F
ACULDADE DEE
NGENHARIA DAU
NIVERSIDADE DOP
ORTOCoatings based on Antimicrobial
Peptides for Prevention of Bone Implant
Associated Infections
Diana Rosa Lopes Oliveira
Mestrado em Engenharia Biomédica Supervisor: Doctor Cláudia Monteiro Co-Supervisor: Doctor Cristina Martins
c
Resumo
Infecções associadas a implantes do osso são um grande problema em medicina.
A imobilização de péptidos antimicrobianos (AMPs) para revestimentos de biomateriais aparece como uma estratégia promissora para prevenir a colonização bacteriana em implantes. Assim, propomos o desenvolvimento de um revestimento antimicrobiano baseado na imobilização cova-lente do péptido MSI-78 (4-20) sobre um filme fino de quitosano.
MSI-78 (4-20) é um péptido curto, com 17 amino ácidos, derivado de um péptido já muito estudado, o MSI-78 (22 amino ácidos), que demonstrou ser tão eficaz e menos tóxico do que o seu originário. O quitosano, por sua vez, foi escolhido por ser um polímero biocompatível e biodegradável com propriedades antibacterianas.
MSI-78 (4-20) modificado com uma cisteína, (Cys-Ahx-MSI-78 (4-20)), foi covalentemente imobilizado sobre os filmes de quitosano usando SM(PEG)8, um agente de reticulação
heterobio-funcional com um grupo éster N-hydroxysuccinimide (NHS) e um grupo maleimida que permitem a conjugação com as aminas do quitosano e o grupo sulfidrilo do péptido, respectivamente.
As superfícies foram caracterizadas usando elipsometria, ângulos de contacto com água e es-pectroscopia de fotoeletrões de raio-x. A atividade antibacteriana foi estudada sujeitando o reves-timento a Staphylococcus epidermidis, uma das estirpes mais prevalentes em infeções associadas a implantes.
Os resultados demonstraram que Cys-Ahx-MSI-78 (4-20) foi imobilizado com sucesso em filmes ultrafinos de quitosano através de um espaçador SM(PEG)8.
O revestimento de quitosano com o péptido MSI-78 (4-20) demonstrou uma elevada ativi-dade antibacteriana e é por isso considerada uma abordagem promissora para combater infeções associadas a implantes.
Abstract
Implant-associated infections are still a major problem in the medical field.
Antimicrobial peptides (AMPs) immobilization on biomaterial coatings appears as a promis-ing strategy to prevent implant bacterial colonization.
Thus, we propose the development of an antimicrobial coating based on the covalent immo-bilization of the peptide MSI-78 (4-20) onto a chitosan thin film. MSI-78 (4-20) is a short AMP, a 17-mer peptide, derived from the well-known MSI-78 (22-mer), which demonstrated to be as effective and less toxic than the parent AMP. Chitosan was chosen as it is a biocompatible and biodegradable polymer with antibacterial properties.
MSI-78 (4-20) modified with a cysteine, (Cys-Ahx-MSI-78(4-20)), was covalently immobi-lized on chitosan films using SM(PEG)8, a heterobiofuncional crosslinker with a N-hydroxysuccinimide
(NHS) ester and a maleimide group that allow conjugation with the amines of the chitosan and the sulfhydryl group of the peptide, respectively.
The surfaces were characterized using ellipsometry, water contact angle and x-ray photoelec-tron spectroscopy. Antimicrobial activity was studied by challenging the coating with Staphylo-coccus epidermidis, one of the most prevalent strains in implant associated infections.
Results demonstrate that Cys-Ahx-MSI-78 (4-20) was successfully immobilized on ultrathin films of chitosan using a spacer SM(PEG)8.
The developed MSI-78 (4-20) chitosan coating demonstrated a great antimicrobial activity, and therefore we consider it a promising approach to fight implant associated infections.
Acknowledgments
First of all, I would like to express my extreme gratitude to my supervisor, Doctor Cláudia Monteiro, for all the support during this project, as well as, for the help that she gave me, not only with the practical part, but also with the research of new information. I would also like to thank her the advices and readiness to answer my questions and doubts.
Additionally, I would also express my thank to my co-supervisor and leader of the work team, Doctor Cristina Martins, for all the support and help that she gave me, in order to understand what I was getting along the investigation.
I place on record my gratitude to the members of the work team, who always tried to help me when it was necessary and, without any doubt, were essential for the success of my work.
I would like to give a special thank to my partners in crime, Inês Borges, Luis Baptista and Micaela Querido for all the good moments that we passed through, all the jokes and confidences throughout the year. Without you, I have no doubt that it would not be the same, for worse of course. You are special people that I would like to maintain for life.
Then, I would like to thank I3S (Instituto de Investigação e Inovação em Saúde) and INEB (Instituto Nacional de Engenharia Biomédica) for the facilities and all the people working there that always helped me when needed. Specially, Ricardo Vidal and Dalila Pedro for all the help and the readiness to solve all the problems that I face with the equipments.
I would also like to express my sincere gratitude to FEUP (Faculdade de Engenharia da Uni-versidade do Porto) for all the transmitted knowledge.
Finally, the last but not the least, I want to thank all the members of my family. To the best parents of the entire world, my wonderful sisters, brothers-in-law and my amazing nieces a giant thank you. You were essential for the success of this stage of my life, despite all the difficulties that we went through this year, we were able to overcome all relying on each other. I love you from the bottom of my heart. Thank you, thank you so much.
To my boyfriend, I do not know how you are able to put up with me. The calls, to ask for urgent help in the middle of the night, the insecurities, but above all the unconditional support and encouragement during this process. I could not ask for a better person by my side.
To those who, directly or indirectly, have contributed to this project, my sincere gratitude.
“If you can’t fly, then run if you can’t run, then walk, if you can’t walk, then crawl, but whatever you do, you have to keep moving forward.
Contents
1 Introduction 1
1.1 Structure of the Dissertation . . . 2
2 Literature Review 3 2.1 Introduction . . . 3
2.2 Staphylococcus epidermidis . . . 3
2.3 Bacterial adhesion and biofilm formation on implant surfaces . . . 4
2.4 Current Strategies . . . 5
2.4.1 Bacteria anti-adhesive surfaces . . . 6
2.4.2 Antimicrobial surfaces . . . 6
2.5 Antimicrobial peptides (AMPs) . . . 6
2.5.1 Mode of antibacterial action . . . 8
2.5.2 Immobilization of AMPs . . . 9
2.5.3 MSI-78(4-20) . . . 11
2.6 Chitosan . . . 11
2.7 Poly(ethylene glycol) (PEG) . . . 14
2.7.1 PEG as a spacer moiety . . . 15
3 Surface Characterization Techniques 17 3.1 Ellipsometry . . . 17
3.2 Water contact angle . . . 18
3.3 X-Ray Photoelectron Spectroscopy (XPS) . . . 19
3.4 Inverted Fluorescence Microscopy . . . 20
4 Materials and Methods 23 4.1 Antimicrobial Peptides (AMPs) . . . 23
4.2 MSI-78(4-20) chitosan coating preparation . . . 23
4.2.1 Substrate preparation . . . 23
4.2.2 Chitosan ultrathin films . . . 24
4.2.3 Peptide immobilization . . . 24
4.3 MSI-78(4-20) surface characterization . . . 26
4.3.1 Ellipsometry . . . 26
4.3.2 Water contact angle measurements . . . 26
4.3.3 X-ray photoelectron spectroscopy (XPS) . . . 26
4.4 Bacterial assays . . . 26
4.4.1 Bacterial strains, media and growth conditions . . . 26
x CONTENTS
5 Results and Discussion 29
5.1 Surface characterization . . . 29
5.1.1 Ellipsometry . . . 29
5.1.2 Water Contact Angle measurements . . . 30
5.1.3 X-Ray Photoelectrons Spectroscopy (XPS) . . . 31
5.2 Antimicrobial activity characterization . . . 33
5.2.1 Viability of adherent bacteria . . . 33
5.2.2 Non-adherent viable bacteria . . . 35
6 Conclusions and Future perspectives 37
List of Figures
2.1 Sequence of events involved in the establishment of an infection on a biomaterial surface. Bacteria are represented as B [1]. . . 4
2.2 Schematic representation of different antimicrobial coatings for implants: (1) coat-ings with anti-adhesive properties, coatcoat-ings with bactericidal effect (2) either act-ing by direct contact with bacteria or (3) coatact-ings that act based on the release of antimicrobial compounds. Adapted from [2]. . . 5
2.3 Classes of antimicrobial peptides. I – peptides with an α-helical structure, II – linear peptides with an extended structure, III – Peptides with a looped structure and IV – β -sheet peptides. Adapted from [3]. . . 7
2.4 Illustration of the mechanisms of action of membrane-active antimicrobial pep-tides (AMPs) (A) — the barrel-stave model, (B) — the carpet model, (C) — the toroidal pore model. Adapted from [4]. . . 9
2.5 Amino acid sequence of the antimicrobial peptide, MSI-78 (4-20). The positively charged amino acids are represented in red. . . 11
2.6 Schematic representation of the chemical preparation of chitosan from chitin. [5] 12
2.7 Chemical structure of SM(PEG)n. Adapted from [6]. . . 15
3.1 Schematic diagram of a nulling ellipsometer [7]. . . 17
3.2 Illustration of contact angles formed by sessile liquid drops on a smooth homoge-neous solid surface [8]. . . 18
3.3 The photoemission process involved for XPS surface analysis. The discs represent electrons and the bars represent energy levels within the material being analysed [9]. 19
3.4 Basic components of a monochromatic XPS system [10]. . . 20
3.5 Schematic diagram of an inverted fluorescence microscope [11]. . . 21
4.1 Representation of spin-coating working equipment [12]. . . 24
4.2 Schematic representation of the chemical reaction used for Cys-MSI-78(4-20) im-mobilization. Conjugation was performed using a PEG heterobiofuncional crosslinker, with N-hydroxysuccinimide (NHS) ester and maleimide groups that allow cova-lent conjugation with the amines of chitosan and the sulfhydryl group of the peptide. 25
4.3 Schematic representation of the modification performed in the surface (not in scale). 25
4.4 Schematic representation of the method used to test antimicrobial activity. . . 27
5.1 Surface characterization of chitosan modified films with SM(PEG)8and
Cys-MSI-78(4-20) using ellipsometry (thickness of the surface). Statistically significant differences are indicated with **** (p<0.0001). . . 30
5.2 Surface characterization of chitosan modified films with SM(PEG)8and
cys-MSI-78 (4-20) using water contact angle measurements. Statistically significant differ-ences are indicated with ** (p<0.01). . . 31
xii LIST OF FIGURES
5.3 XPS high resolution spectra of carbon of the different modified surfaces, Chitosan,
Chitosan_SM(PEG)8, Chitosan_SM(PEG)8_adsorbed MSI-78(4-20) and Chitosan_SM(PEG)8
_Cys-MSI-78(4-20). . . 33
5.4 Antimicrobial activity of the Cys-MSI-78(4-20) modified chitosan film. Quantifi-cation of bacterial adhesion using the Live/Dead Baclight kit. Statistically signif-icant differences are indicated with **** (p<0.0001) (ns/****, dead group com-parison). . . 34
5.5 Representative images of bacterial adhesion to Au, chitosan and chitosan modified surfaces. Images were obtained by MIF at 400×, 40 µm scale . . . 35
5.6 Antimicrobial activity of chitosan modified films with SM(PEG)8and
Cys-MSI-78(4-20) regarding the non-adherent viable bacteria. Statistically significant dif-ferences are indicated with ** (p<0.01). . . 36
List of Tables
5.1 Atomic composition (%) of the different surfaces obtained from XPS element analysis. . . 32
5.2 Relative percentage (%) of the different Carbon bonds obtained from high-resolution XPS spectra. . . 32
5.3 Dead and live bacteria percentage in the modified surfaces. Quantification of cell viability was performed using the Live/Dead Baclight kit. . . 34
Abbreviations and Symbols
AFM Atomic Force Microscopy AMPs Antimicrobial Peptides
CEMUP Centro de Materiais da Universidade do Porto CFUs Colony Forming Units
DA Degree of Acetylation
DAPI 4’,6 - diamidino-2-phenylindole DD Degree of Deacetylation E. coli Escherichia coli
E. faecalis Enterococcus faecalis EKA Electrokinetic analyzer
FT-IRRAS Fourier Transform Infrared Reflection Absorption Spectrosocpy LbL Layer-by-Layer
MDR Multidrug-resistants
MRSA Methicillin resistant staphylococcus aureus MW Molecular Weight
NHS N-hydroxysuccinimide P. aeruginosa Pseudomonas aeruginosa PBS Phosphate Buffer Saline PEG Poly (ethylene Glycol) PEO Poly (ethylene Oxide) PI Propidium iodine P. mirabilis Proteus mirabilis
SAM Self-assembled monolayer S. aureus Staphylococcus aureus SD Standard deviation
SEM Scanning electron microscopy S. epidermidis Staphylococcus epidermidis S. viridans Streptococcus viridans
TCEP Tris (2-carboxyethyl) phosphine TSA Tryptic soy agar
TSB Tryptic soy broth
Chapter 1
Introduction
The implantation of medical devices has been growing aside with the fast development of the medical care. There is a huge variety of medical devices, which range from simple medical instruments like catheters, contact lenses and stents, to more complex ones, such as orthopaedic implants [13]. It is expected that in the future, all individuals will need at least one intervention of this kind once in their lifetime [1]. Chronic diseases associated with the rise in average life expectancy will rise the demands for implanted medical devices ultimately resulting in millions of lives saved worldwide [14].
The increase in the use of implants over the years has resulted in a concomitant rise in bacterial infections, causing high morbidity and contributing to the economic burden globally [14], [15]. In the United States, the annual cost of implants infection increased from 320 million to 566 million dollars during the period from 2001 to 2009 and it is expected that it will exceed 1.62 billion in 2020 [16].
Several strategies have been suggested to reduce the risks of infection on implanted devices, for example, antibacterial coatings with different polymers, quaternary ammonium, metallic com-pounds or coatings impregnated with antibiotics [14]. Nevertheless, such coatings have some drawbacks related to the risk of bacterial resistance development, short-term antimicrobial protec-tion, limited antimicrobial spectrum and/or high toxicity [13], [14].
As such, antimicrobial peptides (AMPs) appear as a promising alternative to create an antimi-crobial coating for implants. AMPs are a class of broad-spectrum antibiotics, with low tendency to induce resistance, high selectivity towards microorganisms and fast killing even at low concen-trations [17].
Taking into account the aforementioned characteristics, we propose the development of an antimicrobial coating, AMP-chitosan thin film, which pretends to be a cost effective, efficient and biocompatible solution that aims to improve the prosthetic performance in combating/preventing infections.
Our aim is to develop a coating based on the small AMP, MSI-78(4-20), which was developed by our team. MSI-78(4-20) is a cost effective short AMP, a 17-mer peptide, derived from the
2 Introduction
well-known pexiganan (MSI-78), a 22-mer AMP. MSI-78(4-20) was shown to be as efficient as MSI-78 and more selective towards bacterial cells [18].
AMP-chitosan thin films will be created through covalent immobilization of MSI-78(4-20) onto chitosan films.
This project involves the following objectives:
• Preparation of AMP-chitosan thin films using the free amine groups of chitosan. Covalent immobilization will be done through a SM(PEG)8spacer, a heterobiofuncional crosslinker
with N-hydroxysuccinimide (NHS) ester and a maleimide group that allow conjugation with the amines of chitosan and the sulfhydryl group of Cys-MSI-78 (4-20).
• Characterization of the antimicrobial activity of AMP-chitosan thin films by quantification of killed bacteria and bacterial adhesion to the coating.
1.1
Structure of the Dissertation
This dissertation is divided in 6 chapters. The current chapter (Chapter 1) gives a brief intro-duction to the topic while presents the objective of the project. Chapter 2 presents a detailed literature review with the most relevant and recent information of the subject in study. Chapter 3
presents some of the surface characterization techniques used in this work as well as its theoretical fundaments and operating mode. Materials and methods used are detailed in Chapter 4. In the closing chapters (Chapter 5and 6) the results and discussion are presented, as well as the main conclusions and future work.
Chapter 2
Literature Review
2.1
Introduction
The increased use of implants over the years has resulted in a concomitant rise in bacterial infections. A large variety of strains has been isolated from infected implants as for example, Staphylococcus aureus (S. aureus), Staphylococcus epidermidis (S. epidermidis), Pseudomonas aeruginosa(P. aeruginosa), Enterococcus faecalis (E. faecalis), Streptococcus viridans (S. viri-dans), Eschericia coli (E. coli) and Proteus mirabilis (P. mirabilis); among which we can find some of the most feared resistant bacterial strains such as Methicillin-resistant staphylococcus aureus(MRSA) and Multidrug-resistant (MDR) P. aeruginosa. [13,19,20].
These microorganisms are introduced mainly by two routes, during prosthesis implantation by contamination from the skin of patients, health-care workers, or other sources in the environment; or derived from temporary bacteraemia. [21,22].
Being one of the most common causes of implant failure, infection occurs with a rate of 3-33% [23]. When bacterial contamination occurs through objects and by airborne bacteria at the time of implantation is considered an immediate infection. On the other hand, the infection is considered late if it has its source in temporary bacteraemia, occuring months or even years after implantation [24,25].
In its early stages implant associated infections might be treated with antibiotics, however most of the cases evolve into chronic infection, which treatment implies a combination of antibi-otics and surgical intervention based on implant removal and surgical debridement of the affected surrounding tissue. Despite this intervention, reinfection occurs in 20% of the cases [23].
2.2
Staphylococcus epidermidis
Staphylococcus epidermidis, is one of the most prevalent strains in implant associated infections and will be used in this study to challenge the developed coating.
This specie is gram-positive and belongs to coagulase-negative group of staphylococcus, as it lacks the enzyme coagulase [26,27]. This bacterium colonizes the skin and mucous membranes
4 Literature Review
of the human body and represents the major part of the normal bacterial flora of this habitat [28]. For this reason, this microorganism can cause nosocomial infections very easily since it facilitates its entrance in the body via injuries or implanted medical devices [29].
S. epidermidisrequires a predisposed host in order to change from a normal inhabitant of the human skin to an infectious agent. It has been regarded as inoffensive but nowadays it is being considered also as pathogenic for drug abusers and immuno-compromised patients [28].
Treatment of infections caused by this bacterium is generally difficult because of increasing re-sistance against many antibiotics and because of the ability to form biofilms [28]. This specimen has the ability to produce slime that protects from phagocytosis making it an almost imperme-able barrier to many antibiotics [28,30,31]. Approximately 80% of S. epidermidis strains from nosocomial infections are resistant to methicillin, and most are also resistant to other antibiotics [28,32].
2.3
Bacterial adhesion and biofilm formation on implant surfaces
The establishment of bacterial infections on implants is influenced by several factors and occurs accordingly to a well-studied sequence of events. The colonization process has been categorized in the following four stages: bacterial attachment, adhesion, aggregation and finally dispersion, as summarized in figure2.1 [1,33]. If bacteria are not able to adhere to the surface, there will be also no chance for them to colonize [33].Figure 2.1: Sequence of events involved in the establishment of an infection on a biomaterial surface. Bacteria are represented as B [1].
Firstly, bacteria approach and attach reversibly to the surface through physico-chemical inter-actions such as Van der Waals forces, hydrophobic interinter-actions and ionic interinter-actions. For these
2.4 Current Strategies 5
kind of interactions bacteria and surface characteristics play an important role. Secondly, mi-croorganisms become irreversibly attached to the surface through structures like adhesins. Then, bacteria start to divide and to form colonies and in a later stage bacteria form biofilm. Once a biofilm is well established, portions of bacterial colony may disperse and colonize other areas of the body [1,2,24,34,35].
A structured biofilm is a microbial community that has the capacity to aggregate and grow on exposed surfaces [36]. The major problem associated with biofilm formation is the difficulty in treating such infections using antibiotics. Once formed, bacteria immersed in biofilms are covered by a polymer matrix, which protects them from phagocytosis, antibiotics, disinfectants and other components of the innate and adaptive immune system and inflammatory system of the host, impairing a successful treatment [37].
2.4
Current Strategies
In order to avoid implant-associated infections, various strategies have been explored. Between them, the most popular is the modification of the biomaterial surface with coatings. Coatings are an excellent choice to improve the antimicrobial performance of an implant. As the existing implants already have good biomechanical properties, it is advantageous to keep the bulk material of the implant and rather alter the surface properties by covering the implant with a coating [38,39]. To do so, several strategies have been developed, such as coatings with anti-adhesive properties and coatings combined with antimicrobial substances [21].
Figure 2.2: Schematic representation of different antimicrobial coatings for implants: (1) coatings with anti-adhesive properties, coatings with bactericidal effect (2) either acting by direct contact with bacteria or (3) coatings that act based on the release of antimicrobial compounds. Adapted
6 Literature Review
2.4.1 Bacteria anti-adhesive surfaces
In order to prevent infection it is important to avoid biofilm formation. As a result, the strat-egy of modifying implant surfaces by making them anti-adhesive has a great potential to prevent infections. Many variables must be considered when designing an anti-adhesive coating, such as surface morphology, physico-chemical properties, environmental conditions and pathogen type [24].
Current approaches to achieve anti-adhesive coatings rely on different strategies using poly-mers including, among others, poly(ethylene oxide) (PEO) [40,41], poly(ethylene glycol) (PEG) [42], Trimethysilane [43], peptide-PEG amphiphilic macromolecules [44], polyacrylamide [45,
46] and polytetrafluoroethylene [47].
In fact, the surfaces that show low adhesion rates are hydrophilic, highly hydrated and non-charged [24].
2.4.2 Antimicrobial surfaces
There are various substances known to possess bactericidal properties such as silver, zinc, copper, chitosan and various bioactive glasses [24,48]. However, the use of some metals is limited due to the possibility of corrosion in physiological environment or to leaching, leading to the release of high concentrations of active ions, causing local toxicity and sometimes accumulation in distant organs [24].
Coatings with antimicrobial effect enable bacterial death either by contact or by drug delivery (leaching). Several compounds have been tested, between them antimicrobial peptides, quorum-sensing inhibitors, essential oils or enzymes, nitric oxide and salicylic acid [19]. However, until now, the release strategy has failed to achieve delivery in a sustained and effective dosage over a relatively prolonged period of time [49]. Additionally, the majority of these coatings have some drawbacks related to the risk of bacterial resistance development, limited antimicrobial spectrum and/or high toxicity [13,50].
In order to surpass some of the limitations described above other alternatives are being con-sidered, as there is an obvious need to prevent biomaterials colonization, while minimizing the development of bacterial resistance, improving long-term stability and reducing cytotoxic effects. The use of antimicrobial peptides on implant coatings represents a promising strategy as it meets these criteria [18, 51, 52, 53, 54, 55, 56]. In order to have a sustained and effective antibac-terial effect over a longer period of time, covalent attachment of antimicrobial peptides is being considered [57].
2.5
Antimicrobial peptides (AMPs)
Antimicrobial peptides (AMPs) are endogenous polypeptides produced by a wide variety of organisms such as animals, plants, bacteria, fungi and viruses, as part of their innate immune sys-tem [56]. Generally, AMPs share some characteristics such as: small size (10-100 amino acids),
2.5 Antimicrobial peptides (AMPs) 7
molecular weights between 1 and 5 kDa, highly cationic character and tendency to adopt amphi-pathic structures [3]. However, AMPs may have differences in the net charge and in secondary structure [55,57,58,59]. Due to its positive charge, AMPs have the tendency to associate with negatively charged microbial surfaces and membranes despite the different secondary structure acquired by the peptides [55]. Antimicrobial peptides exhibit bactericidal, fungicidal, viricidal properties and some have been even described as tumoricidal. AMPs have several advantages: they are less likely to promote bacterial resistance, they are highly selective towards microorgan-isms and promote a fast killing even at low concentrations; properties that make them promising candidates for therapeutic antimicrobial drugs [18,51,58].
Despite peptides similarities in general physical properties, they have different secondary structures. As they are classified regarding their secondary structure, they can be categorized in four groups [3,55,60,61]:
• Group I — Linear peptides with an α-helical structure;
• Group II — Linear peptides with an extended structure, characterized with a predominance of one or more amino acids;
• Group III — Peptides containing a looped structure.
• Group IV — Conformationally more restrained peptides, predominantly consisting of β -strands connected by intramolecular disulfide bridges.
Figure 2.3: Classes of antimicrobial peptides. I – peptides with an α-helical structure, II – lin-ear peptides with an extended structure, III – Peptides with a looped structure and IV – β -sheet
8 Literature Review
2.5.1 Mode of antibacterial action
The exact mechanism of action of AMPs remains a matter of controversy, however there is a consensus about the initial steps of interaction between the AMPs and bacterial membranes. Firstly, as most peptides are positively charged, they are attracted to the negatively charged branes of bacteria (electrostatic interaction) and then, upon interaction with the bacterial mem-brane AMPs adopt an amphipathic structure [55,58].
Three models have been proposed to explain the mode of action of AMPs:
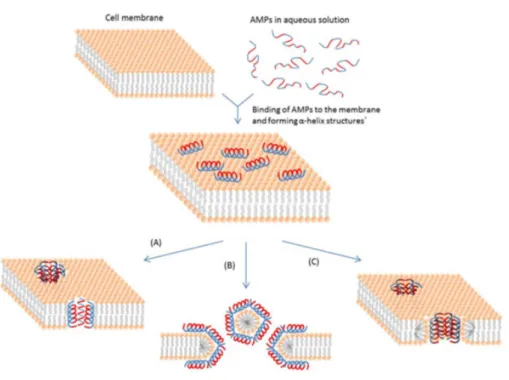
• The barrel-stave model — This mechanism describes the formation of transmembrane channel/pores by bundles of amphipathic α-helices, such that their hydrophobic surfaces interact with the lipid core of the membrane and their hydrophilic surfaces point inward producing an aqueous pore. The transmembrane pore formation involves the binding of peptide monomers to the membrane followed by insertion of the helices into the hydropho-bic core. Progressive recruitment of the additional peptide monomers leads to an increase in pore size that leads to the leakage of cell contents and thereby death of the cell (Figure
2.4A) [55,57,58].
• The carpet model — In this model, the microbial cell membrane is fully covered by a carpet-like cluster of peptides. When a critical concentration is reached, the membrane collapses, and worm holes are formed causing lysis of the microbial cell. It is suggested that the lipid layer bends back on itself. This requires a lateral expansion in the polar head-group region of the bilayer, which is filled up by individual peptide molecules, causing cell lysis (Figure 2.4B) [55,57,58].
• The toroidal pore model — Regarding this model, peptides start to bind to the phospholipid head groups, then peptides insert into the membrane and then cluster into unstructured ag-gregates that span the membrane, creating channels through which ions and larger molecules could leak. The pores are formed as transmembrane clusters, which allow the peptides to cross the membrane without causing significant membrane depolarization. Once inside, the peptides home to their intracellular targets to exert their killing activities (Figure 2.4 C) [55,57,58], [62].
2.5 Antimicrobial peptides (AMPs) 9
Figure 2.4: Illustration of the mechanisms of action of membrane-active antimicrobial peptides (AMPs) (A) — the barrel-stave model, (B) — the carpet model, (C) — the toroidal pore model. Adapted from [4].
2.5.2 Immobilization of AMPs
AMPs can be immobilized onto solid supports by a physical or chemical interaction [14]. Re-garding physical interactions, one of the easiest methods of immobilization is by adsorption [63]. On the other hand, the most popular approach is Layer-by-layer (LBL) assembly of polymeric films [64,65]. In this process, the peptide is sandwiched between alternate layers of polycations and polyanions, resulting on an assembly of n layers of polycations, AMP and polyanions, where n corresponds to the number of layers (thickness) [14,66].
The use of covalent-based immobilization methods of AMPs on surfaces has many advantages compared to the physical functionalization, as AMP leaching is strongly minimised overcoming short-term antimicrobial protection while decreasing toxicity effects [50,67].
AMPs covalent immobilization may be performed through functionalization of the surface, or by using functionalized polymer resins such as poly(ethylene glycol) (PEG) [50,68,69]. In the first situation as the surface is not reactive towards AMPs it requires a first step of functionalization before covalent attachment of the peptides onto the surface, spacers such as PEG are frequently used [50,69,70]. Also the functionalization of a self-assembled monolayer (SAM) with reactive groups enables the coupling of AMPs to the SAM layer. However, not only the length of the SAM spacer will affect AMP activity, but also the orientation of bound AMPs [14]. Regarding
10 Literature Review
adhesive properties and flexibility, which allows rapid and free orientations of bound AMPs; and the water-swelling property that has been reported to be a critical factor for maintaining the activity of bound AMPs [14, 71]. In addition, this approach of covalent conjugation of antimicrobial peptides immobilized onto a hydrophilic polymer has also been proved to produce a robust coating extremely effective in resisting biofilm formation [21].
Although both methods may influence the activity of AMPs, different activity-modulating parameters can be considered to improve the antimicrobial performance of the covalently bound AMPs. The use of a spacer between the AMP and the solid matrices may be crucial to optimize AMPs activity, the kind of spacer, its length and flexibility may influence AMP activity as well as the AMP surface density and also orientation after immobilization [50,57,69].
• Influence of the spacer — Studies that have compared AMP immobilization with and with-out PEGylated spacers have demonstrated that some immobilized AMPs are only bacteri-cidal when a PEGylated spacer was used [50,72]. Bagheri et al., also suggests that the increase in flexibility, associated with longer spacers, maximizes the antimicrobial activity of immobilized peptides, as it provides a parallel peptide orientation and lateral mobility [50]. However, the requirement for a spacer depends on the mode of action of the specific AMP [57].
• Peptide concentration — Peptide surface concentration depends on the immobilization strategy used, as limited accessibility of the peptide reactive groups and different coupling procedures can affect the efficiency of peptide immobilization [57]. Despite some studies indicate that peptide concentrations are not the most critical parameter, others show that the antimicrobial activity was concentration dependent [50, 70]. At the end, this parameter cannot be disregarded.
• Peptide orientation after immobilization — surface binding of peptides can be conducted on different chain positions, such as C-terminal, N-terminal and/or in a relatively random manner [57]. Peptide orientation at the interface is important in peptide-bacteria interac-tions and therefore important to the antimicrobial performance of the immobilized peptides [14,70].
Other important factors such as peptide structure and sequence, as well as, the properties of the surrounding environment (such as pH and salt concentration) can also affect AMPs activity and should also be considered when studying the activity of bound AMPs. In addition, it has also been concluded that immobilized peptides have high long-term stability and resistance to environmental conditions and also reduce potential haemolysis when compared to soluble peptides [57].
In short, understanding the factors of peptide immobilization will enable the design of more effective and functionally stable medical devices and implants [14].
2.6 Chitosan 11
2.5.3 MSI-78(4-20)
Figure 2.5: Amino acid sequence of the antimicrobial peptide, MSI-78 (4-20). The positively charged amino acids are represented in red.
MSI-78(4-20) is a 17-mer peptide, highly cationic (figure 2.5) and it is derived from MSI-78, commercially known as pexiganan [18].
In the project work, the AMP MSI-78 (4-20), designed by our team, will be explored in the context of antimicrobial coatings development. MSI-78 (4-20) seems to have a large spectrum of activity, as it is effective against the positive S. aureus and S. epidermidis and the Gram-negative P. aeruginosa. MSI-78 (4-20) is less toxic than its parent MSI-78, being less haemolytic to red blood cells than MSI-78. MSI-78 (4-20) is unstructed in aqueous solution and forms am-phipatic helices in contact with membrane-mimicking systems. Biophysical studies point to a mechanism of action of MSI-78 (4-20) based on the disruption of the bacterial membrane, similar to the mechanism of action of its parent MSI-78 [18].
The parent MSI-78 (Magainin Pharmaceutical Inc, previously Genaera, Plymouth Meeting, PA, USA) was the first antimicrobial peptide to undergo commercial development. Its antimicro-bial activity, secondary structure and mechanism of action are very well documented. MSI-78, a synthetic 22-mer, demonstrated excellent antimicrobial activity in vitro with a broad-spectrum activity being effective against 3109 bacterial clinical isolates [52].
MSI-78 mechanism of membrane interaction and disruption is based on toroidal pores for-mation in the bacterial membrane [73]. Moreover, it has been shown that MSI-78 forms an antiparallel dimer of amphipathic helices when interacting with the membrane surface [74].
2.6
Chitosan
The aim of this project is to create an antimicrobial coating based on the covalent immobiliza-tion of an AMP on chitosan thin films, in this chapter the properties and the motivaimmobiliza-tions behind the choice of this polymer are exposed.
Chitosan [poly-(β -1/4)-2-amino-2-deoxy-D-glucopyranose] is a natural cationic polymer ob-tained from chitin. Chitin is a polysaccharide, with fibrous structure, generously found in nature as it is the main constituent of the outer skeleton of insects and crustaceans like shrimp, crabs and lobster [75]. This polysaccharide is extracted using a process that involves an acid removal of calcium carbonate (demineralization), followed by an elimination of proteins (deproteinization). After being extracted, chitin has a highly ordered crystalline structure, but also poor solubility and low reactivity [75,5]. When the degree of deacetylation of chitin reaches about 50% the polymer
12 Literature Review
Chitosan, the deacetylated form of chitin, has unique biological characteristics, including bio-compatibility and non-toxicity, which arises its commercial interest [77, 5]. Additionally, it is easily functionalized, allows osteointegration and inhibits bacterial adhesion and growth [17]. It is primarily characterized by its molecular weight (MW) and degree of acetylation (DA). Regard-ing its solubility, chitosan is insoluble in water, but soluble in diluted acid solutions below its pKa (between 6 and 6.5) as for example, acetic acid, formic acid, succinic acid, lactic acid and malic acid [5]. However, its solubility depends also on molecular weight and degree of acetylation [78,75].
The fraction of glucosamine to N-acetyl glucosamine units in the chain is referred to as the degree of deacetylation (DD), which is therefore related with the number of free amine groups (-NH2) [79,80]. The cationic character of chitosan is conferred by the presence of NH3+ groups
[76].
Figure 2.6: Schematic representation of the chemical preparation of chitosan from chitin. [5]
Chitosan is applied in different industries, such as food, medical and textile, due to its antimi-crobial capacity [81]. In the area of health care, more specifically, chitosan may be incorporated into fibers, membranes or hydrogels, to be used in wound dressing, orthopaedic tissue engineering and drug delivery carrier [81]. With respect to the production methods, fibers can be produced by eletrospinning which is a favorable technique for producing continuous polymer fibers and membranes can be produced by casting films or spin-coating [81].
Regarding antimicrobial activity, chitosan has been demonstrated to be effective against algae, gram-positive and gram-negative bacteria, fungi and yeasts [17,75,78,81]. However, several factors can influence bactericidal efficacy of chitosan, such as:
2.6 Chitosan 13
– Species - Chitosan has different inhibitory efficacy against different fungi, Gram-positive and Gram-negative bacteria [81]. While in fungi its antimicrobial activity is by suppressing sporulation and spore germination, in bacteria the antimicrobial ac-tivity of chitosan is not a well understood process, as different bacteria have different cell surface characteristics [82]. Bacteria appear to be, generally, less sensitive to chitosan than fungi [83].
– Cell age - Differences of cell surface electronic negativity varies with the phase of growth, which can lead to different susceptibility of cells towards chitosan [84]. • Intrinsic factors of chitosan (positive charge density, Molecular Weight (MW), degree
of deacetylation (DD), hydrophilic/ hydrophobic character and chelating capacity): – Positive charge density – Higher positive charge density leads to stronger
electro-static interaction with the negatively charged bacterial surface [81]. It has been sug-gested that the positive charge in the amino group of the glucosamine monomer al-lows interactions with the negatively charged microbial cells membranes [85]. This electrostatic interaction results in two-fold interference: i) by promoting changes in the properties of membrane wall permeability, thus provoking internal osmotic imbal-ances and consequently inhibiting the growth of microorganisms, and ii) by hydrolysis of the peptidoglycans in the microorganism wall, leading to the leakage of intracel-lular electrolytes such as potassium ions and other low molecular weight proteina-ceous constituents (e.g. proteins, nucleic acids, glucose, and lactate dehydrogenase) [75,86,87,88,89,90]
– Molecular weight (MW)– Chitosan with different Mw possess a different number of N-acetylglucosamines units, which influences intramolecular and intermolecular interactions, resulting in chitosan with different conformations [39], [81]. Chitosan with low molecular weight has shown to have higher antimicrobial activity effect [91]. – Degree of deacetylation (DD) - With higher DD, the number of free amino groups present in chitosan increases, leading to an increase in positive charge density and consequently higher antimicrobial effect [81].
– Hydrophilic/ hydrophobic character - Antimicrobial agents normally require water for activity, which is the reason why totally dry samples are incapable of releasing their energy stored in chemical bonds to initiate interaction. The hydrophilic character of chitosan determines water solubility. However, the use of chitosan is limited by its poor solubility in water [92]. For that reason, chemical modifications have been done to improve water solubility of chitosan and its derivatives [93,81].
– Chelating capacity - Chitosan possesses high chelating capacity for various metal ions (including Ni2+, Zn2+, Co2+, Fe2+, Mg2+ and Cu2+), where the amine groups are responsible for the uptake of metal cations [94]. In general, such mechanism is
14 Literature Review
groups are unprotonated and the electron pair on the amine nitrogen is available for donation to metal ions [75]. It selectively binds trace metals and thereby inhibits the production of toxins and microbial growth [95].
• Physical state (soluble and solid state):
– Soluble state – Chitosan in solution has an extending conformation, which explains a more effective inhibition of bacterial growth [81].
– Solid state – Solid chitosan only gets in touch with solution through the exposed surface, which leads to a less effective antibacterial effect [81].
• Environmental factors (pH, temperature and time):
– PH - Chitosan is only soluble in an acidic environment and at pH below its pKa (6.3-6.5) the molecule becomes polycationic, which explains the strong antibacterial effect at low pHs [81], [96].
– Temperature and time - Generally, fresh chitosan solutions show higher antibacterial activity than those stored for several weeks, because the glycosidic bonds are cleaved, leading to a decrease in molecular weight and viscosity of the solutions. Chitosan solutions stored at 25◦C possess the same or weaker antibacterial activity than those kept at 4◦C [81].
2.7
Poly(ethylene glycol) (PEG)
The aim of the project is to create an antimicrobial coating by covalent immobilization of an AMP on chitosan films through a PEG spacer. The reasoning behind the use of the PEG spacer is described above. Poly(ehtylene glycol) (PEG), HO-(CH2 CH2O)n-CH2 CH2OH (figure 2.7),
is a widely used biocompatible polymer, with a wide range of solubilities in both organic and aqueous media [97], lack of toxicity [98] and nonbiodegradability. These properties are explained by its chains high mobility, conformational flexibility and water-binding ability [99,100]. PEG is also a non-toxic polymer (approved by FDA for internal consumption) [101]. PEG is ready to chemical modification and attachment to other molecules and surfaces, and when attached to other molecules it has little effect on their chemistry but controls their solubility and increases their size [101]. The terminal hydroxyl groups of the PEG molecule provide a ready site for covalent attachment to other molecules and surfaces [101,97]. Molecules to which PEG is attached usually remain active, demonstrating that bound PEG does not denature proteins or hinder the approach of other small molecules. Bound PEG does, however, retain its ability to repel other large molecules, and thus PEG-modified surfaces and PEG-modified proteins are protein-rejecting [101].
2.7 Poly(ethylene glycol) (PEG) 15
Figure 2.7: Chemical structure of SM(PEG)n. Adapted from [6].
2.7.1 PEG as a spacer moiety
PEG is being used as a spacer molecule for many applications. The length of the chain can be controlled within a desired range by the degree of polymerization of the starting polymer or by oligomers of precise length [102]. The most common application of PEGs is to link two dif-ferent moieties as for example, molecule to molecule or surface to molecule, however, a recent novel application is to replace chain segments within a biopolymer with PEG [103]. Although, it is also possible to link two moieties using a heterobifunctional PEG. Heterobifunctional PEGs have been utilized for grafting the polymer onto solid supports [104], crosslinking two different proteins [105], linking reporter groups to biomaterials and attaching various biologically relevant ligands to membrane-forming lipids [106, 107, 108]. Heterobifunctional PEG derivatives may contain maleimide, iodoacetate, dithiopyridyl, and succinimidyl ester groups [108,109]. Coatings of these highly hydrated polymer chains exhibit a large exclusion volume effect, which leads to in-hibition of protein and bacterial adhesion [21]. The utilization of PEG as a spacer presents several advantages. This polymer can create non-adhesive surfaces due to its non-fouling characteristics, thus preventing non-specific peptide binding to the surface and shielding the peptides from the
Chapter 3
Surface Characterization Techniques
Several characterization techniques were used to analyze the surface of the materials studied in this work: Au, Au_chitosan, Au_chitosan_ PEG, Au_chitosan_PEG_adsorbed MSI-78(4-20) and Au_chitosan_PEG_cys-MSI-78(4-20). The use of different characterization techniques allowed us study the surface regarding thickness, wettability and elemental composition. Moreover, fluo-rescence microscopy allowed the analysis of the antimicrobial properties of the surface.3.1
Ellipsometry
Ellipsometry is a very sensitive optical technique that has been used for years to derive information about surfaces. It deals with the measurement and interpretation of the change in polarization of light upon reflection on a surface, from which it deduces about the physical properties of the material [112,113]. A scheme of the components that constitute a nulling ellipsometer is showed in figure 3.1.
Figure 3.1: Schematic diagram of a nulling ellipsometer [7]. Ellipsometer EP3Nanofilm is characterized by the following features:
• Works on the principle of null ellipsometry, which is equivalent to find the minimum in the signal of a photo-detector;
18 Surface Characterization Techniques
• Allows variable angle incident measurements; • Allows in-situ measurements with a flow cell.
This equipment allows the determination of the refractive index (n) and extinction coefficient (k) of the sample. From these values, it is possible to determine the thickness of the film [112,113]. The refractive index (n) is a measure of the optical density that a particular material has to the propagation of light in comparison to vacuum. The extinction coefficient (k) is a measure of how rapidly the intensity decreases as the light passes through the material [112,113]. This technique can be very useful to determine surface modification of thin films (<100 nm).
3.2
Water contact angle
The contact angle is defined as the angle formed by the intersection of a liquid-solid interface and the liquid-vapor interface. A small contact angle is observed when the liquid spreads on the surface and so, wetting of the surface is favourable. When a large contact angle is observed, the liquid beads on the surface, which means wetting of the surface is unfavourable so the fluid will minimize its contact with the surface and form a compact liquid droplet (figure 3.2) [8].
Figure 3.2: Illustration of contact angles formed by sessile liquid drops on a smooth homogeneous solid surface [8].
Ideally, the shape of a liquid droplet is determined by the surface tension of the liquid. The molecules exposed at the surface do not have neighbouring molecules in all directions to balance forces. As the molecules are pulled inward the liquid voluntarily contracts its surface area, in order to maintain the lowest number of molecules on the surface. This intermolecular force to contract the surface is called the surface tension, and it is responsible for the shape of liquid droplets [8].
Generally, if the water contact angle is smaller than 90◦, the surface is considered hydrophilic and if the water contact angle is larger than 90◦, it is considered hydrophobic [8].
In practice, the phenomenon of the contact angle between a liquid and a solid surface can be explained as a balance between the cohesion and adhesion forces. When a drop of liquid is placed on a surface, it spreads to reach equilibrium between cohesive and adhesive forces with minimum energy. The equilibrium between the forces was described in 1805 by Thomas Young through the following equation (young’s equation):
3.3 X-Ray Photoelectron Spectroscopy (XPS) 19
γlv× cos(Θγ) = γsv− γsl (3.1)
Where:
γlv= liquid-vapor interfacial tension;
γsv= solid-vapor interfacial tension;
γ sl = solid-liquid interfacial tension; θγ= contact angle.
The method of static sessile drop is the simplest one to measure the contact angle and gives information about the surface nature (wettability).
3.3
X-Ray Photoelectron Spectroscopy (XPS)
XPS is a spectroscopic technique to analyse the surface chemistry of a material, based on the pho-toelectric effect, which allows measurements of elemental composition, chemical and electronic state of the components in the material [9].
The photoemission process happens when an atom or molecule absorbs an X-ray photon and then an electron can be ejected from an atomic orbital [114]. The kinetic energy (Ke) of the
electron depends upon the photon energy (hv) and the binding energy (Be) of the electron (i.e., the
energy required to remove the electron from the surface) [9].
Figure 3.3: The photoemission process involved for XPS surface analysis. The discs represent electrons and the bars represent energy levels within the material being analysed [9].
By measuring the kinetic energy of the emitted electrons, it is possible to determine which elements are near a material’s surface, their chemical states and the binding energy of the electron, which is the value of interest. The binding energy depends upon a number of factors, including the following:
20 Surface Characterization Techniques
• The orbital from which the electron is ejected;
• The chemical environment of the atom from which the electron was emitted.
With this technique, the mono-energetic x-rays beam used is in high vacuum. The ultra-high vacuum chamber is an important component since it keeps a sterile environment assuring the absence of other elements during the analysis, and also that all the detected elements belong to the sample in analysis [115].
A scheme of an X-ray photoelectron spectroscopy equipment is represented in figure3.4. The main components of the system are: X-ray source, sample, electrostatic analyser and electron detector.
Figure 3.4: Basic components of a monochromatic XPS system [10].
The binding energy (Be) of an electron on an atom is determined by the following equation:
Be= hv − Ke (3.2)
Where,
hv = energy of the incident X-ray beam; Ke= kinetic energy of the emitted electron;
Be= binding energy of the emitted electron.
3.4
Inverted Fluorescence Microscopy
A fluorescent microscope is a device used to examine the amount and type of fluorescence emitted by a sample. Unlike a conventional microscope, a fluorescent microscope creates readable images through the use of irradiation rather than through reflection [116].
Fluorescence is a process that occurs when susceptible molecules emit light from electron-ically excited states, created by either a physical (absorption of light), mechanical (friction) or chemical mechanism. Fluorescence is the property of some atoms and molecules to absorb light at a particular wavelength and to subsequently emit light of longer wavelength after a brief interval, termed the fluorescence lifetime [116].
3.4 Inverted Fluorescence Microscopy 21
A scheme of a fluorescence microscopy is shown in figure3.5.
Figure 3.5: Schematic diagram of an inverted fluorescence microscope [11].
The category of molecules capable of undergoing electronic transitions that ultimately result in fluorescence are known as fluorescent probes, fluorochromes, or dyes. Fluorochromes that are conjugated to a larger macromolecule, such as a nucleic acid, lipid, enzyme, or protein, through adsorption or covalent bonds are termed fluorophores [116].
DAPI (4’,6-diamidino-2-phenylindole) is an example of a fluorochrome that binds DNA and it is extensively used in fluorescence microscopy for direct counts. When excited with light at a wavelength of 365 nm, the DNA-DAPI complex fluoresces bright blue [117].
LIVE/DEAD Baclight TM Bacterial Viability Kit is a fluorescent stain package composed of a mixture of two nucleic acid-binding stains: SYTO 9 and propidium iodide. These stains differ both in their spectral characteristics and in their ability to penetrate viable bacterial cells [118]. SYTO 9 stains all cells green, while propidium iodide penetrates cells whose cell membrane has been damaged, staining them red [119].
Absorption of light occurs very quickly in discrete amounts termed quanta and corresponds to excitation of the fluorophore from the ground state to an excited state. The energy in a quantum is expressed by the equation:
E= hv = hc/λ (3.3)
Where, E = energy;
h = Planck’s constant;
22 Surface Characterization Techniques
c = Speed of light.
According to the Planck’s Law the radiation energy of an absorbed photon is directly propor-tional to the frequency and inversely proporpropor-tional to the wavelength [116].
Chapter 4
Materials and Methods
4.1
Antimicrobial Peptides (AMPs)
The AMP, MSI-78 (4-20) (KFLKKAKKFGKAFVKIL) Mw (1994.0), was synthesized at the New Peptides in OPorto - University of Porto peptide synthesis facility (POP-UP), from the department of Chemistry and Biochemistry of the Faculty of Sciences of the University of Porto, with purity higher than 95%.
For AMP surface immobilization, Cys-MSI-78 (4-20) (Cys-Ahx-KFLKKAKKFGKAFVKIL), Mw (2211.0), was synthesized with a cysteine amino acid at the N-terminal, to create a reactive sulfhydryl group (SH) that will allow oriented immobilization of the AMP on the surface. An aminohexanoic acid (Ahx) was added between the cysteine amino acid and the N-terminal to act as a spacer allowing a better AMP surface exposure and therefore a more efficient synthesis.
4.2
MSI-78(4-20) chitosan coating preparation
4.2.1 Substrate preparationGold (Au) was used as substrate for coating preparation due to its suitability for characterization techniques. Au substrates obtained from INESC Microsistemas e Nanotecnologias (INESC MN) were prepared by deposition of a chromium layer (5 nm) and a gold layer (25 nm) on silicon wafers (AUREL, Gmbh) by ion beam sputtering as previously described [120]. Chromium was used to improve gold adhesion to silicon. Au substrates used had the dimensions of 1.0 x 1.0 cm2. Au substrates were cleaned as described at Martins, C. et al [121]. Substrates were firstly washed with acetone to remove photoresist, and then washed with ethanol to eliminate all acetone residues. Secondly, Au substrates were cleaned with "Piranha" solution (3 parts of H2O2 and 7
parts of H2SO4) for 5 minutes (attention: this solution reacts violently with many organic materials
and so it should be handled with care). Subsequently, successive cleaning steps were performed with ethanol and Milli Q water and finally, the samples were dried with a gentle stream of argon and stored sealed in petri dishes also saturated with this gas.
24 Materials and Methods
4.2.2 Chitosan ultrathin films
Commercial squid pen chitosan (France Chitine) was purified using the re-precipitation method [122]. Briefly, chitosan was dried in a vacuum oven at 60oC for 24 hours and then hydrated in Milli Q water protected from light for 24 hours at 4oC under slow stirring. Then, glacial acetic acid was added to chitosan suspension and left overnight under slow stirring at room temperature. Chitosan solution was then filtered using a 20 µm filter and subsequently precipitated by addition of small amounts of 1.0 M sodium hydroxide (NaOH) until it reached pH 12. Precipitated chitosan was successively washed with Milli Q water until pH=7, then stored at -80oC for 24 hours and finally lyophilized and milled to obtain a thin powder. The obtained chitosan had a deacetylation degree (DD%) of 94% as determined by FTIR-IRRAS.
Chitosan thin films were prepared by spin coating. The Au substrates were placed in the spin coater and a drop of 150 µL of chitosan solution (0.4% in acetic acid w/v) was dispensed in the center, then the sample spun at 9000 rpm for 1 min [123]. This process was repeated twice to obtain a thicker film of chitosan. The newly prepared ultrathin films were neutralized with 0.1 M NaOH for 5 minutes and washed twice with Milli Q water. Each sample was dried with a gentle stream of argon and stored in sealed plastic Petri dishes saturated with argon until use.
Figure 4.1: Representation of spin-coating working equipment [12].
4.2.3 Peptide immobilization
Immobilization of Cys-MSI-78 (4-20) on chitosan thin films was done using a polyethylene glycol (PEG) heterobiofuncional crosslinker, with N-hydroxysuccinimide (NHS) ester and maleimide groups that allow covalent conjugation with the amines of chitosan and the sulfhydryl group of the peptide. Reaction was performed in two steps, first the crosslinker was immobilized on the chitosan film and subsequently Cys-MSI-78 (4-20) was immobilized through the PEG crosslinker. Prior to chitosan film modification, the samples were hydrated for 30 minutes under slow stirring at 4oC. Immobilization of succinimidyl-(N-maleimidopropionamido)-octaethyleneglycol ester (SM(PEG)8), Mw (689.71) and spacer arm (3.925nm), was carried out by immersing the
chitosan film in a 500 µL solution of SM(PEG)8in phosphate buffer (pH 7.2) for 24 hours under
slow stirring at 4oC. During these period SM(PEG)8 was added several times to the reaction
as NHS-ester reactive group is susceptible to hydrolysis. The first SM(PEG)8 solution had a
concentration of (5 mM), to which 1 mM were added after 4 hours and 2 mM were added after 6 and 8 hours, making a final SM(PEG)8 concentration of 10 mM, if hydrolysis would not be
considered. After, SM(PEG)8 immobilization surfaces were sonicated in Milli Q water in an
4.2 MSI-78(4-20) chitosan coating preparation 25
For Cys-MSI-78(4-20) immobilization, the peptide was dissolved in phosphate buffer (pH= 6.6) and tris(2-carboxyethyl)phosphine (TCEP) (1.1eQ), and left for 1 hour under slow stirring at 4oC. TCEP reducing agent, which is able to reduce peptide disulfide bonds, was used to treat Cys-MSI-78 (4-20) before immobilization as the peptide must have free reduced sulfhydryls to react with the maleimide moiety of the PEG crosslinker. Subsequently surfaces were immersed in 500 µL of TCEP treated Cys-MSI-78(4-20) (1mg/ml) for 24 hours under slow stirring at 4oC.
After immobilization surfaces were sonicated in Milli Q water in an ultrasonic bath for 1 minute to assure removal of Cys-MSI-78 (4-20) not covalently bond to the surface.
Figure 4.2: Schematic representation of the chemical reaction used for Cys-MSI-78(4-20) im-mobilization. Conjugation was performed using a PEG heterobiofuncional crosslinker, with N-hydroxysuccinimide (NHS) ester and maleimide groups that allow covalent conjugation with the amines of chitosan and the sulfhydryl group of the peptide.
26 Materials and Methods
4.3
MSI-78(4-20) surface characterization
4.3.1 EllipsometryAn imaging ellipsometer, model EP3, from Nanofilm Surface Analysis was used to measure the thickness of the coating. This equipment was operated in a polarizer-compensator-sample- an-alyzer mode (null ellipsometry). The light source was a solid-state laser with a wavelength of 532 nm. From these analyses two parameters were determined, refractive index (n = 0.703) and extinction coefficient (k = 2.63), using a delta and psi spectrum with a variation of angle between 63◦ and 73◦. The thickness of the chitosan films was determined considering n = 1.54 and k = 0, for the chitosan film [124]. The measurements were made in four zones for each sample and results are presented as the average of all the measurements on the three independent samples. 4.3.2 Water contact angle measurements
Measurements were performed using the sessile drop method with a contact angle measuring sys-tem from Data Physics, model optical contact angle 15 (OCA 15), equipped with a video CCD-camera and sca 20 software [120]. Images were taken every 2s over 3 minutes after deposition of 4 µ L drop of Milli Q water. Droplet profiles were fitted using the Young-Laplace mathematical func-tion. The water contact angle of each sample was calculated by extrapolating the time-dependent curve to zero. Results are the average of two measurements on the three independent samples. 4.3.3 X-ray photoelectron spectroscopy (XPS)
XPS measurements were carried out on a Kratos Axis Ultra HSA spectrometer from CEMUP (Centro de Materiais da Universidade do Porto). Photoelectrons were analyzed at a take-off angle of 70◦. Regarding the survey, spectra were collected with an analyzer pass energy of 80 eV. As regards to high-resolution C1s, O1s, N1s, S2p spectra, they were collected with an analyzer pass energy of 40 eV. The binding energy (Be) scales were fitted by setting the binding energy of C1s
to 285.0 eV. Sulfur high resolution spectra were fitted 2:1 area ratio and splitting of 1.2 eV [125]. All the spectra were fitted using XPSPEAK fitting software (XPSPEAK41). Element atomic percentages were calculated from the integrated intensities, taking into account the atomic sensi-tivity factors of the instrument data system.
4.4
Bacterial assays
4.4.1 Bacterial strains, media and growth conditions
Staphylococcus epidermidis (S. epidermidis) (ATCC 35984) was obtained from the American Type Culture Collection. Bacteria were grown on tryptic soy agar (TSA, Merck) plates at 37oC overnight and tryptic soy broth (TSB, Merck) at 37oC and 150 rpm overnight (bacterial suspen-sion). Bacterial suspension was adjusted by measuring optical density (600 nm) and then con-firmed by colony forming units (CFUs) counts.
4.4 Bacterial assays 27
4.4.2 Surface antimicrobial activity characterization 4.4.2.1 Sample preparation
Surfaces were washed twice in ethanol (70%) for 30 minutes and thrice in sterile Phosphate Buffered Saline (PBS). Samples were dried in sterile environment and then transferred to a 24-well plate (Sarstedt, Ltd, Newton, USA).
4.4.2.2 Sample incubation with bacteria
Antimicrobial activity was evaluated with a bacterial adhesion assay adapted from Pallavicini et al [126]. A drop of 5 µL of bacterial solution (S. epidermidis ATCC 35984), with an approximate concentration of 1x108CFUs/mL in PBS, was dispensed on each sample and covered with a sterile glass coverslip with a diameter of 1 cm2 to facilitate contact between the surface and bacteria. Then, the samples were incubated at 37oC for 5 hours in a wet environment. The surrounding wells were filled with 1 mL of sterilized PBS, to avoid evaporation.
Figure 4.4: Schematic representation of the method used to test antimicrobial activity.
4.4.2.3 Non-adherent viable bacteria (supernatant) assay
After incubation a volume of 500 µL of PBS was added to resuspend non-adherent bacteria. Serial dilutions of the supernatants from 10−1to 10− 5were performed and plated onto TSA plates. CFU
28 Materials and Methods
4.4.2.4 Viable surface adherent bacteria assay
Viability of adherent bacteria was assessed using the LIVE/DEAD Bacterial Viability Kit (Ba-R
clightTM). After removal of non-adherent bacteria ( 4.4.2.3), surfaces were washed twice with PBS and once with 0.85% Sodium Chloride (NaCL). Then, surfaces were stained with a com-bination of two dyes, red-fluorescent propidium iodide (PI) and green-fluorescent syto 9 for 15 minutes at room temperature, protected from light. Red fluorescence labeled only dead bacte-ria, whereas the green one labeled live bacteria. Finally, surfaces were mounted in slides using VECTASHIELD Mounting Medium for further microscopy observation. Images were obtainedR
with an inverted fluorescence microscope (Axiovert 200 M, Zeiss, Germany) using a magnifica-tion of 400×, corresponding to a surface area of 0.094 mm2 per sample. To quantify the total adherent bacteria, six fields of each sample were obtained and analysed using ImageJ software. The result is presented as bacteria per area, three replicates for each condition were analysed.
4.4.2.5 Statistical analysis
Statistical analysis was performed using ANOVA or Kruskal-Wallis. Data is expressed as the mean ± standard deviation (SD) and p values <0.05 were considered significant, with * corresponding to p<0.05, **corresponding to p< 0.01, *** corresponding to p<0.001 and **** corresponding to p<0.0001.
Chapter 5
Results and Discussion
5.1
Surface characterization
Chitosan thin films with and without peptide were characterized using ellipsometry, water contact angle measurements and x-ray photoelectron spectroscopy.
5.1.1 Ellipsometry
Thickness of chitosan surfaces before and after chemical modification is shown in Figure 5.1. The thickness of chitosan thin films, when subjected to the buffer used for the chemical modifications, was 30.4 ± 2 nm. After being functionalized with SM(PEG)8crosslinker, thickness increased 3.8
nm (± 1.5 nm). Knowing that the spacer arm is 3.9 nm in length, the results suggest a successful functionalization. However, cys-MSI-78 (4-20) immobilization onto chitosan films was not de-tected using this technique, since no significant difference in the thickness was observed between SM(PEG)8modified chitosan film before and after peptide immobilization. This can be explained
either by the flexibility acquired by the peptide when immobilized through SM(PEG)8, or folding
30 Results and Discussion
Figure 5.1: Surface characterization of chitosan modified films with SM(PEG)8 and
Cys-MSI-78(4-20) using ellipsometry (thickness of the surface). Statistically significant differences are indicated with **** (p<0.0001).
5.1.2 Water Contact Angle measurements
Results of the water contact angle measurements are shown in figure5.2. Chitosan thin films (θw
= 68.1◦ ± 1.8◦) became more hydrophilic after modification with SM(PEG)
8 crosslinker (θw =
57.9◦± 4.1◦).
After immobilization of Cys-MSI-78(4-20) water contact angles increased to θw = 67.4◦ ±
4.4◦suggesting a successful immobilization. MSI-78 (4-20) possesses an amphipathic structure, having amino acids of hydrophobic and hydrophilic character distributed along the peptide se-quence, however the presence of hydrophobic amino acids in the C-terminal may be responsible for the observed increase in the water contact angle. This change in surface wettability along the different samples indicates surfaces modifications. For adsorbed MSI-78 (4-20) no signifi-cant difference in water contact angle is observed, when comparing to the sample with SM(PEG)8
5.1 Surface characterization 31
Figure 5.2: Surface characterization of chitosan modified films with SM(PEG)8and cys-MSI-78
(4-20) using water contact angle measurements. Statistically significant differences are indicated with ** (p<0.01).
5.1.3 X-Ray Photoelectrons Spectroscopy (XPS)
From XPS survey spectra it is possible to infer that the relative surface atomic composition of chitosan is in accordance with previous results obtained for chitosan films [127].
After SM(PEG)8immobilization a slight increase in the amount of Carbon and Nitrogen and
a slight decrease in the amount of Oxygen are observed, which can be explained by the fact that these elements (Carbon, Nitrogen and Oxygen) are present in similar proportions in chitosan and SM(PEG)8. Considering results for the high-resolution spectra of Carbon (table 5.2) a decrease of
C1s with binding energies at 285.0 eV (C-C; C-H bonds) and 286.5 eV (C-NH2; C-OH; C-O-C)
is observed, while an increase is observed for C1s with binding energies 288.0 eV. These results confirm SM(PEG)8immobilization, as the decrease of Carbon at 285.0 eV and 286.5 eV indicate
that chitosan was partially covered with other compounds. While Carbon at 288.0 eV (O-C-O; N-C=O) increased significantly due to the presence of N-C=O in SM(PEG)8structure. However, we
expected to see an increase in the C-O-C bonds, characteristic and abundant in the PEG structure, which did not happen, these preliminary results must be confirmed by repeating this analysis.
After Cys-MSI-78(4-20) immobilization an increase in the amount of Carbon and Nitrogen is observed while Oxygen decreases when comparing to the SM(PEG)8sample. Carbon and
Ni-trogen are very abundant in amino acids confirming the modification with AMP. Specifically, the increase in Nitrogen clearly points to a successful immobilization of the peptide. Moreover, Sulfur (S2p) present in the cysteine residue of Cys-MSI-78 (4-20) reinforces the presence of the peptide on the surface. Considering the results for the high-resolution spectra of Carbon ( table 5.2) there
32 Results and Discussion
78(4-20) immobilization as the increase of carbon 285.0 eV indicates the presence of C-C bonds characteristic of the Lysine side chains, abundantly present in Cys-MSI-78 (4-20).
A control sample, where MSI-78(4-20) not modified with a cysteine was incubated with the SM(PEG)8 modified chitosan, was also used for XPS analysis. In this control sample, MSI-78
(4-20) could not react with the maleimide group due to the absence of the sulphydryl group of the cysteine. Therefore for this sample, we expected similar results to the SM(PEG)8 modified
sample. The observed difference both in relative atomic composition and relative percentage of the different Carbon bonds is probably explained by the hydrolysis of the maleimide group of the SM(PEG)8, namely the significant decrease of the C1s with a binding energy at 288.0 eV, due to
the loss of N-C=O bonds. The presence of a residual amount of Sulphur (S2p) in this sample may be explained by a possible contamination of the sample. As previously referred, these results must be confirmed by repeating XPS analysis with new samples.
Table 5.1: Atomic composition (%) of the different surfaces obtained from XPS element analysis. Atomic percentage (%)
C1s N1s O1s S2p
Au_chitosan 66.8 6.4 26.8
-Au_Chitosan_SM(PEG)8 67.4 6.9 25.7
-Au_Chitosan_SM(PEG)8_adsorbed MSI-78 (4-20) 70.1 5.7 24.1 0.1∗
Au_Chitosan_SM(PEG)8_Cys-MSI-78 (4-20) 70.2 8.8 20.8 0.2
* Possible contamination of the sample.
Table 5.2: Relative percentage (%) of the different Carbon bonds obtained from high-resolution XPS spectra.
C1s
285.0 286.5 288.0
C-C; C-H C-NH2; C-OH; C-O-C O-C-O; N-C=O
Au_Chitosan 31 59 10
Au_Chitosan_PEG 24 58 18
Au_Chitosan_SM(PEG)8_adsorbed MSI-78 (4-20) 30 60 10




![Figure 2.7: Chemical structure of SM(PEG)n. Adapted from [6].](https://thumb-eu.123doks.com/thumbv2/123dok_br/15191100.1016898/33.892.353.588.145.369/figure-chemical-structure-sm-peg-n-adapted.webp)
![Figure 3.1: Schematic diagram of a nulling ellipsometer [7].](https://thumb-eu.123doks.com/thumbv2/123dok_br/15191100.1016898/35.892.309.622.773.935/figure-schematic-diagram-nulling-ellipsometer.webp)
![Figure 3.2: Illustration of contact angles formed by sessile liquid drops on a smooth homogeneous solid surface [8].](https://thumb-eu.123doks.com/thumbv2/123dok_br/15191100.1016898/36.892.165.701.598.721/figure-illustration-contact-angles-formed-sessile-homogeneous-surface.webp)
![Figure 3.3: The photoemission process involved for XPS surface analysis. The discs represent electrons and the bars represent energy levels within the material being analysed [9].](https://thumb-eu.123doks.com/thumbv2/123dok_br/15191100.1016898/37.892.319.609.655.897/photoemission-involved-analysis-represent-electrons-represent-material-analysed.webp)
![Figure 3.4: Basic components of a monochromatic XPS system [10].](https://thumb-eu.123doks.com/thumbv2/123dok_br/15191100.1016898/38.892.277.570.421.602/figure-basic-components-monochromatic-xps.webp)