www.bjorl.org
Brazilian
Journal
of
OTORHINOLARYNGOLOGY
ORIGINAL
ARTICLE
Complete
pathologic
response
as
a
prognostic
factor
for
squamous
cell
carcinoma
of
the
oropharynx
post-chemoradiotherapy
夽
Damila
Cristina
Trufelli
a,
Leandro
Luongo
de
Matos
b,∗,
Thaiana
Aragão
Santana
a,
Fábio
de
Aquino
Capelli
c,
Jossi
Ledo
Kanda
c,
Auro
Del
Giglio
a,
Gilberto
de
Castro
Junior
aaDisciplineofOncology,FaculdadedeMedicinadoABC,SantoAndré,SP,Brazil
bDepartmentofPublicHealth(Biostatistics),FaculdadedeMedicinadoABC,SantoAndré,SP,Brazil cDisciplineofHeadandNeckSurgery,FaculdadedeMedicinadoABC,SantoAndré,SP,Brazil
Received26March2014;accepted8October2014 Availableonline21July2015
KEYWORDS
Oropharynx; Carcinoma; Squamouscell; Combined chemotherapy; Neckdissection
Abstract
Introduction:Chemoradiotherapyforsquamouscellcarcinomaoftheoropharynx(SCCO)
pro-videsgoodresultsfor locoregionaldisease control,withhighratesofcompleteclinicaland
pathologicresponses,mainlyintheneck.
Objective:Todetermine whethercomplete pathologic response afterchemoradiotherapy is
relatedtotheprognosisofpatientswithSCCO.
Methods:DatawereprospectivelyextractedfromclinicalrecordsofN2andN3SCCOpatients
submittedtoaplannedneckdissectionafterchemoradiotherapy.
Results:Atotalof19patientswereevaluated.Halfofpatientsobtainedcompletepathologic
response inthe neck.Distant or locoregionalrecurrence occurred inapproximately 42%of
patients,and26%died.Statisticalanalysisshowedanassociationbetweencompletepathologic
responseandlowerdisease recurrencerate(77.8%vs. 20.8%;p=0.017)andgreateroverall
survival(88.9%vs.23.3%;p=0.049).
Conclusion:The presenceofacomplete pathologicresponseafter chemoradiotherapy
posi-tivelyinfluencestheprognosisofpatientswithSCCO.
© 2015Associac¸ãoBrasileira de Otorrinolaringologiae CirurgiaCérvico-Facial. Publishedby
ElsevierEditoraLtda.Allrightsreserved.
夽 Pleasecitethisarticleas:TrufelliDC,deMatosLL,SantanaTA,CapelliFA,KandaJL,DelGiglioA,etal.Completepathologicresponse
asaprognosticfactorforsquamouscellcarcinomaoftheoropharynxpost-chemoradiotherapy.BrazJOtorhinolaryngol.2015;81:498---504.
∗Correspondingauthor.
E-mail:lmatos@amcham.com.br(L.L.deMatos).
http://dx.doi.org/10.1016/j.bjorl.2015.07.009
PALAVRAS-CHAVE
Orofaringe;
Carcinomadecélulas escamosas;
Quimioterapia combinada; Esvaziamento cervical
Respostapatológicacompletacomofatorprognósticonocarcinomaespinocelularde orofaringeapósquimiorradioterapia
Resumo
Introduc¸ão: OtratamentobaseadoemquimirradioterapiadoCarcinomaEspinocelularde
Oro-faringe(CECOF)apresentabonsresultadosnocontrolelocorregionaldadoenc¸acomboastaxas
derespostaclínicaepatológicacompletasespecialmentenopescoc¸o.
Objetivo: Determinar se arespostapatológica completa apósquimiorradioterapia
estárela-cionadaaosprognósticosdospacientescomCECOF.
Método: Osdadosforamobtidosdemaneiraprospectivadarevisãodeprontuáriosdepacientes
comCECOFN2eN3submetidosaesvaziamentocervicalplanejadoapósquimiorradioterapia.
Resultados: Um total de 19 pacientes foram avaliados. Metade dos indivíduos apresentou
resposta patológicacompleta nopescoc¸o.Recidiva àdistância oulocorregionalocorreuem
aproximadamente42%dospacientese26%delesmorreram.Aanáliseestatísticademonstrou
umaassociac¸ãoentrerespostapatológicacompletaemenortaxaderecidiva(77,8%vs.20,8%;
p=0,017)emaiorsobrevivênciaglobal(88,9%vs.23,3%;p=0,049).
Conclusão:A presenc¸a derespostapatológica completa apósquimiorradioterapiainfluencia
positivamentenoprognósticodepacientescomcarcinomaespinocelulardeorofaringe.
©2015Associac¸ãoBrasileiradeOtorrinolaringologiaeCirurgiaCérvico-Facial.Publicado por
ElsevierEditoraLtda.Todososdireitosreservados.
Introduction
An option in thetreatment of locally advanced squamous cell carcinoma of the oropharynx (SCCO) is chemother-apy and radiotherapy combined with organ preservation, alsotargetinglocalandregionalcontrolofthedisease.1---6
Thistherapeuticapproachwasoriginallydevisedtobe fol-lowedbyaplannedneckdissectioninallpatients.7---9Some
authors suggest, however, that this approach should be restricted to patients with N2and N3 stage at diagnosis, or to those patients with N1 stage with partial response aftertreatment.10---12 However,other authors arguethat a
plannedneckdissectionmustbecarriedout,regardlessof theinitialstage,consideringthatthepathological positiv-ityrateaftertreatmentreaches30---40%.10,13 Itisalsowell
establishedthatpatientswithresidualcervicaldiseaseafter chemoradiotherapy have an increasedrisk of locoregional recurrence,aswellasofdistantdisease.14---17
Thus,theaimofthisstudywastoevaluatewhetherthe completepathologicresponseafteracombinedtreatment withchemoradiotherapyisassociatedwiththeprognosisin patientswithlocallyadvancedsquamouscellcarcinomaof theoropharynx.
Methods
Thiswasaprospectivecohortstudyapprovedbythe Institu-tionalEthicsCommitteeunderprotocolNo.098/2008.This studyincludedallpatientswithIVaorIVbstageSCCO (T1-4a, N2-3) consecutively submitted to chemoradiotherapy, followed by planned radical neck dissection 8---12 weeks after the end of the treatment, in the period from Jan-uary2008toDecember2010,andwithcomplete response attheprimarysiteconfirmedbyphysicalexamination, pan-endoscopy, computed tomography (CT) scan, and biopsy,
whenneeded. Completeclinical responsewas considered whentherewasnoevidenceofpersistentdiseaseinthese examinations; complete pathologic response was consid-eredwhenthespecimenobtainedthroughtheplannedneck dissectionshowednopathologicalevidenceofactive malig-nancy(residual tumor). Response assessment wasdefined togetherin a multidisciplinary clinical meeting, including HeadandNeckSurgery,Oncology,Radiology,andPathology Services.Platinum-basedchemotherapywasadministered, withaminimalradiotherapydoseof5000cGyappliedtothe cervical bed. In thisscenario, 19 patients were included, witha minimumoftwoyearsof follow-upguaranteed for allparticipants.
Demographic, clinical, and pathological data were obtainedfrommedicalrecords.ThepTNMstagewasrevised basedonthe seventhedition(2010)of theUnion Interna-tionale Contre le Cancer (UICC) publication. All patients werefollowed monthly, bimonthly, andevery three, four, and six months, respectively for the first, second, third, fourth,andfifthpost-treatmentyear.Humanpapillomavirus (HPV) status was assessed retrospectively at the time of completion of this study through reviewing the paraffin blocksfor thepresence ofp16protein;the specimenwas considered positive when immunoexpression rates were above80%.Noother HPVdetectionmethodologywas per-formed,becauseofunavailabilityatthiscenter.
The primaryoutcome studiedwasprogression-free sur-vival, defined as the time from diagnosis to disease recurrence (locoregional or distant). Secondarily, overall survivalwasstudied,measuredasthetimefromdiagnosis todeathfromanycause.Patientsalivewithoutevidenceof diseaseatthetimeofthisanalysiswerecensoredatthelast follow-up.
Table1 Descriptivedataofpatientsincludedinthisstudy.
Variable Result
Gender
Male 15(78.9%)
Female 4(21.1%)
Age(years)a 55.8±8.1
Primarysite
Softpalate 1(5.3%)
Tonguebase 5(26.3%)
Vallecula 3(15.8%)
Palatinetonsil 8(42.1%)
Lateralwall 2(10.5%)
Habits
Smoking 17(89.5%)
Alcoholism 15(78.9%)
HPV-positivestatus(p16-positive) 1(5.3%)
Pretreatment
Concomitantchemoradiotherapy 12(63.2%)
Inductionchemotherapyfollowed byconcomitantchemoradiotherapy
7(36.8%)
Initialclinicalstage
Tstage
T2 4(21.1%)
T3 8(42.1%)
T4 7(36.8%)
Nstage
N2a 7(36.8%)
N2b 3(15.8%)
N2c 2(10.6%)
N3 7(36.8%)
Completeclinicalresponse 12(63.2%)
Completepathologicalresponse 10(52.6%)
Progression
Locoregional 4(21.1%)
Distant 4(21.1%)
HPV,humanpapillomavirus.
aMean±standarddeviation.
forsurvivalanalysisandforcomparingcurves,respectively. SPSSversion17.0(SPSSInc---Illinois,UnitedStates)wasused forallanalyzes,andthelevelofstatisticalsignificancewas setat5%(p≤0.05).
Results
Nineteen patients, totaling 21 neck dissections, were included (Table1) withamedian of 28 monthsof follow-up.Mostpatientsweremale(78.9%)inthefifthdecadeof life(44---76years),andweresmokersanddrinkers.Onlyone positivep16casewasdetected.Completeclinicalresponse wasobservedin12patients(63.2%)andcomplete patholog-icalresponseinten(52.6%).Eightcases(42.2%)haddisease progressionandfive(26.3%)suffereddisease-relateddeath. Intheunivariateanalysis,noneofthedemographic, clini-cal,orpathologicalvariableswereassociatedwithcomplete pathologicalresponse,asshowninTable2.
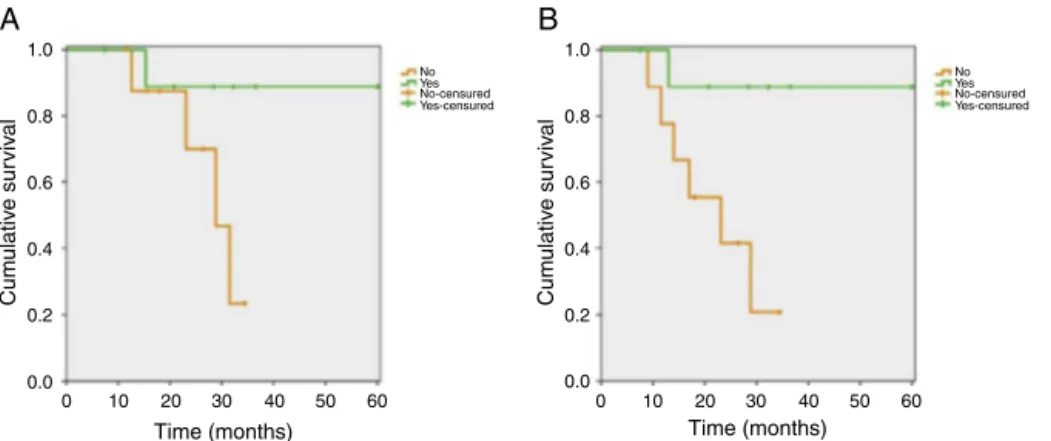
Survival analysis showed that patients with complete pathological responsehadhigher progression-free survival (77.8%vs.20.8%;p=0.017---log-ranktest)andoverall sur-vival (88.9%vs. 23.3%;p=0.049 --- log-rank test)rates, as detailedinFig.1.Themediansforprogression-freesurvival andoverallsurvivalfor patientswithresidualcervical dis-easewere23.1and28.8months,respectively,whilepatients withcompletepathologicresponsedidnotachievedthese mediansoverthe60-monthfollow-up.Bearinginmindthat this was a retrospective study, in the analysis of these findings the power of this estimate was calculated using themethodofcomparisonbetweentwoproportions.Faced withtheobviousdifferencesbetweenKaplan---Meiercurves (88.9%vs.23.3%forprogression-freesurvivaland77.8%vs. 20.8%foroverallsurvival,respectivelyforpatientswithvs. withoutcompletepathologicresponse),theinclusionof19 patientsinthisstudyresultedinananalyticalpowerforthis estimateinexcessof95%.Foraconventionalanalysis(80% testpowerand5%statisticalsignificance),itwasestimated that a sample between eight and 12 patients would suf-ficetodemonstratethesefindings,mainlyduetothegreat differencebetweenthecurves.
Itisalsoimportanttonotethatthestratificationofthe resultsforbothanalyzes(progression-freeandoverall sur-vivals)forpotentialconfoundingvariables(TandNclinical stagesandprevioustreatmentmodalities)didnotalterthe results,which means thatcomplete pathologicalresponse wasabetterprognosticfactorinpatientswithSCCO, regard-lessofothervariables.
Discussion
This study identifiedthat52.6% ofpatients inIVaand IVb stageforsquamouscellcarcinomaoftheoropharynxshow complete pathological response after chemoradiotherapy, a finding similarto other studies. Dhiwakar etal.18
stud-ied selective neck dissection in patients with squamous cellcarcinoma(SCC)of severalsites intheheadandneck withpartialresponseafterchemoradiotherapy,including39 cases (63%)of SCCO.These authorsfound cervical persis-tence in 32 neck dissection specimens (46%); 22 patients (35%) developed recurrent disease (seven at the primary site, 11distant,and fourcases inthe neck, butonly one ipsilateralcase).
AstudycarriedoutattheSloan-KetteringMemorial Can-cer Center19 thatevaluatedplannedneck dissectionin 56
post-chemoradiotherapy patients withhead and neckSCC (71% in the oropharynx) found that presence of a viable tumor in the cervical specimen wasa predictor of lower overall (49%) and disease-free (56%) survival, as well as of lower recurrence-free (40%) survival, when compared to patients with complete cervical pathological response (93%,93%,and75%,respectively).Theauthorsalsoreported that63%of19patientswithaviabletumorrelapsedduring follow-up;amongthese,eightcasesalsodevelopedremote disease. Lango et al.20 found similar results, with37% of
Table2 Univariateanalysis:variablesassociatedwithcompletepathologicalresponse.
Variable Completepathologicalresponse
No Yes p-value
Age(years:mean±standarddeviation) 55.3±9.0 56.4±7.4 0.780a
Gender 0.303b
Male 6(66.7%) 9(90.0%)
Female 3(33.3%) 1(10.0%)
Primarysite 0.543c
Softpalate 1(11.1%) 0(0.0%)
Tonguebase 1(11.1%) 4(40.0%)
Vallecula 2(22.2%) 1(10.0%)
Palatinetonsil 4(44.4%) 4(40.0%)
Lateralwall 1(11.1%) 1(10.0%)
Previoustreatment 1.000b
Concomitantchemoradiotherapy 6(66.7%) 6(60.0%)
Inductionchemotherapyfollowedbyconcomitant chemoradiotherapy
3(33.3%) 4(40.0%)
Cervicalirradiationdose 0.370b
5000cGy 6(66.7%) 4(40.0%)
7000cGy 3(33.3%) 6(60.0%)
Primaryneoplasiadifferentiation 0.289c
Welldifferentiated 1(11.1%) 0(0.0%)
Moderatelydifferentiated 7(77.8%) 10(100.0%)
Poorlydifferentiated 1(11.1%) 0(0.0%)
Initialclinicalstage
Tstage 0.326c
T2 3(33.3%) 1(10.0%)
T3 4(44.4%) 4(40.0%)
T4 2(22.2%) 5(50.0%)
Nstage 0.462c
N2a 3(33.3%) 4(40.0%)
N2b 1(11.1%) 2(20.0%)
N2c 2(22.2%) 0(0.0%)
N3 3(33.3%) 4(40.0%)
Completeclinicalresponse 0.650b
No 4(44.4%) 3(30.0%)
Yes 5(55.6%) 7(70.0%)
a Mann---Whitneytest. b Fisher’sexacttest. c Chi-squaredtest.
Thepresentstudyalsofoundthatthepresenceof resid-ualtumorinthecervical specimenobtainedfromplanned neck dissection in SCCO patients treated with chemora-diotherapywasassociated withloweroverall survival and disease-freerates,regardlessofotherpossibleconfounding variables---againafindingwhichissimilartothoseobserved by other authors.2,10,11,21 It should also benoted that the
presentstudyincluded19consecutivepatients,whichmay atfirstbeconsideredalimitedsample;however,thepower calculatedforthemainconductedestimates(differencesin progression-freeandoverallsurvivals)exceeded95%, show-ingstatisticalsignificanceforthesefindings.
Krstevskaetal.22studiedchemoradiotherapyasprimary
treatmentinpatientswithIII-andIV-stageSCCOandfound
that recurrence-free, disease-free, and overall survivals were41.7%,33.2%,and49.7%,respectively.Claymanetal.23
1.0
0.8
0.6
0.4
0.2
0.0
0 10 20 30 Time (months)
40 50 60
Cum
ulativ
e sur
viv
al
No Yes No-censured Yes-censured
1.0
0.8
0.6
0.4
0.2
0.0
0 10 20 30 Time (months)
40 50 60
Cum
ulativ
e sur
viv
al
No Yes No-censured Yes-censured
A
B
Figure1 Overallsurvival(A)andprogression-free(B)curves stratifiedfor pathologicalresponse(88.9%vs.23.3%;p=0.049 ---log-ranktestforoverallsurvivaland77.8%vs.20.8%,p=0.017---log-ranktestforsurvivalfreeofdiseaseprogression).
identified in patients withcomplete clinical response for theirprimarytumoraftertreatment.Allpatientsrequiring salvageneckdissectionaswellasresectionoftheprimary tumorbecauseofdiseasepersistencesufferedlocoregional recurrenceduringfollow-up---anenormousresultwhen com-paredto patients not submittedto any rescue procedure (12%)ortothosetreatedonlywithneckdissection(7%).In thesamestudy,disease-freeandoverallsurvivalrateswere 49.2%and78.4%,respectively,fortheentirecohort.A sig-nificantincreasewasobservedinoverallsurvivalspecifically for patientsundergoingsalvage neck dissection(vs. those withoutnodaldissection)whoachievedcompleteresponse inthe primarysite,but withpartial responsein theneck afterchemoradiotherapy.Theauthorsalsorecommendthat patientswithcompleteclinicalorradiologicalresponse,and eventhosewhohadabulkycervicaldisease,shouldonlybe monitored,withoutplannedneckdissection,becausenone ofthe29patientswithnegativeresultsbyCTandmagnetic resonanceimagingsufferedneckrecurrence.
Returning tothis subject, the literature suggeststhat, if there is clinical and radiological evidence of a com-plete locoregional response after chemoradiotherapy, the chanceofresidualcervicaldiseasewillbelessthan20%.21
Forthesepatients,positronemissiontomography with flu-orodeoxyglucose(18-FDG PET-CT) is the primary ancillary testtobeusedinacervicalassessment.Somestudies advo-cate,asaprinciple,theuseofdissectioninthesepatients; butthemostrecentstudiesdonotrecommendneck dissec-tioninN2-andN3-stage patientswithevidenceofclinical orimagingresponse(CTand/or18-FDGPET-CT),considering thelowresidualdiseaserateandthataplannedprocedure would not lead to improvement in overall and disease-free survival rates. Thus, it is recommended that neck dissection is performed only asa rescue procedure.8,23---31
Despitetheliteratureevaluated,thisstudyestablishedthat clinical response (assessed by physical examination, pan-endoscopy,andcomputedtomography)wasnotassociated withcomplete pathologic response; therefore, a planned neckdissection couldstillbean indicationin this patient group.
Asdemonstrated,someinstitutionsandprotocolsargue thata 18-FDG PET-CT negativeresult for the neck is suf-ficient for an expectant management strategy. In theory, thiswouldobviatetheneedforaplannedneckdissection.
However,a recentlypublishedstudy32 reviewed243 cases
ofpatientswithsquamouscellcarcinomaofheadandneck (70%oftheoropharynx)submittedtoPET-CTpriortotheir plannedneckdissection(112N0-clinicalstagepatientsand 131 patients witha clinically positive neck). The authors found that the sensitivity, specificity, positive predictive value,negativepredictivevalue,andaccuracyrateswere, respectively, 57%, 82%, 59%, 80%, and 74% for N0-clinical stagepatients;and93%,70%,96%,58%,and91%forpatients withevidenceoflymphnodedisease.Thus,theseauthors concludedthatPET/CThaslowefficacyindetecting cervi-cal metastasesof N0-clinical stagepatients, comparedto individualswithapositiveneck;andthatthemethodisnot beneficialfor stagingof N0-clinicalstagepatients, dueto the highrates of false-positiveandfalse-negative results. Anotherimportantpointtobeconsideredisthe inaccessi-bilityofthisdiagnosticmodalityinmanyoncologycenters. This further underscores the importance of this study in determiningthe planoftreatment for thispopulation. An expectantstrategycanstillbeadoptedinpatientswith com-pleteclinicalresponseaftertreatment,withgoodlong-term disease-freesurvivalrates.27
The diversity of treatment strategies found for these patientsdenotesthecomplexityofthissubject.Inaddition, JavidniaandCorsten33 calculatedthe‘‘numberneededto
treat’’(NNT)inpatientswithadvancedheadandneckSCC (N2andN3stages)basedonasystematicreviewof15 stud-ieswithatotalof817patients.The calculatedNNTvalue was7.5; i.e., topreventonecase of deathfromcervical recurrenceafterchemoradiotherapy, 7.5casesof planned neck dissection should be performed, demonstrating the cost-effectivenessoftheprocedure.
Goguenet al.34 studied 105 neck dissection specimens
afterchemoradiotherapy,including83casesofSCCO(79%), and found thatthe presenceof positive lymphnodeswas significantlyassociatedwithadecreaseinthe progression-freesurvival,aswellasinoverallsurvival.
Cupinoetal.35specificallystudiedpatientswithstageIV
follow-up,respectively.Despitethesesignificantoncological responses,thestudyissomewhatdebatable,duetothe het-erogeneityofdissectedcervicallevelsandthelownumber ofcases.Estelleretal.36includedadiversifiedgroupofhead
andneckSCC,with34.2%adjustedfive-yearoverallsurvival rate after rescue surgery. In an earlier study,24 the same
groupfoundthatN3-stagepatientswereatincreasedriskof cervicalresidualdiseaseafterchemoradiotherapy;andthat N2-stagepatients withcompleteresponseaftertreatment (evaluated byphysical examination,neck CT,and PET-CT) didnotrequireplannedneckdissection,becausesucha pro-cedure doesnotincrease overallsurvival anddisease-free rates.
Conclusion
Thecompletepathologicresponseafterchemoradiotherapy positively influences the prognosis of patients with squa-mous cellcarcinoma ofthe oropharynx, withbetterrates ofsurvivalfreeofdiseaseprogression,aswellasofoverall survival ---results similartothosefound in the literature. However,morestudies,especiallywithlargerseries,should beperformedtoestablishatherapeuticguidelinebasedon moreconsistentscientificevidence.
Conflicts
of
interest
Theauthorsdeclarenoconflictsofinterest.
References
1.Al-SarrafM, LeBlancM, Giri PG,Fu KK, Cooper J, VuongT, etal.Chemoradiotherapyversusradiotherapyinpatientswith advancednasopharyngealcancer:phaseIIIrandomized Inter-groupstudy0099.JClinOncol.1998;16:1310---7.
2.BonnerJA,HarariPM,GiraltJ,CohenRB,JonesCU, SurRK, etal.Radiotherapypluscetuximabforlocoregionallyadvanced headandneckcancer:5-yearsurvivaldatafromaphase3 ran-domisedtrial, and relationbetween cetuximab-inducedrash andsurvival.LancetOncol.2010;11:21---8.
3.DenisF,GaraudP,BardetE,AlfonsiM,SireC,GermainT,etal. Finalresultsofthe94-01FrenchHeadandNeckOncologyand RadiotherapyGrouprandomizedtrialcomparingradiotherapy alonewithconcomitantradiochemotherapyinadvanced-stage oropharynxcarcinoma.JClinOncol.2004;22:69---76.
4.ForastiereAA,GoepfertH,MaorM,PajakTF,WeberR, Morri-sonW, etal. Concurrentchemotherapyandradiotherapyfor organpreservationinadvancedlaryngealcancer.NEnglJMed. 2003;349:2091---8.
5.Ribeiro Salles Vanni CM, de Matos LL, Faro Junior MP, Ledo KandaJ,CerneaCR,GarciaBrandaoL,etal.Enhanced morbid-ityofpectoralismajormyocutaneousflapusedforsalvageafter previouslyfailedoncologicaltreatmentandunsuccessful recon-structiveheadandnecksurgery.SciWorldJ.2012:2012384179.
6.PintoFR,MatosLL,GumzSegundoW,VanniCM,RosaDS,Kanda JL.Tobaccoandalcoholuseafterheadandneckcancer treat-ment:influenceofthetypeofoncologicaltreatmentemployed. RevAssocMedBras.2011;57:171---6.
7.BarkleyHTJr,FletcherGH,JesseRH,LindbergRD.Management ofcervicallymphnodemetastasesinsquamouscellcarcinoma ofthetonsillarfossa,baseoftongue,supraglotticlarynx,and hypopharynx.AmJSurg.1972;124:462---7.
8.Hamoir M, FerlitoA, Schmitz S, Hanin FX, Thariat J, Wey-nandB, et al. The role ofneck dissection in thesetting of
chemoradiationtherapyforheadandnecksquamouscell carci-nomawithadvancedneckdisease.OralOncol.2012;48:203---10.
9.deBreeR,vanderWaalI,DoornaertP,WernerJA,Castelijns JA,LeemansCR.Indicationsandextentofelectiveneck dis-section in patients withearly stage oral and oropharyngeal carcinoma:nationwidesurveyinTheNetherlands.JLaryngol Otol.2009;123:889---98.
10.MendenhallWM,MillionRR,CassisiNJ.Squamouscellcarcinoma oftheheadandnecktreatedwithradiationtherapy:therole ofneckdissectionforclinicallypositivenecknodes.IntJRadiat OncolBiolPhys.1986;12:733---40.
11.MendenhallWM,VillaretDB,AmdurRJ,HinermanRW,Mancuso AA. Plannedneckdissectionafterdefinitiveradiotherapyfor squamous cellcarcinoma of theheadand neck. Head Neck. 2002;24:1012---8.
12.Ensley JF, Jacobs JR, Weaver A, Kinzie J, Crissman J, Kish JA, et al. Correlation between response to cisplatinum-combination chemotherapy and subsequent radiotherapy in previouslyuntreatedpatientswithadvancedsquamouscell can-cersoftheheadandneck.Cancer.1984;54:811---4.
13.Boyd TS, Harari PM, Tannehill SP, Voytovich MC, Hartig GK, Ford CN, et al. Plannedpostradiotherapy neck dissection in patients with advanced head and neck cancer. Head Neck. 1998;20:132---7.
14.Robbins KT, Doweck I, Samant S, Vieira F. Effectiveness of superselectiveandselectiveneckdissectionforadvancednodal metastasesafterchemoradiation.ArchOtolaryngolHeadNeck Surg.2005;131:965---9.
15.LavertuP,AdelsteinDJ,SaxtonJP,SecicM,WanamakerJR, Eli-acharI,etal.Managementoftheneckinarandomizedtrial comparing concurrent chemotherapy and radiotherapy with radiotherapyaloneinresectablestageIIIandIVsquamouscell headandneckcancer.HeadNeck.1997;19:559---66.
16.SimonC,GoepfertH,RosenthalDI,RobertsD,El-NaggarA,Old M,etal.Presenceofmalignanttumorcellsinpersistentneck diseaseafterradiotherapyfor advancedsquamouscell carci-nomaoftheoropharynxisassociatedwithpoorsurvival. Eur ArchOtorhinolaryngol.2006;263:313---8.
17.StensonKM,HuoD,BlairE,CohenEE,ArgirisA,HarafDJ,etal. Plannedpost-chemoradiation neckdissection:significance of radiationdose.Laryngoscope.2006;116:33---6.
18.Dhiwakar M,RobbinsKT, VieiraF,RaoK,MaloneJ.Selective neckdissectionasanearlysalvageinterventionforclinically persistentnodaldiseasefollowingchemoradiation.HeadNeck. 2012;34:188---93.
19.GanlyI,BockerJ,CarlsonDL,D’ArpaS,ColemanM,LeeN,etal. Viabletumorinpostchemoradiationneckdissectionspecimens asanindicatorofpooroutcome.HeadNeck.2011;33:1387---93.
20.Lango MN, Andrews GA, Ahmad S, Feigenberg S, Tuluc M, GaughanJ,etal.Postradiotherapyneckdissectionforheadand necksquamouscellcarcinoma:patternofpathologicresidual carcinomaandprognosis.HeadNeck.2009;31:328---37.
21.PellitteriPK,FerlitoA,RinaldoA,ShahJP,WeberRS,LowryJ, etal.Plannedneckdissectionfollowingchemoradiotherapyfor advancedheadandneckcancer:isitnecessaryforall.Head Neck.2006;28:166---75.
22.Krstevska V, Stojkovski I, Zafirova-Ivanovska B. Concurrent radiochemotherapy inlocally-regionally advanced oropharyn-gealsquamouscellcarcinoma:analysisoftreatmentresultsand prognosticfactors.RadiatOncol.2012:2012.
23.ClaymanGL,Johnson CJ2nd, Morrison W, GinsbergL, Lipp-manSM.Theroleofneckdissectionafterchemoradiotherapy for oropharyngeal cancerwithadvancednodal disease. Arch OtolaryngolHeadNeckSurg.2001;127:135---9.
25.Jang NY, Lee KW, Ahn SH, Kim JS, Kim IA. Neck control afterdefinitiveradiochemotherapywithoutplannedneck dis-sectioninnode-positiveheadandneckcancers.BMCCancer. 2012:2012.
26.Lau H, Phan T, Mackinnon J, Matthews TW. Absence of planned neckdissection for the N2---N3 neckafter chemora-diation for locallyadvancedsquamous cell carcinomaofthe headand neck. Arch OtolaryngolHead NeckSurg. 2008;134: 257---61.
27.DaMostoMC,LupatoV,RomeoS,SpinatoG,AddonisioG,Baggio V,etal.Isneckdissectionnecessaryafterinductionplus concur-rentchemoradiotherapyincompleteresponderheadandneck cancerpatientswithpretherapyadvancednodaldisease.Ann SurgOncol.2013;20:250---6.
28.Moukarbel RV, Fung K, Venkatesan V, Franklin JH, Pavamani S, Hammond A, et al. The N3 neck: outcomes following primary chemoradiotherapy. J Otolaryngol Head Neck Surg. 2011;40:137---42.
29.Lopez Rodriguez M, Cerezo Padellano L, Martin Martin M, Counago LorenzoF.Neckdissectionafterradiochemotherapy inpatientswithlocoregionallyadvancedheadandneckcancer. ClinTranslOncol.2008;10:812---6.
30.Soltys SG, Choi CY, Fee WE, Pinto HA, Le QT. A planned neck dissection is not necessary in all patients with N2-3
head-and-neckcanceraftersequentialchemoradiotherapy.Int JRadiatOncolBiolPhys.2012;83:994---9.
31.SuzukiM,KawakitaD,HanaiN,HirakawaH,OzawaT,TeradaA, etal.Thecontributionofneckdissectionforresidualneck dis-easeafterchemoradiotherapyinadvancedoropharyngealand hypopharyngealsquamous cellcarcinoma patients.IntJClin Oncol.2012.
32.OzerE,NaibogluB,MeachamR,RyooC,AgrawalA,Schuller DE.ThevalueofPET/CTtoassessclinicallynegativenecks.Eur ArchOtorhinolaryngol.2012;269:2411---4.
33.JavidniaH,CorstenMJ.Numberneeded totreatanalysisfor plannedneckdissectionafterchemoradiotherapyforadvanced neckdisease.JOtolaryngolHeadNeckSurg.2010;39:664---8.
34.GoguenLA,ChapuyCI,LiY,ZhaoSD,AnninoDJ.Neck dissec-tionafterchemoradiotherapy:timingandcomplications.Arch OtolaryngolHeadNeckSurg.2010;136:1071---7.
35.Cupino A, Axelrod R, Anne PR, Sidhu K, Lavarino J, Kung B,et al.Neck dissectionfollowed bychemoradiotherapyfor stageIV(N+)oropharynxcancer.OtolaryngolHeadNeckSurg. 2007;137:416---21.