ISSN 0104-6632 Printed in Brazil
www.abeq.org.br/bjche
Vol. 33, No. 04, pp. 907 - 917, October - December, 2016 dx.doi.org/10.1590/0104-6632.20160334s20150376
Brazilian Journal
of Chemical
Engineering
LEACHING Zn FROM THE LOW-GRADE ZINC
OXIDE ORE IN NH
3
-H
3
C
6
H
5
O
7
-H
2
O MEDIA
Ma Ai-yuan
1,2,3,4, Peng Jin-hui
1,2,3,4, Zhang Li-bo
1,2,3,4, Shiwei Li
1,2,3,4*,
Yang Kun
1,2,3,4and Zheng Xue-mei
1,2,3,41Yunnan Provincial Key Laboratory of Intensification Metallurgy, Kunming 650093, China.
Phone: + 86 871 65174756; Fax: + 86 871 65138997 E-mail: lswei11@163.com; lishiweikmust@163.com
2
National Local Joint Laboratory of Engineering Application of Microwave Energy and Equipment Technology, Kunming, Yunnan 650093, China.
3
Key Laboratory of Unconventional Metallurgy, Ministry of Education, Kunming 650093, China.
4
Faculty of Metallurgical and Energy Engineering, Kunming University of Science and Technology, Kunming 650093, China.
(Submitted: March 25, 2015 ; Revised: June 15, 2015 ; Accepted: July 21, 2015)
Abstract - In this research, the effect of different citric acid concentrations, ammonia concentration, temperature, leaching time, stirring speed and liquid-to-solid ratio on the zinc leaching from low-grade zinc oxide ore in a NH3-H3C6H5O7-H2O system were studied. The results showed that the zinc leaching rate is only
4.7% when the citric acid concentration is 0 M, and the leaching efficiency of Zn increased with increasing citric acid concentration. Under the conditions: citric acid concentration of 1.0 M, ammonia concentration of 6 M, temperature of 25 °C, leaching time of 60 min, stirring speed of 300 rpm and the starting solid-to-liquid ratio of 1:5, 81.2% of Zn is leached. The mineralogical changes of the low-grade zinc oxide ores during the processes were characterized by X–ray fluorescence (XRF), X–ray powder diffraction (XRD), Scanning Electron Microscopy associated with Energy Dispersive Spectroscopy (SEM–EDS) and Fourier transform infrared spectroscopy (FT–IR). From the evidence we deduced that citric ions complexed with zinc ions, forming a Zn-citrate complex. As a result, the zinc leaching rate was improved without the risk of pollution or pretreatment. This makes it as a good choice for a more ecological treatment of hemimorphite.
Keywords: Low-grade zinc oxide ores; Hemimorphite; Ammonia leaching; Citric acid.
INTRODUCTION
Considering the huge reserves of low grade zinc oxide ores with high alkaline gangue (CaO and MgO) in the world (Li et al., 2010), which is hard to handle by the traditional zinc smelting methods, re-covering zinc from low grade oxide zinc ores has been a matter of discussion recently. The leach-sol-vent extraction-electro-winning process route is the most common method of extraction of zinc from low-grade zinc oxide ores (de Wet and Singleton, 2008). This is a material which can be processed by flotation (Irannajad et al., 2009; Navidi Kashani and
Rashchi, 2008; Li et al., 2013) and acid-leaching methods (Irannajad et al., 2013; Xu et al., 2010; Xu
908 Ma Ai-yuan, Peng Jin-hui, Zhang Li-bo, Shiwei Li, Yang Kun and Zheng Xue-mei
Brazilian Journal of Chemical Engineering
The application of alkaline leaching techniques has become an increasingly important aspect in the recovery of base and precious metals from complex low grade zinc oxide ores (Santos et al., 2010; Ding
et al., 2010; Yin et al., 2010). However, alkaline
leaching processing is often confronted with the problem of low-grade complex ores, especially zinc silicate. The low solubility of these ores does not usually allow for the recovery of the target metal, even by direct chemical leaching in many leaching reagents (Zhao et al., 2009). Extracting zinc from hemimorphite by leaching in sodium hydroxide solu-tion requires high temperature, pressure and alkalin-ity (Chen et al., 2009). Currently, hydrometallurgy in ammonia has been considered as a prospective me-dium for the leaching of complex zinc ores of both oxide and sulfide types (Li et al., 2014).
The use of organic acids for the extraction of metals has been studied by many researchers. It was reported that low molecular weight citric acid has been found to be effective in removing heavy metals by forming soluble complexes and chelates with metal ions (Chen et al., 2003; Hongki et al., 2013), and there is no concern about environmental prob-lems after the treatment. Citric acid was found to be more active than H2SO4. In hydrometallurgical treat-ments, the ability to recover metals or metal oxides depends on their chemical reactivities (Larba et al., 2013). So citric acid seems more appropriate to use as a less costly and more environmentally friendly leachant.
Several papers have been published on the coor-dination of citric acid and transition metals, lead citrate (Kourgiantakis et al., 2000), tungsten citrate (Zhang et al., 2003), aluminum citrate (Matzapetakis
et al., 1999) and germanium citrate (Willey et al.,
2001). Based on previous work, a new hydrometal-lurgical process for low-grade zinc oxide ore is put
forward. In this work, the effect of the concentration of citric acid, ammonia concentration, temperature, leaching time, stirring speed and liquid-to-solid ratio on the leaching of zinc from low grade zinc oxide ore in the NH3-H3C6H5O7-H2O system was studied. The aim of this work was to develop a new hydro-metallurgical technology, which intends to provide a green and economic method to extract zinc from low-grade zinc oxide ores.
MATERIALS AND METHODS
Experimental Materials
The low-grade zinc oxide ores used were given by Lanping County of Yunnan Province, China. The main chemical composition of the low-grade zinc oxide ores obtained by chemical analysis is listed in Table 1. It can be observed that silica (SiO2) and a high content of alkaline gangues (CaO + MgO) are the main components of the ore. The zinc content in this ore, around 6.01%, is considered to be low for a successful pyrometallurgical treatment for zinc re-covery (Dutra et al., 2006), which leads to the greater interest in the hydrometallurgical treatment. The XRD spectrum of the low-grade zinc oxide ore is shown in Figure 1. The XRD analysis shows that silica (SiO2), calcium carbonate (CaCO3), lead carbonate (PbCO3), and hemimorphite (Zn4Si2O7(OH)2·H2O) are the main chemical components. Table 2 for the mineral com-position in zinc shows that the ore contains mainly 67.55% hemimorphite (Zn4Si2O7(OH)2·H2O), 29.78% zinc carbonate (ZnCO3), 1.53% zinc sulfide (ZnS), and 1.05% Franklinite ( ZnFe2O4). Other zinc-con-taining phases, which, on the basis of the chemical analysis, could be present (Table 1) are probably be-low the detection limit.
Table 1: Chemical analysis of the main elements present in the zinc oxide ore.
Element O Si Ca Fe Zn Pb Al C Mg S
Weight
(mass %) 46.73 13.03 9.19 8.11 6.01 6.41 4.43 4.30 0.52 0.45
Table 2: Main mineral composition of zinc in ore sample.
Phase composition (Zn4Si2O7(OH)2·H2O) ZnCO3 ZnS ZnFe2O4
Zn Content (mass %) 4.06 1.79 0.092 0.063
E n lo in an zi
Scanning Energy Dispe
iques were u ow-grade zin n Figure 2.
nalyze the e inc oxide or
Electron M ersive Spectr used for the nc oxide ore Three typic element dist re. It is seen
Fig
Figure 1: T
Microscopy a roscopy (SEM
morphologic e, and the res al phases w tribution in n that Figure
gure 2: SEM
The XRD pat associated w M / EDS) te cal study of sults are sho were selected the low-gra e 2b (point 1
M image (a) an
ttern of the lo with
ech-the own d to ade 1 in
the som in pa tai gre co
nd EDS patte
ow-grade zin e black parts me other me the ore sam rts) reveals ining compo
ey parts), it exist.
erns (b)-(d) i
nc oxide ore. s) shows tha etal element mple. Figure
the region ounds. From can be foun
in different a
at Zn, Si, O, ts are mutua e 2c (point 2 ns enriched
Figure 2d ( und that Si,
area.
Al, Fe, K an ally embedde 2 in the whi
in lead-co (point 3 in th
91 E 1 a n th m th am ac ti le Z fi T ac
η
w (g (L th lo F lo T M tu 10 Experimenta The exper 49 μm) fract Two-step l 300 mL clo etic stirrer, a he leaching p The gener ments was ashe conical fl mmonia (6 M cid were pla ime 1 h and eaching expe Zn content in
iltrate was a The leaching ccording to t
0 Zn Zn Z
C
V
m C
η
=
×
×
where
η
Zn (% g/L) the zinc L) the leachi he low-grade ow-grade zinFigure 3: Flo
ow-grade zin Thermodyna Medium Citric acid ural formula al Method riments used tion as mater leaching exp osed glass rea
and Figure 3 process.
ral procedur follows: 20 g ask, 100 mL M) with diffe aced in the re d magnetical eriments we n the solution analyzed by recovery of the following 0
100%
ZnV
×
%) is the leac c concentrati ng volume, e zinc oxide nc oxide ore.
ow diagram nc oxide ore l
amic Analysi
contains thre is shown in
Ma Ai-yuan, P
d the −100 + rial.
periments we actor equipp shows the f re for the le g of samples L of the solu erent concent eactor at 298 lly stirred at ere performe n was determ EDTA titrim f zinc (ηZn) g equation (E
ching recove ion of leach
0 Zn
C
(%) the ore, m (g) tof the leach leaching.
is for the Zin
ee COO- grou n Figure 4.
Peng Jin-hui, Zha
Brazilian Jou
+200 mesh ( re conducted ped with a m
flow diagram eaching expe
was placed i ution contain trations of ci .15 K, leach t 300 rpm. T ed in triplica mined. Zn in
metric meth was calcula Eq. (1)):
ry of zinc, C hing solution
zinc content the mass of
hing process
nc - Citric A
up and its str The ionizat
ang Li-bo, Shiwe
urnal of Chemica
74-d in mag-m of eri-into ning itric hing The ate. the hod. ated (1) Zn C n, V t of the for Acid ruc-tion con pK sol of car an dis et sol be 6 C K 6 C K 6 C K
a f La pre H2 H( ion mo spe of tur al. et Fig (C Ef tio
ei Li, Yang Kun a
al Engineering
nstants of c Ka2=4.76 ; pK
lution has a s ionized citr rboxyls are d, upon diss stilled water,
al., 2013). In lution. The d expressed a
6 8 7 2
a1
H O H O
7.4 10−
+ ↔
= ×
6 7 7
a2
H O (aq) H
1.7 10 − − + = × 2 6 6 7
a3
H O (aq) H
4.0 10 − − + = × The fraction function of p apidus, 2014
edominates b 2(Cit)− specie (Cit)2− appea n. Because ore than 9, a
ect to Eq. (4 zinc citrate re. The comp , 2005) and
al., 1983).
gure 4: Th
6H8O7).
RES
ffect of Citri
The study f on on the ex
and Zheng Xue-m
itric acid are Ka3=6.40, and strong acidity ric acid are
contained i sociation of , 3 M H+ is
n fact, not al dissociation
s follows (D
3 ( aq ) 6
4 1
H O C
, pK 3.
+
−
↔ +
=
2 3 (aq )
5 2
H O H O
, pK 4 +
−
↔
=
2 3 ( aq
7 3
H O H O
, pK
+
−
↔
=
ns of each ci pH at 298.15
), where it c below pH = es dominates ar and, initia the pH of t basic reactio ). Few studie complexes w pounds found
[Zn(II)(C6H
he structural
SULTS AND
ic Acid Conc
for the effec xtraction of
mei
re as follows d show that y. The main related to t in one C6H f 1 mol citri
theoretically all the H+ is r reaction of Demir et al., 2
6H O (aq),7 7
.13
−
2 ) C H O6 6 7 (a
4.76 −
+
3 q ) C H O6 5 7 (a
6.40
− +
itrate species 5 K (Zárate-can be note 3 and betwe s; only above ating at pH
the reaction on was carrie es focused o
with non-mo d are C12H14O H5O7)2 ·4NH
l formula o
D DISCUSSI
centration
ct of citric a zinc was c
s: pKa1=3.13 the citric ac existing form the pH. Thr H8O7 molecu
c acid in 1 y produced (
released to th citric acid ca 2006): (2 q), (3 aq), (4
s are shown -Gutiérrez an
d that H3(C en 3 and 5 th e pH = 5 do
= 7, the Cit medium w ed out with r n the structu olecular stru O16Zn3 (Che 4+ ] (Swanso
of citric ac
ION
cid concentr carried out b
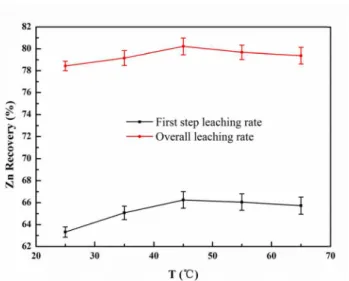
v ra F o co n zi sp 2 in ex d in d ef in m le in ce in o re ti m kn ac b w ra F re le tw le arying conc ange. The a Figure 5, whi
f Zn increas oncentration amely 0 and inc were ex pectively. Th 4.57% to 81 ncreased fro xtraction as t ue to the citr ng the C5H7O
issolution of fficiencies w ng, under the monia, 1.0 M eaching time n stirring spe As seen in entration inc ng the proce usly consum esulting in a ion at low c maximum rec nown to driv cid, facilitati The down e the product winning or pr ation of impu
Figure 5: Eff
ecovery from eaching rate wo steps; the eaching rate
centrations in average leac ich shows th sed with the n. At the low d 0.05 M, o xtracted in t he zinc extr .27% when t om 0.05 to the citric aci tric acid conc
O5COO- acti f zinc contai were obtained e following le M in citric ac
e, 298.15 K in eed.
n Figure 5, an creases the di ess of leach med as it ge
a possible in citric acid c covery. An i ve the reactio ing an increa nstream proc tion of zinc b recipitation o urities.
fect of conce m low-grade represents th e first step l in the first st
n the 0-1.0 ching rates hat the leach e increase of w citric acid only 4.73%
the two-stag raction was the citric aci 1.0M. Incre id concentrat centration ef ivity, that re ining ore. 81 d for Zn by tw
eaching cond id concentra n temperatur n increase in issolution ra ing, citric a ets converted nsufficient ci concentration increase in c on of zinc res ase in leachin cessing of le by solvent ex of Zn2+ after
entration of C e zinc oxide he total leach leaching rate tep as shown
M citric a are shown ing efficienc f the citric a concentratio and 24.57% e leaching, improved fr d concentrat easing the z tion increase ffect of incre esults in furt 1.27% leach wo-stage lea dition: 6 M a ation, 60 min re, and 300 r
citric acid c te of zinc. D acid is contin
d into produ itric acid sit ns, limiting concentration
sidue with ci ng.
each liquor c xtraction elect
chemical se
C6H8O7 on z e ores. Over
hing rate in e represents n in Figure 3.
acid in cies acid ons, % of re-rom tion zinc s is eas-ther hing ach- am-n iam-n rpm on- Dur- nu-uct, tua-the n is itric can tro- epa-zinc rall the the . Ef by the zin aci 25 rat mo lea to in Fig rec Ef on tio of mo bil der sul H3 cre ma rat zin wi Ef
ffect of Amm
Zinc oxide y two-step le e total ammo nc. The leac id concentra °C; stirring tio of 5:1. Th
As shown i onia concent aching effici 80.21%. Ho a stable rang
gure 6: Effe
covery from
ffect of Temp
An investig n zinc extrac ons were the
effect of am onia concent lity of zinc i r the same c lts are shown The zinc 3C6H5O7-H2O eased temper ained in a co tion under th nc extraction ith increased
ffect of Leac
Figure 8 sh
monia Conce
ore samples eaching to in onia concentr ch condition ation of 0.7 speed of 30 he results are in Figure 6, trations from
ency increas owever, the s
ge of approxi
ect of ammo low-grade zi
perature
gation into th ction was als
same as tho mmonia conc tration was a n the NH3-H conditions w n in Figure 7
leaching e O system in
rature. The z onstant range he studied te n was found temperature
ching Time
hows the ef
entration
s were leach nvestigate th tration on the ns were as f 75 M; temp 00 rpm; and e shown in Fi , with the in m 2.0 M to 8 sed quickly solubility of
imately 6 M.
onia concent inc oxide ore
he influence o so performe ose detailed centration, e adjusted to 6 H3C6H5O7-H2 was measure 7.
efficiency i ncreased stea zinc leaching e, which was temperature. d to exhibit
e.
ffect of leac
hed for 60 m e influence e extraction follows: citr erature set liquid-to-sol igure 6. ncrease of am
.0 M, the zin from 23.06 zinc remaine .
tration on zin es.
of temperatu ed. The cond
in the sectio except the am 6 M. The sol
2O system u d, and the r in the NH adily with i g efficiency r s close to sat In this stud a little impa
91 le m o p li o in re d d F lo F fr E ac ti ra le 12 eaching effic min to 90 min
f 0.75 M; am erature set a iquid-to-solid
f Zn increase ng time incr eaction was a
emonstrated uration is su
Figure 7: Effe
ow-grade zin
Figure 8: Ef
rom low-grad
Effect of Stir
Under the cid concentr ion of 6 M, t atio of 5:1, eaching effic
ciency of Zn n, maintainin mmonia conc at 25 °C; stir
d ratio of 5: ed from 61.3 reased from almost comp that the dur ufficient to ac
fect of temper nc oxide ores
ffect of leach de zinc oxide
rring Speed
technical pa ration of 0.7 temperature o
the effects ciency were
Ma Ai-yuan, P
n for leaching ng citric acid centration of rring speed o :1. The leac 36% to 79.53 20 min to 9 pleted in 60 m ration of abou chieve maxim
rature on zinc s.
hing time on e ores.
arameters as 75 M, ammo
of 25 °C and of stirring studied, and
Peng Jin-hui, Zha
Brazilian Jou
g time from d concentrat f 6 mol/L; te of 300 rpm; a hing efficien 3% as the lea
90 min and min. The resu
ut 60 to 80 m mum efficien
c recovery fr
n zinc recov
s follows: cit onia concent d liquid-to-so speed on z d the results
ang Li-bo, Shiwe
urnal of Chemica
20 tion em-and ncy ach-the ults min ncy. rom very tric tra-olid zinc are dis lea 78 to a s rpm of thi cre thi du cre Bu sol fic spe Fig fro Ef usi tio 0.7 rpm lea inc an the of wa ter sys the rap mo
ei Li, Yang Kun a
al Engineering
splayed in F aching effici
.44% as the 300 rpm, an significant ef m. This is b
hemimorphi in diffusion ease the stirr ickness attac ucing the thi
ease the reac ut the stirrin lid and the d ciency canno eed exceeds
gure 9: Effe
om low-grade
ffect of Liqu
The leachin ing various ons were as 75 M; tempe
m; and amm ach time of 1
Figure 10 s creases signi increased li e zinc leachin
approximate ard a possible ristics of zinc stem. At a lo e zinc ion co pidly. Howev ost invariable
and Zheng Xue-m
Figure 9. It ency of Zn i stirring spee nd that the sti ffect on the r ecause the a ite on the su layer at the ring speed re ched to the
ckness of th ction rate an ng cannot d diffusion laye ot be substan
350 rpm.
ect of stirrin e zinc oxide
uid-to-Solid R
ng efficienci liquid-solid follows: cit erature of 25 monia concen
h. The result shows that th ificantly from
quid/solid ra ng efficiency ely 5:1. This e cause of th c oxide ore i ow liquid-sol
oncentration ver, the zinc e in the range
mei
can be obse increased fro ed increased tirring speed recovery of ammonia lea urface of the
solid-liquid reduces the d
solid surfac he diffusion nd the disso destroy adhe
er, so the zin ntially increa
ng speed on ores.
Ratio
ies of zinc w d ratios. The
tric acid con 5 °C; stirring
ntration as 6 ts are shown he zinc leach m 65.75% to atio from 3 t y remained in observation he difficult le in the NH3-H
lid ratio (fro n in the solu c leaching ef e of 5:1 to 7:1
erved that th om 63.72%
from 200 rp does not hav zinc after 30 aching proce
solid forms d interface. I diffusion lay ce, thereby r layer, and i olving proces
esion betwee nc leaching e
ased when th
zinc recove
were evaluate e leach cond ncentration g speed of 30 6 mol/L and n in Figure 10
hing efficienc o 80.85% wi to 7. Howeve n a stable rang n may point t
aching chara H3C6H5O7-H2 om 3:1 to 5:1 ution increase fficiency is a 1. The possib
ca lo d m b F re F st sy te ro F ci w v ri
ause of this ow liquid-so
iffusion proc mainly in the y chemical r
Figure 10: E
ecovery from
FT-IR Analy
In order to tructure, we ynthesized c echniques. T oom tempera
Figure 11: FT
The FT-In itric acid and were recorded ibrationally ic and symm
problem is olid ratio m
cess and the e concentrati reaction cont
Effect of liq m low-grade z
sis
o obtain more studied the compound u The experime
ature are show
T-IR of differe nfrared spectr d different ci d in KBr an active carbox metric vibra
that the ex might be con
e diffusion re ion gradient trol in the lat
quid-to-solid zinc oxide or
e informatio e vibrational
using FT-IR ental IR spec wn in Figur
ent citric acid ra (400-4000 itric acid lea nd revealed t xylate group ations for t
xtraction rate ntrolled by esistance ex , but is mai ter stage.
d ratio on z res.
n on the crys spectra of R spectrosco ctra recorded re 11.
d concentratio 0 cm−1) of so aching solutio the presence ps. Antisymm he carboxyl e at the ists inly zinc stal the opic d at ons. olid ons e of met-late gro sen str car Th υs 13 vib wh 13 ric rev wi me (C the ord my ban the ing shi dit at dro an ion va res co ass tio eff gro can aci car po RC M Ch Le XR du ter by pat
oups of the nt in the s retching vibr
rboxylate ca he correspon (COO−) for 50-1550 cm bration peak hich shifted 97 cm-1) com c stretching
vealed the io ith metal cati etric and ant
OO−)), was e citrate carb dinated to z yrsi et al., 2 nd at 3496 c e citrate ligan g intermolec
ifted to lowe tion, the com 3496 cm−1, oxyl group o d involved i nized and thu
lues (Zárate-spect, it cou ordination to sembly.
Steer and G on might be
fects from L oups attache n play an ids are only rboxylate an otentially alte
-COO +MOU
– metal; R –
haracterizat
eaching Res RF
The X-ray ue was perfor rns of the le y 1 M citric a ttern of leac
coordinated spectra. In ations υas (C arbonyls in th nding symme the same gr m−1 (Kaliva e
ks (VC=O: 17 to lower fre mpared with
vibrations a onized COO−
ions. The dif isymmetric s greater than boxylate grou
inc in a mo 2001). Presen
cm−1 shows t nd is protona cular H bond er frequency mpound gives attributed to of water. We
n coordinati us undergo d -Gutiérrez an uld employ o zinc ion(s) Griffiths (201 better expla ewis acid/ba ed to the car important ro
partially dis nion (RCOO− er the extracti
RCOOM
U
– organic sub
tion of the L
idue Chara
characteriza rmed after th eached residu acid are show ch residue ob
d citrate liga particular, COO−) were p
the range 15 etric stretch roups were p
et al., 2006). 740 cm−1) o equencies (1 free citric ac and symmet − groups from
fference betw stretches, ( n 200 cm-1, i ups were eith onodentate fa nce of a bro that the hydr ated. Becaus d, the absor y (Che et al., s a strong ab o the vibrati e found that ion to zinc m deprotonation nd Lapidus,
all of its ca possibly pro 13) noted tha ained by sub ase theory. T rboxylic acid role. Althoug ssociated ‘we −) shown in tion capabilit 2 M+H O bstituent grou Leaching Res acterization
ation of the he process. T ue obtained wn in Figure
btained was
nds were pr antisymmetr present for th
62-1630 cm ing vibration present aroun The carbox of citric aci 1579 cm-1 an cid. Asymme tric vibration m AC chelate
ween the sym (υas (COO− )-indicating th her free or c fashion (Tsar oad absorptio
roxyl group se of the exis rption band , 2005). In a bsorption ban
ion of the h the citrate li may further b
n at higher p 2014). In th arboxylates omoting a ne
at zinc extra bstituent grou The substitue
d functionali gh carboxyl eak acids’, th
Eq. (5) cou ty.
(5
up.
sidue
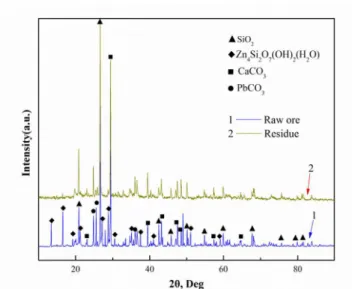
by XRD an
leaching res The XRD pa with leachin 12. The XR
matched wi re-ric
91 th ac d P S re le ac m st la co b at 3 p ac in m sp d o w 14
hat of the raw cid, the peak iminished s PbCO3 dimin iO2 and CaC ecovery of Z eaching, the
cterized as n may be used tock feed for andfill, if av
ontents, subj ility study in Chemical t 6.0 M am 00 rpm, soli erature of 2 cid leaching The result ng citric aci markedly wh pondingly. T ue are quar xide mineral with the disso
Table
w ore. Obviou for hemimor significantly. ished margin CO3 were not Zn is directl leaching res non-hazardou
as material r steel maki vailable, dep ject to an eco n each case.
composition mmonia conc
id-liquid rati 5 °C after 0 are shown in s in Table 3 id concentra hile the Zn The main mi
rtz and sma ls of iron, le olution of sili
3: Analysis Concentration (M) Zn (wt.%) Si (wt.%) Fe (wt.%)
Ma Ai-yuan, P
usly, in the pr rphite (Zn4Si
However, nally and the t diminished ly related to sidue can the us solid wast for road con ng plants, or ending on th onomic and ns of the res entration, st io of 5:1and 0.05, 0.45 an
n Table 3. showed that ation, Si an content de inerals in the ll amounts ead and calci
ica.
Figure
raw ore
of the leach
n
Peng Jin-hui, Zha
Brazilian Jou
resence of ci i2O7(OH)2·H2
the peaks e peaks for b d. As the over
o hemimorph erefore be ch
te. This resid nstruction or
r just placed he heavy me technical fea sidues obtain irring speed d leaching te
nd 1.0 M cit t, upon incre nd Fe enrich
ecreased cor e leaching re of undissolv ium, associa
12: XRD pa e. hing residue 0.05 4.61 13.62 8.40
ang Li-bo, Shiwe
urnal of Chemica
itric 2O) for both rall hite har-due r as d in etal asi-ned d of em-tric eas-hed rre- esi-ved ated M ch Ele siv use res sho of ind ion las bri (Fi po the (Fi con Ca the me dis lea
atterns of the
after leachi
ei Li, Yang Kun a
al Engineering orphology o
To obtain m aracteristics ectron Micro ve Spectrosc ed for the m sidue leached
own in Figu The elemen interesting a dicative of th ns overlap, th st image in w ight particles igure 13a po oint 3) on th
e ions of O igure 13d), C nfirm the hi a, Pb and Al e distribution etal with th scussed abov aching residu
e leaching res
ing in differ
0.45
1.74 14.69 8.98
and Zheng Xue-m
of the Leach
more informa of the main oscopy assoc copy (SEM
morphologic d by 1 M citr ure 13. nt distribution
aspects. The he signal int his may or m which only on s (Figure 13 oint 2) and w e distributio O (Figure 13
Ca (Figure 1 igh content i oxides and S n map for Zn
e low conte ve. The zinc ue, and can b
sidue and
ent citric ac
mei
hed Residue
ation about th metal eleme ciated with E / EDS) tec cal study of ric acid, and n maps reve
intensity of tensity of ea may not lead ne colour can 3a point 1), white particle on maps, cor 3b), Si (Fig 13e), and Pb in the leach Si minerals. F
n indicates t ent, validatin
did not get e be leached by
cid concentr 1.0 1.23 15.02 9.31 he distributio ents, Scannin Energy Dispe chniques we f the leachin
the results a aled a numb f each image ach ion. Whe to areas in th n be seen. Th grey particl es (Figure 13 rresponding gure. 13c), A b (Figure 13
ing residue Figure 13g f that this is th ng the resul enriched in th y citric acid.
Figure 13: (a) SEM image of the leaching residue, EDS mapping of O (b), Si (c), Al (d), Ca (e), Pb (f), Zn (g).
CONCLUSIONS
The dissolution behavior of low grade zinc oxide ores in the NH3-H3C6H5O7- H2O system was investi-gated.
(1) The concentration of citric acid was found to have an important role in low-grade zinc oxide ore dissolution, maximizing at 1 M, and a zinc leaching efficiency of about 81.2% was obtained, under the fol-lowing leaching conditions: ammonia 6 M, tempera-ture of 25 °C, leaching time of 60 min, stirring speed of 300 rpm and solid to liquid ratio of 1:5. The zinc leaching efficiency increased with the increase in cit-ric acid concentration, liquid-solid mass ratio, leach-ing time, stirrleach-ing speed and ammonia concentration.
(2) The FT-IR spectra reflect the changes in the structural group units and justify the leaching mecha-nism for zinc dissolution, and the complex demon-strated that the Zn (II) ion is coordinated. Three strong FT-IR bands at 1579 cm-1 and 1397cm-1 were
found and they were assigned to a zinc–citrate com-plex in the aqueous solution.
(3) The results of XRD, IR, and SEM/EDS ex-periments indicate that hemimorphite is soluble, and the oxides of Si, Ca, Pb, Fe and Al are the main minerals in the leaching residue that are undissolved.
ACKNOWLEDGEMENTS
916 Ma Ai-yuan, Peng Jin-hui, Zhang Li-bo, Shiwei Li, Yang Kun and Zheng Xue-mei
Brazilian Journal of Chemical Engineering NOMENCLATURE
Zn
η
leaching rate of zinc, (%) ZnC zinc concentration of leaching solution (g/L)
V the leaching volume, (L) 0
Zn
C
the zinc content of the low-grade zinc oxide ore (%)m the mass of the low-grade zinc oxide ore (G)
REFERENCES
Che, P., Fang, D. Q., Zhang, D. P., Feng, J., Wang, J. P., Hu, N. H., Meng, J., Hydrothermal synthesis and crystal structure of a new two-dimensional zinc citrate complex. Journal of Coordination Chemistry, 58(17), 1581-1588 (2005).
Chen, A. L., Zhao, Z. W., Jia, X. J., Long, S., Huo, G. S., Chen, X. Y., Alkaline leaching Zn and its concomitant metals from refractory hemimor-phite zinc oxide ore. Hydrometallurgy, 97(3-4), 228-232 (2009).
Chen, Y. X., Lin, Q., Luo, Y. M., He, Y. F., Zhen, S. J., Yu, Y. L., Tian, G. M., Wong, M. H., The role of citric acid on the phytoremediation of heavy metal contaminated soil. Chemosphere, 50(6), 807-811 (2003).
de Wet, J. R., Singleton, J. D., Development of a viable process for the recovery of zinc from ox-ide ores. Journal of the South African Institute of Mining and Metallurgy, 108(5), 253-259 (2008).
Demir, F., Laçin, O., Dönmez, B., Leaching kinetics of calcined magnesite in citric acid solutions. Industrial & Engineering Chemistry Research, 45(4), 1307-1311 (2006).
Ding, Z. Y., Yin, Z. L., Hu, H. P., Chen, Q. Y., Dis-solution kinetics of zinc silicate (hemimorphite) in ammoniacal solution. Hydrometallurgy, 104(2), 201-206 (2010).
Dutra, A. J. B., Paiva, P. R. P., Tavares, L. M., Alka-line leaching of zinc from electric arc furnace steel dust. Minerals Engineering, 19(5), 478-485 (2006).
Ejtemaei, M., Gharabaghi, M., Irannajad, M., A re-view of zinc oxide mineral beneficiation using flotation method. Advances in Colloid and In-terface Science, 206(SI), 68-78 (2014).
He, S. M., Wang, J. K., Yan, J. F., Pressure leaching of high silica Pb–Zn oxide ore in sulfuric acid me-dium. Hydrometallurgy, 104(2), 235-240 (2010).
He, S. M., Wang, J. K., Yan, J. F., Pressure leaching of synthetic zinc silicate in sulfuric acid medium. Hydrometallurgy, 108(3-4), 171-176 (2011). Irannajad, M., Ejtemaei, M., Gharabaghi, M., The
effect of reagents on selective flotation of smith-sonite-calcite-quartz. Minerals Engineering, 22 (9-10), 766-771 (2009).
Irannajad, M., Meshkini, M., Azadmehr, A. R., Leaching of zinc from low grade oxide ore us-ing organic acid. Physicochemical Problems of Mineral Processing, 49(2), 547-555 (2013). Steer, J. M., Griffiths, A. J., Investigation of
car-boxylic acids and non-aqueous solvents for the selective leaching of zinc from blast furnace dust slurry. Hydrometallurgy, 140, 34-41 (2013). Kaliva, M., Kyriakakis, E., Gabriel, C., Raptopoulou, C. P., Terzis, A., Tuchagues, J. P., Salifoglou, A., Synthesis, isolation, spectroscopic and structural characterization of a new pH complex structural variant from the aqueous vanadium(V)-peroxo-citrate ternary system. Inorganica Chimica Acta, 359(14), 4535-4548 (2006).
Kourgiantakis, M., Matzapetakis, M., Raptopoulou, C. P., Terzis, A., Salifoglou, A., Lead–citrate chemistry. Synthesis, spectroscopic and struc-tural studies of a novel lead(II)–citrate aqueous complex. Inorganica Chimica Acta, 297(1-2), 134-138 (2000).
Larba, R., Boukerche, I., Alane, N., Habbache, N., Djerad, S., Tifouti, L., Citric acid as an alterna-tive lixiviant for zinc oxide dissolution. Hydro-metallurgy, 134, 117-123 (2013).
Li, M. X., Jian, S., Zhao, W. J., Study on pre-concen-tration and Calcium Removal of low grade zinc oxide ore. Applied Mechanics and Materials, 316-317, 830-833 (2013).
Li, C. X., Xu, H. S., Deng, Z. G., Li, X. B., Li, M. T., Wei, C., Pressure leaching of zinc silicate ore in sulfuric acid medium. Transactions of Nonfer-rous Metals Society of China, 20(5), 918-923 (2010).
Li, Q. X., Chen, Q. Y., Hu, H. P., Dissolution mecha-nism and solubility of hemimorphite in NH3-(NH4)2SO4-H2O system at 298.15 K. Journal of Central South University, 21(3), 884-890 (2014). Li, L., Ge, J., Wu, F., Chen, R. J., Chen, S., Wu, B.
R., Recovery of cobalt and lithium from spent lithium ion batteries using organic citric acid as leachant. Journal of Hazardous Materials, 176 (1-3), 288-293 (2010).
alumi-num citrate complex. Inorganic Chemicals, 38(4), 618 -619 (1999).
Navidi Kashani, A. H., Rashchi, F., Separation of oxi-dized zinc minerals from tailings: Influence of flotation reagents. Minerals Engineering, 21(12-14), 967-972 (2008).
Park, H., Jung, K., Alorro R. D., Yoo, K., Leaching behavior of copper, zinc and lead from contami-nated soil with citric acid. Materials Transac-tions, 54(7), 1220-1223 (2013).
Santos, F. M. F., Pina, P. S., Porcaro, R., Oliveira, V. A., Silva, C. A., Leão, V. A., The kinetics of zinc silicate leaching in sodium hydroxide. Hydro-metallurgy, 102(1-4), 43-49 (2010).
Swanson, R., Ilsley, W. H., Stanislowski, A. G., Crys-tal structure of zinc citrate. Journal of Inorganic Biochemistry, 18(3), 187-194 (1983).
Tsaramyrsi, M., Kavousanaki, D., Raptopoulou, C. P., Terzis, A., Salifoglou, A., Systematic synthesis, structural characterization, and reactivity studies of vanadium(V)–citrate anions [VO2(C6H6O7)]22−, isolated from aqueous solutions in the presence of different cations. Inorganica Chimica Acta, 320(1-2), 47-59 (2001).
Willey, G. R., Somasunderam, U., Aris, D. R., Err-ington, W., Ge(IV)–citrate complex formation: Synthesis and structural characterisation of GeCl4(bipy) and GeCl(bipy)(Hcit) (bipy=2,2´-bipyridine, H4cit=citric acid). Inorganica Chimica Acta, 315(2), 191-195 (2001).
Xu, H. S., Wei, C., Li, C. X., Fan, G., Deng, Z. G.,
Li, M. T., Li, X. B., Sulfuric acid leaching of zinc silicate ore under pressure. Hydrometal-lurgy, 105(1-2), 186-190 (2010).
Xu, H. S., Wei, C., Li, C. X., Fan, G., Deng, Z. G., Zhou, X. J., Qiu, S., Leaching of a complex sul-fidic, silicate-containing zinc ore in sulfuric acid solution under oxygen pressure. Separation and Purification Technology, 85, 206-212 (2012).
Yang, J. L., Zhang, H. M. Wang, G. F., Ma, S. J., Zhang, M., Sulfuric acid leaching on low grade oxide ore. Advanced Materials Research, 826, 122-125 (2013).
Yin, Z. L., Ding, Z. Y., Hu, H. P., Liu, K., Chen, Q. Y., Dissolution of zinc silicate (hemimorphite) with ammonia–ammonium chloride solution. Hydrometallurgy, 103(1-4), 215-220 (2010). Zhao, Z. W., Long, S., Chen, A. L., Huo, G. S., Li,
H. G., Jia, X. J., Chen, X. Y., Mechanochemical leaching of refractory zinc silicate (hemimor-phite) in alkaline solution. Hydrometallurgy, 99(3-4), 255-258 (2009).
Zhang, H., Zhao, H., Jiang, Y. Q., Hou, S. Y., Zhou, Z. H., Wan, H. L., pH- and mol-ratio dependent tungsten(VI)–citrate speciation from aqueous solutions: Syntheses, spectroscopic properties and crystal structures. Inorganica Chimica Acta, 351, 311-318 (2003).