Bioprocess Engineering 11 (1994) 23 28 9 Springer-Verlag 1994
Strategy
reaction
for the simulation of batch reactors when the
is accompanied by enzyme deactivation
F. X. Malcata
enzyme-catalyzed
Abstract The balance equations pertaining to the modelling of
batch reactors performing an enzyme-catalyzed reaction in the presence of enzyme deactivation are developed. The functional form of the solution for the general situation where both the rate of the enzyme-catalyzed reaction and the rate of enzyme deactivation are dependent on the substrate concentration is obtained, as well as the condition that applies if a maximum conversion of substrate is sought. Finally, two examples of practical interest are explored to emphasize the usefulness of the analysis presented. List of symbols C e mol/m 3 Q,o mol/m3 Cs mol/m 3 Cs, o mol/m 3 Cs, min mol/m 3 k S/S kl s/s k 2 1/S K,~ mol/m 3 p mol/(m 3 s ) q mol/m 3 t s
concentration of active enzyme initial concentration of active enzyme concentration of substrate
initial concentration of substrate minimum value for the concentration of substrate
first order rate constant associated with conversion of enzyme/substrate complex into product
first order deactivation constant of enzyme (or free enzyme)
first order deactivation constant of enzyme in enzyme/substrate complex form
Michaelis-Menten constant time derivative of Cs
auxiliary variable
time elapsed after reactor startup
Greek symbols ~b SiS mol/m 3 mol/m 3 ~, sis ~ ' m3/(mol s)
univariate function expressing the dependence of the rate of enzyme deactivation on Cs
dummy variable of integration dummy variable of integration univariate function expressing the dependence of the rate of substrate depletion on Cs
derivative of ~ with respect to Cs
Received 28 June 1993 F. X. Malcata
Escola Superior de Biotecnologia, Universidade Cat61ica Portuguesa, Rua Dr. Ant6nio Bernardino de Almedia, 4200 Porto, Portugal
1
Introduction
Batch reactors used to perform enzyme-catalyzed reactions are fairly common in the biochemical (and fine chemical) industry. In order to be able to properly design such reactors a priori and
to properly optimize their utilization a posteriori, efficient
techniques of mathematical modelling are required that take into account various possibilities for the kinetic mechanisms pertaining to the enzyme-catalyzed reaction and to the thermal deactivation of the enzyme. A variety of uni- and multisubstrate mechanisms for the former have been comprehensively described in the literature [1], and similar observation holds for unimolecular mechanisms associated with the latter in the presence of macroheterogeneity [2] or microheterogeneity [3]. The assumption of substrate-dependent rate expressions for either the enzyme-catalyzed reaction and the deactivation of the enzyme has been successfully employed before, e.g. in the determination of the optimum temperature path in the case of batch reactors performing enzyme-catalyzed reactions [4]. The combination of some of the aforementioned mechanisms for the enzyme-catalyzed reaction with the material balances for various reactor configurations aiming at optimized reactor designs has also been reported to considerable extent [5-lo]. The existence of constraints in terms of the maximum conversion of substrate ever attainable when enzyme deactivation is present was emphasized elsewhere for the case of CSTR cascades [n-s3]. However, the integration of both the enzyme-catalyzed and the enzyme deactivation reactions with the material balances pertaining to the reactor has not deserved extensive attention so far.
This communication attempts to partially fill this gap by providing a general mathematical strategy for the simulation of biochemical reactors operated batchwise when both the enzyme- catalyzed reaction and the enzyme deactivation reaction take place; its applicability is tested via the discussion of two selected examples.
2
Theory
The unsteady-state mass balances to enzyme undergoing deactivation following first order kinetics with respect to the enzyme, and substrate undergoing depletion also following first order kinetics with respect to the enzyme in a well-stirred reactor operated batchwise can be written as:
dCe
-
O{cAc~,
O)
dt
Bioprocess Engineering n 0994)
24
with initial condition:
t=O, C~=Q,o,
and dCs
- - O { C s } C ~ ,
dt
with initial condition
t=O, Cs=Cs, o, (4)
respectively, where C E and Cs are the molar concentrations of active enzyme and substrate, respectively, t is time elapsed since startup of the reactor, 4 is a univariate expression relating the rate of enzyme deactivation with substrate concentration, and O is a univariate expression relating the rate of substrate depletion with substrate concentration.
Equation (1) may be replaced by the expression obtained by division of Eq. O) by Eq. (3):
dee
4{
Cs}dG 0{
c A '(5)
and, therefore, Eq. (2) should be replaced by:
Cs=Cs, o, CE=CE, o 9
Rewriting Eq. (3) as:
1 dC s C E =
0{ Cs} d t "
and differentiating Eq. (7) with respect to t, one gets: ( d C E \ / d C s \ O' l d C s \ 2 1 (d2Cs~_
d-~s) t--dT-)--~Tt--&- ) +~ t,
dt 2 ] - 0 , where: dOO'-
dCs "Combination of Eqs. (8) and (5) yields:
(d
-<i7r)+4
2Cs~ (dCs'~ O' fdCs~ 2 \ d t } O \ d t / I =o,which now requires two initial conditions, viz. Eq. (4) and
des
t=O, T = -O{ Cs, o} CE, o.
Equation (lo) is a second-order, second-degree ordinary differential equation which can replace Eq. (3). Solution of Eq. (lo) may proceed through the introduction of the new dependent variable p, defined as:
des
p ~ - ~ - , 02)
which, combined with Eq.
(10),
leads to [14] :(
dp'~ O'G=G,o, P= - 9 {
G,o}
G,o.
Equation (13) has the form of a general first order ordinary differential equation, which has the following solution: (2) cs,0cs 4'{ 4} ) /
,4{ ~1
CE 0+ ] - - 05) p - - - O { c s } [ , d~ . %(3) Recalling Eq. (12), one finally observes that: 4{{}
+fg 4{~} '
(16)
which can be rewritten as:
(~{~) (d~ d(
--
Q~
dt= I (~7)
Cs
The auxiliary variable q is defined as:
( - p )
q ~- 6 (18)
Recall Eq. 06), which can be written in the alternative form:
cs,0 d~
(6) t = * ( fCs, o4{~} ) " (19)
Cs 0{(} CE,0-- d{
For t = 0% one will obtain the maximum ever attainable (7) conversion of substrate, Cs, mln. Since the integration range is
finite, inspection of the denominator of the integrating function in Eq. (19) indicates that, in such situation, Cs, min should satisfy the following condition:
(8)
Cso 4{ 4}
r minI=OVC<o - 5 ~ d ~ = 0 . (20)
Cs,mln ~ } g /
The first condition is trivial, since for every type of kinetics for (9) substrate consumption by an enzyme-catalyzed reaction, the rate of reaction is obviously nil when the concentration of substrate is zero. The second condition is much more constraining. It arises from the fact that there are two competing factors in the reacting system, the deactivation of enzyme, which leads to (lO) a decrease in the number of active enzyme molecules with time,
and the rate of the enzyme-catalyzed reaction, which leads to a decrease in the number of substrate molecules with time; hence, longer reaction times imply more extensive conversion of (11) substrate per unit molecule of active enzyme but also fewer
molecules of enzyme left active, which may lead to the existence of a maximum for the conversion of substrate. It should be emphasized that Cs, m~n > O i f 4 is a weaker function on Cs than is
irrespective of the value of CE,0, which is equivalent, in physical terms, to saying that 4{Cs}IO{Cs} becomes unbounded when Cs tends to zero; the equality Cs,~i~ = o can be guaranteed a priori only if the enzyme is actually reactivated by the presence of substrate at whichever concentration, i.e. if
4{ Cs} < o; in the remaining cases, e.g. 4{ Cs} 10{ Cs} equal to a constant or limc,~o 4{ Cs} 10{ Cs} equal to a finite value, the decision on which solution of Eq. (2o) holds depends on the (13) numerical values of the parameters in question.
The applicability of the foregoing general analysis is explored (14) below in two examples of practical interest.
F. X. Malcata: Modelling batch enzyme reactors with deactivation
3
First numerical example
Assume that the rate of deactivation is independent of the concentration of substrate. In this situation, ~b{ Cs} reduces to a constant, and Eq, (17) becomes:
/
1 \q{cs, o}
d~/t - - - ~
- - ,
(21)-tr
o}) ,~{Cs} U
or, using Eqs. (15) and (18):
t
4qCs, o} i ~
(22)
t = -
In 1 C~,o c, "Assuming Michaelis-Menten kinetics for the substrate consumption and first order deactivation of enzyme, the result is:
k,
ci~ K~+ (
d ~ } ,(23)
where kl is the first order deactivation constant, K~ is the Michaelis-Menten parameter i.e. the dissociation constant of the enzyme/substrate complex, and k is the first order constant associated with transformation of the enzyme/substrate complex into product and concomitant regeneration of the free enzyme form. After simple manipulation of Eq. (23), one finally gets: k~t= - l n f l k'Cs'~ ( K ~ l n (Cs, o ) Cs \ ]
Plots of Cs/Cs, o vs. k~ t are available in Figs. la to lC for a number of values of parameters kC E, o / ( k ~ C s, o ) and k~/ C s, o and K J C s, o .
Equation (20) now takes the form:
Cso k~(Km+~)
j"
d~=C~,o,
which is equivalent to:
_ _ t C s , Km
1ii
o l --Cs'min+l ~ ' kCEo--0.
Cs, o ( Cs, min ) Cs, o kl Cs, o
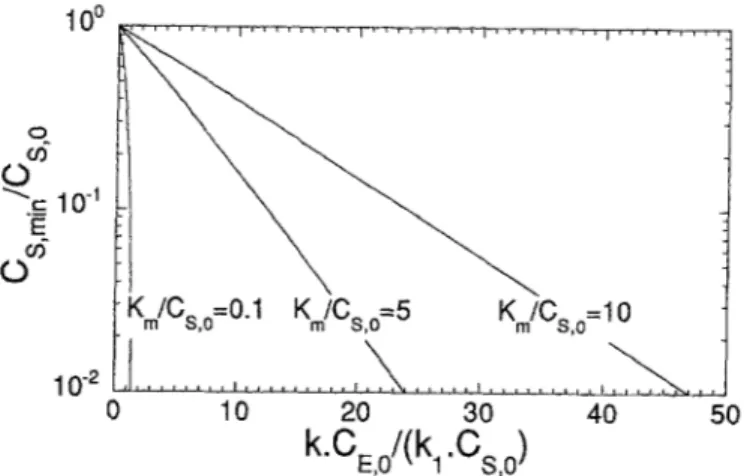
Plots of CS, mi~/Cs, o are available in Fig. 2 as functions of parameters kCE, o/(klCs, o) and K,dCs, o.
(25)
(26)
4
Second numerical example
Assume now that the free enzyme deactivates via a first order irreversible process with constant kl and that the enzyme in the enzyme/substrate complex form deactivates via a first order irreversible process characterized by constant k2, which, in general, will be different from kl; assume in addition that the substrate consumption follows Michaelis-Menten kinetics as in the previous example. In this situation, Eqs. (1) and (3) take the form: dC~ k l K m § s CE, (27) dt K~ + Cs and dCe k Cs C~, (28) dt K m + C s
25
1.0
0.8
~0.6
0
0.4
0.2
0 L
0
1.0
0.8
,~0.6
o
0~0.4
0.2
0 0
k'CE, o/(k 1 -Cs,o)=I
k-CE,o/(k 1 .Cs,o)=5 ~
1 2 3k 1 .t
I i I" ~ k.CE,o/(k ~ .Cs,o) = 1
k-CE,o/(k 1 -CE,o)=5
~ ~ ~ .
k.CE,o/(kl .Cs,o)=l 0
1 2 3k 1.t
I 4 5 4 5 1.0 ~ 0 . 6o
O m 0.40.8 ~
~
k'CE'~176
l
~ ~ - - - - ~
k.CE,o/(k, .Cs,o )=5
o F /
k Co,,o o
0
i
,O
0
1
2
3
4
5
k 1.t
Fig. la~c. Plots of the dimensionless substrate concentration, Cs/Cs, o, vs. the dimensionless time, kl t, for (a) K,~/Cs, o = o.1, (b) K,,/Cs.o = 1 and (c) Km/Cs, o=lo
Bioprocess Engineering 11 (1994) 26 100 6O ("~,- 10 "I E 0 10 2
9 Km/Cs,o=0.1 Km/Cs,o=5 Km/Cs,o=lO
0 10 20 30 40 50
k.CE,o/(k 1 .Cs, o)
Fig. 2. Log-lin plot of the dimensionless minimum substrate concentration,
Cs, min/Cs, o, VS. the dimensionless parameter kCe, o/(k~Cs, o) for
KmlCs, o=O,1, KmlCs, o=5, and KJCs, o=m
respectively, so Eq. (16) becomes: K m + ~
cs k - - - ~ d (
t = - j (29)
,~ k~Km+k2~
Cs,o CE, o + jQo
-k~
" d~
Algebraic manipulation of Eq. (24) yields:
1
I~iT--,/klKm )
1 Q k \ v, + k 2 d[
+ j , (30)
which, upon integration and rearrangement, becomes:
klt= --ln {1--klCs'o
(Km In ~Cs.o'~+k2 ( 1 C s
"]~+
kCE, o \Cs, o ( Cs J kl\
Cs, oJ/IJ "'"(
k2) i
617+ 1 - ~
k C E
0Km
k2
(31)c~/C~,o k~ C~'os +~s.o
in{ 17} + kll ( ' 7 - 1 ) Plots ofCs/Cs, o
vs. k~ t are available in Figs. 3a to 3f for a n u m b e r of values of parametersk Qo/(k~. Cs, o), k2/k~,
andkmlCs, o.
Equation (zo) takes in this situation the form:
Cs, o
k~K~+k2~,
d ~ = C ~ o , (32)S -
Cs, min
which can be rewritten a s :
Km ( l n (
Cso ] k2[
C s ~ ' ~ \
kCe, o
s,o. - - ~
Plots of Cs, rnin/Cs, o a r e available in Fig. 4 as functions of parameters
[kC ~,o/(k~Cs, o) ] I(K~lCs, o)
andk flkl.
5
Discussion and conclusions
It is interesting to note that in both examples studied the minimum conversion of substrate ever attainable is a bivariate function as apparent from inspection of Eqs. (26) and (33); if one moves from example 1 to example 2, parameter
kCE, ol(ks Cs, o)
should be substituted by the lumped parameter[kC~,o/
(ks Cs, o ) l / ( g J C s ,
o),
whereas parameterKm/Cs, o
should be substituted by parameterk2/ks.
Inspection of Fig. 2 indicates that the normalized value of ln(Cs, msn ) is essentially a linear function of parameterkCe, o / (kl Cs, o),
with a common vertical intercept at unity and a slope that decreases as the dimensionless value of K m increases. With respect to Fig. 4, it is apparent that(a) for k 2/k~ = o, in (Cs, min ) is always a decreasing linear function of
kCe, o/(k~gm),
and that (b) such linear behavior is also observed for other values ofkz/kl
provided that parameterkQo/(k~g~)
takes large values.Careful inspection of Eq. (31) allows one to conclude that if ks = k2, then example 2 reduces to example 1. This fact should be expected because it implies that the rate of deactivation of enzyme is the same irrespective of the forms it takes, and it is known that the relative amounts of either free or conjugated forms of enzyme are direct functions of the concentration of the substrate. It should be noted that reactivation of the enzyme by substrate implies that the intrinsic activity of the enzyme actually increases when substrate is present, as compared with the complete absence thereof; this is not the case explored in example 2 for
k21ks<l
because in the latter situation the presence of substrate leads only to a decrease of the rate of deactivation of enzyme when substrate exists in large amounts with respect to its complete absence. The lines depicted in Figs. 3a to 3f associated withk2/kl
= 0 correspond to the situation where the presence of substrate at any concentration prevents inactivation of enzyme.In Figs. i and 3, the lines plotted extend from unity to a certain lower asymptotic limit that can be obtained in every case from Fig. 2 or Fig. 4, respectively. Although in a few of the several cases depicted in Figs. 1 and 3 it seems that the lines extend virtually to zero substrate concentration, such is never the case because the value of Cs, min is actually finite and positive, as implied by the fact that q5 is always a weaker function on
Cs
than is ~ in all the cases depicted. As expected, whenkC~,o/(kiCs, o)
increases, i.e. when either
k/k~
orCs, o/Ce, o
increases, the minimum concentration of substrate attainable decreases because the effect ofklt
on the enhancement of the rate of the enzyme-catalyzed reaction is stronger than said effect on the enhancement of the enzyme deactivation reaction. Given any set of values forkCE, ol (klCs, o )
and Km/ Cs, o, both increases of k2lk tfrom zero to unity, and from unity to a higher value lead to decreases in Cs, mi n , thus implying that detrimental effects of the presence of substrate on the activity of the enzyme always constrain the best performance of the batch reactor. Furthermore, the effect of
k2/kl
is diluted whenK,,/Cs, o
andkCE, ol(klCs, o)
are large.The analysis presented in this communication is relevant for the simulation of batch reactors that perform enzyme-catalyzed reactions because it includes the effect of thermal deactivation, a factor that very often places severe constraints on the technical and commercial feasibility of such reactors; The reasoning was developed in such way as to possess the advantage of being
F. X. Malcata: Modelling batch enzyme reactors with deactivation 1.0 0.8 ~-0.6 0 0.4 0.2 1.0 0 0 i i k2/kl=O k2/kl =1 ' k2/kl= 0 -= ' 1 --I k2/k 1 =5 0.8 ~-0.6 0 0 ~ 0 . 4 k 1 .t k2/k t = 10 - k~k~ =5 k2/k 1 = 1 k2/k 1 =0 0 . 2 b 0 _1 0 1 1.0 0.8 ~ 0 . 6
0
O3o 0,4
0.2 0 0 J- 0 1 I I - - - -2 3k 1
.t [ - - i -- l kz/k 1 =10 k2/k 1 =5 ke/k~=l k2/k 1 =0 3 4 5k 1 .t
Fig. 3a-f. Plots of the dimensionless substrate concentration, CslCs, o, vs.
the dimensionless time, kl t, for: (a) kC~,o / (k~Cs, o) = t and K~ / Cs, o = o.1; (b) kCE, o/(k~Cs, o)=l and K,~/Cs, o =l; (c) kC~,o/(k~Cs.o)=t and g~/Cs, o=lO;
0
o
1 ~ 1 .- /k I =20O
10-2 [-. , I , I . . . , , 0 5 10 15 20 25k.Ce.0l(k
1 , K )
Fig. 4. Log-lin plots of the dimensionless minimum substrate concentration,
Cs, mln / Cs, o, VS, the dimensionless parameter [ kC E, ol ( k~ C s, o ) ] I ( K J C s, o) for
k2/k~ =o, k2/k1=5, k2/k~=z2, and k~/k~ =20 1 . 0 - - 0.8 ~-0.6 0 0 ~ 0.4 0.2 1.0 0.8 ~-0.6 0 0 ~ 0 . 4 0.2 1.0 0.8 ~ 0 . 6
0
O.4 0.2 d T l B Ik /kt=lC
kJk~=5
k2/kl=1 k2/k~=O 0 2 3 4 5k 1.t
l
e 0 1 2 k2/k 1 = 10 k2/k 1 =5 k2/kl=1 k2/kl = 0 k~ .t i .... i k2/k ~ = 10 k2/k ~ =5 kJkl=l k2/k 1 =0 5O0
1 2 4k 1.t
(d) kC~,o/(klCs, o)=lo and Km/Cs, o=o.1; (e) kCE, o/(kiCs, o)=lo and K~/Cs, o=l; and (f) kC~,o/(klCs, o)=to and Km/Cs, o=io
d i m e n s i o n l e s s , a n d h a v i n g the final f o r m of the s o l u t i o n of the s i m u l t a n e o u s m a t e r i a l b a l a n c e s to s u b s t r a t e a n d enzyme, viz. Eq. (19), r e q u i r e e v a l u a t i o n of an i n t e g r a l r a t h e r t h a n s o l u t i o n of a set of two differential e q u a t i o n s , viz. Eqs. (1) a n d (3).
References
1. Segel, I, H.: Enzyme Kinetics, Wiley, New York (t96~)
2. Henley, ]. P,; Sadana, A.: Deactivation theory. Biotechnol. Bioeng. 28 (1986) 1277
3. Malhotra, A.; Sadana, A.: Effect of activation energy microheterogeneity on first-order enzyme deactivation. Biotechnol. Bioeng. 3o (1987) lo8 4- Sadana, A.: A generalized optimum temperature operations criterion
for a deactivating immobilized enzyme batch reactor. AIChE J. 25 (1979) 535
5. Luyben, K. C.; Tramper, l.: Optimal design for continuous stirred tank reactors in series using Michaelis-Menten kinetics. BiotechnoL Bioeng, 24 (1982) 1217
Bioprocess Engineering 11 (1994)
28
6. Malcata, F. X.: Optimal design on an economic basis for continuous stirred tank reactors in series using Michaelis-Menten kinetics for Ping-Pong reactions. Can. J. Chem. Eng. 66 (1988) 168
7. Malcata, F. X.: CSTR's in biochemical reactions - an optimization problem. Chem. Eng. Ed. 23 (1989) 112
8. Malcata, F. X.: A heuristic approach for the economic optimization of a series of CSTR's performing Michaelis-Menten reactions. Biotechnol. Bioeng. 33 (1989) 251
9. Gooijer, C. D.; Hens, H. J. H.; Tramper, J.: Optimum design for a series of continuous stirred tank reactors containing immobilized biocatalyst beads obeying intrinsic Michaelis-Menten kinetics. Bioproc. Eng. 4 (1989) 153 lo. Malcata, F. X.; Cameron, D. C.: Optimal design of a series of CSTR's
performing reversible reactions catalyzed by soluble enzymes: a theoretical study. Biocatalysis 5 (1992) z33
tl. Malcata, F. X.: On the maximum conversion of substrate during biochemical reactions performed by a series of CSTRs in the presence of enzyme deactivation. I. Chem. Eng. Japan z3 (199o) 37z
12. Lopes, T. I.; Malcata, F. X.: Optimal design of a series of CSTR's for biochemical reactions in the presence of enzyme deactivation. J. Chem. Eng. Japan z6 (199z) 94
13. Paiva, A. L.; Malcata, P. X.: Optimal temperature and concentration profiles in a cascade of CSTR's performing Michaelis-Menten reactions with first order enzyme deactivation. Bioprocess Eng. (1994) [in press] 14. Stephenson, G.: Mathematical Methods for Science Students, Longman,
![Fig. 4. Log-lin plots of the dimensionless minimum substrate concentration, Cs, mln / Cs, o, VS, the dimensionless parameter [ kC E, ol ( k~ C s, o ) ] I ( K J C s, o) for k2/k~ =o, k2/k1=5, k2/k~=z2, and k~/k~ =20 1](https://thumb-eu.123doks.com/thumbv2/123dok_br/15650340.1058814/5.897.68.448.83.772/fig-plots-dimensionless-minimum-substrate-concentration-dimensionless-parameter.webp)