Car
la Magalhães
A New
Appr
oach for Cancer
Therapy Based on Marine Chemiluminescence
M.
ICB
AS
2018
A New Approach for Cancer
Therapy Based on Marine
Chemiluminescence Car la Mar isa P acheco Magalhães INS TITUT O DE CIÊNCIAS BIOMÉDICAS ABEL S ALAZAR MESTRADO ONCOLOGIA
A New Approach for Cancer Therapy Based on Marine
Chemiluminescence
Carla Marisa Pacheco Magalhães
M
2018I
Carla Marisa Pacheco Magalhães
A New Approach for Cancer Therapy Based on Marine
Chemiluminescence
Dissertação de Candidatura ao grau de Mestre em Oncologia –
Especialização em Oncologia Molecular, submetida ao Instituto de
Ciências Biomédicas Abel Salazar da Universidade do Porto
Orientador – Doutor Luís Pinto da Silva
Categoria – Bolseiro de Pós-Doutoramento
Afiliação – Faculdade de Ciências da Universidade do Porto
Co-orientador – Joaquim C.G. Esteves da Silva
Categoria – Professor Catedrático
III
Agradecimentos
Ao Prof. Dr. JCG Esteves da Silva, pela oportunidade de integrar uma equipa prestigiada de trabalho, na qual pude fortalecer as minhas capacidades de trabalho e desenvolver o meu conhecimento científico. Desta forma, sinto-me preparada para o mundo do trabalho, sendo capaz de responder positivamente a qualquer desafio que o futuro me proporcionará.
Ao meu orientador, Luís Pinto da Silva, por todos os conhecimentos transmitidos, pela paciência e disponibilidade em ensinar-me e corrigir todos os meus erros. Pela dedicação ao projeto e todas as ideias incríveis que só uma mente brilhante poderia ter. Por desenvolver a minha capacidade cognitiva e fazer-me pensar mais além. E por último, pela paciência com os meus problemas pessoais e conselhos valiosos, considero-o não só um colega de trabalho, mas também um amigo que levo comigo para a vida.
À Prof. Dr. Cármen Jerónimo pela possibilidade de integrar o Mestrado em Oncologia, permitindo desenvolver as minhas capacidades na área, assim como integrar uma equipa de renome com alguns dos melhores profissionais. Agradeço também a disponibilidade para resolver problemas que foram surgindo ao longo deste percurso.
Ao ICBAS, por toda a disponibilidade e vontade de auxiliar os seus alunos em todas as questões.
Aos restantes colegas de trabalho, à Diana, ao Paulo, à Ara, ao Ricardo, ao Guilherme, ao Abdo, ao El Hadi, ao José, ao Luís e ao André por toda ajuda e disponibilidade que sempre demonstram. Pela boa disposição e por me proporcionarem bom ambiente de trabalho. Por todas as piadas e momentos musicais. Por todos os conselhos e ajuda pessoal com que pude sempre contar, levo-os a todos no meu coração.
Aos meus pais, por toda a paciência comigo e por todo o apoio que me proporcionaram durante deste período.
IV Ao João por me acompanhar em todas as fases da minha vida e apoiar todas as minhas decisões. Por ouvir sempre as minhas preocupações e acreditar sempre em mim. Por tornar tudo mais fácil.
Ao Centro de Investigação em Química da Universidade do Porto (CIQUP), onde este trabalho foi realizado, pelas condições realizadas. Este trabalho foi feito no âmbito dos projetos NORTE-01-00145-FEDER-000028, POCI-01-0145-FEDER-006980 e PTDC/QEQ-QFI/0289/2014, financiados por fundos nacionais (FCT) e comunitários (FEDER).
V
Abreviations
ABDA- 9,10-anthracenediyl-bis(methylene)dimalonic acid Act- Activator
AMP- Adenosine monophosphate ATP- Adenosine triphosphate BET- Back electron transfer BL- Bioluminescence
BRET- Bioluminescent resonance energy transfer BS- Broken-symmetry
CASPT2- Complete-active-space second-order perturbation theory CIEEL- Chemically induced electron-exchange
CL- Chemiluminescence CO2- Carbone dioxide
CRET- Chemiluminescent resonance energy transfer CT- Charge transfe
DMSO- Dimethyl sulfoxide ET- Electron transfer
FRET- Fluorescence resonance energy transfer HPLC- High Performance Liquid cromatrography ICIC- Interstate crossing-induced chemiexcitation
IEFPCM- Integral equation formalism polarizable continuum model
IRC- Intrinsic reaction coordinates
LDH- Lactate desidrogenase MS- Multistate
m-THPC- Meta-tetra-hydroxyphenyl-chlorine
MTT- 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide NIR- Near infra-red
PDT- Photodynamic therapy PS- Photosensitizer
RLU- Relative light units
ROS- Reactive oxygen species SA- State-average
TD- Time-dependent TS- Transition states
VI
Abstract
Photodynamic therapy (PDT) is a procedure with the potential for superseding common cancer treatments, due to its minimally invasive character. It consists on the destruction of target tumor cells mainly by singlet oxygen, which is produced by light-induced activation of a photosensitizer. However, due to problems in light-penetration through tissues, this treatment is only applied to small tumors and/or the skin (or just under), as well on the surface of organs/cavities. PDT is unable to deal with metastasized cancer due to its localized nature. Thus, the goal of this work is to use new compounds as the basis for self-activating photosensitizers, which would be able to overcome the current limitations of PDT.
To this end, the novel photosensitizers will be based on marine Coelenterazine, that is able to emit chemiluminescence in the absence of a catalyst. More specifically, excited states are produced in the absence of an external light source. Thus, the problems typically associated with light-penetration into tissues are eliminated. The absence of a catalyst also facilitates the use of Coelenterazine chemiluminescence on PDT, as only the Coelenterazine should be administered to the patient, eliminating the difficulty of delivering several reaction components to the same cellular compartment without reacting during delivery.
Besides, Coelenterazine is not only an excitation source (through chemiluminescence), but also acts as a photosensitizer by itself. That is, the proposed chemiluminescence substrate produces chemiexcited triplet excited states able to
produce singlet oxygen by interaction with molecular O2.
While Coelenterazine presents some advantages to development of self-activated PSs, there are some problems that must be overcome before that. Namely, chemiluminescence reactions have low quantum yields and the Coelenterazine system is known for producing chemiexcited singlet excited states instead of triplet ones (the desired ones for producing singlet oxygen). These problems are difficult to be solved due to lack of knowledge and mechanistic insight into Coelenterazine. To address this, the present project pursued two different avenues of research (which can be considered also as the two main objectives of this thesis):
• Use of a combined spectroscopic, chromatographic and theoretical approach to obtain mechanistic insight into the chemiluminescence of Coelenterazine and related molecules, with emphasis on the chemiexcitation process, in different environments.
• Synthesis of novel Coelenterazine-based photosensitizers, their characterization (with focus on their ability of producing singlet oxygen), and the evaluation of their
VII
in vitro cytotoxicity towards tumor cells. This study can be considered as a
proof-of-concept phase.
The chemiexcitation step of Coelenterazine and Cypridina luciferin were studied by using a combined spectroscopic and computational approach in model DMSO solutions. It was found that the chemiluminescence of these molecules is pH-dependent: the total light output decreases with increasing pH, while the reaction rate has an opposite behavior. Further studies attributed these differences in the chemical equilibria of the dioxetanone intermediate. The thermolysis of neutral dioxetanone leads to a more efficient chemiexcitation efficiency. This results from neutral dioxetanone having access during its thermolysis to a degeneracy region, in which singlet ground and excited states are degenerated. Anionic dioxetanone does not appear to have access to this region.
While previous studies of the chemiluminescence of Coelenterazine has explored extensively the substitution of the imidazopyrazinone core at difference positions, the effect exerted by the solvent has been relatively unexplored. To bridge this gap in knowledge, it was studied comparatively the chemiluminescence of Coelenterazine and two derivatives in different aprotic solvents, by using a combined spectroscopic, chromatographic and theoretical approach. It was found that only DMSO and DMF allow for significant chemiluminescence at acidic pH. This was explained by the inability of most solvents to deprotonate the imidazopyrazinone core, preventing the oxygenation step of the chemiluminescent reaction.
So far, different researchers have tried to explain the chemiexcitation mechanism based on either electron transfer or charge transfer steps, which resulted in chemiexcitation by charge annihilation. However, recent evidence has discredited these hypotheses. Here was pursued an alternative explanation for the efficient chemiexcitation of dioxetanone molecules. The new mechanism is based on the degree
of interaction between the keto and CO2 moieties of dioxetanone. If favorable, the
detachment of CO2 to produce the light emitter is delayed, and the reacting molecules
have access to a zone of degeneracy between ground and excited states, which favors chemiexcitation.
Six novel Coelenterazines were synthesized with the goal that they can act both as a self-activation molecule and as photosensitizer. To this end, the derivatives were characterized spectroscopically in terms of chemiluminescence and the ability to produce singlet oxygen. Proof-of-concept was provided by performing in vitro toxicity assays in tumor cell lines. In conclusion, novel photosensitizers were produced with the ability of self-activation, without the need for either photoexcitation or energy transfer steps. This opens the door for using PDT for treating tumors irrespective of their size and localization in the body.
IX
Resumo
A terapia fotodinâmica (TFD) é uma potencial terapia no tratamento do cancro devido ao seu caracter minimamente invasivo. Este tratamento consiste na destruição de células tumorais alvo através de oxigénio singleto, que é produzido pela ativação do fotossensibilizador (FS), na presença de luz. No entanto, devido a problemas de penetração da luz nos tecidos, este tratamento apenas é aplicado a pequenos tumores e/ou na pele, bem como na superfície de órgãos/cavidades. A TFD é então incapaz de tratar cancros metastizados devido à natureza da sua localização. Posto isto, o objetivo deste trabalho é utilizar novos compostos como base para fotossensibilizadores auto-ativáveis, que serão capazes de superar as limitações correntes da TFD.
Para tal, o novo FS será baseado na Coelenterazina marinha que é capaz de emitir quimioluminescência (QL) na ausência de um catalisador. Mais concretamente, estados excitados são produzidos na ausência de uma fonte de luz externa. Logo, os problemas associados com a penetração de luz nos tecidos são eliminados. A ausência de catalisador também facilita o uso da QL da Coelenterazina na TFD, uma vez que apenas a Coelenterazina seria administrada ao paciente, eliminando as dificuldades associadas à libertação de todos os componentes reacionais no mesmo compartimento celular sem reagir durante a libertação.
Além disso, a Coelenterazina não é apenas a fonte de excitação (através da QL), mas também atua como FS. Isto é, o substrato QL proposto produz estados tripleto
excitados capazes de produzir oxigénio singleto através de interação com O2.
Apesar da Coelenterazina apresentar algumas vantagens para o
desenvolvimento de FS auto-ativáveis, há alguns problemas que têm ainda que ser ultrapassados. Nomeadamente, as reações QL têm baixo rendimento quântico e o sistema da Coelenterazina é conhecido pela produção de estados singleto excitados em vez de estados tripleto (os necessários para a produção de oxigénio singleto). Estes problemas são difíceis de resolver devido à falta de conhecimento e informação mecanística sobre a Coelenterazina. Para abordar este problema, o projeto presente segue duas vias de investigação (que podem ser considerados os dois objetivos principais desta tese):
• Uso de uma abordagem espetroscópica, cromatográfica e teórica combinada para obter informação mecanística sobre a QL da Coelenterazina e moléculas relacionadas, com ênfase no processo de quimioexcitação (QE), em diferentes meios. Este estudo foi feito através da compreensão dos mecanismos básicos da QL da Coelenterazina.
X • Síntese de novos FS baseados na Coelenterazina, a sua caracterização (com foco na sua capacidade de produzir oxigénio singleto) e a avaliação da sua citotoxicidade in vitro em células tumorais. Este estudo pode ser considerado como uma fase de prova de conceito.
O passo de QE da Coelenterazina e Cypridina luciferina foi estudado através de uma abordagem espetroscópica e computacional combinada em soluções modelo de DMSO. Foi descoberto que a QL destas moléculas é dependente do pH: o output total de luz diminuí com o aumento de pH, enquanto que a velocidade da reação apresenta o comportamento oposto. Estas diferenças foram atribuídas ao equilíbrio químico do intermediário dioxetanona. Mais especificamente, a termólise da dioxetanona neutra leva a uma QE mais eficiente. Isto resulta da dioxetanona neutra ter acesso a uma região de degeneração, na qual o estado fundamental singleto e estados excitados estão degenerados. A espécie aniónica não tem acesso a esta região.
Enquanto estudos prévios da QL da Coelenterazina exploraram extensivamente a substituição do core imidazopirazinona em diferentes posições, o efeito exercido pelo solvente não tem sido explorado. Para colmatar esta falta de conhecimento, foi feito um estudo comparativo da QL da Coelenterazina e dois derivados em diferentes solventes apróticos, através de uma abordagem espetroscópica, cromatográfica e teórica combinada. Foi descoberto que apenas o DMSO e o DMF permitem uma QL significativa a pH ácido. Isto foi explicado pela incapacidade da maioria dos solventes de desprotonar o core imidazopirazinona, o que previne a oxigenação da reação QL.
Até agora, diferentes investigadores têm tentado explicar os mecanismos de QE baseando-se em transferência eletrónica ou transferência de carga, o que resulta em QE por aniquilação de carga. No entanto, evidências recentes desacreditaram estas hipóteses. Neste trabalho propusemos uma explicação alternativa para a QE eficiente das moléculas de dioxetanona baseado no grau de interação entre os fragmentos ceto
e CO2 da dioxetanona. Quando favorável, a separação do CO2 para produzir a molécula
emissora de luz é retardada e as moléculas reagentes têm acesso a uma zona de degeneração entre o estado fundamental e os estados excitados, o que favorece a QE.
Seis novas Coelenterazinas foram sintetizadas com o objetivo de atuarem como
molécula auto-ativável e como FS. Os derivados foram caracterizados
espetroscopicamente e foi avaliada a capacidade de produção de oxigénio singleto. Prova de conceito foi adquirida pela realização de ensaios in vitro de toxicidade em linhas celulares tumorais. Para concluir, novos FS foram produzidos com capacidade de auto-ativação, sem a necessidade de fotoexcitação ou passos de transferência de energia. As descobertas abrem então uma porta para o uso TFD no tratamento de tumores independentemente do seu tamanho e localização no corpo.
XII Index
1. Introduction ... 1
1.1. Photodynamic therapy ... 1
1.2. General Mechanisms of Chemi- and Bioluminescence ... 3
1.3. Chemiexcitation Mechanisms Responsible for CL/BL Emission ... 5
1.4. Mechanisms of the Systems Already Studied for Photodynamic Therapy ... 7
1.4.1. Luminol reaction ... 7
1.4.2. Firefly luciferin reaction ... 8
1.4.3. Coelenterazine reaction ... 8
1.5. Application of Chemi- and Bioluminescence to Photodynamic Therapy ... 11
1.5.1. Firefly Luciferin-Mediated Photodynamic Therapy ... 11
1.5.2. Coelenterazine-Mediated Photodynamic Therapy ... 11
1.5.3. Luminol-Mediated Photodynamic Therapy ... 12
1.6. Pitfalls of Chemi-/Bioluminescence-Mediated Photodynamic Therapy ... 14
1.7. Other Strategies for Deep Photodynamic Therapy ... 15
1.8. Objectives of this work ... 17
1.9. Structure of this work ... 19
2. Mechanistic insight Cypridina luciferin and Coelenterazine chemiexcitation . 21 2.2.1. Kinetic chemiluminescent assay... 21
2.2.2. Measurement of chemiluminescence and fluorescence spectra ... 22
2.2.3. Measurement of UV-visible spectrum ... 22
2.2.4. Theoretical investigation of Cypridina Dioxetanone ... 22
2.3.1. Chemiluminescence of Cypridina Luciferin in DMSO ... 23
2.3.2. Theoretical Investigation of Cypridina Dioxetanone ... 26
2.3.3. Chemiluminescence of Coelenterazine in DMSO ... 30
2.3.4. Theoretical Investigation of Coelenterazine Dioxetanone ... 36
2.3.5. UV-Vis spectrum of Coelenterazine ... 37
3. Comparative Study of the Chemiluminescence of Coelenterazine, Coelenterazine-e and Cypridina Luciferin ... 40
3.2. Materials and methods ... 40
3.2.1. Kinetic chemiluminescent assays for Cypridina luciferin, Coelenterazine, Coelenterazine-e and Coelenteramide ... 40
3.2.2. Measurement of fluorescence spectra for Cypridina luciferin, Coelenterazine, Coelenterazine-e and Coelenteramide ... 41
3.2.3. Chromatographic assays ... 41
3.2.4. Theoretical study of chemiexcitation mechanism of model dioxetanones in different solvents ... 41
XIII
3.2. Results and discussion ... 42
4. Theoretical study of chemiexcitation mechanism of model dioxetanones from TD-DFT and Multi-Reference Calculations ... 58
4.2. Materials and methods ... 58
4.3. Results and Discussion ... 60
5. Synthesis and evaluation of self-illuminating photosensitizers ... 69
5.2. Materials and methods ... 69
5.2.1. Synthesis Methodology ... 69
5.2.2. Kinetic chemiluminescent assay... 69
5.2.3. Measurement of chemiluminescence and fluorescence spectra ... 70
5.2.4. Fluorescent assay for detection of singlet oxygen ... 70
5.2.5. In vitro assays for the cellular viability ... 70
5.3. Results and discussion ... 70
5.3.1. Chemiluminescence and fluorescence of Compounds 3, 4 and 5 in DMSO . 70 5.3.2. Fluorescent assay for detection of singlet oxygen ... 72
5.3.3. In vitro toxicity assays ... 73
6. Conclusions and future work ... 78
7. References ... 81
XIV
Index of figures
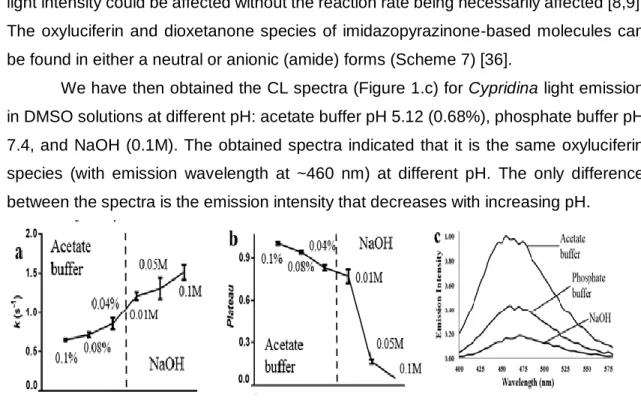
Figure 1- Rate constants (k, in s-1) at acidic and basic pH in DMSO solutions (a). Plateau of light intensity (normalized values) at and basic pH (b). Chemiluminescence spectra in DMSO (c). ... 24
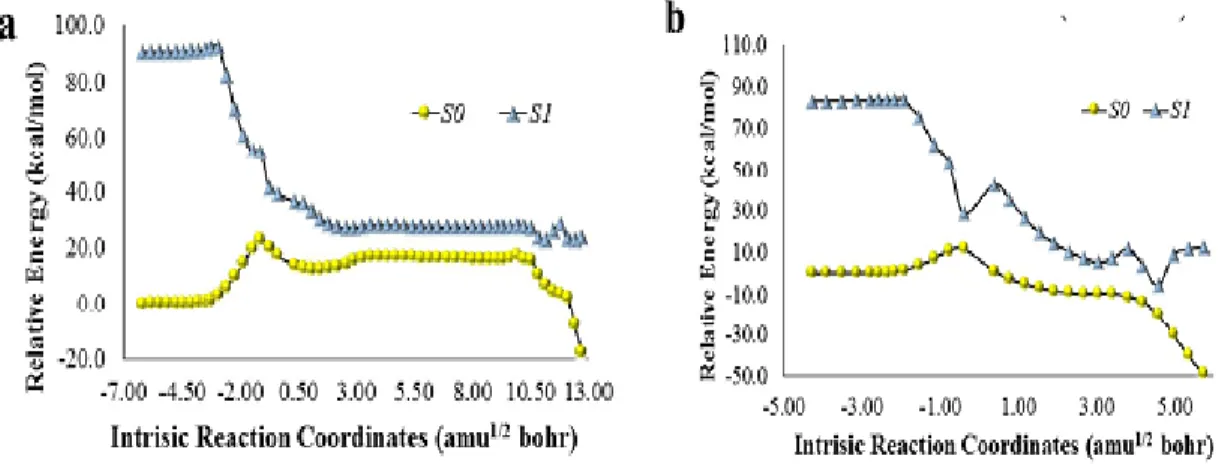
Figure 2- Potential energy curves of S0 and S1 states, as a function of intrinsic reaction
coordinates, of model (Scheme 9, R2=R3=CH3) neutral (a) and anionic (b) dioxetanone.
... 27 Figure 3- Electron spin density of the dioxetanone and imidazopyrazinone moieties of
anionic (a) and neutral (b) model Cypridina dioxetanone (Scheme 9, R2=R3=CH3), as a
function of intrinsic reaction coordinates. ... 28
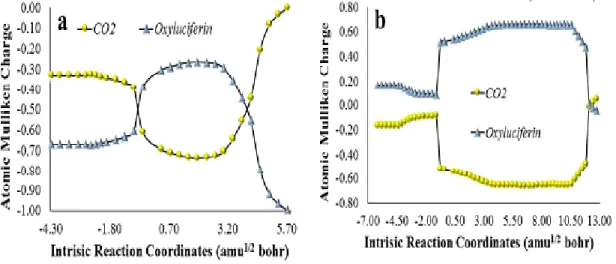
Figure 4- Atomic Mulliken charge of the CO2 and oxyluciferin moieties of anionic (a) and
neutral (b) model Cypridina dioxetanone (Scheme 9, R2=R3=CH3), as a function of
intrinsic reaction coordinates. ... 30
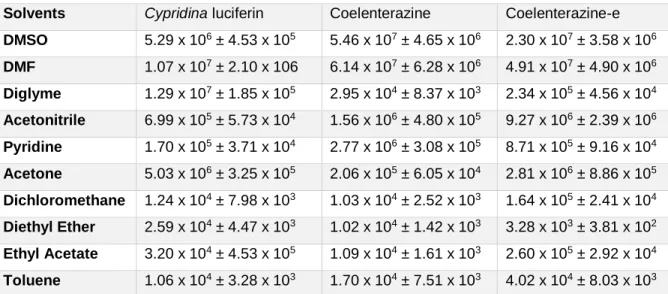
Figure 5- Calculated area (in RLU) of the CL profile of Coelenterazine, between 0 and
85 seconds, with increasing concentrations. Values were obtained at acidic (by addition of acetate buffer pH 5.12), neutral (phosphate buffer pH 7.4) and basic pH (by addition of NaOH). ... 32
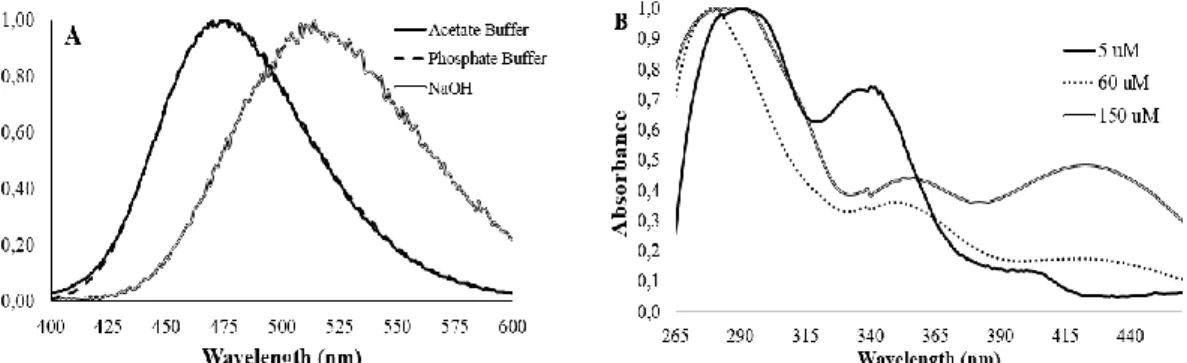
Figure 6 - CL spectra (normalized values) of the light-emitting reaction of Coelenterazine
(3 µM) in DMSO (A). UV-Vis spectra (normalized values) obtained in DMSO (in the presence of acetate buffer pH 5.12, 0.68%), with increasing concentrations of Coelenterazine (B). ... 34
Figure 7- Atomic Mulliken charge of the CO2 and Coelenteramide moieties of an anionic and neutral model Coelenterazine dioxetanone (a phenol and methyl groups at the C6 and C2, respectively), as a function of intrinsic reaction coordinates. Calculations were made at ωB97XD/6-31G(d,p) level of theory in implicit diethyl ether, as described in reference [45]... 37
Figure 8- CL profile (light emission as a function of time) for Coelenterazine in
DMSO/DMF (A) and acetonitrile/pyridine (B), with addition of acetate buffer pH 5.12. These profiles are also representative for the other imidazopyrazinones here studied (Cypridina luciferin and Coelenterazine-e). Fluorescence spectra (normalized) for Coelenteramide in DMF and acetonitrile (C), with addition of acetate buffer pH 5.12. . 44
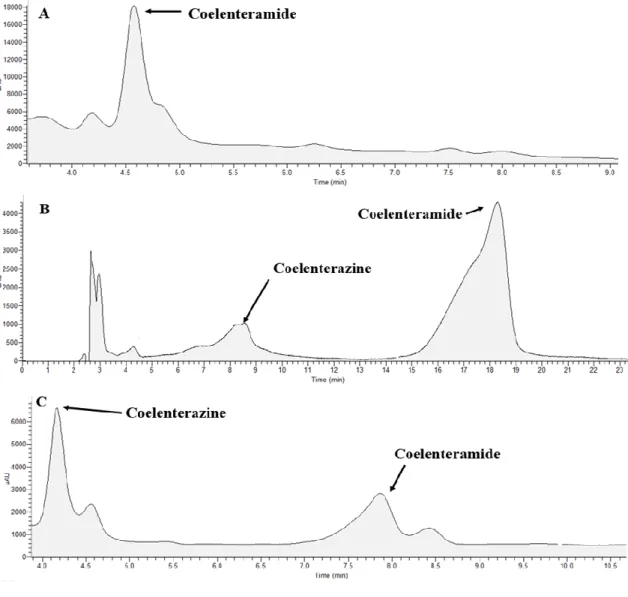
Figure 9- Chromatograms obtained for the reaction mixtures of Coelenterazine CL in
DMF (A), acetonitrile (B) and diethyl ether (C), all with addition of acetate buffer pH 5.12 (0.68%). The mobile phase was composed of water (70%) and acetonitrile (30%) for reaction mixtures in acetonitrile and diethyl ether. For reaction mixtures in DMF the percentage of acetonitrile was increased to 40%. The flux was of 0.35 mL per minute. ... 47
Figure 10- Chromatograms obtained for the reaction mixtures of Coelenterazine CL in
DMF (A), acetonitrile (B) and diethyl ether (C), all with addition of NaOH 0.1M. The experimental conditions were the same as the ones indicated in Figure 9... 48
Figure 11- Potential energy curves for S0 and S1 states, as a function of intrinsic reaction
coordinates, of model neutral dioxetanone (Scheme 9; R1 = indole, R2 = R3 = CH3). The
calculations were made at the TD UωB97XD/6-31+G(d,p) level of theory, in implicit DMF (A), acetonitrile (B) and diethyl ether (C). ... 50
Figure 12- Potential energy curves for S0 and S1 states, as a function of intrinsic reaction
coordinates, of model anionic dioxetanone (Scheme 9; R1 = indole, R2 = R3 = CH3). The
calculations were made at the TD UωB97XD/6-31+G(d,p) level of theory, in implicit DMF (A), acetonitrile (B) and diethyl ether (C). ... 51
XV
Figure 13- Calculated area of the CL profile (up to 85 seconds) obtained in the
chemiluminescent reaction of Coelenterazine (1µM), in either DMF, DMSO and acetonitrile (A). Fluorescence intensity of Coelenteramide (1 µM), in either DMF, DMSO and acetonitrile (B). To the mixtures were added either acetate buffer pH 5.12 (0.68%), NaOH 0.1M or NaOH 0.3 M. ... 54
Figure 14- Hole-electron distribution for the S0 → S1 chemiexcitation at the TS structure
for the thermolysis reaction of a neutral dioxetanone model (Scheme 10, R1 = phenol, R2
= R3 = CH3), at the TD UωB97XD/6-31+G(d,p) level of theory in implicit DMF (A) or
diethyl ether (B). ... 55
Figure 15- Transition dipole moment for the S0 → S1 chemiexcitation at the TS structure
for the thermolysis reaction of a neutral dioxetanone model (Scheme 10, R1 = phenol, R2
= R3 = CH3), at the TD UωB97XD/6-31+G(d,p) level of theory in implicit DMF (A) or
diethyl ether (B). ... 56
Figure 16- Active orbitals included in the MS-CASPT2 calculations for the thermolysis
of -CH2CH3 dioxetanone. ... 59
Figure 17- Active orbitals included in the MS-CASPT2 calculations for the thermolysis
of -CH2CF3 dioxetanone. ... 59
Figure 18- Potential energy curves of S0 and S1 states, as a function of intrinsic reaction coordinates, of model dioxetanones (Scheme 11). The curves were calculated at the
ωB97XD/6-31+G(d,p) level of theory. Dioxetanone species: -CH3 (A), -OH (B), -CH2CH3
(C) and -CH2CF3 (D). ... 62
Figure 19- Potential energy curves of the S0, S1 and S2 states as a function of intrinsic reaction coordinates of model dioxetanones (Scheme 11). The calculations were made at the MS-CASPT2/ANO-RCC-VDZ, on top of geometries obtained in
ωB97XD/6-31+G(d,p) optimizations. Dioxetanones species: -CH2CH3 (A) and -CH2CF3 (B). ... 63
Figure 20- ESP atomic charges, as a function of intrinsic reaction coordinates, of model
dioxetanones. The charge densities were calculated at the ωB97XD/6-31+G(d,p) level
of theory. Dioxetanone species: -CH3 (A), -OH (B), -CH2CH3 (C) and -CH2CF3 (D). .... 64
Figure 21- E(ζ), ∆∆E(ζ)strain and ∆∆E(ζ)int values plotted as a function of intrinsic
reaction coordinates, during the thermolysis of model dioxetanones (Scheme 11). They
were calculated at the ωB97XD/6-31+G(d,p) level of theory. Dioxetanone species: -CH3
(A), -OH (B), -CH2CH3 (C) and -CH2CF3 (D). ... 65
Figure 22- Variation of the O-C-O angle (in Å), as a function of intrinsic reaction
coordinates, of the CO2 moieties of model dioxetanones during their thermolysis
reactions. Dioxetanone species: -CH3 (A) and -CH2CH3 (B). ... 67
Figure 23- CL profile (light emission as a function of time) for Compounds 3, 4 and 5
and respective area of chemiluminescence profile. ... 71
Figure 24- Fluorescence spectra (normalized) for light emitter species of the
Compounds 3, 4 and 4. ... 72
Figure 25- CL spectra of the light-emitting reaction of Compounds 3 and 5. ... 72 Figure 26- Analysis of the fluorescence of an oxygen singlet probe (ABDA), with
increasing concentrations of the studied compounds. Values were obtained at neutral (phosphate buffer pH 7.4). ... 73
Figure 27- Percent viability measured by MTT reduction assays for Compounds 1 (24
and 48h) and 2 (24H), at increasing concentrations. The percentage values above some bars refer to toxicity measured with the LDH assay, when statistically significative. Percent viability was obtained by comparing with control cell viability, considered as 100%. Data represents the mean ± SD of measurements of three independent experiments. Results of the one-way ANOVA factorial analysis. ... 74
Figure 28- Percent of cytotoxicity measured by MTT assay (bars) and percentage of
XVI compounds. Percent viability was obtained by comparing with control cell viability, considered as 100%. Data represent the mean ± SD of measurements of three independent experiments. Results of the one-way ANOVA factorial analysis. ... 75
XVII
Index of schemes
Scheme 1 - The Type I and II mechanisms of action of PDT. ... 1 Scheme 2- Representation of the three sub-groups of peroxide-containing CL/BL
substrates. ... 3
Scheme 3- Reaction mechanisms for the thermal decomposition of dioxetanones... 4 Scheme 4- Representation of the intermolecular CIEEL (a) and revised CIEEL
mechanisms (b) for dioxetanones CL. ... 5
Scheme 5 - Schematic representation of the chemi- and bioluminescent reactions
studied as excitation sources in PDT. ... 7
Scheme 6- General structure of imidazopyrazinones, and structures for relevant
examples of natural imidazopyrazinones. ... 9
Scheme 7- General mechanism for the CL reaction of Coelenterazine. ... 10 Scheme 8- Comparison between the conventional CL-mediated PDT and the approach
proposed in this dissertation... 17
Scheme 9- Reaction mechanism of Cypridina CL. ... 25 Scheme 10- Schematic representation of Coelenterazine and solvent molecules and
its respective state of protonation. ... 52
Scheme 11- Representation of known cyclic peroxides involved on chemi- and
XVIII
Index of tables
Table 1- Rate constants (k, in s-1) and plateau of light intensity (normalized values) of Cypridina CL in DMSO, with addition of phosphate buffer pH 7.4 or NaOH (0.1M). .... 23
Table 2- Activation energies, with thermal corrections, (in kcal mol-1) for the thermolysis reaction of neutral and anionic model Cypridina dioxetanones (Scheme 9), at the
ωB97XD/6-31+G(d,p) level of theory in DMSO. The R1 substituent is an indole moiety in
all models (Scheme 9). ... 26
Table 3- Rate Constants (k, in s-1) and Plateau of Light Intensity (in Relative Light Units, RLU) of Coelenterazine Chemiluminescence in DMSO, with Addition of Acetate Buffer pH 5.12, Phosphate Buffer pH 7.4 or NaOH (0.1 M). ... 31
Table 4- Ratios between Coelenterazine, Coelenteramide and the proposed dimer, in
DMSO solutions with increasing concentrations of Coelenterazine (in the presence of acetate buffer pH 5.12, 0.68%). The ratios were calculated by measuring the UV-Vis spectra of these solutions (Figure 7.B), and by considering the absorbance of the peaks attributed to each species, based on previous findings by us and other authors. [84–87] ... 38
Table 5- Calculated area (in RLU) of the CL profile of different imidazopyrazinones,
between 0 and 85 s, with addition of acetate buffer pH 5.12. ... 42
Table 6- Light intensity maxima (in RLU) of the CL profile of different
imidazopyrazinones, between 0 and 85 s, with addition of acetate buffer pH 5.12. ... 43
Table 7- Initial velocities (in RLU s-1) under steady-state conditions for the CL reaction of different imidazopyrazinones, with addition of acetate buffer pH 5.12. ... 43
Table 8- Activation energies (Eact, in kcal mol-1) for the thermolysis reaction of four model dioxetanone species (Scheme 11), at the ωB97XD/6-31+G(d,p). ... 60
1
1. Introduction
1.1. Photodynamic therapy
Photodynamic therapy (PDT) is a therapeutic approach used for treating esophageal, skin, non-small cell lung cancers [1,2]. To induce cytotoxicity, this therapy induces the synthesis of reactive oxygen species (ROS). ROS can destroy cancer cells by inducing immunostimulation, apoptosis, vessels shutdown and necrosis [2]. A non-toxic photosensitizer (PS), presence of molecular oxygen and light (of specific wavelength) are three fundamental requirements of PDT [1–3]. The excitation of PS induces the cytotoxic effects, once light is irradiated in the target site. More specifically, photo-excitation occurs from the singlet ground state to an excited singlet state. Then, that excited state, via intersystem crossing, crosses to long-lived triplet states, inducing ROS production [3,4].
There are two pathways to form ROS (Scheme 1) [3,4]: the Type I and II reaction. On the first one, the triplet state of the PS transfers an electron or a hydrogen-atom to biological molecular oxygen or substrates, resulting in the formation of radical species. The latter one is the most prevalent one in PDT, in which the triplet state of the PS
transfer its energy to molecular O2, leading to the formation of singlet O2.
Scheme 1 - The Type I and II mechanisms of action of PDT.
PDT has some advantages over other cancer treatments (such as surgery, radiotherapy and chemotherapy), as the PS only undergoes activation under light excitation under specific wavelengths. This feature of PDT allows for reduced side-effects. However, the depth of light penetration into biologic tissues is smaller than 1 cm, [1–4]. Therefore, PDT can only be used on tumors located on the lining of internal organs/cavities and under the skin. Given all this, PDT is a localized therapy, being unsuccessful against metastatic tumors [1].
Ground State Singlet excited states Triplet excited states Intersystem Crossing
Radicals and
H
2O
2Singlet
Oxygen
LightPS
PS*
2 Consequently, it is essential to design PSs that can be activated intracellularly without the need for external light sources. This achievement can increase the potential of PDT in successful cancer therapy. Several researchers have been trying to accomplish this goal through the creation of “self-illuminating” PDT systems based on either chemiluminescent resonance energy transfer (CRET) or bioluminescent RET (BRET) [1–6]. Such processes consist on non-radiative energy from chemi- or bioluminescent donors to an appropriate acceptor molecule.
Bioluminescence (BL) is defined as the light emission in enzyme-catalyzed reactions in living organisms [7–10]. This phenomenon is observed in fireflies, worms, insects and bacteria. BL is originated from the oxidation of a molecule termed luciferin, in a reaction catalyzed by an enzyme, luciferase. BL can be considered a sub-type of chemiluminescence (CL), consisting in the production of light as a result of chemical reactions [8]. Many BL/CL systems have been studied [8,9,11–15] and both have numerous advantages as high quantum yields, specificity and speed of reaction. Consequently, these systems have been useful in microbial detection, bioimaging, analytical determination, and gene reporter [16,17].
3
1.2. General Mechanisms of Chemi- and Bioluminescence
Light emission from CL/BL systems results from enzyme-catalyzed reactions that are divided into two classes: luciferase-luciferin reactions [8,9,18,19] and photoprotein systems [19,20]. In luciferase-luciferin reactions (the most prevalent in bioluminescent systems), the luciferase enzyme catalyzes the oxidation of luciferin, generating an
electronically excited singlet state product. This product, oxyluciferin, relaxes to the S0
state by photon emission. The luciferin, luciferase and oxyluciferin show differences between bioluminescent species [8,9,21].
Photoprotein systems are present only in marine organisms. In these systems occurs the formation of a stable enzyme-substrate [8,9,18,19] , which is formed between the apoprotein and an oxygenated marine luciferin (2-hydroxyperoxycoelenterazine). The light emission is due to the decomposition of the stable complex, which occurs when calcium ions bind to the photoprotein [22].
The efficiency of light emission of CL/BL reactions is described as quantum yield,
which is determined by three different factors [8,9]: yield of the S0 conversion of luciferin
into oxyluciferin; chemiexcitation yield of singlet luminophores; fluorescent quantum yield of the emitter. Commonly, BL reactions have higher quantum yields, with some reactions reaching yields of 45-61% [23]. Given the efficient conversion of chemical energy into light emission, the relative non-toxicity of luciferin molecules and relatively simple chemistry of these systems (among other characteristics), several CL/BL systems gained several biomedical, pharmaceutical and bioanalytical applications (besides PDT) [16,23].
Most of CL substrates possess a peroxide bond (-O-O-), which provides a pathway for the production of excited state products [14,24,25]. These peroxide substrates are sub-divided into three groups, as seen in Scheme 2 [8,9,11,26].
Scheme 2- Representation of the three sub-groups of peroxide-containing CL/BL
substrates.
Dioxetanes and dioxetanones decompose thermally into an optically-active excited state, which is the most important feature for a CL molecule. This was verified
4 by thermochemical calculations (heats of reaction and activation energies varying
between 70-90 and 20-30 kcal mol-1, respectively).
There are two reaction mechanisms proposed for the ground state decomposition of these peroxides [9,11,27]: the stepwise-biradical mechanism and the concerted mechanism (Scheme 3). In the first one, the reaction is initiated by homolytic cleavage of the peroxide bond, producing a biradical transition state. The C-C bond breaking occurs only after the formation of the transition state; the one based on a concerted thermolysis, in which both O–O and C–C bond breaking occurs simultaneously.
5
1.3. Chemiexcitation Mechanisms Responsible for CL/BL Emission
The Chemically Induced Electron-Exchange Luminescence (CIEEL, Scheme 4) explains the efficient generation of singlet excited states by CL/BL [28,29]. Namely, a radical ion pair is generated by an electron transfer (ET) from an oxidazable electron-rich moiety (or activator, Act) to the peroxide. This ion pair then suffers back electron transfer (BET) from the carbonyl radical anion to the radical cation, forming excited states with high efficiency. This mechanism can be called intramolecular or intermolecular CIEEL. The first, when the Act is part of the organic peroxide and the latter if the Act is another molecule.
Nevertheless, the validity of the CIEEL theory has been questioned since the CL of main examples of CIEEL (diphenoyl peroxide and dimethyldioxetanone) was re-examined [30,31]. The studies revealed these peroxides presented relatively low CL quantum yields, despite undergoing a CIEEL decay. Given the failure of this mechanism, different authors have suggested different mechanisms in which the formation of excited states is explained by charge (CT) and back charge transfer (BCT), instead of full electron transfer (Scheme 4). Such mechanisms are generally termed Charge Transfer Induced Luminescence (CTIL) [30,32,33]. Nevertheless, these new formulations of CIEEL still clash with the known data. Theoretical calculations showed that CT/BCT also occurred when no Act moiety was found [9,11]. Additionally, the presence of Act moieties does not guarantee the efficient generation of singlet excited states [24,30].
Scheme 4- Representation of the intermolecular CIEEL (a) and revised CIEEL
mechanisms (b) for dioxetanones CL.
Taking these finding into account, our group have developed the Interstate Crossing-Induced Chemiexcitation (ICIC) mechanism [11,34,35]. While ET/CT steps appear to have an important and beneficial effect on the activation barrier for the
6 thermolysis, these processes seem to have an opposite effect on the chemiexcitation step. Theoretical calculations have showed that a more efficient chemiexcitation is achieved when the reacting molecules can access a region of the PES, where the ground and excited states are degenerated/near-degenerated [11,32,34–36].
Despite the ongoing discussion regarding the mechanism responsible for efficient chemiexcitation, three key structural moieties were identified for the different CL/BL substrates (Scheme 3) [8,34]. The first is the peroxide bond that initiates the thermally-activated singlet chemiexcitation. Despite some differences, this type of bond is a constant in this type of process. The second moiety is an electron-rich moiety, responsible for tuning the activation energy of the thermolysis [11,34,35]. This is also a ubiquitous moiety, except for Latia luciferin in which is thought that the enzyme provides the moiety in the form an amino-acid. [16] Finally, the last moiety is an ionizable group, that finely tunes the activation energy for the thermolysis [11,32,34–36].
7
1.4. Mechanisms of the Systems Already Studied for Photodynamic Therapy
1.4.1. Luminol reaction
The oxidation of luminol in basic solutions is the most efficient CL reaction (Scheme 5) [37–39]. It is observed with the addition of hydrogen peroxide and oxidant catalysts (such as Cu (II), Fe (II), periodate ions, Co (II), or hydrogen peroxidase). The CL reaction is triggered by the interaction of luminol with hydroxide anions, leading to the formation of a dianion [37–39]. The dianion reacts with oxygen to yield the aminophthalate ion in an emissive state, which is responsible for the emission of bright blue light (with a maximum of 425 nm) [37–39].
Scheme 5 - Schematic representation of the chemi- and bioluminescent reactions
8
1.4.2. Firefly luciferin reaction
While luminol oxidation is the most well-described CL reaction, the luciferase-catalyzed oxidation of firefly luciferin is the most studied BL system (Scheme 5). The luciferase enzyme catalyzes the adenylation between luciferin and adenosine triphosphate (ATP), leading to the formation of an adenylyl intermediate. Then, this
intermediate is oxidized by O2, leading to the formation of the chemiexcited luminophore
(oxyluciferin), and to the release of adenosine monophosphate (AMP) and carbon
dioxide (CO2).
One of the interesting features of this BL system is its pH-sensitive light emission [8,9,40]. While at basic pH the emission peaks at ~560 nm, it shifts to a maximum of ~620 nm at acidic pH. This phenomenon occurs due to pH-induced changes on the active site microenvironment, which leads to changes in the intermolecular interactions formed between oxyluciferin and active site molecules [7,41,42]. Another relevant feature for the application of this system to photodynamic therapy is its flash pattern of light emission [21,43,44]. The in vitro emission, at relatively high substrate concentration, commences with a brief flash that quickly decays to negligible levels. This pattern is caused by the production of inhibitory products, with one of them being the light emitter itself: oxyluciferin (Ki = 0.50 ± 0.03 µM) [21,43,44]. However, the most important inhibitor is dehydroluciferyl-adenylate (Ki = 3.8 ± 0.7 nM), an oxidation by-product of the bioluminescent reaction [21,43,44].
1.4.3. Coelenterazine reaction
Coelenterazine is a common marine luciferin, which is used by most BL marine organisms as a substrate for luciferase-luciferin and photoprotein-based reactions [22]. Several luciferases from natural sources have demonstrated activity by using Coelenterazine as a substrate [22]. However, Renilla reniformis, Gaussia and Metridia
longa luciferases are the ones which have been used in practical applications of
luciferase-Coelenterazine reactions.
Coelenterazine is part of the imidazopyrazinone class of CL/BL molecules, in which also are included Cypridina and Watasenia luciferins (Scheme 6). This class connects many luminescent substrates found in marine organisms [23,45]. The luminescent reactions of imidazopyrazinone-based compounds have been described as
follows (Scheme 7) [13,22,46,47]: the imidazopyrazinone scaffold reacts with O2, which
quickly leads to the generation of a dioxetanone intermediate. Upon decomposition of this latter species into chemiexcited oxyluciferin, visible light is emitted due to radiative
decay of the chemiluminophore to the ground state. Besides O2, the imidazopyrazinone
9 generate visible light. In this way, these species have also been used as CL probes for reactive oxygen species [47].
In the specific case of Coelenterazine, the oxyluciferin species is called Coelenteramide (Scheme 7). Upon chemiexcitation, this luminophore is responsible for emission of blue-green light with a spectral peak at 480 nm.
Scheme 6- General structure of imidazopyrazinones, and structures for relevant
10
11
1.5. Application of Chemi- and Bioluminescence to Photodynamic Therapy
1.5.1. Firefly Luciferin-Mediated Photodynamic Therapy
Theodossiou and co-workers tested the potential application of BL to PDT by using the firefly luciferase-luciferin system as an intracellular excitation source for Rose Bengal [6]. They transfected cell lines with a Fluc gene, adding latter both luciferin and the PS to the cell cultures. These tested system led to a 90% toxicity, thus supporting the use of firefly BL as an excitation source [6].
Later, Schipper and colleagues have re-assessed the use of the firefly system in PDT [48], affirming that it was not clear if the photon output of Fluc transfected cells could cause similar photodynamic effects to those induced by light doses typically
administrated in PDT (around 50 mW/cm2, and above 1 J/cm2) [48]. Contrary to the
previous study, the photon output obtained was only 1.2x10-9 mW/cm2 [6]. Thus,
according to this study, two different PS (hypericin and Rose Bengal) were not capable to produce a cytotoxic effect [48]. However, while Theodossiou and co-workers used luciferin concentrations of 500 µM [6], Schipper and colleagues used significantly lower concentrations (as high as 20 µM) [48]. Thus, the different results obtained in these studies are not unexpected.
1.5.2. Coelenterazine-Mediated Photodynamic Therapy
The Renilla reniformis luciferase-Coelenterazine BL system was also tested as an intracellular excitation source in PDT. Lai and co-workers [49] were the first group that have studied this system in the context of PDT. To absorb the photons emitted by Coelenterazine BL via BRET, they have conjugated luciferase with quantum dots. This approach allows the use of the BRET reaction with a higher number of PS, as the emission wavelength of the quantum dots is more easily tunable than that of BL.
These BL-quantum dots conjugates were used to excited the marketed PS meta-tetra-hydroxyphenyl-chlorine (m-THPC, Foscan®), in mice treated with human lung adenocarcinoma epithelial A549 cells [49]. The results were promising since in the mice treated with the conjugates, the tumor growth was significantly reduced. Besides that, when compared to treated with m-THPC/Coelenterazine (4.2% inhibition) or luciferase/Coelenterazine/quantum dot (23.3% inhibition) the isolated tumor sizes of
PDT-treated groups (luciferase/quantum dots/m-THPC/Coelenterazine) was
meaningfully lower. However, this study also showed that the luciferase-quantum dot conjugates can be cytotoxicity by themselves, reducing its clinical applicability. The conjugates were demonstrated to decrease the degree of vascularization, leading to suppression of tumor growth.
12 The authors had also aimed towards the evaluation of the efficiency of bioluminescence-mediated PDT compared with conventional external light irradiation [49]. They have observed, at the same concentration of PS, that the BRET-induced PDT
yielded an irradiation of 0.6-0.8 J/cm2. This was enough to produce a significant
photodynamic effect in vivo, despite being lower than the light doses used in clinical PDT
(above 1 J/cm2). Additionally, the photon output measured (0.6-0.8 J/cm2) is significantly
higher than the measured values by Schipper et al. in the study of firefly
luciferin-mediated PDT (1.03 x 10-4 mJ/cm2) [48,49].
In conclusion, the work of Lai and co-workers supported the use of Coelenterazine BL, when coupled to quantum dots, being an alternative for excitation source for PDT [49]. As the induced cytotoxicity was not significant, further optimization of the system will be needed. The decrease of efficiency of the system can be due to a great number of energy transfer steps, as there are two energy transfer steps: the BRET step, in which the energy is transferred from the BL reaction to the nanoparticles; the second step is a FRET process from the quantum dots to the PS.
Recently it was reported a Renilla reniformis luciferase-Coelenterazine BL-mediated PDT [50]. Given the lower energy output obtained when compared to the required for conventional PDT, Kim and co-workers wanted to assess if BL is a good excitation source to be used in PDT [48–50]. Thus, they developed luciferase-quantum dot conjugates to excite intracellularly chlorine e6 (Ce6). Mice’s were treated with three tumor cell lines: colorectal (CT26), melanoma (B16F10) and lung cancer cells (LLC). The authors began with the calculation of the efficiency of BRET from Coelenterazine to the quantum dots (60-65%). It is interesting to note that while Ce6 molecules (concentration
of 100 µM) are activated 4 x 107 times per minute by a flux from a 660 nm laser light with
2.2 mW, the same PS is activated 3 x 108 per minute by the
luciferase/Coelenterazine/quantum dot conjugates [50]. Hence, BRET energies in the order of 100 µJ can generate a more efficient activation in the cellular membrane than a laser energy.
1.5.3. Luminol-Mediated Photodynamic Therapy
The well-known luminol system was first studied by Firer and co-workers, towards its use in PDT [1]. They added luminol, hydrogen peroxide and a catalyst (ferrous sulphate) to murine hybridoma cells. The aim was to evaluate the efficiency of luminol for the activation of the PS haematoporphyrin (Hp) [1]. The results showed potential, as the activation of Hp by luminol resulted in an approximate 100% cytotoxicity [1]. However, the system revealed some biocompatibility issues, as luminol induced by itself about 15% cytotoxicity [1].
13 Besides that, a similar luminol system (containing hydrogen peroxide and horseradish peroxidase) was assessed by Wang et al. These researchers analyzed the potential of luminol to activate the PS oligo (p-phenylene vinylene) (OPV) [51]. Less than 10% in vitro cellular viability was observed with increasing OPV concentration [51]. Still, the CL system was also toxic to normal human cells, induced 30% toxicity by itself towards HeLa cells [51]. Similarly to other studies, [1,51] this system has some biocompatibility issues. Furthermore, the results indicated that this system has no specificity for tumor cells. The therapeutic efficacy of luminol was also evaluated by Wang and co-workers in HeLa cell tumor-bearing nude mice [51]. They observed ~ 30% of tumor inhibition ratio by the CL-mediated PDT group in comparison with the CL system alone.
The most recent report involving luminol [52] consisted on the construction of a nanoconjugate. In it, semiconducting polymer dots (Pdots) incorporating the PS m-THPC and amphiphilic Janus dendrimers were used to conjugate horseradish peroxidase and aminated folic acid onto the surface of the Pdots [52]. The potential for PDT was assessed in vitro by incubating the Pdots nanoconjugates with C6 glioma, MCF-7 breast cancer and NIH 3T3 fibroblasts cells. [52]. When the concentration of the nanoconjugates was 10μg/mL, 72%, 32%, and 17% cell viabilities were observed for the NIH 3T3, C6, and MCF-7 cells, respectively. It should be noted that the difference in cell viabilities between non-cancerous (NIH 3T3) and cancerous (C6 and MCF-7) cells can be attributed to aminated folic acid present in the surface of Pdots, given the overexpression of folate receptors in tumor cells [52].
14
1.6. Pitfalls of Chemi-/Bioluminescence-Mediated Photodynamic Therapy
The studies previous discussed showed the potential of CL/BL systems for acting as intracellular excitation sources in PDT, opening the way for addressing the current limitations of clinical PDT. However, the cytotoxicity induced by the studied CL/BL systems is not high enough for their use in clinical PDT and require further optimization. This insufficient cytotoxicity can be attributed to their mechanism of use. It is required that the CL/BL substrate and the catalyst are in the same microenvironment, to generate the required photon output. Nevertheless, it is not easy that all components are delivered to the same cellular compartment without reacting during delivery. Additionally, CRET and BRET require donor-acceptor proximity, so the chosen PS should be in that same cellular compartment as the luminophore.
The effectiveness of CL/BL-mediated PDT is dependent on four sequential steps: CL/BL → CRET/BRET to the PS → intersystem crossing → ROS production. These steps can lead to a decrease in the overall yield of PDT. Also, of note is that PDT has high selectivity, as the photodynamic effect affect only tissue containing the PS and irradiated by light. As these systems have no tumor-specificity, substituting laser photo-excitation by CL/BL activation will result in the loss of this advantage. This is especially of concern once some of the studied systems (as that of luminol) presented significant cytotoxicity by themselves. So, the use of these systems in PDT will require the development of new strategies for providing tumor-selectivity.
15
1.7. Other Strategies for Deep Photodynamic Therapy
Besides CL/BL, some authors have been testing other types of excitation sources for deep PDT. These strategies generally consist on an indirect excitation of the chosen PS by FRET via photo-conversion nanoparticles. Thus, different nanoparticles have been developed for deep PDT with indirect excitation of the PS by near infra-red (NIR) light and X-ray, based on physical absorption, covalent conjugation and surface coating. NIR light (from 650 to 1350 nm) is more suitable for deep PDT than UV and visible light, as it provides a higher penetration depth in biological tissues. Organic dyes and upconversion nanoparticles have been used to convert one- and two-photon NIR light into UV/visible light to excite PS in deep PDT [53–55]. Lanthanide-doped upconversion nanoparticles have better optical properties than other fluorescent compounds (such as organic dyes). First, they present high photostability, large anti-Stokes shift, sharp multi-wavelength emission bandwidth, weak auto-fluorescence and deep tissue penetration [53]. Second, they can convert low-energy NIR (generally at 980 nm) light into high energy UV-Vis light [56],[58]. Thus, their use allows the excitation of PS in deeper locations in the body than conventional light sources, once NIR light has a higher depth of penetration. Despite these beneficial properties, upconversion nanoparticles present only relatively low quantum yields [53]. Besides that, the use of NIR light may lead to heat damage to the tissues. The conservative limit for skin exposure to 980 nm laser is
726 mW/cm2, however this power density is too low to provide the photodynamic effect
needed [53]. In this wise, the use of upconversion nanoparticles does not resolves all the problems of conventional PDT.
Another attempt to overcome the issues of conventional PDT has been the use of X-rays, ionizing radiation with photon energy between keV to MeV. Contrary to UV-Vis and NIR light, X-rays have an unlimited depth of penetration into the body, thus have been widely used in CT imaging and radiotherapy. Based on this, X-ray-mediated PDT has the potential to be used to excite a PS inside tumors, irrespective of their localization in the body. Moreover, a single X-ray treatment is able to induce a synergistic PDT and radiotherapeutic effect [57]. On the other hand, X-rays are not able to activate the PS directly due to the energy difference between X-ray (photon energy of keV-MeV) and the energy required to excited different PS (in the order of eV). Therefore, scintillating nanoparticles [53,58] and X-ray excited persistent luminescence nanoparticles [53,58] have been explored to convert high energy ionizing radiation into photons of the appropriated energy to excite PS. The use of such nanoparticles did show potential for X-ray-mediated PDT in tumors localized deeper in the body. However, the photo-conversion of these nanoparticles is still low [53]. Once the energy of this type of radiation is high, this approach can cause considerable damage to surrounding normal tissues,
16 leading to serious side-effects. It is also difficult to develop scintillating-PS conjugates with good stability, enhanced tumor retention and optimal FRET efficiency.
In summary, while alternative approaches to CL/BL-mediated PDT have been proposed, they do not provide better results. The “PS-excitation” output is still too low for use in clinical PDT, these systems do not present any specific tumor-selectivity, and these chosen excitation sources (NIR light and X-ray radiation) may lead to damage to normal tissues and cause serious side-effects. Moreover, the use of these systems requires external excitation equipment that CL/BL do not. Thus, CL/BL -mediated PDT is still one of the alternatives with the highest potential for generalizable antineoplastic PDT in a clinical environment, without limitations regarding tumor-localization.
17
1.8. Objectives of this work
The main goal of this work is to develop self-illuminating PS based on CL, so to be no need for external light sources: the PS can convert thermal energy into excitation energy via a chemical reaction. Thus, the problems typically associated with light-penetration into tissues is eliminated. The choice for a CL system instead of BL (which are known for higher quantum yield) is to eliminate the need for a luciferase or a photoprotein to catalyze the reaction. Namely, only the CL substrate must be administered to the patient, eliminating the difficulty of delivering several reaction components to the same cellular compartment without reacting during delivery. To ensure that losses during energy transfer steps are minimized, our objective is that the CL is not only the excitation source, but also acts as the PS by itself. That is, instead of ROS production being triggered by the chemiexcited production of singlet excited states (during CL) that transfer their energy to the PS towards its activation (Scheme 8), the proposed CL substrate produces chemiexcited triplet excited states during the CL
reaction, able to produce ROS by interaction with molecular O2 (Scheme 8). The
proposed scheme is significantly simplified, which is expected to minimize efficiency losses.
Scheme 8- Comparison between the conventional CL-mediated PDT and the approach
18 The new PS will be based on marine Coelenterazine (described in section 1.4.3.), which besides being a BL substrate in the presence of luciferase/photoprotein, is also able be used in CL reactions without a catalyst. Namely, it reacts with superoxide anion to rapidly form a dioxetanone intermediate, with then decomposes in a chemiexcited luminophore [47,59]. That is, superoxide anion activates the CL reaction of Coelenterazine, with no need for other co-factors or catalysts. This gives a two-fold advantage for Coelenterazine to act as a self-activated PS in PDT:
1. Tumor cells are under significant oxidative stress, one of the reasons for this being the over-expression of superoxide anion [60,61]. Thus, the need of the Coelenterazine CL reaction for superoxide can be used as a tumor-selective factor.
2. As superoxide anion is already over-expressed in tumor cells, it is already present in the cells of the patient. Therefore, only the Coelenterazine-based PS needs to be administered to the patient, which significantly simplifies the procedure.
While Coelenterazine presents some advantages to development of self-activated PSs, there are some problems that must be overcome before that. Namely, CL reactions have low quantum yields (especially when compared with BL reactions) [24]. Furthermore, the Coelenterazine CL system is known for producing chemiexcited singlet excited states [23] instead of triplet ones (the desired ones for producing ROS species
by interacting with O2). In short, the major challenges for achieving the proposed goals
are to increase the efficiency of chemiluminescence and modulate the chemiexcited triplet to singlet product ratio. These problems are difficult be solved, however, due to lack of knowledge and mechanistic insight into Coelenterazine and related imidazopyrazinones. To address this, the present project pursued two different avenues of research (which can be considered also as the two main objectives of this thesis):
• Use of a combined spectroscopic, chromatographic and theoretical approach to obtain mechanistic insight into the CL of Coelenterazine and related imidazopyrazinones, with emphasis on the chemiexcitation process, in different environments. Such study was made towards the understanding of the basic mechanics of Coelenterazine CL.
• Synthesis of novel Coelenterazine-based PSs, their characterization (with focus on their ability of producing ROS), and the evaluation of their in vitro cytotoxicity towards tumor cells. This study can be considered as a proof-of-concept phase.
19
1.9. Structure of this work
The remainder of this Thesis is divided into two sections: the presentation and discussion of results; the conclusions and future perspectives. Each section is composed of chapters, corresponding to scientific outputs produced during this work, in the form of published and submitted papers in specialized peer-review international journals, patents, book chapters, oral communications and scientific posters:
Papers Published and Submitted:
1. C.M. Magalhães, J.C.G. Esteves da Silva, L. Pinto da Silva, Comparative Study of the Chemiluminescence of Coelenterazine, Coelenterazine-e and Cypridina Luciferin with an Experimental and Theoretical Approach. J. Photochem.
Photobiol. B 2018, Submitted.
2. L. Pinto da Silva, C.M. Magalhães, Mechanistic Insights into the Efficient Intramolecular Chemiexcitation of Dioxetanones from TD-DFT and Multi-Reference Calculations. Int. J. Quantum Chem. 2018, Submitted.
3. C.M. Magalhães, J.C.G. Esteves da Silva, L. Pinto da Silva, Study of Coelenterazine luminescence: electrostatic interactions as the controlling factor for efficient chemiexcitation. J. Lumin. 2018, 199, 339-347.
4. L. Pinto da Silva, R.F.J. Pereira, C.M. Magalhães, J.C.G. Esteves da Silva, Mechanistic Insight into Cypridina Bioluminescence with a Combined Experimental and Theoretical Chemiluminescent Approach. J. Phys. Chem. B
2017, 121, 7862-7871.
Patents:
1. Patent PPP59213 (Pending): Chemiluminescent Imidazopyrazinones-based photosensitizers with available singlet and triplet excited states.
Book Chapters:
1. L. Pinto da Silva, C.M. Magalhães, P.J.O. Ferreira, D.M.A. Crista, Chemi- and Bioluminescence in Self-Illuminating Photodynamic Therapy, In Photodynamic Therapy (PDT): Principles, Mechanisms and Applications. Nova Science Publishers, Inc., 2017. ISBN: 978-1-53611-912-1.
Oral Communications:
1. “Study of the Chemiexcitation Step of Coelenterazine Luminescence”. Oral
presentation at IJUP, February 2018, Faculdade de Medicina da Universidade do Porto, Porto, Portugal.
20
2. “A New Approach for Cancer Therapy Based on Marine Chemiluminescence”.
Oral presentation at 2nd SYMPOSIUM OF THE MSc IN ONCOLOGY, May 2018,
Instituto Português de Oncologia (IPO), Porto, Portugal.
Poster:
1. “Development of Self-Excitable Photosensitizers for Photodynamic Therapy of
Cancer”. C. Magalhães, P. Ferreira, A. Montenegro, E. Borges, L. Pinto da Silva, J. Esteves da Silva. Poster presentation at VIII Meeting of the Paul Ehrlich Euro-PhD Network, July 2018, Faculdade de Ciências da Universidade do Porto, Porto, Portugal.
The Results and Discussion section encompasses four chapters corresponding to the characterization of the chemiexcitation mechanism of Coelenterazine and
Cypridina luciferin (section 2.), the study of the solvent effect on the CL reaction of
Coelenterazine, Coelenterazine-e and Cypridina luciferin (section 3.), elucidation of a general mechanism for the chemiexcitation of dioxetanone molecules (section 4.), and the synthesis and characterization of novel PSs (section 5.).
The last section presents the main conclusions of the work performed, as well as perspectives of future research.
21
2. Mechanistic insight Cypridina luciferin and
Coelenterazine chemiexcitation
2.1. Aim
The chemiexcitation step of Coelenterazine and related imidazopyrazinones during the CL reaction is still far from understood. This can be explained by the instability of the dioxetanone intermediate, which thermolysis leads to chemiexcitation, which impairs its experimental characterization. Theoretical modelling can circumvent this limitation by providing reliable and accurate insight into the thermolysis of this peroxide. However, the lack of experimental data that can be used as reference makes the interpretation of theoretical data rather difficult.
Herein, the chemiexcitation step of Coelenterazine and Cypridina luciferin (Scheme 6 and 7) CL was studied by using a combined spectroscopic and computational approach. The energy and kinetic CL profiles of these reactions were obtained with luminometric and fluorescence methods. These experiments were made in model dimethyl sulfoxide (DMSO) solutions, as imidazopyrazinones have been found to
chemiluminesce readily in aprotic solvents by interacting with O2 [13]. Thus, DMSO and
other aprotic solvents are useful media for studying CL reactions. The thermolysis and chemiexcitation mechanisms of Coelenterazine and Cypridina CL were studied with an accurate density functional theory (DFT)-based approach. This combined experimental-computational approach allowed the rationalization of the CL mechanism for these molecules. It should be noted while that the major focus of this Thesis is on Coelenterazine, Cypridina luciferin was also studied to ensure that the conclusions here obtained are also valid for other imidazopyrazinone-based CL substrates, which can be found in about eight phyla of luminescent organisms.
2.2. Material and Methods
2.2.1. Kinetic chemiluminescent assay
Cypridina luciferin and Coelenterazine were dissolved in methanol. Kinetic
chemiluminescent assays were performed in a homemade luminometer using a Hamamatsu HC135-01 photomultiplier tube, at ambient temperature.
To prepare the light reactions was used a solution of DMSO. To this solvent was added either acetate buffer pH 5.12 (0.68%), phosphate buffer pH 7.4 (0.075 M) or NaOH (0.1 M). The injection of the DMSO solution reaction into a tube containing Coelenterazine or Cypridina luciferin initiated the reaction. Increasing concentrations of
22 Coelenterazine was used. The final solution of Cypridina luciferin had a concentration of 4.3 µM. To integrate and record the light, it was used 0.1 s intervals. The Graphpad software package (Version 7.03 for Windows) allowed to analyze the resulting data.
2.2.2. Measurement of chemiluminescence and fluorescence spectra
A Horiba Jovin Yvon Fluoromax 4 spectrofluorometer was used to measure the chemiluminescence spectra for Coelenterazine and Cypridina luciferin. The integration time was of 0.1s and for the emission monochromators were used slit widths of 29 nm. Quartz cells were used. The spectra were obtained in solutions with a final volume of 500 L, being the Coelenterazine concentration of 3 µM. The spectra were obtained in solutions with a final volume of 500 L, being the Cypridina luciferin concentration of 12.3 µM. Besides, the fluorescence spectra of Cypridina was obtained, using a slit of 5 nm.
2.2.3. Measurement of UV-visible spectrum
UV spectra were obtained in DMSO solutions with addition acetate buffer pH 5.12 (0.68%), increasing the levels of the target imidazopyrazinone (5, 60 and 150 µM).
2.2.4. Theoretical investigation of Cypridina Dioxetanone
S0 geometry optimizations and frequency calculations were performed at the
ωB97XD/6-31G(d,p) level of theory, [62]. The U approach was used with a broken-symmetry (BS) technology, which mixes the HOMO and LUMO, making an initial guess for a biradical [35,36,63–65]. Intrinsic reaction coordinates (IRC) were carried out to assess if the obtained transition state (TS) connect the desired reactants and products.
The energies of the S0 IRC-obtained structures were re-evaluated by single-point
calculations at the ωB97XD/6-31+G(d,p) level of theory. The S1 state was calculated by
using the time-dependent (TD) DFT approach, at the TD ωB97XD/6-31+G(d,p) level of theory. ωB97XD is a long-range-corrected hybrid exchange-correlation functional, [35,36,63–65] which provides accurate estimates for π → π*, n → π* local excitation, and CT and Rydberg states.
ωB97XD has already been used in the study of other imidazopyrazinone-based dioxetanones, which facilitates the comparison of results here obtained with previous work.[35,65] Geometry optimizations, frequency and IRC calculations were made in vacuo, while single-point calculations were made in implicit DMSO. This was achieved with the Polarizable Continuum Model using the integral equation formalism variant (IEFPCM) [66]. All calculations were made with the Gaussian 09 program package.