras Cardiol. 2005; 85: 388-96.
16. Menezes Ada S Jr, Moreira HG, Daher MT. Analysis of heart rate variability
in hypertensive patients before and after treatment with angiotensin II-converting enzyme inhibitors. Arq Bras Cardiol. 2004; 83: 169-72.
17. Kleiger RE, Miller JF, Bigger JT Jr, Moss AJ. The multicenter post infarction research group. Decreased heart rate variability and its association with increased mortality after acute myocardial infarction. Am J Cardiol. 1987; 59: 256-62.
18. Topal E, Ozdemir R, Barutcu I, Aksoy Y, Sincer I, Akturk E, et al. The effects of trimetazidine on heart rate variability in patients with slow coronary artery flow. J Electrocardiol. 2006; 39: 211-8.
19. Szabo B, van Veldhuisen DJ, van der Veer N, Brouwer J, De Graeff PA, Crijns HJ. Prognostic value of heart rate variability in chronic congestive heart failure secondary to idiopathic or ischemic dilated cardiomyopathy. Am J Cardiol. 1997; 79 (7): 978-80.
20. Fragasso G, Palloshi A, Puccetti P, Silipigni C, Rossodivita A, Pala M, et al. AA randomized clinical trial of trimetazidine, a partial free fatty acid oxidation inhibitor, in patients with heart failure. J Am Coll Cardiol. 2006; 48: 992-8.
21. Sisakian H, Torgomyan A, Barkhudaryan A. The effect of trimetazidine on left ventricular systolic function and physical tolerance in patients with ischaemic cardiomyopathy. Acta Cardiol. 2007; 62: 493-9.
22. Fragasso G, Piatti PM, Monti L, Palloshi A, Setola E, Puccetti P, et al. Short andShort and long term beneficial effects of trimetazidine in patients with diabetes and ischaemic cardiomyopathy. Am Heart J. 2003; 146: E1-E8.
23. Birand A, Kudabierdieva GZ, Batyraliev TA, Akgul F, Usal A. Effects of trimetazidine on heart rate variability and left ventricular systolic performance in patients with coronary artery disease after percutaneous transluminal angioplasty. Angiology. 1997; 48: 413-22.
24. Aronson D, Mittleman MA, Burger AJ. Measures of heart period variabilityMeasures of heart period variability as predictors of mortality in hospitalized patients with decompensated congestive heart failure. Am J Cardiol. 2004; 93: 59-63.
25. Shlyakhto EV, Almazov VV, Nifontov EM, Vakhrameyeva IV, Rudomanov OG, Zakharov DV, et al. Antianginal effects of trimetazidine and left ventricular function improvement in patients with stable angina pectoris. Am J Cardiovasc Drugs. 2002; 2: 119-24.
26. Bricaud H, Brottier L, Barat JL, Combe C, Boussens B, Bonnet J. Cardioprotective effects of trimetazidine in severe ischemic cardiomyopathy. Cardiovasc Drugs Ther. 1990; 4: 861-5.
27. Marzilli M. Cardioprotective effects of trimetazidine: a review. Curr Med Res Opin. 2003; 19: 661-72.
28. Lopaschuk GD, Stanley WA, Lopaschuk CC. Metabolic approach in heart failure: the rationale for metabolic interventions. Heart Metab. 2005; 27: 5-10.
29. Fragasso G, Spoladore R, Bassanelli G, Cuko A, Montano C, Salerno A, et al. New directions in the treatment of heart failure: targeting free fatty acid oxidation. Curr Heart Fail Rep. 2007; 4: 236-42.
30. Lagadic-Gossmann D, Le Prigent K, Feuvray D. Effects of trimetazidine on pHi in the rat isolated ventricular myocyte. Br J Pharmacol. 1996; 117: 831-8.
31. Renaud JF. Internal pH, Na+, and Ca2 regulation by trimetazidine during
cardiac cell acidosis. Cardiovasc Drugs Ther. 1988; 1: 677-86.
32. Wolff AA, Rotmensch HH, Stanley WC, Ferrari R. Metabolic approaches to the treatment of ischemic heart disease: the clinicians’ perspective. Heart Fail Rev. 2002; 7: 187-203.
left ventricular ejection fraction. Long-term and larger sample studies are necessary to evaluate whether these findings may have clinical implications on mortality and morbidity.
Potential Conflict of Interest
No potential conflict of interest relevant to this article was reported.
Sources of Funding
There were no external funding sources for this study.
Study Association
This study is not associated with any post-graduation program.