Effect
of
alendronate
on
bone-specific
alkaline
phosphatase
on
periodontal
bone
loss
in
rats
Paula
Goes
a,
Iracema
M.
Melo
a,
Caio
S.
Dutra
a,
Ana
Patrı´cia
S.
Lima
a,
Vilma
Lima
b,*
aDepartmentofDentistryClinic,SchoolofDentistry,FederalUniversityofCeara´,BrazilbDepartmentofPhysiologyandPharmacology,FacultyofMedicine,FederalUniversityofCeara´,Fortaleza,Ceara´,Brazil
1.
Introduction
Periodontitisisachronicinflammatorydiseaseofdestructive andnon-reversibleaction,that,ifnottreated,cancausetooth mobilityleadingtosubsequenttooth loss.1Various
mecha-nisms are related to aetiopathogenesis of periodontitis; however, factors associated to immunoinflammatory host responseaffect mainlydevelopment anduse ofdrugs that mightpromoteamodulationforthisresponse.1
The mechanism of bone resorption in periodontitis is mediatedbyosteoclasts.Thesecellsareoriginatedbyblood precursorsfrombonemarrow,andareactivatedbyvarious mediators, especially cytokines, such as tumour necrosis factor(TNF)andinterleukin(IL)-1,whichinduceanincreaseof receptoractivatorofnuclearfactork-Bligand(RANKL)onthe osteoblast surface,2favouring RANK–RANKL linkage, which
resultsinosteoclastactivationandosteoclastogenesis. On the resorption site, osteoclasts attach to the bone matrixthroughavb1integrin,formingasealingzone.3Later,
a
r
t
i
c
l
e
i
n
f
o
Articlehistory: Accepted14July2012
Keywords: Alendronate
Bone-specificalkalinephosphatase Alveolarboneloss
Inflammation
a
b
s
t
r
a
c
t
Objective: The studyaimsto evaluate theeffect of alendronate(ALD) onbone-specific alkalinephosphatase(BALP)serumlevelsonperiodontalbonelossinWistarrats.
Design: Periodontitiswasinducedbyligaturearoundtheuppersecondmolarin36male Wistarrats(200g).Groupsofsixanimalsreceived0.9%saline(SAL)orALD(0.01;0.05; 0.25mgkg 1,s.c.),over11days;thentheyweresacrificedandtheirmaxillaewereremoved tobedefleshedandstainedformacroscopicorhistopathologicalanalysis.Bloodsamples were collected for BALP, transaminases and total alkaline phosphatase (TALP) serum dosage,andhaematologicstudy.Ratswereweigheddaily.
Results: PeriodontitisinductioncausedreductionofBALP,intensealveolarboneloss(ABL), cementumandperiodontalligamentdestructionsandintenseleucocyteinfiltrationseen microscopically. Systemically,periodontitisinduced leucocytosis,weightloss andTALP reduction.ALD(0.25mgkg 1)preventedBALPreduction(19.17
1.36Ul 1)whencompared to SAL (13.61.5), as well as prevented ABL, by 57.2%, when compared to SAL (4.80+0.18mm2),whichwascorroboratedbyhistologicalfindings(ALD0.25mgkg 1=1.5 (1–2)andSAL=3(2–3))(p<0.05).ALDdidnotaltertransaminasesbutreducedTALPlevels (p<0.05).ALD0.25mgkg 1reduced6th-hneutrophilia(2.500.22cell103mm 3)and 7th-(12.290.66)and11th-daylymphomonocytosis(15.740.52)whencomparedtoSAL (5.200.28;18.241.05;and23.211.48,respectively).ALDdidnotaltertheweightloss.
Conclusion: ALDpreventedBALPreductionandABLandreducedinflammatoryinfiltrate, withoutcausingsystemicalterations.
#2012ElsevierLtd.Allrightsreserved.
*Correspondingauthorat:FederalUniversityofCeara´,DepartmentofPhysiologyandPharmacology,RuaCoronelNunesdeMelo,1127 -RodolfoTeo´filo,Fortaleza,Ceara´,Brazil.Tel.:+558599892199;fax:+558533668232.
E-mailaddresses:villima@yahoo.com.br,vilma@ufc.br(V.Lima).
Available
online
at
www.sciencedirect.com
journalhomepage:http://www.elsevier.com/locate/aob
0003–9969/$–seefrontmatter#2012ElsevierLtd.Allrightsreserved.
theyorganisetheircytoskeleton,andthen exhibitaruffled bordercalledtheresorptiveorgan.Bythen,agreatamountof acidvesiclesarereleasedon theresorptionsite,which are associatedtoaprotonpumpinordertostarthydroxyapatite crystaldissolution.3
Thenitrogen-containingbisphosphonates(nBPs)are phar-macologicalagentsthatpossessachemicalstructuresimilar topyrophosphate,whichprovidesastrongaffinitytocalcium. This structure promotes chelation to circulating calcium, binding it to the bone mineral surface.4 Amongst
bispho-sphonates,sodiumalendronate(ALD)standsout duetoits highaffinitytobonetissue.
ThemechanismofactionofnBPisbasedontheinhibition ofthe enzymefarnesyldiphosphate synthase(FPPS).5FPPS
stimulatesthe isoprenylation ofsmall guanosine-50 -tripho-sphatases(GTPases),whichsignalisetoproteinsthat,when activated, regulate alterations on osteoclast morphology, cytoskeletonarrangement,vesicletraffic5andruffledborder.
Whenthevesiculartrafficandruffledborderareinhibited,the activitiesthatelicitboneresorptionarealsoreduced.Finally, whenFPPSconcentrationreaches100mM,osteoclast apopto-sisinduction begins. Thus, nBPs areindicatedas excellent boneresorptioninhibitors.5
Theenzymealkalinephosphatasehasbeenknownformany years.6Alkalinephosphataseisametalloenzymeanchoredto
thecellmembrane,anditisdistributedparticularlyintheliver, bowel,placentaandbone.6Bone-specificalkalinephosphatase
(BALP), an isoenzyme of alkaline phosphatase, has been implicatedintheprocessesofboneformation6anditisthe
majorenzymeinvolvedinremovinginorganicpyrophosphate, aninhibitorofbonemineralisation.6
BecauseBALPisanexoenzymethatfacestheextracellular compartment,itisconceivablethatitsactivityandfunction canbemodulated byenvironmentalconditions.6Therefore,
weaimedtoevaluatetheeffectofALDonBALPonperiodontal bonelossinWistarrats.
2.
Materials
and
methods
2.1. Animalselection
Thirty-sixmaleWistarrats(Rattusnorvegicus)weighing180– 220g,fromourownanimalfacilities,wereusedinthisstudy. Theanimalswereacclimatisedforatleast1weekbeforethe beginningoftheexperimentandwerehousedundernormal laboratory conditions with laboratory chow and water available ad libitum. Experimental protocols were executed following ethicalprinciples for laboratory animal use,and wereapprovedbyinstitutionalEthicalCommitteeofAnimal Research and European Convention for the Protection of VertebrateAnimalsusedforExperimentalandOtherScientific Purposes (Protocol no. 101/2009). All efforts were made to reduceanimalnumber,theirpain,sufferingandstress.
2.2. Modelofexperimentalperiodontitis
Theratsweredividedintofourgroups,withsixanimalseach. Themodelofligature-inducedperiodontitisusedconsistedof insertionofnylonligaturearound thecervixofsecondleft
upper molar of rats anaesthetised with chloral hydrate (Vetec1, Duque de Caxias, RJ, Brazil).7,8 The ligature was
placedthroughtheproximalspaceoftherespectivetooth,and was knotted onthebuccal sideofthetooth,resultingina subgingivalpositionpalatinallyandinasupragingival posi-tionbuccallyoftheligature.Thecontralateralrightsidewas usedastheunligatedcontrol.Animalswereobserveduntilthe 11thday,theperiodofthemostintensealveolarboneloss, when they were then sacrificed. All ligature-induced peri-odontitiswasmaderandomly.
2.3. Experimentalgroups
2.3.1. Salinegroup
Thiscontrolgroupwasconstitutedbysixratssubmittedto periodontitis.Theanimalsreceived0.5mlof0.9%sterilesaline solution subcutaneously (s.c.), 30min before ligature and, afterthat,daily,foran11-dayperiod,whentheywerethen sacrificed.
2.3.2. ALDgroups
Theanimalsweresubdividedinthreegroupsofsixanimals each,whichreceivedALDsubcutaneously(Fosamax1,Merck,
Sa˜oPaulo-SP,Brazil)dissolvedin0.9%sterilesalinesolutionin thedosesof0.01,0.05and0.25mgkg 1,respectively,30min beforeligature,anddailyuntilthe11thday.
2.4. Morphometricstudyofbonetissue
On the 11thday,afterperiodontitisinduction,the animals weresacrificedandtheirmaxillaewereremovedandfixedin 10% neutralbufferedformalin(Reagen1,Riode Janeiro,RJ,
Brazil),for24h.Followingthat,themaxillaewereseparatedin half,dissectedandstainedwith1%aqueousmethyleneblue (Vetec1,DuquedeCaxias,RJ,Brazil)andplacedonmicroscope
slides.8,9 Then, they followed to photographic registration
usingadigitalcamera,Nikon1(D40,Melville,NY,USA).The
measurementoftheresorptionareawasmadebyadelimited region,involvingtheocclusalborderofthevestibularsideof the hemimaxilla until bone border. These areas were evaluated by ImageJ1 software (Software ImageJ 1.32j,
NationalInstitutesofHealth;EUA)inaccordanceto method-ologydescribedbyGoesetal.8
2.5. Histologicalanalysisofalveolarbone
Extra groups of six animals with periodontitis that had received saline or ALD (0.25mgkg 1) were sacrificed as described aboveand hadtheirmaxillaeexcised.The speci-menswerefixedin10%neutralbufferedformalinandwere demineralised in 10% ethylene diamine tetraacetic acid (EDTA) (DinaˆmicaQuı´mica Contemporaˆnea1, Diadema, SP,
from0to3,basedontheintensityoffindings,asfollows:Score 0: absence of or only discrete cellular infiltration, few osteoclasts,preservedalveolarprocessandcementum;Score 1:moderatecellularinfiltration,presenceofsomeosteoclasts, some but minor alveolar process resorption and intact cementum; Score 2: accentuated cellular infiltration, large number of osteoclasts, accentuated degradation of the alveolarprocess and partial destruction ofcementum;and Score3:accentuatedcellularinfiltrateandtotaldestructionof alveolarprocessandcementum.9
2.6. SerumdosageofBALP
Blood samples were collected from the orbital plexus of anaesthetisedanimals(salineandALD)beforetheexperiment and on the 11th day. The BALP was evaluated using the thermoactivationmethod,byheatingthesampleat568Cfor 10min,10 since BALP is a thermosensible isoform of total
alkalinephosphatase(TALP).BALPserumlevelswereobtained bythesubtractionofheatedalkalinephosphatasefromTALP serumlevels.Themethodologyusedtoevaluatetheenzymes’ serum levels followed the manufacturers’ directions (Labt-est1,LagoaSanta-MG,Brazil).
2.7. Serumdosageoftransaminases(ASTandALT)and TALP
On the baseline and on the 11th day of the assay, blood sampleswerecollected fromtheorbitalplexuses of anaes-thetised animals (saline and ALD). Liver function was evaluatedthroughserumdosageoftransaminases:aspartate aminotransferase(AST)andalanineaminotransferase(ALT). TALP serum levels werealso evaluated. Specific kits were used,andmethodologyfollowedthemanufacturer’s instruc-tions(Labtest1,LagoaSanta-MG,Brazil).
2.8. Haematologicstudy
Themethodusedtoanalysewhitebloodcellcounts,aswellas itssubpopulation(neutrophilandmononuclearcells),wasas follows:20mlofblood,takenfromtherattail,wasaddedto 380mlofTurksolution. Totalwhite blood cell countswere performed using a Neubauer chamber and the differential countsweremadeusingsmearsstainedbyrapidInstantProv Stain Set (Newprov Produtos para Laborato´rio; Pinhais-PR, Brazil).Aleucogramofthegroupsofanimals(salineandALD) wasperformedbeforeperiodontitisinduction,atthe6thhour and2nd,7thand11thdaysaftertheligature.
2.9. Corporalmassvariation
Animalsfrom salineand ALDgroupshad their body mass measuredbeforeperiodontitisinductionandafterthat,daily until the 11th day. Values were expressed as body mass variation(g)comparedtotheinitialbodymass.
2.10. Statisticalanalysis
Thedataarepresentedasmeanstandarderrorofthemean (SEM)ormedian(andrange),whereappropriate.Analysisof
variance(ANOVA),followedbyBonferroni’stestorStudent’st -test,wereusedtocompare means,andKruskal–Wallisand Dunntestswereusedtocomparemedians.Ap<0.05value was considered as indicating significant differences. All calculationswereperformedusingGraphPadPrism5software (GraphPad,Inc.,SanDiego,CA,USA).
3.
Results
3.1. Morphometricstudyofbonetissue
Themacroscopicanalysisofalveolarboneshowedthat11day ligature-inducedperiodontitiscausedintenseboneresorption
(Table1),associatedwithrootexpositionandfurcationlesion
(Fig. 1(d)). ALD, at the lowest dose (0.01mgkg 1), did not protectalveolarbone(p>0.05)whencomparedtosaline.ALD athigherdoses(0.05and0.25mgkg 1)wasabletosignificantly inhibit bone loss by 33.5% and 57.2%, respectively, when comparedtosaline(p<0.05).Although theanimalstreated with ALD (0.25mgkg 1) had not presented alveolar bone preservation similar to normal hemimaxilla (Fig. 1(a)), the periodontalaspectwasdifferentfromsaline(Fig.1(g)).
3.2. Histologicalanalysisofalveolarbone
Forthehistological analysis,anotherassay wasperformed, and then thehemimaxillae wereprocessedforhistological analysis (Table 1). It was observed that alveolar bone and cementum resorptions were associated to an important inflammatory infiltrate (p<0.05) on animals submitted to periodontitis (Table 1; Fig. 1(e) and (f)), when comparedto normalperiodontium(Table1;Fig.1(b)and(c))(p<0.05).ALD (0.25mgkg 1)treatmentsignificantlyattenuatedthe inflam-matory infiltrate and preserved periodontal ligament, root cementumandalveolarbone(Table1;Fig.1(h)and(i)),when comparedtosaline(p<0.05).
3.3. SerumdosageofBALP
SerumdosagesofBALPwereanalysed(Fig.2).Salinepresenteda significant decrease by 45.6% on BALP serum levels (13.621.56Ul 1) when compared to its baseline (25.041.43Ul 1). The treatment with ALD (0.01 and 0.05mgkg 1)causedareductionofBALPserumlevels,although notsignificant(p>0.05),by17.6%(19.922.97Ul 1)and19.5% (21.622.39Ul 1),respectively,whencomparedtoits respec-tive baseline (ALD 0.01=24.191.62; ALD 0.05mgkg 1= 26.672.15Ul 1). The treatment with ALD (0.25mgkg 1)
inducedasignificantdecreaseby28.1%(19.171.36Ul 1)for this enzyme after 11days of ligature-induced periodontitis whencomparedtoitsbaselinedata(26.672.15Ul 1); howev-er,thetreatmentwiththehighestdoseofALDpreventedBALP reductionby17.5%,whencomparedtosalineafter11daysof periodontitis(p<0.05).
3.4. SerumdosageoftransaminasesandTALP
Onthe11thday,forASTandALT,therewasnostatistical difference in the saline group when compared to its respectivebaseline.However,asignificantdecreaseinTALP serumlevelswas observedintheanimalsfromthe saline groupafter11days,whencomparedtoitsbaselinedata.The treatment with ALD did not cause significant alteration (p>0.05) in AST and ALT serum levels, but it reduced
(p<0.05)TALPserumlevelswhencomparedtoitsrespective baselinedata.
3.5. Haematologicstudy
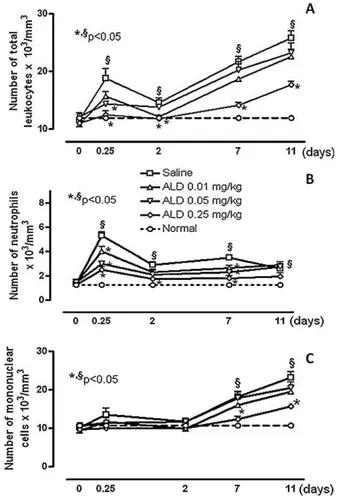
Regarding total leucocyte counts, it was observed that periodontitiscausedleucocytosisatthe6thhourafterligature Table1–Macroscopicandhistologicalanalysisofnormalhemimaxillaorsubmittedtoperiodontitisreceivingsalineor ALD.
Normal Saline ALD0.01mgkg 1 ALD0.05mgkg 1 ALD0.25mgkg 1
Macroscopicanalysis MeanSEM
– 4.80.2 4.10.4 3.20.5* 2.1
0.1*
Histologicalanalysis Scores
0(0–0) 3(2–3)# – – 1.5(1–2)*
(–)Indicatesthattherewasnoanalysis.Formacroscopicanalysis,valuesrepresentthemeanfollowedbyS.E.Mofaminimumof6animalsper groupbyAnovaandBonferronitest.Forhistologicalanalysis,valuesrepresentthemediansfollowedbyscoresvariation(lower–higher)ofa minimumof6animalspergroupbyKruskal–WallisandDunntest.
* Statisticallysignificantdifferencewhencomparedtosaline.
# Statisticallysignificantdifferencewhencomparedtonormalhemimaxillae(p<0.05).
Fig.1–Macroscopicandmicroscopicaspectrespectivelyofnormalperiodontium(A)–(C)andperiodontiumofratsubmitted
toperiodontitisreceivingsaline,showingmacroscopicboneresorption(D)andalveolarboneandcementumresorption,
andinflammatorycellinfiltrationseeninhistopathology(E)and(F).(G)(H)and(I)illustratethereductionofinflammation andalveolarbonelossinperiodontiumofratstreatedwithALD(0.25mgkgS1)for11days(macroscopicoriginal
(18.771.66 leucocytes103mm 3) (Fig. 3(a)), when
com-paredtoitsbaselinedata(11.560.31).Thisleucocytosiswas marked (p<0.05) by neutrophilia (5.200.28 neutrophil103mm 3), when compared to its baseline (1.370.08)(Fig.3(b)).Following,onthe2ndday,therewas adecreaseintotalleucocytecount;however,the basalcell countswerenotachieved.Newleucocytosiswasobservedon the 7th (21.730.87 leucocytes103mm 3) and 11th (25.841.23)days,withpredominationofmononuclearcells (7th day=18.241.05;11th day=23.211.48 mononuclear cells103mm 3)whencomparedtoitsbaseline(10.19
0.25) (Fig.3(c)).AlldosesofALDpreventedneutrophiliaatthe6th hour (ALD 0.01=4.000.42; ALD 0.05=2.980.21; ALD 0.25=2.500.22), when compared to saline (5.200.28) (p<0.05) (Fig. 3(b)).However, onlyALD (0.25mgkg 1) pre-ventedmononuclearcellpeaksonthe7th(12.290.66)and 11th(15.740.52)days(Fig.3(c)).
3.6. Corporalmassvariation
Periodontitiscausedbodyweightlossnotedonthe3rdday afterligatureplacementwhencomparedtonormalanimals. Afterthat,animalsshowedgainofweightandatendencyto follow the normal animal corporal mass curve. Animals
treatedwithALDshowedasimilarcorporalmasspatternto saline. ALD did not alter initial loss of weight, when compared to saline. After the 3rd day, gain of mass was observed accompanying animals from the saline group (Fig.4).
Fig.2–EffectofALDonbone-specificalkalinephosphatase.
BarsrepresentmeanWSEMofBALP(U/l)ofaminimumof
6animalspergroup.(*)indicatesstatisticallysignificant differencewhencomparedtosaline11daydata.(§) indicatesstatisticallysignificantdifferencewhen
comparedtoitsrespectivebaselinedata[Two-wayAnova;
BonferronitestandStudent’st-test](p<0.05).
Fig.3–EffectofALDonleukocytecounts.Pointsrepresent meanWSEMoftotalleukocytes(A),neutrophils(B), mononuclearcells(C)T103mmS3ofaminimumof6 animalspergroup.(*)indicatesstatisticallysignificant differencewhencomparedtosaline.(§)indicates statisticallysignificantdifferencewhencomparedtoits baselinedata[AnovaandBonferronitest](p<0.05).
Table2–SerumdosageofASTandALTandTALPofanimalssubmittedtoperiodontitisandreceivingsalineorALD.
Days Groups
Saline ALD0.01mgkg 1 ALD0.05mgkg 1 ALD0.25mgkg 1
AST(U/l) 0 44.512.13 40.612.97 45.443.92 47.443.33
11 48.644.74 38.722.50 46.043.86 42.513.52
ALT(U/l) 0 18.443.89 19.193.81 17.363.27 19.324.18
11 22.033.44 19.911.30 21.572.72 16.021.99
TALP(U/l) 0 95.611.21 96.511.52 97.071.97 93.061.09
11 70.141.74§ 77.29
1.99§ 75.75
2.11§ 69.64
1.71§
ValuesrepresentmeanSEMof6animalspergroup.
§ Statisticallysignificantdifferencewhencomparedtoitsrespectivebaselinedata[Two-wayAnova;BonferronitestandStudent’st-test]
4.
Discussion
In the present study, it was seen that ligature-induced periodontitis caused intense alveolar bone resorption and periodontalinflammation,asdemonstratedbymacroscopic andhistologicalanalyses.Inaddition,asignificantdecreasein BALPandTALPserumlevelswasobserved,andnochangein ASTandALTserumlevels.Periodontitiscausedleucocytosis markedbyneutrophiliaatthe6thhandmarkedby lympho-monocytosisonthe7thand11thdays.Inaddition,aninitial weightlossfollowedbytendencytoaccompanynormalrat corporal mass curve was observed. Treatment with ALD preventedalveolarboneresorptionofanimalssubmittedto ligature-inducedperiodontitis,confirmedinmacroscopicand histologicalanalyses,whencomparedtosaline.ALD,atthe higherdose, prevented thereduction ofBALPserum levels whencomparedtosaline,and didnotaltertransaminases’ serumlevels.Besides,ALDprevented6th-hneutrophilia,as wellas lymphomonocytosis observedon the 7th and 11th days.ALDdidnotpreventtheinitialweightloss,althoughthe animalshadshown gainofcorporalmasssimilartosaline corporalmasscurve.
IthasbeendescribedthatALDisrapidlyeliminatedfrom plasma,andmainlydistributedtothebone,whereabout60% ofthedoseislocalisedinbonetissueofrats.11Accordingly,
Azumaet.al.12observedtheconcentrationof[14 C]-alendro-nate in several bone tissues at various times after the 0.05mgkg 1 IV dose. The maximal concentration in long boneandlumbarspineoccurredabout8and24h, respective-ly,aftertheIVdose,andtheconcentrationofALDinboneat 2hvariedinadose-dependentmanner,when0.05–15mgkg 1 of [14C]-alendronate was injected IV. Furthermore, reports fromtheliteraturehaveshownthatnBPsnotonlyactedon osteoclastboneresorption, but alsoaffected the behaviour and metabolismofother bone-related cells,suchas osteo-blasts,osteocytesandmacrophages.13,14Therefore,weaimed
toevaluateBALPserumlevelsaftertreatmentwithALD.BALP, an isoform of TALP, acts specifically as a bone formation marker. Its mechanism of action is based on inorganic pyrophosphatehydrolysis,removingthisosteogenicinhibitor, while it creates inorganic phosphate, required for the
generation and deposition of hydroxyapatite.15 BALP is
secretedfromosteoblast membranetowardmatrixvesicles, allowing the mineralisationprocessto occur.15Itis known
thatmammalian-tissueBALPisstronglyactivatedbydivalent cations such asMg2+and Zn2+, and hasanactive siteand contains twoZn2+ ionsthatstabiliseitstertiarystructure.14
Theintestinalandplacentalisoenzymesarelessinfluencedby thesecations.16
Inthisstudy,wehaveshownthatthelowestdosesofALD (0.01and0.05mgkg 1)preventedthereductionofBALPserum levels,whencomparedtoitsbaselinedata.Ontheotherhand, the highest dose of ALD (0.25mgkg 1) prevented BALP reductionwhencomparedtosalineafter11daysof periodon-titis,but itwassignificantlydifferentonBALPserumlevels when compared toits baseline. Although slight, the lower levelofBALPaftertreatmentwithALDmayberelatedtotwo aspects:thechemicalstructure,whichiscloselylinkedtothe anti-resorptiveeffectofthisdrug,anditsconcentration.17,18
nBPs,likeALD,havetworadicalslinkedtothecarbonatom, one,calledR1thathasahydroxylgroup(–OH)andimproves mineralaffinity,andtheotherone,calledR2,whichincreases nBP potency to inhibit bone resorption.14 This chemical
structureelicitsthedevelopmentofastructuralmotifcalled ‘bonehook’thatbindstothemineralbychelationofdivalent cations.18 Therefore, considering that BALPneeds divalent
cations to become activated and that the ALD bone hook reducestheofferofthesecations,ourpresentobservations suggestthatthehighestdoseofALDinhibitedBALPactivity through divalent cation chelation within the bone hook structure.Thissuggestionisbasedonapreviousreportwhere BALPinhibitionwasreversedbyanexcessofZn2+orMg2+.13
However,itwasseenthatlowerdosesofALDprevented BALP reduction while the highest dose did not, when comparedtoitsrespectivebaseline;therefore,wecaninfer thatALDmayhaveadose-dependenteffectonBALPserum levels. In fact, reports from the literature had already confirmedourfinding.17,18ForStilletal.17atlower
concentra-tions (10 9 to 10 7M), ALD increased the formation of fibroblastic colonies,suggestinga mildanabolic effect. The treatmentwithhighconcentrations(10 4M)ofALDcauseda total inhibitionofcolonyformation. Itwasalsofoundthat intermediate concentrations (10 6M) ofALDdecreased the formationofcoloniesdisplayingosteoblastic characteristics suchasalkalinephosphataseexpression,collagen accumula-tionandcalcification.ItwasalsoobservedbyVaismanetal.18
that low doses of nBPs (10 10 to 10 5M) stimulated BALP activity, whereas high concentrations (10 4M) inhibited it. Levelsof10 4MofALDareestimatedtobefoundinvivoat resorptionlacunaeinexperimentalanimalmodels.Thus,our present observations are physiologically relevant in the context of alocal action ofnBPs used inthe treatment of differentbonediseases,suchasperiodontitis.
In order to corroborate BALP serum level results, we evaluatedthebone-sparingactionofALDonmorphometric andhistologicalanalyses.Asignificantboneprotectionwas observedwhenthehighestdoseofALDwasused.Thealveolar bone protection performed by ALD after ligature-induced periodontitishasbeendemonstratedinpreviousreports,in studiesusingthesimilarmethodology.19,20This
anti-resorp-tiveeffectmaybeexplainedbytheattractionofALDtothe Fig.4–EffectofALDoncorporalmassvariation.Points
representmeanWSEMofaminimumof6animalsper
group.(*)indicatesstatisticallysignificantdifferencewhen
comparedtosaline[AnovaandBonferroni’stest]
boneand itsinterferenceonenzymeactivity.21,22nBPs,like
ALDinhibitFPPS,amevalonatepathwayenzymeresponsible forisoprenylationofsmallGTPases,suchasRab,Rac,Rasand Rho.23ThesesmallGTPasesaresignallingproteinsthat,when
activated,regulateseveralstructuralpropertiesimportantfor osteoclast function, including morphology, cytoskeletal ar-rangement,vesiculartraffickingandmembraneruffling.24,25
Bythetimethatvesiculartraffickingandmembraneruffling areinhibited bone resorptionis alsoreduced, due toFPPS inhibitionandconsequentGTPasesisoprenylationdecrease. Therefore,FPPS inhibitionseems to be responsible forthe pharmacologiceffectsofthenBPsattissuelevel.26
Themacroscopicaspectwascorroboratedbyhistological analysis,demonstratingpartialpreservationofalveolarbone, cementumandperiodontalligamentaswellasreductionof inflammatoryinfiltrateinanimalsreceivingALD.Beyondthe anti-resorptive action, ALD has shown anti-inflammatory activity,byinhibitionofpro-inflammatorycytokinesrelease, suchasIL-1,IL-6andTNF,andofnitricoxide(NO).27–29This
anti-inflammatoryactivity may alsorebound on ALD anti-resorptive action, since IL-1 and TNF, mainly stimulate expressionofRANKL,aTNFfamilycytokine,whichisessential forosteoclastogenesisinduction.30
TreatmentwithALDseemedtobesafe.Animalstreated withALDshowedinitialweightloss,similartosaline,which mayhavebeencausedbyligatureplacement.7,9Afterthat,it
wasseenthatALDtherapydidnotinduceadditionallossof weight, according to previous data.20 ALD therapy did not
cause significant changes in AST and ALT serum levels, suggesting thatALDdoes notinterfere withliverfunction, whichwasexpected,sincethisdrugisnotmetabolisedinthe liver.31 Studies in patients who received liver transplant
demonstrated that ALD has been well tolerated without deleteriouseffects on liverfunctiontests.32Patients taking
ALD and diagnosed with primary biliary cirrhosis did not present significant hepatic effects regarding biochemical parameters of liver disease.33 Our study also revealed
significantinhibitionofTALP serumlevels after11days of periodontitisinanimalsreceivingeithersalineorALD.This inhibitionmaybeduetothereductionoftheboneisoform, sinceBALPrepresentsabout90%oftheTALP.16
Wealsoobservedthat ALDprevented neutrophiliaand lymphomonocytosis.Thesefindingsareinaccordancewitha previousreportinwhichALDtreatmentinducedasignificant decreaseintotalwhitebloodcell,neutrophilandlymphocyte counts,inpatientswithPaget’sdisease.34Thereductionin
neutrophil count may effect neutrophil migration and activity,onceitwasseenthatALDdecreasedonneutrophil influxusing a carrageenan-induced peritonitis model and reducedmyeloperoxidaseactivityaswell.20Inaddition,the
reductioninperipheralmononuclearcells,whichincludes monocytesandlymphocytes,wasalsoanimportantfinding considering that circulating monocytes can migrate and differentiate locally on osteoclasts,thereby exerting bone resorption activity.22 Thus, the reduction of mononuclear
cellsmaycontributetothebone-sparingeffectofALDinthis model.
Insummary,ourresultsdemonstratedthatALDprevented BALPreductionandABL,andreducedinflammatoryinfiltrate, withoutcausingsystemicalterations.
Funding
This work was supported by Brazilian grants from the ConselhoNacionaldeDesenvolvimentoCientificoe Tecnolo´-gico(CNPq,Grants471407/2009-7),Coordenac¸a˜ode Aperfei-c¸oamentodePessoaldeNı´velSuperior(CAPES)andFundac¸a˜o CearensedeApoioaoDesenvolvimentoCientı´ficoe Tecnolo´-gico(FUNCAP,Grants247.01.00/09).
Competing
interests
Nonedeclared.
Ethical
approval
Theexperimentalprotocolswereexecutedfollowingethical principlesforlaboratoryanimaluse inaccordancewiththe EuropeanConventionfortheProtectionofVertebrateAnimals usedforExperimentalandOtherScientificPurposes,andthey wereapprovedbyInstitutionalEthicalCommitteeofAnimal Research(ProcessNo.101/2009).
r
e
f
e
r
e
n
c
e
s
1. GiannobileWV.Host-responsetherapeuticsfor periodontaldiseases.JournalofPeriodontology
2008;79(Suppl.8):1592–600.
2. CochranDL.Inflammationandbonelossinperiodontal disease.JournalofPeriodontology2008;79(Suppl.8):1569–76.
3. VaananenK.Mechanismofosteoclastmediatedbone resorption—rationaleforthedesignofnewtherapeutics.
AdvancedDrugDeliveryReviews2005;57(7):959–71.
4. RussellRG.Bisphosphonates:modeofactionand pharmacology.Pediatrics2007;119(Suppl.2):150–62.
5. KimmelDB.Mechanismofaction,pharmacokineticand pharmacodynamicprofile,andclinicalapplicationsof nitrogen-containingbisphosphonates.JournalofDental Research2007;86(11):1022–33.
6. WhyteMP.Physiologicalroleofalkalinephosphatase exploredinhypophosphatasia.AnnalsoftheNewYork AcademyofSciences2010;1192:190–200.
7. LimaV,VidalFDP,RochaFAC,BritoGAC,RibeiroRA.Effects ofTNF-ainhibitorspentoxifyllineandthalidomideon alveolarbonelossinshort-termexperimentalperiodontal diseaseinrats.JournalofPeriodontology2004;75(1):162–8. 8. GoesP,LimaAPS,MeloIM,RegoRO,LimaV.Effectof
atorvastatinonligature-inducedperiodontitisinWistar rats:radiographicandmacroscopicanalysis.BrazilianDental Journal2010;21(3):193–8.
9. LimaV,BezerraMM,AlencarVBM,VidalFD,daRochaFA,de CastroBritoGA,etal.Effectsofchlorpromazineonalveolar bonelossinexperimentalperiodontaldiseaseinrats.
EuropeanJournalofOralSciences2000;108(2):123–9.
10.MossDW,WhitbyLG.Asimplifiedheat-inactivation methodforinvestigatingalkalinephosphataseisoenzymes inserum.ClinicaChimicaActa1975;61(1):63–71.
11.LinJH,DugganDE,ChenIW,EllisworthRL.Physiological dispositionofalendronate,apotentantiosteolytic
12.AzumaY,SatoH,OueY,OkabeK,OhtaT,TsuchimotoM, etal.Alendronatedistributedonbonesurfacesinhibits osteoclasticboneresorptioninvitroandinexperimental hypercalcemiamodels.Bone1995;16(2):235–45.
13.RussellRG.Bisphosphonates:thefirst40years.Bone
2011;49(1):2–19.
14.RogersMJ,CrockettJC,CoxonFP,Mo¨nkko¨nenJ.Biochemical andmolecularmechanismsofactionofbisphosphonates.
Bone2011;49(1):34–41.
15.BalcerzakM,HamadeE,ZhangL,PikulaS,AzzarG,Radisson J,etal.Therolesofannexinsandalkalinephosphatasein mineralizationprocess.ActaBiochimicaPolonica
2003;50(4):1019–38.
16.AndersonRA,BosronWF,KennedyFS,ValleeBL.Roleof magnesiuminEscherichiacolialkalinephosphatase.
ProceedingsoftheNationalAcademyofSciencesoftheUnited StatesofAmerica1975;72(8):2989–93.
17.StillK,PhippsRJ,ScuttA.Effectsofrisedronate,
alendronate,andetidronateontheviabilityandactivityof ratbonemarrowstromalcellsinvitro.CalcifiedTissue International2003;72(2):143–50.
18.VaismanDN,McCarthyAD,CortizoAM.Bone-specific alkalinephosphataseactivityisinhibitedby
bisphosphonates:roleofdivalentcations.BiologicalTrace ElementResearch2005;104(2):131–40.
19. DuartePM,deAssisDR,CasatiMZ,SallumAW,Sallum EA,NocitiJrFH.Alendronatemayprotectagainst increasedperiodontitis-relatedbonelossin estrogen-deficientrats.JournalofPeriodontology2004;75(9):1196–202. 20.MenezesAM,RochaFA,ChavesHV,CarvalhoCB,Ribeiro
RA,BritoGA.Effectofsodiumalendronateonalveolarbone resorptioninexperimentalperiodontitisinrats.Journalof Periodontology2005;76(11):1901–9.
21.PapapoulosSE.Bisphosphonateactions:physicalchemistry revisited.Bone2006;38(5):613–6.
22.RussellRG,WattsNB,EbetinoFH,RogersMJ.Mechanismsof actionofbisphosphonates:similaritiesanddifferencesand theirpotentialinfluenceonclinicalefficacy.Osteoporosis International2008;19(6):733–59.
23. LuckmanSP,HughesDE,CoxonFP,GrahamR, RussellG,RogersMJ.Nitrogen-containing
bisphosphonatesinhibitthemevalonatepathwayand preventpost-translationalprenylationofGTP-binding proteins,includingRas.JournalofBoneandMineralResearch
1998;13(4):581–9.
24.AlakangasA,SelanderK,MulariM,HalleenJ,LehenkariP, Mo¨nkko¨nenJ,etal.Alendronatedisturbsvesicular traffickinginosteoclasts.CalcifiedTissueInternational
2002;70(1):40–7.
25.PavlosNJ,XuJ,RiedelD,YeohJS,TeitelbaumSL, PapadimitriouJM,etal.Rab3Dregulatesanovelvesicular traffickingpathwaythatisrequiredforosteoclasticbone resorption.MolecularandCellularBiology2005;25(12):5253–69.
26.FisherJE,RogersMJ,HalasyJM,LuckmanSP,HughesDE, MasarachiaPJ,etal.Alendronatemechanismofaction: geranylgeraniol,anintermediateinthemevalonate pathway,preventsinhibitionofosteoclastformation,bone resorption,andkinaseactivationinvitro.Proceedingsofthe NationalAcademyofSciencesoftheUnitedStatesofAmerica
1999;96(1):133–8.
27.GiulianiN,PedrazzoniM,PasseriG,GirasoleG. BisphosphonatesinhibitIL-6productionbyhuman osteoblast-likecells.ScandinavianJournalofRheumatology
1998;27(1):38–41.
28.MakkonenN,SalminenA,RogersMJ,FrithJC,UrttiA, AzhayevaE,etal.Contrastingeffectsofalendronateand clodronateonRAW264macrophages:theroleofa bisphosphonatemetabolite.EuropeanJournalof PharmaceuticalSciences1999;8(2):109–18.
29.Mo¨nkko¨nenJ,Simila¨ J,RogersMJ.Effectsoftiludronateand ibandronateonthesecretionofproinflammatorycytokines andnitricoxidefrommacrophagesinvitro.LifeSciences
1998;62(8):PL95–102.
30.TakayanagiH.Inflammatorybonedestructionand osteoimmunology.JournalofPeriodontalResearch
2005;40(4):287–93.
31.LambrinoudakiI,ChristodoulakosG,BotsisD.
Bisphosphonates.AnnalsoftheNewYorkAcademyofSciences
2006;1092:397–402.
32.AtamazF,HepgulerS,AkyildizM,KarasuZ,KilicM.Effects ofalendronateonbonemineraldensityandbonemetabolic markersinpatientswithlivertransplantation.Osteoporosis International2006;17(6):942–9.
33.ZeinCO,JorgensenRA,ClarkeB,WengerDE,KeachJC, AnguloP,etal.Alendronateimprovesbonemineraldensity inprimarybiliarycirrhosis:arandomized placebo-controlledtrial.Hepatology2005;42(4):762–71.