www.jped.com.br
ORIGINAL
ARTICLE
Fecal
microbiota
analysis
of
children
with
small
intestinal
bacterial
overgrowth
among
residents
of
an
urban
slum
in
Brazil
夽
Carolina
Santos
Mello,
Mirian
Silva
do
Carmo
Rodrigues,
Humberto
Bezerra
de
Araújo
Filho,
Lígia
Cristina
Fonseca
Lahoz
Melli,
Soraia
Tahan,
Antônio
Carlos
Campos
Pignatari,
Mauro
Batista
de
Morais
∗UniversidadeFederaldeSãoPaulo(UNIFESP),DepartamentodePediatria,DisciplinadeGastroenterologiaPediátrica,SãoPaulo, SP,Brazil
Received12December2016;accepted2August2017 Availableonline16October2017
KEYWORDS Fecalmicrobiota; Environmental exposure; Child
Abstract
Objective: Toanalyzethefecalmicrobiotacompositionofchildrenlivinginanurbanslumin Brazil,withorwithoutsmallintestinalbacterialovergrowth,andtoinvestigatetheoccurrence ofstuntingandanemia.
Methods: A total of 100children were studied, aged 5---11years,from the municipality of Osasco,SãoPaulo.Smallintestinalbacterialovergrowthwasscreenedthroughhydrogenand methane breathtest withlactulose.Weightandheightwere measured,andthe height-for-ageandbodymass-for-ageanthropometricindexeswerecalculated.Theoccurrenceofanemia wasinvestigatedbycapillaryhemoglobin.Analysisofbacterialphylum,genus,andspecieswas performedbyreal-timepolymerasechainreactioninfecalsamples.
Results: Smallintestinalbacterialovergrowthwasidentifiedin61.0%ofthechildren.Alower meanofheight-for-ageZ-score([−0.48±0.90]vs.[−0.11±0.97];p=0.027),aswellas cap-illaryhemoglobin([12.61±1.03g/dL]vs.[13.44±1.19g/dL];p<0.001)wasdemonstratedin childrenwithSIBOwhencomparedwithchildrenwithoutsmallintestinalbacterialovergrowth. ChildrenwithsmallintestinalbacterialovergrowthpresentedahigherfrequencyofSalmonella
spp.,whencomparedtothosewithoutsmallintestinalbacterialovergrowth(37.7%vs.10.3%;
p=0.002).HighercountsoftotalEubacteria(p=0.014)andFirmicutes(p=0.038)wereobserved inchildrenwithoutsmallintestinalbacterialovergrowth;however,ahighercountofSalmonella
(p=0.002)wasfoundinchildrenwithsmallintestinalbacterialovergrowth.
夽
Pleasecitethisarticleas:MelloCS,RodriguesMS,FilhoHB,MelliLC,TahanS,PignatariAC,etal.Fecalmicrobiotaanalysisofchildren withsmallintestinalbacterialovergrowthamongresidentsofanurbansluminBrazil.JPediatr(RioJ).2018;94:483---90.
∗Correspondingauthor.
E-mail:maurobmorais@gmail.com(M.B.deMorais). https://doi.org/10.1016/j.jped.2017.09.003
Conclusion: Childrenwho livedinaslumandwerediagnosedwithsmallintestinalbacterial overgrowthshowedlowerH/AZ-scoresandhemoglobinlevels.Furthermore,differenceswere observedinthefecalmicrobiotaofchildrenwithsmallintestinalbacterialovergrowth,when comparedtothosewithoutit;specifically,ahigherfrequencyandcountofSalmonella,and lowercountsofFirmicutesandtotalEubacteria.
©2017SociedadeBrasileiradePediatria.PublishedbyElsevierEditoraLtda.Thisisanopen accessarticleundertheCCBY-NC-NDlicense(http://creativecommons.org/licenses/by-nc-nd/
4.0/).
PALAVRAS-CHAVE Microbiotafecal; Exposic¸ãoambiental; Crianc¸a
Análisedamicrobiotafecaldecrianc¸ascomsobrecrescimentobacterianono intestinodelgadodemoradorasdeumafavelaurbananoBrasil
Resumo
Objetivo: Analisar acomposic¸ãoda microbiota fecal de crianc¸as moradorasde uma favela urbananoBrasil,comesemsobrecrescimentobacterianonointestinodelgado,einvestigara ocorrênciadedéficitdecrescimentoeanemia.
Métodos: Foramestudadas100crianc¸as,comidadeentre5e11anos,nacidadedeOsasco,São Paulo.Sobrecrescimentobacterianonointestinodelgadofoipesquisadoportesterespiratório dohidrogênio e metanonoarexpirado com lactulose.Forammensurados peso,estatura e calculados os índicesantropométricosestatura para idade eíndice de massa corporal para idade.Foiinvestigadaaocorrênciadeanemia,pelaavaliac¸ãodahemoglobinacapilar.Aanálise dosfilos,gêneroseespéciesbacterianasem amostrasdefezesfoirealizadaporpolymerase chainreactionemtemporeal.
Resultados: Sobrecrescimentobacterianonointestinodelgadofoidiagnosticadoem61,0%das crianc¸as avaliadas.FoiverificadamenormédiadoescoreZdoíndiceestatura paraidade (-0,48±0,90vs.-0,11±0,97DP)edehemoglobina capilar(12,61±1,03vs.13,44±1,19g/dL)no grupodecrianc¸ascomsobrecrescimentobacterianonointestinodelgado,quandocomparadas àquelas semsobrecrescimento bacterianono intestinodelgado (p<0,05). Nascrianc¸as com sobrecrescimentobacterianonointestinodelgadofoiobservadamaiorfrequênciadeSalmonella spp.,quandocomparadasàquelassemsobrecrescimentobacterianonointestinodelgado(37,7%
vs.10,3%;p=0,002).MaiorcontagemdeEubactériastotais(p=0,014)eFirmicutes(p=0,038) foiobservadanascrianc¸assemsobrecrescimento bacterianonointestinodelgado, enquanto queas crianc¸as comsobrecrescimentobacteriano nointestinodelgado apresentarammaior contagemdeSalmonella(p=0,002).
Conclusão: Nascrianc¸ascomdiagnósticodesobrecrescimentobacterianonointestinodelgado verificaram-semenoresvaloresdeestaturaparaidadeedehemoglobina.Foramconstatadas diferenc¸asnamicrobiotafecaldascrianc¸ascomsobrecrescimentobacterianonointestino del-gado,especificamente,maiorfrequênciaecontagemdeSalmonellaspp.emenorescontagens deFirmicuteseEubactériastotais.
©2017SociedadeBrasileiradePediatria.PublicadoporElsevierEditoraLtda.Este ´eumartigo OpenAccesssobumalicenc¸aCCBY-NC-ND(http://creativecommons.org/licenses/by-nc-nd/4. 0/).
Introduction
Over the last few years, several studies have been car-riedoutaimingtobroadentheknowledgeaboutthehuman intestinal microbiota composition. The stool contains a large biomass of bacterial cells, representing a combina-tionofmucosalbacteriaandthosetransientlypresentinthe intestinallumen.1However,littleis knownaboutthe
bac-terialcommunities thatadhere toand colonize the small
intestine, because of the technical difficulties to collect
samplesfor analysisofthe intestinalcontentsin this
gas-trointestinaltractregion.2
An increase in the amount of bacteria in the small
intestine, especially of species common to the colon,
characterizessmallintestinalbacterialovergrowth(SIBO).3
This clinical condition is often associated with
envi-ronmental enteropathy, recently renamed environmental
enteric dysfunction,4 in individuals exposed to unhealthy
environments.5 Thus, morphological and functional
alter-ations of the small intestine can be observed, derived
fromalocalinflammatoryprocess4,5 throughtheaction of
pathogenicbacteria,especiallyGram-negative,3triggering
a picture of chronic malabsorption of nutrients and
con-sequentgrowthdeficit in children,4---6 evenwhentheyare
asymptomatic.4,7
Respiratorytestsareanon-invasivealternativeforSIBO
investigation.8Inhealthyindividuals,hydrogenandmethane
productionoccurspredominantlybyanaerobicbacterial
fer-mentationinthelargeintestine.InthepresenceofSIBO,the
intestine,throughtheactionofcontaminatingbacteria.8In
this context,a study carried out by the present research
group7 inchildrenexposed topoor livingconditionsfound
thatthosediagnosedwithSIBOhadahigherfermentation
potential not only in the smallintestine, but also in the
colon, suggesting a situation of dysbiosis throughout the
entiregastrointestinaltractinthepresenceofthisclinical
condition.
Considering that the intestinal microbiota composition
canbeinfluencedbytheenvironmentandtheliving
condi-tionstowhichtheindividualisexposed9 andthenegative
consequencesof theenvironmental entericdysfunctionin
childhood,4,5 thepresentstudyaimedtoanalyzethefecal
microbiotacomposition ofchildren withandwithout SIBO
livinginanurbansluminBrazil,aswellastoinvestigatethe
occurrenceofgrowthdeficitandanemiainthesechildren.
Methods
Design
This wasacross-sectional study carriedoutin thecity of Osasco,metropolitanregionofSãoPaulo,Brazil.Thestudy populationconsistedofchildrenoflowsocioeconomic sta-tus, living in an urban slum, constituting a convenience sample.
Inclusion criteria were age between 5 and 11 years, absenceof diarrhea(liquid stools),andnon-useof antibi-oticsforatleastonemonth.Failuretoperformarespiratory test and/ornon-delivery of stoolsample constituted sam-plelosses.Childrenwithclinicalevidenceofseverechronic diseases(e.g.,heartdisease)werenotincludedinthestudy. Withthehelpofacommunityleader,participantswere invitedtothestudy.Atotalof122children,accompaniedby theirparents,volunteeredtoparticipate;however, 22did notmeetthecriteriaforstudyenrollment.
This projectwasapprovedbytheResearchEthics Com-mitteeoftheUniversidadeFederaldeSãoPaulo. Asigned informedconsentwasobtainedfromeachparticipant’s par-ents/guardiansatthetimeofstudyenrollment.
Housingconditions,anthropometricsand hemoglobinlevelmeasurement
Informationwasobtainedonthehousingconditionsfromthe parents/guardians.Tomeasureweightandheight,adigital scale(FilizolaSAPesagemeAutomac¸ão,SãoPaulo,Brazil) wasused, witha 150kg capacityand sensitivity of 100g, andaverticalanthropometer(SecaGmbhCo.Kg.,Hamburg, Germany)with190cmmeasuringcapacityandsensitivityof 0.1cm.Theheight-for-age(H/A)andbodymass index-for-age(BMI/A)Z-scoreswereobtained.10
Capillaryhemoglobinlevels,obtainedfromablood
sam-plecollectedbydigitalpulppuncture,weredeterminedin
a portable photometer (Hemocue®, Ängelholm, Sweden),
considering anemia asthe presence of hemoglobin levels
below11.5g/dL.11
Breathtestwithlactulose
Thebreathtestwasperformedafter8hoffastingandoral hygienewithantisepticsolution.Afterthecollectionof a baseline expired air sample, 10g of lactulose7,12 (Daiichi
Sankyo,SãoPaulo,Brazil)wereadministeredorallyina10%
aqueoussolution.Newsamplesofexpiredairwerecollected
at15,30,45,60,90,120,and180minafterlactulose
inges-tion.Sampleswerecollectedinasingleforcedexpiration,
in hermetically sealed bags. Hydrogen (H2) and methane
(CH4)concentrations were measured by gas
chromatogra-phy(MicroLyzerSC,QuintronInstrumentCo.Inc.,Wisconsin,
USA).
SIBOwascharacterizedbyanincreaseinthe
concentra-tionsofH2≥20ppmand/orofCH4≥10ppmintheexpired
air,inrelationtotheconcentrationsinfastingsamples,up
to60minafterthetest.7,12
Real-timepolymerasechainreaction(PCR)
Stool collection was performed by the children’s par-ents/guardians, after receiving instructions. The samples werestoredin auniversal stoolcollectorandthen stored ina domestic freezer for upto24h (betweenevacuation anddelivery).Inthelaboratory,afecalaliquotof approxi-mately1gwastransferredtoasterilecryotubecontaining ASLbufferfromtheQiaAmpminiStoolkit(Qiagen,Hilden, Germany) and kept at −20◦C until DNA extraction; the
bacterial genomic DNA was extracted from the samples accordingtothe protocol suggested bythe manufacturer. ThepurifiedDNAwasdilutedinabuffersolutiontoafinal volume of 200L. DNA quantification was performed on
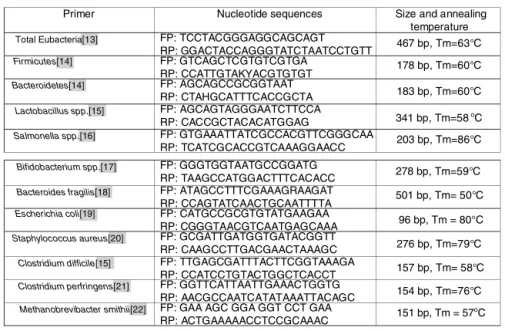
aNanodroop 1000 spectrophotometer(ThermoScientific ---Waltham,USA).AllDNAsamplesweredilutedtothe concen-trationof20ng/Landstoredat−20◦C.Theprimers(Fig.1)
were used for identification and quantification of total
Eubacteria,13 FirmicutesandBacteroidetes14 phyla,
Lacto-bacillus spp.,15 Salmonella spp.,16 Bifidobacterium spp.17
genera, and the following species: Bacteroides fragilis,18
Escherichia coli,19 Staphylococcus aureus,20 Clostridium difficile,15 Clostridium perfringens,21 and Methanobre-vibactersmithii.22DNAfromallfecalsampleswassubmitted
tothereal-timePCRassay.
Allreactionswerecarriedoutinduplicate,inafinal
vol-umeof10Lcontaining5LofRotor-geneSYBRGreenPCR
MasterMix(Qiagen---Hilden,Germany),0.2L(10pmol/L)
of the forward and reverse primers of each bacteria,
0.5Lof the DNA sample (20ng/L), and4.1Lof DEPC
(diethylpyrocarbonate)water(Qiagen---Hilden,Germany).
Thermocyclingwasperformed ontheRotor-geneQ
equip-ment (Qiagen --- Hilden, Germany) under the following
conditions:5min at 95◦C, followed by 40 cyclesof 95◦C
for10sand60◦Cfor15s.Theproductdissociationcyclefor
themeltingcurvewas95◦Cfor1mandonephaseforthe
meltingcurvethatrangedfrom70◦Cto95◦C,withagradual
increaseinthetemperatureof1◦
C/s.
Aninternalreactioncontrolwascarriedoutforall
sam-ples,using primersdesigned todetect total Eubacteria,23
workingasastandardfortherelativequantificationoftotal
bacterialDNA.Asnegativecontrol,areactioncontainingall
Primer Nucleotide sequences Size and annealing temperature
Total Eubacteria[13] FP: TCCTACGGGAGGCAGCAGT
RP: GGACTACCAGGGTATCTAATCCTGTT 467 bp, Tm=63°C Firmicutes[14] FP: GTCAGCTCGTGTCGTGA
RP: CCATTGTAKYACGTGTGT 178 bp, Tm=60°C Bacteroidetes[14] FP: AGCAGCCGCGGTAAT
RP: CTAHGCATTTCACCGCTA 183 bp, Tm=60°C Lactobacillus spp.[15] FP: AGCAGTAGGGAATCTTCCA
RP: CACCGCTACACATGGAG 341 bp, Tm=58oC Salmonella spp.[16] FP: GTGAAATTATCGCCACGTTCGGGCAA
RP: TCATCGCACCGTCAAAGGAACC 203 bp, Tm=86°C
Bifidobacterium spp.[17] FP: GGGTGGTAATGCCGGATG
RP: TAAGCCATGGACTTTCACACC 278 bp, Tm=59°C Bacteroides fragilis[18] FP: ATAGCCTTTCGAAAGRAAGAT
RP: CCAGTATCAACTGCAATTTTA 501 bp, Tm= 50°C Escherichia coli[19] FP: CATGCCGCGTGTATGAAGAA
RP: CGGGTAACGTCAATGAGCAAA 96 bp, Tm = 80°C Staphylococcus aureus[20] FP: GCGATTGATGGTGATACGGTT
RP: CAAGCCTTGACGAACTAAAGC 276 bp, Tm=79°C Clostridium difficile[15] FP: TTGAGCGATTTACTTCGGTAAAGA
RP: CCATCCTGTACTGGCTCACCT 157 bp, Tm= 58°C Clostridium perfringens[21] FP: GGTTCATTAATTGAAACTGGTG
RP: AACGCCAATCATATAAATTACAGC 154 bp, Tm=76°C Methanobrevibacter smithii[22] FP: GAA AGC GGA GGT CCT GAA
RP: ACTGAAAAACCTCCGCAAAC 151 bp, Tm = 57
o
C
Figure1 Designofprimersusedinthestudy.
Thestandardcurveforallanalyseswasperformedbythe amplificationofaTopoTAplasmid(Invitrogen®,USA),which containedthegenefragmentforeachbacterium,previously amplifiedbyconventionalPCR,anditsspecificitywas con-firmed by sequencingand alignment in the BLAST system (Canablast®,Canada).
Statisticalanalysis
TheMann---WhitneyorStudent’st-testwasusedtoanalyze theresultswhencomparingtwoindependentgroupsfor con-tinuousnumericalvariables,whilethechi-squaredtestwas usedfor categoricalvariables. The calculationswere per-formedusingtheSigmaStatprogram(Systatsoftware,Inc, version3.1,USA)settingthelevelforrejectionofthenull hypothesisat5%.
Results
SIBO was diagnosed in 61/100 (61.0%) children. Table 1
presents the demographic and anthropometric data,
fre-quencyofanemia,andmeanhemoglobinvalues.Thegroup
of children with SIBOpresented lower valuesof H/Aand
hemoglobinZ-scores (p<0.05) whencomparedwith those
withoutSIBO.AnassociationwasalsofoundbetweenSIBO
andabsenceofrunningwatersupplyatthehousehold.
Table 2 shows the hydrogen and methane production,
obtainedfromthebreathtestwithlactulose,andexpressed
asindividualareas under thecurve. It wasobserved that
children with SIBO showed a higher hydrogen production
duringthefirsthourof thetest (p=0.002),presumablyin
the smallintestine. This difference wasnot verified with
methaneconcentrations.Between60and180min,aperiod
during which gas production occurs predominantly in the
large intestine, children with SIBO showed higher
hydro-genandmethaneconcentrations;however,thedifferences
didnot reachstatistical significance (p=0.081 and0.098,
respectively).
The following were identified in all children (100.0%):
Bacteroides fragilis, Escherichia coli, Lactobacillus spp.,
Bifidobacteriumspp., andMethanobrevibacter smithii.As
for the other genera and species analyzed, variable
fre-quencieswereobservedinchildrenwithandwithoutSIBO,
respectively: Salmonellaspp. (37.7%vs.10.3%; p=0.002),
Staphylococcusaureus(52.5%vs.41.0%;p=0.267), Clostrid-iumdifficile (44.3% vs. 41.0%;p=0.751), andClostridium perfringens(91.8%vs.92.3%;p=0.928).
A higher total count of Eubacteria (p=0.014) and the
Firmicutes phylum(p=0.038)wasverifiedin thegroup of
childrenwithoutSIBO;however,ahigherSalmonellacount
(p=0.002)wasobservedinchildrenwithSIBO.The
quantifi-cationofbacterialphyla,genera,andspecies,accordingto
thepresenceorabsenceofSIBO,ispresentedinTable3.
Discussion
Inthepresentstudy,differenceswereobservedinthefecal microbiota composition of children with SIBO living in an urbanslum;moreprecisely,higherfrequencyandcountsof Salmonella spp. and lowercounts of Firmicutesand total EubacteriawereobservedinchildrenwithSIBOwhen com-paredtothosewithoutit.
Inapreviousstudy carriedoutby thepresentresearch team,afindingthatmotivatedthestudyofthefecal micro-biota of children exposed to poverty and diagnosed with SIBO was a differentiated pattern of fermentation in the colon,characterizedbythehigherproductionofhydrogen inthebreathtest.9This resultledtotheassumptionthat
individuals withSIBO possiblyhave a situation of
dysbio-sisinthedifferentintestinalsegments,andnotonlyinthe
smallintestine.However,thispatternofhigherproduction
ofhydrogenandevenmethaneinthecolonofchildrenwith
SIBO,althoughsuggestive,wasnotconfirmedbythepresent
results.
Thestudyoftheintestinalbacterialcompositionismade
possible by the analysis of fecal samples.1 In turn,
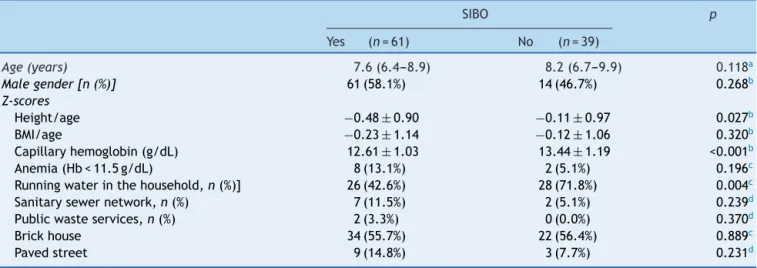
Table1 Anthropometricdataandlivingconditionsofchildrenlivinginanurbanslum,withorwithoutsmallintestinebacterial overgrowth(SIBO).
SIBO p
Yes (n=61) No (n=39)
Age(years) 7.6(6.4---8.9) 8.2(6.7---9.9) 0.118a
Malegender[n(%)] 61(58.1%) 14(46.7%) 0.268b
Z-scores
Height/age −0.48± 0.90 −0.11± 0.97 0.027b
BMI/age −0.23±1.14 −0.12±1.06 0.320b
Capillaryhemoglobin(g/dL) 12.61± 1.03 13.44± 1.19 <0.001b
Anemia(Hb<11.5g/dL) 8(13.1%) 2(5.1%) 0.196c
Runningwaterinthehousehold,n(%)] 26(42.6%) 28(71.8%) 0.004c
Sanitarysewernetwork,n(%) 7(11.5%) 2(5.1%) 0.239d
Publicwasteservices,n(%) 2(3.3%) 0(0.0%) 0.370d
Brickhouse 34(55.7%) 22(56.4%) 0.889c
Pavedstreet 9(14.8%) 3(7.7%) 0.231d
BMI,bodymassindex;Hb,hemoglobin.
a Mann---Whitneytest,expressedasmedian(25thand75thpercentiles). b Student’st-test(one-tailedanalysis),expressedasmean±standarddeviation. c Chi-squaretest.
d Fisher’sexacttest.
Table2 AreaunderthecurveoftheconcentrationinPPM/min,ofhydrogen(H2)andmethane(CH4)obtainedfromthebreath
testwithlactuloseofchildrenlivinginanurbanslum,withorwithoutsmallintestinebacterialovergrowth(SIBO)duringthe first60min,between60and180minandtheentiretestperiod.
WithSIBO (n=61) WithoutSIBO (n=39) pa
H2 0---60min 750.0(528.75---960.0) 472.5(307.5---712.5) 0.002
60---180min 3292.5(2126.25---4398.75) 2550.0(1410.0---4080.0) 0.098 0---180min 3978.75(2662.5---5257.5) 2902.50(1807.50---4800.0) 0.048
CH4 0---60min 1072.5(217.5---1680.0) 840.0(0.0---1417.5) 0.146
60---180min 1920.0(442.5---4665.0) 1680.0(0.0---2910.0) 0.081 0---180min 2857.5(630.0---6198.75) 2535.0(0.0---4327.5) 0.069
a Mann---Whitneytest,expressedasmedian(25thand75thpercentiles). PPM,partspermillion.
Table3 Bacterialphylum,genus,andspecies(colonyformingunits:CFU/goffeces)thatrepresentthefecalmicrobiotaof childrenlivinginurbanslums,withorwithoutsmallintestinebacterialovergrowth(SIBO).
WithSIBO(n=61) WithoutSIBO(n=39) pa
TotalEubacteria UFC/g(×1014) 1.42(0.26---5.25) 3.62(0.97---24.68) 0.014
PhylumBacteroidetes UFC/g(×109) 1.55(0.51---2.29) 1.73(0.50---3.23) 0.344
Bacteroidesfragilis UFC/g(×1010) 1.08(0.15---5.16) 2.15(0.27---14.02) 0.145
PhylumFirmicutes UFC/g(×108) 0.68(0.25---2.31) 1.60(0.52---3.73) 0.038
Lactobacillusspp. UFC/g(×107) 6.39(1.66---25.5) 6.51(2.46---31.93) 0.777
Clostridiumdifficile UFC/g(×103) 0.0(0.0---1.18) 0.0(0.0---1.19) 0.956
Clostridiumperfringens UFC/g(×105) 0.49(0.10---6.30) 0.96(0.16---5.20) 0.628
Staphylococcusaureus UFC/g(×105) 0.10(0.0---4.42) 0.0(0.0---5.47) 0.672
Bifidobacteriumspp. UFC/g(×105) 5.63(0.97---31.93) 3.18(0.64---10.96) 0.249
Salmonellaspp. UFC/g(×102) 0.0(0.0---1.64) 0.0(0.0---0.0) 0.002
Escherichiacoli UFC/g(×109) 1.08(0.19---9.74) 1.50(0.38---33.47) 0.381
Methanobrevibactersmithii UFC/g(×107) 4.18(1.15---8.71) 2.24(0.57---9.31) 0.347
content(jejunalaspirate),wouldbenecessaryforthe char-acterizationof thesmall intestinemicrobiota, considered thegoldstandardinthediagnosisofSIBO.3,8,24However,the
invasivecharacteristics and thehigh cost8 of thismethod
make itunfeasible for the evaluation of asymptomatic or
non-specificindividuals,inadditiontothefactthatitsuse
maynotbeethicallyappropriateforresearchpurposes.24
In healthy individuals, the bacterial colonization in
the proximal small intestine (102CFU/g of intestinal
contents) is smallwhen compared with that in the colon
(1010---1012CFU/gfeces).Thelowerbacterialdensityinboth
thestomachandsmallintestineisduetotheactionof
gas-tricjuiceanddigestiveenzymes,in additiontoperistaltic
movements,aspartofthemigratingmotorcomplex(MMC)
observed in these segments.24 Conversely, the
character-ization of SIBO is usually associated with qualitative and
quantitativechangesinbacterialgeneraandspeciesinthe
smallintestine.3
The bacteria involved in the occurrence of SIBO are
mainlyGram-negative,whichhavelipopolysaccharide(LPS)
intheircellmembranes.LPSisassociatedwiththeonsetof
alocalinflammatory process,causingmucosallesionsand
increasedintestinalpermeability,3,6withaconsequent
mal-absorptionsyndrome4---6 and high nutrient fermentation in
thecolon.3AninhibitoryactionofMMCisalsoattributedto
thebacterialLPS,whichwouldcauseastasisoftheluminal
contentintheinterdigestiveperiod,favoringtheexcessive
growth,in thesmallintestine, ofbacteriacommontothe
colon.24
Intestinalentericdysfunctionisassociatedwithinfection
by potentially pathogenic microorganisms, which
perme-ates a condition of intestinal dysbiosis.5 Salmonella and
Escherichiacoli25arespecieswithhighpathogenicpotential
strains,veryoftenwithdiarrheaasagastrointestinal
symp-tom.In thepresent study,ahigh frequencyof Salmonella
spp. anda highercountin fecal sampleswasobserved in
childrenwithSIBO when comparedwiththosewithout it,
a result that indicates a higher number of asymptomatic
carriersthanexpected.
AlowerquantificationofFirmicuteswasobservedinthe
SIBOgroup.Accordingtosomeauthors,agreater
variabil-ityinintestinalbacterialcompositionmayreflectagreater
resistancetopathogeninvasion.26Intestinalbacterial
diver-sityappearstoconferresilienceand,consequently,greater
stabilityofthebacterialecosystem.27 However,thehigher
quantificationofabacterialphylumisnotnecessarily
asso-ciated with a greater number or diversity of colonizing
bacterialgeneraandspecies.Similarly,thehigher
quantifi-cationoftotalEubacteriainthegroupofchildrenwithSIBO
canbereflectahigherbacterialconcentration.
Thegeneticvariabilityofthemicroorganismsthatmake
upthemicrobiotaofindividualswithandwithoutSIBOcould
be identified with the use of new generation sequencing
technologies, which may be the subject of future
stud-ies.The techniqueusedinthepresentstudy constitutesa
limitingfactor,sinceitonlyallowstheassessmentofsome
pre-selectedbacterialgroups.
The presentstudy didnotfindother differencesinthe
fecalmicrobiotacompositioninchildrenwithandwithout
SIBO.Basedonthisobservation,thepowerofthetestwas
analyzed;theresults ofEscherichia colicounts were
con-sideredfor thecalculation, sincetheinterpretation of its
resultsshowsbiologicalplausibilityinthepresenceofSIBO.
Consideringthestatisticaltest(Mann---Whitney)andeffect
size(d=0.45),calculatedfromthemeansandstandard
devi-ationsoftheEscherichiacolicount(CFU/goffeces)ofboth
groups,itwasobservedthatthepower(1−ˇ)forthis
anal-ysiswas56.6%.Toobtain apowerof80%,maintainingthe
effectsizeand ˛=5%,a totalof 164individuals wouldbe
required (Software G*Power, version 3.1.9.2). Therefore,
this alsoconstitutesa study limitation,which mayjustify
thenon-attainmentofstatisticalevidenceinsomeanalyses.
TheoccurrenceofSIBOinchildrenexposedtounhealthy
housing conditions and to vehicles of contamination is
the main indicator of environmental enteropathy.7 In the
present study, the prevalence of SIBO (61.0%) was high,
being significantly higher than that observed in other
studies.7,12Inthiscontext,theloweraccesstorunningwater
inthehouseholdsofchildrenwithSIBOisnoteworthy.A
pre-vious study,7 carriedout in this samecommunity, showed
that41.2%ofthehouseholdshadaclandestinewater
sup-ply;inthegroupofchildrenwithSIBO,thepresenceoffecal
coliformswasverifiedin80.8%ofanalyzedwatersamples.
In environmental enteropathy, the presence of small
intestinal villous atrophy, cryptic hyperplasia, and
lym-phoplasmacytic infiltrate in the lamina propria can be
observed.5Macronutrientandmicronutrientsmalabsorption
is characterized, and these digestive-absorptive
dysfunc-tionsmaybeassociatedwiththeoccurrenceofshortstature
inchildrenfromdevelopingcountries.24
Differentauthors,when studying thebehavior of
envi-ronmental enteropathybiomarkers in childrenexposed to
poor living conditions in the Northeast of Brazil6 and in
Bangladesh,28 found a reduction in the intestinal barrier
actionandabsorptivefunctionduetotheincreasein
intesti-nal permeability verifiedby serum levelsof zonulin6 and
lactulose and mannitol absorption test,6,28 respectively.
An association with the systemic inflammatory response
inducedbymicrobialproducts,suchasLPS,6wasalso
ver-ified; thebiomarkerswereshowntobefactorsassociated
withgrowthdeficitsinchildren.However,thesedataneed
tobeanalyzedwithcaution,duetothecomplexityofthe
mechanismsinvolvedintheintestinalandsystemic
inflam-matoryresponse.28
Thegreater susceptibilityof childrenliving in slumsto
nutritional disorders has been already well demonstrated
intheliterature.7,9However,thepresentfindingsalso
indi-cate ahigher frequencyof low nutritionalstaturein SIBO
patients,whencomparedtothosewithoutSIBO,whenboth
groupsareexposedtothesameriskfactors.
AstudycarriedoutinBangladeshwith902-year-old
chil-drenlivinginpovertyfoundthatthemainfactorassociated
withSIBOwasthereductionintheH/AZ-score,in
compari-sontothebirthparameters,regardlessofwhetherchildren
hadornotrecentorfrequentdiarrhealdisease.29
Anotherresultthat,similartoshortstature,reinforces
theoccurrenceofamalabsorptionsyndrome,wasthelower
meanlevelsof hemoglobinfound inthe groupof children
withSIBO.Anotherstudy30carriedoutwithchildrenlivingin
aslumfoundanassociationbetweentheoccurrenceof
ane-miaandanintestinalfunctionabnormality,characterizedby
lowerabsorptionofd-xylose.
Itisimportanttoemphasizetheoriginalityofthepresent
enteric dysfunction and its consequences, more precisely
the association with SIBO and changes in the intestinal
microbiota.ItshouldbeemphasizedthefindingoflowerH/A
andhemoglobinlevelsinchildrenwithSIBO,whencompared
tothosewithoutit,eventhoughtheylivedinthesameurban
slum.Thisresultmaysuggestthatexposureto
microorgan-ismswithhighpathogenicpotential,characterizedhereby
thehigherfrequencyandcountsof Salmonellainchildren
withSIBO,couldrepresent animportantfactor associated
withthedevelopmentofshortstatureandanemia.
Much remains to be elucidated about bacterial
com-munitiesandtheir interactionswiththe human organism.
However,basedonthe hypothesisthat individuals
suscep-tibletobacterialcontaminationby potentiallypathogenic
species may present serious damage in their health and
nutrition, it is necessary to emphasize the importanceof
effective public policies for the improvement of housing
conditions and basic sanitation of the vulnerable
popula-tion,thuscontributingtotheeradicationofenvironmental
enteropathy.
Funding
Fundac¸ão de Amparo à Pesquisa do Estado de São Paulo (FAPESP).
Conflicts
of
interest
Theauthorsdeclarenoconflictsofinterest.
References
1.EckburgPB,BikEM,BernsteinCN,PurdomE,DethlefsenL, Sar-gentM,etal.Diversityofthehumanintestinalmicrobialflora. Science.2005;308:1635---8.
2.Boojiink CC, Zoetendal EG, Kleerebezem M, de Vos WM. Microbialcommunitiesinthehumansmallintestine:coupling diversitytometagenomics.FutureMicrobiol.2007;2:285---95.
3.Pyleris E, Giamarellos-Bourboulis EJ, Tzivras D, Koussoulas V, Barbatzas C, Pimentel M. The prevalence of overgrowth by aerobic bacteria in the small intestine by small bowel culture:relationshipwithirritablebowelsyndrome.DigDisSci. 2012;57:1321---9.
4.CraneRJ,JonesKD,BerkleyJA.Environmentalenteric dysfunc-tion:anoverview.FoodNutrBull.2015;36:S76---87.
5.OwinoV,AhmedT,FreemarkM,KellyP,LoyA,ManaryM,etal. Environmentalentericdysfunctionandgrowthfailure/stunting inglobalchildhealth.Pediatrics.2016;138:e20160641.
6.GuerrantRL,LeiteAM,PinkertonR,MedeirosPH,Cavalcante PA,DeBoerM, etal. Biomarkers ofenvironmental enteropa-thy,inflammation,stunting,andimpairedgrowthinchildrenin NortheastBrazil.PLOSONE.2016;11:e0158772.
7.MelloCS,TahanS,MelliLC,RodriguesMS,deMelloRM, Scalet-skyIC,etal.Methaneproductionandsmallintestinalbacterial overgrowthinchildrenlivinginaslum.WorldJGastroenterol. 2012;18:5932---9.
8.GasbarriniA,CorazzaGR,GasbarriniG,MontaltoM,DiStefano M,BasiliscoG,etal.MethodologyandindicationsofH2-breath testingingastrointestinaldiseases:theRomeConsensus Con-ference.AlimentPharmacolTher.2009;29:1---49.
9.MelloCS,RodriguesMS,Araújo-FilhoHB,MelliLC,TahanS, Pig-natariAC,etal.Gut microbiotadifferences inchildrenfrom
distinctsocioeconomiclevelslivinginthesameurbanareain Brazil.JPediatrGastroenterolNutr.2016;63:460---5.
10.WorldHealthOrganization(WHO).WHOAnthroPlusforpersonal computersmanual:softwareforassessinggrowthoftheworld’s childrenandadolescents.Geneva:WHO;2009.
11.WorldHealth Organization(WHO).Irondeficiency anaemia ---assessment, prevention and control.A guide for programme managers.Geneva:WHO/NHD/01.3;2001.
12.LeibyA,MehtaD,GopalareddyV,Jackson-WalkerS,HorvathK. Bacterialovergrowthandmethaneproductioninchildrenwith encopresis.JPediatr.2010;156:766---70.
13.Nadkarni MA,Martin FE, Jacques NA, Hunter N. Determina-tion of bacterialload by real-timePCR using a broad-range (universal) probe and primers set. Microbiology. 2002;148: 257---66.
14.XuP,LiM,ZhangJ,ZhangT.Correlationofintestinal micro-biotawithoverweightand obesityinKazakhschoolchildren. BMCMicrobiol.2012;12:283---9.
15.RinttiläT, Kassinen A, Malinen E,KrogiusL, Palva A. Devel-opment of an extensive set of 16S rDNA-targeted primers for quantification of pathogenic and indigenous bacteria in faecal samplesbyreal-timePCR. JAppl Microbiol. 2004;97: 1166---77.
16.Rahn K, De Grandis SA, Clarke RC, McEwen SA, Galán JE, Ginocchio C,et al. Amplification o fan invAgene sequence ofSalmonellatyphimuriumbypolymerasechainreactionasa specificmethodofdetection ofSalmonella.Mol CellProbes. 1992;6:271---9.
17.Langendijk PS, Schut F, Jansen GJ, Raangs GC, Kamphuis GR, Wilkinson MH, et al. Quantitative fluorescence in situ hybridizationofBifidobacterium spp.withgenus-specific16S rRNA-targetedprobesanditsapplicationinfecalsamples.Appl EnvironMicrobiol.1995;61:3069---75.
18.Matsuki T, Watanabe K, Fujimoto J, Miyamoto Y, Takada T, Matsumoto K,etal. Developmentof16SrRNA-gene-targeted group-specificprimersforthedetectionand identificationof predominantbacteriainhumanfeces.ApplEnvironMicrobiol. 2002;68:5445---51.
19.Huijsdens XW, Linskens RK, Mak M, Meuwissen SG, Vandenbroucke-Grauls CM, Savelkoul PH. Quantification ofbacteria adherentto gastrointestinalmucosabyreal-time PCR.JClinMicrobiol.2002;40:4423---7.
20.BrakstadOG,AasbakkK,MaelandJA.Detectionof Staphylococ-cusaureusbypolymerasechainreactionamplificationofthe nucgene.JClinMicrobiol.1992;30:1654---60.
21.KatoN,KimSM,KatoH,TanakaK,WatanabeK,UenoK,etal. Identification of enterotoxin-producing Clostridium perfrin-gensbythepolymerasechainreaction.KansenshogakuZasshi. 1993;67:724---9.
22.Johnston C, Ufnar JA, Griffith JF, Gooch JA, Stewart JR. A real-time qPCR assay for the detection of the nifH geneof
Methanobrevibacter smithii, a potentialindicator ofsewage pollution.JApplMicrobiol.2010;109:1946---56.
23.NakagawaT,UemoriT,AsadaK, KatoI,HarasawaR. Achole-plasmalaidlawiihastRNAgenesinthe16S-23Sspacerofthe rRNAoperon.JBacteriol.1992;174:8163---5.
24.Donowitz JR, Petri WA Jr. Pediatric small intestinal bacte-rial overgrowth in low-income countries. Trends Mol Med. 2015;21:6---15.
25.Rezania S, Amirmozaffari N, Tabarraei B, Jeddi-Tehrani M, Zarei O, Alizadeh R, et al. Extraction, purification and characterization of lipopolysaccharide from Escherichia coli
and Salmonella typhi. Avicenna J Med Biotechnol. 2011;3: 3---9.
27.McCann KS. The diversity-stability debate. Nature. 2000;405:228---33.
28.Campbell RK, Schulze KJ, Shaikh S, Mehra S, Ali H, Wu L, etal.Biomarkersofenvironmentalentericdysfunctionamong children in rural Bangladesh. J Pediatr Gastroenterol Nutr. 2017;65:40---6.
29.Donowitz JR, Haque R, Kirkpatrick BD, Alam M, Lu M, Kabir M, et al. Small intestine bacterial overgrowth and
environmental enteropathy in Bangladeshi children. MBio. 2016;7:e02102---2115.