ContentslistsavailableatScienceDirect
Molecular
Immunology
j ourna l h o m e pa g e :w w w . e l s e v i e r . c o m / l o c a t e / m o l i m m
Dogs
immunized
with
LBSap
vaccine
displayed
high
levels
of
IL-12
and
IL-10
cytokines
and
CCL4,
CCL5
and
CXCL8
chemokines
in
the
dermis
Juliana
Vitoriano-Souza
a,
Nádia
das
Dores
Moreira
a,
Daniel
Menezes-Souza
b,
Bruno
Mendes
Roatt
a,
Rodrigo
Dian
de
Oliveira
Aguiar-Soares
a,
Fernando
Augusto
Siqueira-Mathias
a,
Jamille
Mirelle
de
Oliveira
Cardoso
a,
Rodolfo
Cordeiro
Giunchetti
a,b,
Renata
Guerra
de
Sá
c,
Rodrigo
Corrêa-Oliveira
b,
Cláudia
Martins
Carneiro
a,d,
Alexandre
Barbosa
Reis
a,d,e,∗aLaboratóriodeImunopatologia,NúcleodePesquisasemCiênciasBiológicas/NUPEB,UniversidadeFederaldeOuroPreto,OuroPreto,MinasGerais,Brazil bLaboratóriodeImunologiaCelulareMolecular,InstitutodePesquisasRenéRachou,Fundac¸ãoOswaldoCruz,BeloHorizonte,MinasGerais,Brazil cLaboratóriodeBioquímicaeBiologiaMolecular,NúcleodePesquisasemCiênciasBiológicas/NUPEB,UniversidadeFederaldeOuroPreto,OuroPreto,
MinasGerais,Brazil
dDepartamentodeAnálisesClínicas,EscoladeFarmácia,UniversidadeFederaldeOuroPreto,OuroPreto,MinasGerais,Brazil eInstitutoNacionaldeCiênciaeTecnologiaemDoenc¸asTropicais-INCT-DT,CNPq,Brazil
a
r
t
i
c
l
e
i
n
f
o
Articlehistory:
Received27March2013
Receivedinrevisedform22May2013
Accepted23May2013
Available online 1 August 2013
Keywords:
Caninevisceralleishmaniasis
Cytokines Chemokines Vaccine Saponin Adjuvants
Real-timePCR
a
b
s
t
r
a
c
t
Thecomplex interplaybetweencytokinesand chemokinesregulates innateand adaptive immune responses againstpathogens; specifically, cytokine and chemokine expressiondrivesactivation of immuneeffectorcellsandtheirrecruitmenttotissueinfectionsites.Herein,weinoculateddogswith Leishmaniabraziliensisantigensplussaponin(theLBSapvaccine),aswellaswiththevaccinecomponents, andthenusedreal-timePCRtoevaluatethekineticsofdermalexpressionofmRNAsofcytokines(IL-12, IFN-␥,TNF-␣,IL-4,IL-13,TGF-andIL-10)andchemokines(CCL2,CCL4,CCL5,CCL21andCXCL8)1,12,24 and48hafterinoculation.Wealsoevaluatedthecorrelationbetweencytokineandchemokine expres-sionanddermalcellularity.TheLBSapvaccineinducedhighlevelsofIL-12andIL-10expressionat12and 24h,respectively.Furthermore,weobservedpositivecorrelationsbetweenIL-12andIL-13expression, IFN-␥andIL-13expression,andIL-13andTGF-expression,suggestingthatamixedcytokine microen-vironmentdevelopedafterimmunizationwiththevaccine.Inoculationwiththesaponinadjuvantalone inducedachemokineandcytokineexpressionprofilesimilartothatobservedintheLBSapgroup.CCL4 andCXCL8chemokineexpressionwasupregulatedbytheLBSapvaccine.CCL5expressionwasinitially highestintheLBSapgroup,butat48h,expressionwashighestintheLBgroup.Informationaboutthe kineticsoftheimmuneresponsetothisvaccinegainedusingthisdogmodelwillhelptoelucidatethe mechanismsofandfactorsinvolvedinaprotectiveresponseagainstLeishmaniainfectionandwillaidin establishingrationalapproachesforthedevelopmentofvaccinesagainstcaninevisceralleishmaniasis.
© 2013 Elsevier Ltd. All rights reserved.
1. Introduction
Human visceral leishmaniasis are heterogeneous diseases caused by obligate intracellular protozoan parasites of the genusLeishmaniaandaretransmittedbythebiteofthefemale
∗Correspondingauthorat:LaboratóriodeImunopatologia,NúcleodePesquisas
emCiênciasBiológicas,ICEBII,MorrodoCruzeiro,UniversidadeFederaldeOuro
Preto,35400-000OuroPreto,MinasGerais,Brazil.Tel.:+553135591694;
fax:+553135591680.
E-mailaddresses:alexreis@nupeb.ufop.br,alexreisufop@gmail.com(A.B.Reis).
phlebotominesandfly(Desjeux,2004).Dogsinfectedwithcanine visceralleishmaniasis(CVL)constitutethemaindomesticreservoir andplaya centralroleinthetransmissioncycleoftheparasite tohumans(Deane,1995).Therefore,acaninevaccinemaybethe mostpractical and effectivemethodfor reducing theincidence ofhumanvisceralleishmaniasis,andmayalsoprovideabasisfor thedevelopmentofasimilarvaccineforhumans(Gradoni,2001; Hommeletal.,1995;Mauel,2002).
In the search for a potential vaccine against CVL, various approachesinvolvingthedogmodelhavebeenemployed,andthe useofpurifiedfractionsfromparasiteextracts(e.g.,fucose man-noseligandantigen)(Borja-Cabreraetal.,2004;daSilvaetal.,2000)
0161-5890/$–seefrontmatter© 2013 Elsevier Ltd. All rights reserved.
56 (2013) 540–548 541
andfromparasitecultures(excreted/secretedantigens)(Lemesre etal.,2005,2007)have shownparticularpromise.Additionally, remarkableresultshave beenobtainedfollowing vaccinationof dogswithkilledparasitevaccines(Giunchettietal.,2007,2008a; Lasrietal.,1999;Mayrinketal.,1996;Moreiraetal.,2009;Panaro etal.,2001;Vitoriano-Souzaetal.,2008;Roattetal.,2012).These vaccinesrepresentanexcellenttoolforimmunoprophylaxisand controlofCVL,consideringtheirwide spectrumofantigenicity, aswellas theircost and safety(Giunchettietal., 2007,2008a; Lasrietal.,1999;Mayrinketal.,1996;Moreiraetal.,2009;Panaro etal.,2001; Vitoriano-Souzaetal., 2008).Moreover,inanimals thatreceivesaponinasanadjuvant,themajoradversereaction isminorlocalswelling,indicatingthatindogs,overalltolerance to the candidate vaccines appears to be adequate (Giunchetti etal.,2007,2008a,b;Moreiraetal.,2009;Vitoriano-Souzaetal., 2008).
Understanding the cellular immune responses of dogs to infectionwithLeishmaniaparasites iscrucial for vaccinedesign (Strauss-Ayalietal.,2007).InvolvementofIL-10intheimmune responseofLeishmania-infecteddogshasnotyetbeenestablished insomeorgans,becauseexpressionofIL-10mRNAisnotelevated inantigen-stimulatedperipheralbloodmononuclearcellsduring thecourseofanexperimentalinfectionorinthebonemarrowof naturallyinfecteddogs(Quinnelletal.,2001;Santos-Gomesetal., 2002).Lageetal.(2007)suggestedthatseverityCVLalsoismarked bythebalancedsplenicproductionoftype1and2cytokineswith thepredominantaccumulationofIL-10andIFN-␥asaconsequence ofincreasedparasiticloadandprogressionofthesymptoms. Fur-thermore,Menezes-Souzaetal.(2011)showedthatdogswithhigh skinparasitismexhibitedpredominantlyimmunoregulatory pat-ternofimmuneresponsecharacterizedbyincreasedexpressionof IL-10andTGF-.
IL-4mRNAisexpressedinthebone marrowofsome symp-tomaticdogs(Quinnelletal.,2001),andhighIL-4expressionwas recentlydetectedlocallyinskinlesionsofL.infantum–infected dogs(Brachelenteetal.,2005).Thus,thesecontradictoryfindings havebeenproventhattheroleofIL-4intheCVLprogressionstill unclearneedingmorestudiestoclarifytheirrealfunctioninthe naturalhistoryofthecaninedisease(Menezes-Souzaetal.,2011). Interferon-␥ expression in antigen-stimulated peripheral blood mononuclearcells,skinlesionsandbonemarrowofinfecteddogs hasbeenshowntobeelevated(Quinnelletal.,2001;Brachelente et al., 2005; Chamizo et al., 2005; Strauss-Ayali et al., 2005). Thus,thisIFN-␥increasedisresponsibleforthemainmechanism involvedinprotectiveimmuneresponseofdogsinfectedwithL. (L.)infantum,throughactivationofmacrophagestokillintracellular amastigotes(Vouldoukisetal.,1996).
The potential roles of chemokines in Leishmania infection include host defense functions such as leucocyte recruitment, participationincell-mediatedimmunity,cellactivationand anti-Leishmaniaactivity(RotandvonAndrian,2004).Chemokinesplaya vitalroleintheregulationofimmunitytovariousdiseasesaffecting theskin,includinghumancutaneousleishmaniasis(Guptaetal., 2011).However,theimmunomodulatoryprocesses triggeredby chemokines in assessments of the immunogenicity of vaccines againstCVLhavenotbeenwellstudied,andthereforeweaddressed thisissueinthepresentstudy.
SuccessfulimmunizationagainstinfectionbyLeishmaniaspp. requires theparticipationof both innate and adaptive immune responses.Thecytokineandchemokine microenvironment pro-motes immediate recruitment of cells that drive the immune response.Inthisprospectivestudy,weinvestigatedthekinetics oftheexpressionofcytokineandchemokinemRNAsintheskinof dogsafterimmunizationwithLeishmaniabraziliensisantigensplus saponinasanadjuvant(theLBSapvaccine),aswellasinoculation withtheseparatecomponentsofthevaccine.
2. Materialsandmethods
2.1. Animals
The details of the proposed study were presented to, and approvedby,theEthicalCommitteefortheUseofExperimental AnimalsoftheUniversidadeFederaldeOuroPreto, OuroPreto, MG, Brazil.As subjects, we used 12 mongrel dogs(males and females),8–12monthsold,whichwerebornandraisedinthe ken-nelsoftheCenterofAnimalScience,UniversidadeFederaldeOuro Preto.Thedogsweretreatedwithananthelminticandvaccinated againstrabies(Tecpar,Curitiba,PR,Brazil),caninedistemper,type2 adenovirus,coronavirus,parainfluenza,parvovirusandleptospira (VanguardHTLP5/CV-L;PfizerAnimalHealth,NewYork,NY,USA). Twodayspriortotheexperiments,thedorsalareaofallanimals wasshaved,andnolocalreactionswereobserved.
2.2. Immunizationprotocol
Thedogsweresubdividedintofourgroupsofthreedogsper group.TheSapgroupwasinoculatedwith1mgofsaponin(Sigma ChemicalCo., St.Louis, MO,USA), theLBgroupwasinoculated with600gofL.braziliensistotalproteins,theLBSapgroupwas inoculatedwith600gofL.braziliensistotalproteinsand1mgof saponin,andtheSgroup(control)wasinoculatedwithan equiva-lentvolumeofsterile0.9%salinesolution.Adjuvantsandantigens weredilutedwithsterile0.9%salinesolutiontoatotalvolumeof 250L.Followingapplicationofageneralanaesthetic(Thiopentax; Cristália,Itapira,SP,Brazil;7mg/kgofbodyweight),theinoculants wereintradermallyinjectedintotheshaveddorsalareaofthe ani-mals.Atintervals,skinbiopsieswerecollectedfromtheinoculation areasandstoredat−80◦CuntilrequiredforRNAanalysis.
Descrip-tiveandkinectstudiesofthebiopsiedtissuewereperformedat1, 12,24and48h.Thehistologicalanalysiswasperformedaccording toVitoriano-Souzaetal.(2008).Briefly,ahistologicalexamination (morphometricanalysisandleucocytedifferentialcounting)of sec-tionsstainedwithhaematoxylinandeosin(HE).Thekineticsofcell migrationwasevaluatedwithinthreeskinlayers(outerdermis, innerdermisandhypodermis)andthepredominanceofcellsin theinflammatoryinfiltrateandtheirdistributionwithintheskin layerswereassessed.Aquantitative(morphometric)analysisof theinflammatorycellspresentintheskinlayerswasperformed by acquiring digital images of pre-marked areas. Images were capturedat40 and 100× magnificationusinga LeicaDM5000B micro-camera (Leica Microsystems-Switzerland Ltd., Heerbrugg, Switzerland)and LeicaApplicationSuitesoftware(version2.4.0 R1), andanalyzed withtheaidofLeica QWin(V3)software.In ordertoidentifywhichtypesofcellswererecruitedtothesites ofdifferentinoculationssites,theinflammatorycells(neutrophils, eosinophils,macrophagesandlymphocytes)werecountedandthe resultswereexpressedin percentages.Herein,correlation anal-ysisbetweenthe cellularityand thecytokines and chemokines mRNA expression in the skin of these same animals was per-formed.
2.3. ExtractionoftotalRNAandsynthesisoffirst-strandcDNAs
542 56 (2013) 540–548
Table1
Sequencesofforward(F)andreverse(R)primersusedforreal-timePCRquantificationofmRNAexpressioninskinbiopsiesofimmunizeddogs.
Gene Primersequence(5′–3′) Productlength(bp) Reactionefficiency(%) R2 GenBankaccessionno. GAPDH
F TTCCACGGCACAGTCAAG 115 99.1 0.996 AB038240
R ACTCAGCACCAGCATCAC IL-12p40
F CAGCAGAGAGGGTCAGAGTGG 109 96.5 0.989 U49100
R ACGACCTCGATGGGTAGGC IFN-␥
F TCAACCCCTTCTCGCCACT 113 95.4 0.967 AF126247
R GCTGCCTACTTGGTCCCTGA TNF-␣
F CGTCCATTCTTGCCCAAAC 94 97.2 0.983 DQ923808
R AGCCCTGAGCCCTTAATTC IL-4
F CACCTCCCAACTGATTCCAA 123 96.9 0.991 AF239917
R CTCGCTGTGAGGATGTTCAA IL-13
F CCTCCTCAGAGCAAAGTG 148 96.7 0.973 AF244915
R CCCAGCACAAACAAAGAC IL-10
F AGAACCACGACCCAGACATC 129 97.1 0.993 U33843
R CCACCGCCTTGCTCTTATTC TGF-1
F AGGATCTGGGCTGGAAGTG 134 95.1 0.981 L34956
R CGGGTTGTGCTGGTTGTA CCL2
F CCTGCTGCTATACACTCA 91 96.3 0.979 U29653
R GCTTCTTTGGGACACTTG CCL4
F TCCTACTGCCTGCTGCTT 76 95.9 0.981 AB183194
R GCTGGTCTCAAAGTAATCTGC CCL5
F TTCTACACCAGCAGCAAG 136 98.7 0.991 AB098562
R TTCTACACCAGCAGCAAG CCL21
F AGTCTGGCAAGAAGGGAAAG 60 96.7 0.972 AB164433
R GGGTCTGTGGCTGTTCAGT CXCL8
F ACACTCCACACCTTTCCAT 116 98.7 0.977 AF048717
R GGCACACCTCATTTCCATTG
bymeasuringtheOD ratioat 260/280nm usingNanovue® (GE Healthcare, USA). To confirm the integrity of extracted RNA, electrophoresisofafractionofeachRNAsamplewasperformed onadenaturing agarosegeland verifiedboth the18Sand 28S ribosomal RNA(rRNA) bands. Theprocedureincluded a DNAse treatment step, and the efficiency of this step was evaluated byPCRamplificationofthecDNAreactionmixturewithoutthe additionoftheThermoScriptenzyme.EachquantitativePCRrun wasperformedwithtwointernalcontrolsassessingbothpotential contaminationbygenomicDNA(noreversetranscriptaseadded) and reagent purity (no cDNA added). First-strand cDNAs were synthesizedfrom1.0goftotalRNAusingtheThermoScript RT-PCRSystem(InvitrogenBrasil,SãoPaulo,SP,Brasil)witholigo-dT primersaccordingtothemanufacturer’sinstructions.
2.4. Designofprimersforgeneevaluation
PrimersweredesignedwiththeaidofGeneRunner(ver.3.05, HastingSoftwareInc.)usingspecificcaninesequencesobtained fromGenBank(http://www.genbank.nlm.nih.gov).Thesequences oftheprimersemployedarelistedinTable1.Theprimerswere synthesizedbyEurogentec(Southampton,UK)andreconstituted innuclease-freewater.
2.5. Real-timePCR
Real-time PCR was performed on an ABI Prism 7000 DNA SequenceDetectionSystemusing10LofSYBRGreenPCRMaster
Mix(PEAppliedBiosystems,FosterCity,CA,USA),4Lof100mM primersand8LofcDNAdilutedat1:5carriedoutinafinal vol-umeof20L.Thesampleswereincubatedat95◦Cfor10minand
thensubmittedto40cyclesof95◦Cfor15sand60◦Cfor1min,
duringwhichtimefluorescencedatawerecollected.Theefficiency ofeachpairofprimerswasevaluatedbyserialdilutionofcDNA accordingtothe protocoldeveloped byPE Applied Biosystems. Toevaluategeneexpression,weperformedthreereplicate anal-yses,andtheamountoftargetRNAwasnormalizedwithrespect totheendogenous control (referencegenes) geneGAPDH. Data weregeneratedbymeansofthe2−Ctmethodusingthemean valueoftheCtofthecontrolgroupasthecalibrator(Livakand Schmittgen,2001).PCRproductswereclonedwithpGEM-TEasy Vector(Promega)andsequencedtocheckspecificityusinganABI 3100AutomatedSequencer (PEApplied Biosystems)and a Dye TerminatorKit.
2.6. Statisticalanalysis
56 (2013) 540–548 543
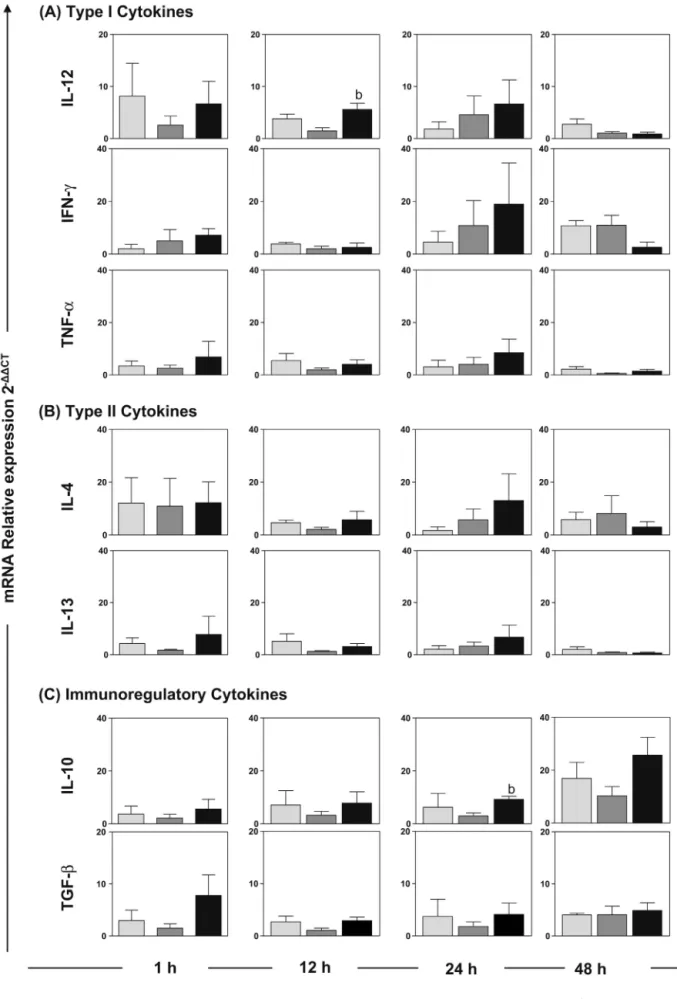
Fig.1.RelativeexpressionofmRNAsoftypeI(IL-12,IFN-␥andTNF-␣),typeII(IL-4andIL-13)andimmunoregulatory(IL-10andTGF-)cytokinesinthedermisofdogs
1,12,24and48hafterinoculationwithL.braziliensisantigens(LBgroup; ),saponin(Sapgroup; )orL.braziliensisantigensplussaponin(LBSapgroup;).Significant
544 56 (2013) 540–548
3. Results
3.1. CytokinemRNAexpressionprofilesintheskinofdogsafter immunization
EvaluationofcytokinemRNAexpressionisessentialfor improv-ingourunderstandingoftheprotectivecellularimmuneresponse inducedbyvaccines againstcanineleishmaniasis. Therefore,we evaluatedtheexpressionofIL-12,IFN-␥,TNF-␣,IL-4,IL-13,
TGF-andIL-10mRNAsinthedermisofdogs1,12,24and48hafter inoculationwiththeLBSapvaccineoritscomponents(Fig.1).At 12h,expressionofIL-12intheLBSapgroupwashigherthanthat intheSapgroup,and,interestingly,theexpressionofIL-10was alsohigherintheLBSapgroupthanintheSapgroup.Nosignificant differencesintheexpressionofIFN-␥,TNF-␣,IL-4,IL-13orTGF-
wereobserved(Fig.1).
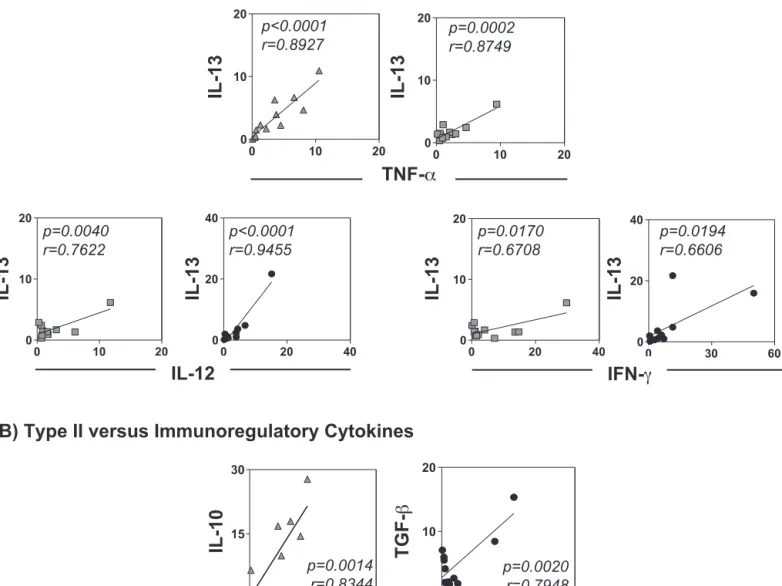
Wecarriedoutadetailedanalysisofthecorrelationsbetween type1,type2andimmunoregulatorycytokines(Fig.2)andfound thatintheLBgroup,therewasapositivecorrelationbetweenIL-13
andTNF-␣(p<0.0001,r=0.8927).IntheSapgroup,therewere pos-itivecorrelationsbetweenIL-13andTNF-␣(p=0.0002,r=0.8749), betweenIL-13andIL-12(p=0.0040,r=0.7622) andbetween IL-13andIFN-␥(p=0.0170,r=0.6708).IntheLBSapgroup,positive correlationswereobservedbetweenIL-13andIL-12(p<0.0001, r=0.9455)andbetweenIL-13andIFN-␥(p=0.0194,r=0.6606).In theLBgroup,apositivecorrelationwasobservedbetweenIL-10 and IL-4(p=0.0014, r=0.8344), and intheLBSap groupa posi-tivecorrelationwasobservedbetweenTGF-andIL-13(p=0.0020, r=0.7948).
3.2. ChemokinemRNAexpressionprofilesintheskinofdogsafter immunization
We evaluated the expression of the mRNAs of CCL2, CCL4, CCL5,CCL21andCXCL8(IL-8)chemokines1,12,24,and48hafter inoculationtheLBSapvaccineoritsindividualcomponents(Fig.3). At24hafterinoculation,expressionofCCL4mRNAintheLBSap groupwas11.6timesthatintheLBgroup(p<0.05).At1hafter
IL-10
IL-4
IL-13
0 10 20
0 10 20
p<0.0001
r=0.8927
0 20 40
0 10 20
p=0.0020
r=0.7948
TNF-
α
0 10 20
0 10 20
p=0.0002
r=0.8749
IL-13
0 20 40
0 10 20
p=0.0170
r=0.6708
0 30 60
0 20 40
p=0.0194
r=0.6606
0 10 20
0 10 20
p=0.0040
r=0.7622
0 20 40
0 20 40
p<0.0001
r=0.9455
IFN-
γ
IL-12
p=0.0014
r=0.8344
(A) Type I versus Type II Cytokines
0 10 20
0 15 30
(B) Type II versus Immunoregulatory Cytokines
TGF-β
IL-13
IL-13
IL-13
IL-13
IL-13
Fig.2.Correlationsbetweenrelativeexpressionof(A)typeIandIIcytokinemRNAsand(B)typeIIandimmunoregulatorycytokinemRNAsinthedermisofdogsinoculated
withL.braziliensisantigens(LBgroup; ),saponin(Sapgroup; )orL.braziliensisantigensplussaponin(LBSapgroup;䊉).Pearson’scorrelationindexes(r)andp-values
56 (2013) 540–548 545
Fig.3. RelativeexpressionofmRNAofchemokinesCCL2,CCL4,CCL5,CCL21andCXCL8inthedermisofdogs1,12,24and48hafterinoculationwithL.braziliensisantigens
(LBgroup; ),saponin(Sapgroup; )orL.braziliensisantigensplussaponin(LBSapgroup;).Significantdifferences(p<0.05)comparedwithLB,SapandLBSapare
indicatedbytheletters“a,”“b”and“c,”respectively.
inoculation,CCL5expressionwassignificantlyupregulatedinthe LBSap groupin comparison withexpression in theLB and Sap groups (4.0-fold and 6.8-fold,respectively; p<0.05). At12 and 24hafterinoculation,CCL5expressionintheLBgroupwashigher thanthatintheSapgroup(28.2-foldand72.1-fold,respectively; p<0.05). Also, 48h after inoculation, we observed increases in expressionof these chemokine (CCL5)in the LBgroup relative toexpressionintheSapandLBSapgroups(6.9-foldand5.6-fold, respectively;p<0.05).Inaddition,CXCL8expressionwas signif-icantlyhigherintheLBSapgroupthanintheLBandSapgroups
24h(13.9-foldand6.4-fold,respectively;p<0.05)and48hafter inoculation(13.6-foldand4.7-fold,respectively;p<0.05).There werenosignificantdifferencesinexpressionofCCL2andCCL21 betweenthegroups.
3.3. Correlationofinflammatorycellsintheouterandinner dermiswithcytokineandchemokineexpression
546 56 (2013) 540–548
Neutrophils
Neutrophils
Neutrophils
Neutrophils
Neutrophils
Outer dermis
Inner dermis
0 50 100
0 2 4
p=0.0005
r=0.8917
CCL5
0 40 80
0 2 4
CCL5
0 40 80
0 30 60
p=0.0240
r=0.6434
IL
-4
Macrophages
Neutrophils
mRNA Relative expression 2
-ΔΔCT
Number of cells (%)
0 40 80
0 10 20
p=0.0479
r=0.5803
IL-13
0 40 80
0 2 4
p=0.0030
r=-0.8020
CCL5
0 40 80
0 20 40
p=0.0138
r=0.6859
IL-12
0 40 80
0 20 40
p=0.0125
r=0.6930
IL-13
0 40 80
0 30 60
p=0.0015
r=0.8085
IFN
-γ
0 40 80
0 20 40
p=0.0040
r=0.7615
TNF
-α
Lymphocytes
0 40 80
0 10 20
CCL5
p=0.0345
r=-0.6117
0 40 80
0 30 60
p=0.0351
r=0.6103
IL-12
p=0.0083
r=-0.7464
Fig.4.CorrelationsbetweenrelativeexpressionofcytokineandchemokinemRNAsandpercentagesofmacrophagesandneutrophilsintheouterandinnerdermisofdogs
afterinoculationwithL.braziliensisantigens(LBgroup; ),saponin(Sapgroup; )orL.braziliensisantigensplussaponin(LBSapgroup;䊉).Pearson’scorrelationindexes
(r)andp-valuesareshownonthegraphs.
(Fig.4).Weobservedimportantcorrelationsbetweenchemokine and cytokineexpression and cellularityin the outerdermis. In the Sap group, macrophages and CCL5 expression were posi-tivelycorrelated(p=0.0005,r=0.8917),andneutrophilsandCCL5 expression were negatively correlated (p=0.0083, r=−0.7464). IntheLBSapgroup,neutrophilswerepositivelycorrelatedwith IFN-␥(p=0.0015,r=0.8085),IL-12(p=0.0351,r=0.6103)andIL-4 (p=0.0240,r=0.6434)expression.
Intheinnerdermis,weobservedapositivecorrelationbetween neutrophilsandIL-13(p=0.0479,r=0.5803)intheLBgroup.As in theouter dermis, there wasa negative correlationbetween CCL5and neutrophils(p=0.0030,r=−0.8020)in theSapgroup. Inthe LBSapgroup,we observed positivecorrelations between neutrophilsandIL-12(p=0.0138,r=0.6859)andbetweenTNF-␣
(p=0.0040,r=0.7615)andIL-13(p=0.0125,r=0.6930),aswellas anegativecorrelationbetweenlymphocytesandCCL5(p=0.0345, r=−0.6117).
4. Discussion
ThesearchfornewimmunoprophylacticsagainstCVLshould focusonprospectiveantigenscapableofinterveningintheimmune systeminsuchawayastoinduceaspecificimmunologicalevents related to parasite elimination. The development of immunity againstinfectiousagentssuchasLeishmaniaspp.requiresthe con-certedactionofcomponentsofboththeinnateandtheadaptive immunesystems(Teixeiraetal.,2005).Thedifferentialregulation oftype1andtype2responsesandtheassociationofThcytokines andimmunefunctionareimportanttostudytheadaptiveimmune responsetoLeishmaniainfections(Menezes-Souzaetal.,2011).
56 (2013) 540–548 547
of neutrophilsto theskinof animalsimmunized withSap and LBSaparesimilartothereactionsobservedinanimalsthatexhibit resistancetoLeishmaniainfection(Vitoriano-Souzaetal.,2008). Basedontheseresultsitispossibletohypothesizethatneutrophils functionatthefrontlineoftheimmunesystem,responding imme-diatelyupon request, anddirect themigration ofother cellsto theinoculation area somehours afterwards (macrophages and lymphocytes).It appears,therefore, thatneutrophils participate effectivelyintheadaptiveimmuneresponsefromthevery begin-ningoftheprocess(Vitoriano-Souzaetal.,2008).Inthissense,the investigationofthekineticsofcellmigrationtotheinoculationarea isextremelyrelevantsincethenumberandtypesofcellsrecruited immediatelyafterinoculationwillstimulatetheinnateimmune systemandwillinfluencethedevelopmentofacquiredimmunity. Toexpandourinvestigationoftheimmuneresponsetriggered bythecomponentsoftheLBSapvaccine,weevaluatedexpression ofcytokine andchemokine mRNAsin theskinof dogs,seeking biomarkersofimmunogenicityinthecontextoftheinnateimmune response.Wecarriedoutakineticsstudyoftherelativeexpression ofthemRNAsoftype I,IIandimmunoregulatorycytokinesand chemokinesinducedbythevaccineanditsseparatecomponents atthesiteofinoculationafter1,12,24and48h.Additionally,we evaluatedthecorrelationsbetweencytokineandchemokinemRNA expressionandcellularityintheouterandinnerdermis.
WeanalyzedexpressionofIL-12,IFN-␥,TNF-␣,IL-4,IL-13,
TGF-andIL-10mRNAsinthedermisofdogsimmunizedwiththeLBSap vaccineandfoundthatexpressionofIL-12andIL-10mRNAsat12 and24h,respectively,washigherintheLBSapgroupthaninthe Sapgroup.Moreover,weobservedpositivecorrelationsbetween expressionofthemRNAsoftypeI(IL-12,IFN-␥,TNF-␣)andtype IIcytokines(especiallyIL-13)in thegroupsinoculated withthe separatevaccinecomponentsand withtheLBSapvaccineitself. Theseresultssuggestthatafteradministrationofeachinocula,a microenvironmentofmixedtypeIandIIcytokinesdevelopsinthe skinofanimals.Cytokinesplaya decisiverolein theregulation oftheimmuneresponsedirectedagainstL.infantum, determin-ingtheprevention orprogression of theinfection(Carrillo and Moreno,2009).Severalstudieshavedemonstratedthatamixed cytokinepatterncanbeassociatedwithresistanceor susceptibil-ityin both vaccineand Leishmania-infection models(Raziuddin andAbdalla,1994;Peruhype-Magalhaesetal.,2006).Inmurine leishmaniasis,severalresearchershaveobservedthatIL-13 synthe-sispromotesinitialIFN-␥productionandinfluencestheassembly andmaturationoftissuegranuloma.However,suchexperiments havenot addressedthemechanism(s)bywhich IL-13regulates theexpressionofanti-leishmanialtype1response(Murrayetal., 2006).Menezes-Souzaetal.(Menezes-Souzaetal.,2011) demon-strated that asymptomatic Leishmania-infected animals present highexpressionofIL-13withconcomitantIFN-␥production.The authorssuggestthatinflammatorycytokineprofiles,particularly thosedrivenbyIFN-␥,TNF-␣andIL-13,couldbeusedas biomark-ersforasymptomaticclinicalformsinCVL.
InstudiesofHIVinfectionswasdemonstratedeffectsofboth IL-13andIFN-␥onHIV-1macrophageinfections(Meylanetal.,1993; Kornbluthetal.,1989),toenhanceAgpresentingfunction(deWaal Malefytetal.,1993;Marshalletal.,1997),andtobeassociatedwith improveddiseasestatus(increasedCD4ordecreasedHIVviralload) suggestacontributionbythesecytokinesinmaintainingimmune functionfollowingHIV-1infection(Robertetal.,1999).
In this study, we observed increased expression of some chemokinesintheskinofdogsinoculatedwiththevariousvaccine components.Isimportanttonotethatchemokinesproducedatthe siteofinfectionarecriticalindeterminingthecellsthatinfiltrate thesiteandthusindefiningtheeventualoutcomeofthedisease (Teixeiraetal.,2006).Inaddition,Leishmaniaparasitesalsopossess achemotacticfactorthatselectivelyattractsneutrophilswhichcan
serveashostcellsintheearlystagesofinfection(vanZandbergen etal.,2002).
Our datashowedincreasedexpressionofCCL4 mRNAin the LBSapgroup24hafterinoculation.Inaddition,significant upregu-lationofCCL5mRNAexpressionwasobserved1hafterinoculation intheLBSapgroup.Attheotherbiopsytimes,thelevelsofCCL4 andCCL5mRNAswerehigherintheLBgroupthanintheother groups.ThesedatademonstratetheabilityoftheLBSapvaccineto inducetheproductionofchemokines(CCL4andCCL5),whichare importantintherecruitmentofimmunecellstotheinoculation site.Thesechemokinespreferentiallyrecruitmonocytestotheskin, suggestingahoststrategyforcontrollingparasitismduringongoing CVLinfection(Menezes-Souzaetal.,2011).Inaddition,the expres-sionofCCL5mayberelatedtoresistancetoinfectionbyLeishmania followingthecascadeofeventsleadingtoparasitecontrolduring L.majorinfection(Santiagoetal.,2004).
Inthisstudy,weobservedhigherlevelsofCXCL8expressionin theskinofdogsvaccinatedwiththeLBSapvaccinethaninthedogs intheothergroups.InthefirstfewhoursofLeishmaniainfection, CXCL8productionamplifiestherecruitmentofneutrophilstothe infectionsite(vanZandbergenetal.,2002;Badolatoetal.,1996; Venuprasadetal.,2002).Neutrophilsrepresentthefirstgroupof leukocytestoarriveatthesiteofinfection,andtheyarethought toserveasTrojanhorsesforeventualentryofintomacrophages, theultimatecellularhostforLeishmania.However,theinitialinflux ofneutrophilsseemstobebeneficialforLeishmaniasurvivalinthe infectedtissue(vanZandbergenetal.,2004).
Theinteractionsofcytokinesandchemokineswithvariouscells in theskinlayers after immunizationare importantfor under-standingthe mechanisms bywhich adaptive immunityagainst Leishmaniainfectionisactivated.Ourcorrelationanalysisbetween celltypesandchemokineexpressionintheouterdermisshoweda positivecorrelationbetweenmacrophagesandCCL5andanegative correlationbetweenneutrophilsandCCL5intheSapgroup.These dataconfirmtheroleofCCL5intherecruitmentofmacrophages andothermonocytes.IntheLBSapgroup,neutrophilswere posi-tivelycorrelatedwithincreasedexpressionofIFN-␥,IL-12andIL-4 mRNAs,andamixedprofileofcytokineswasobservedintheskin.In theinnerdermis,neutrophilsandIL-13expressionwerepositively correlatedintheLBgroup.Aswasthecaseintheouterdermis,CCL5 expressionandneutrophilswerenegativelycorrelatedintheSap group.IntheLBSapgroup,neutrophilswerepositivelycorrelated withexpressionofIL-12,TNF-␣andIL-13mRNAs.
The developmentof immunitydepends on themigration of appropriate cell populations to infected sites, which in turn dependsontheexpressionofcytokinesandchemokines. Addition-ally,theknowledgeofthecellprofileofthelocalinflammatory infiltratecanprovidesupplementaryinformationconcerningthe immune response in the microenvironment of the inoculation (Stewartetal.,1984).Takentogether,ourcurrentresultsallowus toconcludethatintheskinmicroenvironmentofdogsimmunized withLBSapvaccine,therewasamixedcytokineprofileanda dis-tinctiveexpressionprofileforCCL4,CCL5andCXCL8mRNAs.Our datacanbeexpectedtocontributetotheestablishmentofa ratio-nalstrategyforthedevelopmentofvaccinesandimmunological therapiesagainstvisceralleishmaniasis.
Acknowledgements
548 56 (2013) 540–548
gratefulfortheuseoffacilitiesatCEBIO,UniversidadeFederalde MinasGerais,andRedeMineiradeBioterismo(FAPEMIG),andfor supportwiththeprovisionofexperimentalanimals.
References
Badolato,R.,Sacks,D.L.,Savoia,D.,Musso,T.,1996.Leishmaniamajor:infectionof
humanmonocytesinducesexpressionofIL-8andMCAF.Experimental Parasi-tology82,21–26.
Borja-Cabrera,G.P.,CruzMendes,A.,ParaguaideSouza,E.,HashimotoOkada,L.Y.,
de,A.T.F.A.,Kawasaki,J.K.,etal.,2004.Effectiveimmunotherapyagainstcanine
visceralleishmaniasiswiththeFML-vaccine.Vaccine22,2234–2243.
Brachelente,C.,Muller,N.,Doherr,M.G.,Sattler,U.,Welle,M.,2005.Cutaneous
leishmaniasisinnaturallyinfecteddogsisassociatedwithaThelper-2-biased immuneresponse.VeterinaryPathology42,166–175.
Carrillo,E.,Moreno,J.,2009.Cytokineprofilesincaninevisceralleishmaniasis.
Vet-erinaryImmunologyandImmunopathology128,67–70.
Chamizo,C.,Moreno,J.,Alvar, J.,2005.Semi-quantitativeanalysisofcytokine
expressioninasymptomaticcanineleishmaniasis.VeterinaryImmunologyand Immunopathology103,67–75.
daSilva,V.O.,Borja-Cabrera,G.P.,CorreiaPontes,N.N.,deSouza,E.P.,Luz,K.G.,
Palat-nik,M.,etal.,2000.AphaseIIItrialofefficacyoftheFML-vaccineagainstcanine
kala-azarinanendemicareaofBrazil(SaoGoncalodoAmaranto,RN).Vaccine 19,1082–1092.
deWaalMalefyt,R.,Figdor, C.G.,Huijbens,R.,Mohan-Peterson,S.,Bennett,B.,
Culpepper,J.,Dang,W.,Zurawski,G.,deVries,J.E.,1993.EffectsofIL-13on
phenotype,cytokineproduction,andcytotoxicfunctionofhumanmonocytes: comparisonwithIL-4andmodulationbyIFN-gorIL-10.JournalofImmunology 151,6370.
Deane,L.M.,Deane,M.P.,1995.Leishmaniosevisceralurbana(nocãoenohomem)
emSobral,Ceará.Hospital,75–87.
Desjeux,P.,2004.Leishmaniasis:currentsituationandnewperspectives.
Compar-ativeImmunology,MicrobiologyandInfectiousDiseases27,305–318.
Giunchetti,R.C.,Correa-Oliveira,R.,Martins-Filho,O.A.,Teixeira-Carvalho,A.,Roatt,
B.M.,deOliveiraAguiar-Soares,R.D.,etal.,2007.Immunogenicityofakilled
Leishmaniavaccinewithsaponinadjuvantindogs.Vaccine25,7674–7686.
Giunchetti,R.C.,Reis,A.B.,daSilveira-Lemos,D.,Martins-Filho,O.A.,Correa-Oliveira,
R.,Bethony,J.,etal.,2008a.Antigenicityofawholeparasitevaccineas
promis-ingcandidateagainstcanineleishmaniasis.ResearchinVeterinaryScience85, 106–112.
Giunchetti,R.C.,Correa-Oliveira,R.,Martins-Filho,O.A.,Teixeira-Carvalho,A.,Roatt,
B.M.,deOliveiraAguiar-Soares,R.D.,etal.,2008b.AkilledLeishmaniavaccine
withsandflysalivaextractandsaponinadjuvantdisplaysimmunogenicityin dogs.Vaccine26,623–638.
Gradoni,L.,2001.Anupdateonantileishmanialvaccinecandidatesandprospects
foracanineLeishmaniavaccine.VeterinaryParasitology100,87–103.
Gupta,G., Dey, R.,Bhattacharyya, S., Majumdar, S.,2011. Role of exogenous
chemokinesasimmunotherapeutictoolagainstvisceralleishmaniasis.Science AgainstMicrobialPathogens:CommunicatingCurrentResearchand Technolog-icalAdvances,605–612.
Hommel,M.,Jaffe,C.L.,Travi,B.,Milon,G.,1995.Experimentalmodelsfor
leishman-iasisandfortestinganti-leishmanialvaccines.AnnalsofTropicalMedicineand Parasitology89(Suppl.1),55–73.
Kornbluth,R.S.,Oh,P.S.,Munis,J.R.,Cleveland,P.H.,Richman,D.D.,1989.
Inter-feronsandbacteriallipopolysaccharideprotectmacrophagesfromproductive infectionbyhumanimmunodeficiencyvirusinvitro.JournalofExperimental Medicine169,1137.
Lage,R.S.,Oliveira,G.C.,Busek,S.U.,Guerra,L.L.,Giunchetti,R.C.,Correa-Oliveira,R.,
etal.,2007.Analysisofthecytokineprofileinspleencellsfromdogsnaturally
infectedbyLeishmaniachagasi.VeterinaryImmunologyandImmunopathology 115,135–145.
Lasri,S.,Sahibi,H.,Sadak,A.,Jaffe,C.L.,Rhalem,A.,1999.Immuneresponsesin
vaccinateddogswithautoclavedLeishmaniamajorpromastigotes.Veterinary Research30,441–449.
Lemesre,J.L.,Holzmuller,P.,Cavaleyra,M.,Goncalves,R.B.,Hottin,G.,Papierok,G.,
2005.Protectionagainstexperimentalvisceralleishmaniasisinfectionindogs
immunizedwithpurifiedexcretedsecretedantigensofLeishmaniainfantum promastigotes.Vaccine23,2825–2840.
Lemesre,J.L.,Holzmuller,P.,Goncalves,R.B.,Bourdoiseau,G.,Hugnet,C.,Cavaleyra,
M.,etal.,2007.Long-lastingprotectionagainstcaninevisceralleishmaniasis
usingtheLiESAp-MDPvaccineinendemicareasofFrance:double-blind ran-domisedefficacyfieldtrial.Vaccine25,4223–4234.
Livak,K.J.,Schmittgen,T.D.,2001.Analysisofrelativegeneexpressiondatausing
real-timequantitativePCRandthe2(-DeltaDeltaC(T))Method.Methods25, 402–408.
Marshall,J.D.,Robertson,S.E.,Trinchieri,G.,Chehimi,J.,1997.PrimingwithIL-4and
IL-13duringHIV-1infectionrestoresinvitroIL-12productionbymononuclear cellsofHIV-infectedpatients.JournalofImmunology159,5705.
Mauel,J.,2002.VaccinationagainstLeishmaniainfections.CurrentDrugTargets:
Immune,EndocrineandMetabolicDisorders2,201–226.
Mayrink,W.,Genaro,O.,Silva,J.C.,daCosta,R.T.,Tafuri,W.L.,Toledo,V.P.,etal.,
1996.PhaseIandIIopenclinicaltrialsofavaccineagainstLeishmaniachagasi
infectionsindogs.MemoriasdoInstitutoOswaldoCruz91,695–697.
Menezes-Souza,D.,Correa-Oliveira,R.,Guerra-Sa,R.,Giunchetti,R.C.,
Teixeira-Carvalho,A.,Martins-Filho,O.A.,etal.,2011.Cytokineandtranscriptionfactor
profilesintheskinofdogsnaturallyinfectedbyLeishmania(Leishmania)chagasi presentingdistinctcutaneousparasitedensityandclinicalstatus.Veterinary Parasitology177,39–49.
Meylan,P.R.,Spina,C.A.,Richman,D.D.,Kornbluth,R.S.,1993.Invitrodifferentiation
ofmonocytoidTHP-1cellsaffectstheirpermissivenessforHIVstrains:amodel systemforstudyingthecellularbasisofHIVdifferentialtropism.Virology193, 256–267.
Moreira, N., Giunchetti, R.C., Carneiro, C.M., Vitoriano-Souza, J., Roatt, B.M.,
Malaquias,L.C.,etal.,2009.Histologicalstudyofcellmigrationinthedermis
ofhamstersafterimmunisationwithtwodifferentvaccinesagainstvisceral leishmaniasis.VeterinaryImmunologyandImmunopathology128,418–424.
Murray,H.W.,Tsai,C.W.,Liu,J.,Ma,X.,2006.VisceralLeishmaniadonovaniinfection
ininterleukin-13−/− mice.InfectionandImmunity74,2487–2490.
Panaro,M.A.,Acquafredda,A.,Lisi,S.,Lofrumento,D.D.,Mitolo,V.,Sisto,M.,etal.,
2001.Nitricoxideproductionbymacrophagesofdogsvaccinatedwithkilled
Leishmaniainfantumpromastigotes.ComparativeImmunology,Microbiology andInfectiousDiseases24,187–195.
Peruhype-Magalhaes,V.,Martins-Filho,O.A.,Prata,A.,SilvaLde,A.,Rabello,A.,
Teixeira-Carvalho,A.,etal.,2006.Mixedinflammatory/regulatorycytokine
pro-filemarkedbysimultaneousraiseofinterferon-gammaandinterleukin-10and lowfrequencyoftumournecrosisfactor-alpha(+)monocytesarehallmarksof activehumanvisceralLeishmaniasisduetoLeishmaniachagasiinfection.Clinical andExperimentalImmunology146,124–132.
Quinnell,R.J.,Courtenay,O.,Shaw,M.A.,Day,M.J.,Garcez,L.M.,Dye,C.,etal.,2001.
Tissuecytokineresponsesincaninevisceralleishmaniasis.JournalofInfectious Diseases183,1421–1424.
Raziuddin,S.,Abdalla,R.E.,el-Awad,E.H.,al-Janadi,M.,1994.Immunoregulatoryand
proinflammatorycytokineproductioninvisceralandcutaneousleishmaniasis. JournalofInfectiousDiseases170,1037–1040.
Roatt,B.M.,Aguiar-Soares,R.D.,Vitoriano-Souza,J.,Coura-Vital,W.,Braga,S.L.,
Correa-Oliveira,R.,etal.,2012.PerformanceofLBSapvaccineafter
intrader-malchallengewithL.infantumandsalivaofLu.longipalpis:immunogenicity andparasitologicalevaluation.PLoSONE7,e49780.
Robert,T.,Bailer,A.H.,Sun,Junwei,Joseph,B.Margolick,Martin,Melissa,Kostman,
Jay,Montaner,LuisJ.,1999.IL-13andIFN-gsecretionbyactivatedTcellsin
HIV-1infectionassociatedwithviralsuppressionandalackofdiseaseprogression. TheJournalofImmunology,7534–7542.
Rot,A.,vonAndrian,U.H.,2004.Chemokinesininnateandadaptivehostdefense:
basicchemokinesegrammarforimmunecells.AnnualReviewofImmunology 22,891–928.
Santiago,H.C.,Oliveira,C.F.,Santiago,L.,Ferraz,F.O.,deSouza,D.G.,de-Freitas,
L.A.,etal.,2004.InvolvementofthechemokineRANTES(CCL5)inresistance
toexperimentalinfectionwithLeishmaniamajor.InfectionandImmunity72, 4918–4923.
Santos-Gomes,G.M.,Rosa,R.,Leandro,C.,Cortes,S.,Romao,P.,Silveira,H.,2002.
Cytokineexpressionduringtheoutcomeofcanineexperimentalinfectionby Leishmaniainfantum.VeterinaryImmunologyandImmunopathology88,21–30.
Stewart,R.J.,Holloway,L.J.,Isbister,W.H.,1984.Peritonealneutrophilia:apotential
indicatorofthesurgicalacuteabdomen.AustralianandNewZealandJournalof Surgery54,565–568.
Strauss-Ayali,D.,Baneth,G.,Shor,S.,Okano,F.,Jaffe,C.L.,2005.Interleukin-12
aug-mentsaTh1-typeimmuneresponsemanifestedaslymphocyteproliferation andinterferongammaproductioninLeishmaniainfantum-infecteddogs. Inter-nationalJournalforParasitology35,63–73.
Strauss-Ayali,D.,Baneth,G.,Jaffe,C.L.,2007.Splenicimmuneresponsesduring
caninevisceralleishmaniasis.VeterinaryResearch38,547–564.
Teixeira,C.R.,Teixeira,M.J.,Gomes,R.B.,Santos,C.S.,Andrade,B.B.,Raffaele-Netto,
I.,etal.,2005.SalivafromLutzomyialongipalpisinducesCCchemokineligand
2/monocytechemoattractantprotein-1expressionandmacrophage recruit-ment.JournalofImmunology175,8346–8353.
Teixeira, M.J., Teixeira, C.R., Andrade, B.B., Barral-Netto, M., Barral, A., 2006.
Chemokinesinhost–parasiteinteractionsinleishmaniasis.Trendsin Parasito-logy22,32–40.
vanZandbergen,G.,Hermann,N.,Laufs,H.,Solbach,W.,Laskay,T.,2002.Leishmania
promastigotesreleaseagranulocytechemotacticfactorandinduce interleukin-8releasebutinhibitgammainterferon-inducibleprotein10productionby neutrophilgranulocytes.InfectionandImmunity70,4177–4184.
vanZandbergen,G.,Klinger,M.,Mueller,A.,Dannenberg,S.,Gebert,A.,Solbach,
W.,etal.,2004.Cuttingedge:neutrophilgranulocyteservesasavectorfor
Leishmaniaentryintomacrophages.JournalofImmunology173,6521–6525.
Venuprasad,K.,Banerjee,P.P.,Chattopadhyay,S.,Sharma,S.,Pal,S.,Parab,P.B.,
etal.,2002.Humanneutrophil-expressedCD28interactswithmacrophageB7
toinducephosphatidylinositol3-kinase-dependentIFN-gammasecretionand restrictionofLeishmaniagrowth.JournalofImmunology169,920–928.
Vitoriano-Souza,J.,Reis,A.B.,Moreira,N.D.,Giunchetti,R.C.,Correa-Oliveira,R.,
Carneiro,C.M.,2008.Kineticsofcellmigrationtothedermisandhypodermis
indogsvaccinatedwithantigeniccompoundsofLeishmaniabraziliensisplus saponin.Vaccine26,3922–3931.
Vouldoukis,I.,Drapier,J.C.,Nussler,A.K.,Tselentis,Y.,DaSilva,O.A.,Gentilini,M.,
etal.,1996.Caninevisceralleishmaniasis:successfulchemotherapyinduces