UNIVERSIDADE DE TRÁS-OS-MONTES E ALTO DOURO
Desenvolvimento e validação de um método para determinação de fenóis
voláteis: Aplicação ao estudo do impacto de produtos enológicos na
diminuição dos teores de 4-etilfenol e 4-etilguaiacol em vinho tinto
Dissertação de Mestrado em Enologia
Juliana Nair Milheiro Ferreira
Orientadores: Professor Doutor Fernando Nunes
Professora Doutora Fernanda Cosme
Vila Real, 2015
Discriminação de Vinhos M onovarietais de
acordo com a sua composição fenólica, por
FTI R-ATR e HPLC
!!
Relatório Final de Estágio Licenciatura em Bioquímica
! ! !
Juliana Milheiro Ferreira!
Nº 35302
II
UNIVERSIDADE DE TRÁS-OS-MONTES E ALTO DOURO
Desenvolvimento e validação de um método para determinação de fenóis
voláteis: Aplicação ao estudo do impacto de produtos enológicos na
diminuição dos teores de 4-etilfenol e 4-etilguaiacol em vinho tinto
Dissertação de Mestrado em Enologia
Juliana Nair Milheiro Ferreira
Orientadores: Professor Doutor Fernando Nunes
Professora Doutora Fernanda Cosme
Vila Real, 2015
Discriminação de Vinhos M onovarietais de
acordo com a sua composição fenólica, por
FTI R-ATR e HPLC
!!
Relatório Final de Estágio Licenciatura em Bioquímica
! ! !
Juliana Milheiro Ferreira!
Nº 35302
Instituição Universidade de Trás-os-Montes e Alto Douro Curso Mestrado em Enologia
Título Desenvolvimento e validação de um método para determinação de fenóis voláteis: Aplicação ao estudo do impacto de produtos enológicos na diminuição dos teores de 4-etilfenol e 4-etilguaiacol em vinho tinto Autor Juliana Nair Milheiro Ferreira
Orientadores Professor Doutor Fernando Nunes Professora Doutora Fernanda Cosme
Este trabalho foi expressamente elaborado como
dissertação original para o efeito de obtenção de grau
de Mestre em Enologia
“As doutrinas apresentadas no presente trabalho são da
exclusiva responsabilidade do autor”
RESUMO
Os fenóis voláteis como o 4-etilfenol e o 4-etilguaiacol podem estar presentes no vinho tinto, por isso, são uma preocupação em enologia. Estes fenóis afetam a qualidade do vinho e são responsáveis por um defeito sensorial denominado de “odor animal” ou carácter “Brett”. A Brettanomyces/Dekkera produz o 4-etilfenol e o 4-etilguaiacol a partir de ácidos hidroxicinâmicos. Esta levedura cresce essencialmente depois da fermentação alcoólica e da fermentação malolática, que ocorre durante a maturação do vinho em barricas. Neste estudo, um método baseado na extracção líquido-líquido, utilizando o solvente de extracção pentano/ éter dietílico, e determinação por cromatografia gasosa acoplado à espetrometria de massa foi optimizado e validado para que fosse possível identificar e quantificar o etilfenol e o 4-etilguaiacol em vinhos tintos. Os resultados indicam que o método da extracção líquido-líquido é adequado para o propósito, com boas características analíticas numa gama de concentrações apropriada para as concentrações destes fenóis voláteis existentes nos vinhos. Vinte produtos enológicos, alguns convencionais e outros não convencionais à base de polímeros sintéticos, minerais, proteínas, polissacarídeos e resina, foram utilizados de maneira a se analisar a sua eficiência na remoção do 4-etilfenol e do 4-etilguaiacol do vinho tinto. Apesar do carvão alterar algumas características importantes do vinho, este foi o que obteve os melhores resultados, diminuindo significativamente as concentrações totais de 4-etilfenol e 4-etilguaicol (57% em média) e no espaço de cabeça do vinho (75% em média). A albumina de ovo também levou a uma diminuição dos teores destes fenóis voláteis (19% em média do seu conteúdo nos vinhos e 30% no espaço de cabeça). A cola de peixe, carboximetilcelulose e quitosana permitiram uma redução da concentração destes fenóis voláteis no espaço de cabeça (27%, 15% e 27%, respectivamente), no entanto não diminuindo o seu conteúdo total nos vinhos. Estes últimos produtos enológicos tiveram um menor impacto nas características cromáticas dos vinhos tratados quando comparados com o carvão ativado. Estes resultados fornecem uma informação importante para a indústria do vinho na seleção de produtos enológicos para a remoção do 4-etilfenol e 4-etilguaiacol de forma a aumentar a qualidade do vinho.
Palavras-chave:
Vinho tinto; 4-etilfenol; 4-etilguaiacol; cromatografia gasosa; extracção líquido-líquido; produtos enológicos; compostos fenólicosABSTRACT
Volatile phenols like 4-ethylphenol and 4-ethylguaiacol in wine have been a concern in oenology nowadays. These phenols affect wine quality, since they are responsible for a sensorial defect described as “animal odour” or “Brett” character. Brettanomyces/Dekkera produces 4-ethylphenol and 4-ethylguaiacol from hydroxycinnamic acids. These yeasts grow up essentially after alcoholic and malolactic fermentation, during wine maturation in oak wood. In this study a method based on liquid-liquid extraction, using pentane/ ether diethyl as extraction solvent, and determination for gas chromatography coupled to mass spectrometry was optimized and validated in order to identify and quantify ethylphenol and 4-ethylguaiacol in red wines. The results indicate that the liquid-liquid extraction method is suitable for the intended propose with good analytical characteristics in a range of concentrations appropriate for the amount of volatile phenols present in wines. Twenty oenological products, some of them conventional and another unconventional, based on synthetic polymers, minerals, proteins, polysaccharides and resins, were used in order to analyse their efficiency in 4-ethylphenol and 4-ethylguaiacol removal from red wine. Although carbon changes some important characteristics of wine, it was the one which had the best results, decreasing significantly total concentrations of ethylphenol and 4-ethylguaiacol (57% in average) and in headspace of wine (75% in average). Egg albumin also decreased levels of these volatile phenols (19% in average in wine content and 30% in headspace). Isinglass, carboxymethylcellulose and chitosan reduced the headspace concentration of these volatiles in headspace (27%, 15% and 27% respectively), although they do not decrease their content in wines. These last oenological products had a less impact on chromatic characteristics of treated wines when compared with activated carbon. These results take important information for wine industry in the selection of oenological products for 4-ethylphenol and 4-ethylguiacol removal in order to improve wine quality.
Keywords:
Red wine; 4-ethylphenol; 4-ethylguaiacol; gas chromatography; liquid-liquid extraction; fining agents; phenolic compoundsCONTENTS
RESUMO VI
ABSTRACT VII
Contents VIII
Index of figures XII
Index of tables XIII
Acknowledgements XIV
I. GENERAL INTRODUCTION 1
1. Phenolic characterization of red grapes and wine 2
2. Wine quality 3
3. Characterization of Brettanomyces/Dekkera 4
4. Volatile phenols production 5
5. The presence of Brettanomyces in wine 7
5.1. Growth and control of Brettanomyces during winemaking 7
6. Chemical treatments 8
6.1. Sulphur dioxide 8
6.2. Dimethyl dicarbonate (DMDC) 9
7. Detection of Brettanomyces in wine 9
8. Fining agents 10
8.1. Polysaccharide source 10
8.1.1. Carboxymethylcellulose (CMC) 10
8.1.3. Chitosan 12
8.1.4. Arabic gum 13
8.1.5. Alginic acid 13
8.1.6. Microcrystalline cellulose 14
8.1.7. Locust bean gum 14
8.1.8. Gellan gum 15 8.1.9. Pectin 16 8.2. Protein source 16 8.2.1. Isinglass 16 8.2.2. Egg albumin 17 8.2.3. Gelatines 18 8.2.4. Mannoproteins 18 8.2.5. Potassium caseinate 19 8.3. Mineral source 20 8.3.1. Bentonite 20 8.4. Synthetic source 21 8.4.1. Polyvinylpolypyrrolidone (PVPP) 21 8.5. Other sources 22 8.5.1. Activated carbon 22 8.5.2. Shellac 23
9. AIM OF THE STUDY 24
II. SCREENING OF CONVENTIONAL AND UNCONVENTIONAL FINING AGENTS FOR REDUCING 4-ETHYLPHENOL AND 4-ETHYLGUIACOL LEVELS IN RED WINE
37
Abstract 38
1. Introduction 38
2. Experimental 39
2.1. Wine sample 39
2.2. Reagents and chemicals 39
2.3. Liquid-liquid extraction procedure 39
2.4. Method validation 40
2.4.1. Calibration curve and linearity 40
2.4.2. In-house method validation 40
2.4.3. Detection and quantification limits (LOD and LOQ) 40
2.4.4. Repeatability and intermediate repeatability 40
2.4.5. Accuracy and recovery 41
2.4.6. Existence of matrix effects 41
2.5. GC-MS analysis 41
2.6. Fining experiments 42
2.7. Solid phase microextraction (SPME) procedure 43
2.8. High performance liquid chromatography (HPLC) analysis of anthocyanins and phenolic acids
44
2.9. Quantification of non-flavonoids, flavonoids and total phenols 44
2.11. Statistical analysis 45
3. Results and Discussion 45
3.1. Method optimization 45
3.2. Method validation 46
3.3. Effect of different fining agents on total concentration of 4-ethylphenol and 4-ethylguaiacol in red wine
49
3.4. Effect of different fining agents on headspace concentration of 4-ethylphenol and 4-ethylguaiacol in red wine
49
3.5. Effect of different fining agents on chromatic characteristics, colour and phenolic compounds
50
4. Conclusion 61
References 61
INDEX OF FIGURES
Figure 1.1 – Structure of anthocyanins. 3
Figure 1.2 – Structure of (1) benzoic and (2) cinnamic acids. 3
Figure 1.3 – B. bruxellensis. 5
Figure 1.4 – Production of ethylphenols from their precursors. 6
Figure 1.5 – Carboxymethylcellulose chain. 10
Figure 1.6 – Structure of (a) chitin and (b) chitosan. 12
Figure 1.7 – Structure of locust bean gum. 15
Figure 1.8 – Structure of repeating unit of gellan. 15
Figure 1.9 – Structure of montmorillonite. 20
Figure 1.10 – Structure of PVPP. 21
Figure 1.11 – Structure of shellac. (a) Polyesters; (b) single esters. 23 Figure 2.1 – GC-MS chromatogram for the liquid-liquid extraction of low
concentration (100 μg/L) in standard addition to red wine. ethylphenol (EP), 4-ethylguiacol (4-EG), 3,4-dimethylphenol (3,4-DMP).
INDEX OF TABLES
Table 2.1 – Fining agents used in this study. 43
Table 2.2 – Validation parameters of liquid-liquid extraction method. 46 Table 2.3 – Recovery study of 4-ethylphenol and 4-ethylguaiacol in red wine. 48 Table 2.4 – Comparison between different extraction methods for volatile phenols
in red wine.
50
Table 2.5 – Percentages of total and headspace concentrations reduction of 4-ethylphenol and 4-ethylguiacol by conventional and unconventional fining agents.
52
Table 2.6 – Effect of the first group of fining agents on colour intensity, hue, total phenolic index, total anthocyanins, non-flavonoids, flavonoids, total phenols, polymerized pigments, coloured anthocyanins and total pigments of both unfined and fined red wine
55
Table 2.7 – Effect of the first group of fining agents on monomeric anthocyanins determined by HPLC of both unfined and fined red wine
56
Table 2.8 – Effect of the first group of fining agents on catechin and phenolic acids determined by HPLC of both unfined and fined red wine
57
Table 2.9 – Effect of the second group of fining agents on colour intensity, hue, total phenolic index, total anthocyanins, non-flavonoids, flavonoids, total phenols, polymerized pigments, coloured anthocyanins and total pigments of both unfined and fined red wine
58
Table 2.10 – Effect of the second group of fining agents on monomeric anthocyanins determined by HPLC of both unfined and fined red wine
59
Table 2.11 – Effect of the second group of fining agents on catechin and phenolic acids determined by HPLC of both unfined and fined red wine
ACKNOWLEDGEMENTS
Concluída mais uma importante etapa na minha vida, resta-me agradecer às pessoas que me acompanharam e me apoiaram ao longo desta jornada.
Posto isto, agradeço:
Ao Professor Fernando Nunes e à Professora Fernanda Cosme pela oportunidade que me proporcionaram e pela excelente orientação, fazendo com que o trabalho fosse levado a cabo com sucesso.
Ao Professor Luís Filipe Ribeiro pela ajuda e partilha de conhecimentos.
Aos meus pais, por todo o incentivo e palavras de apoio. Estiveram presentes nos momentos menos bons e fizeram com que tudo se tornasse mais fácil.
À minha irmã pelo apoio incondicional e por sempre ter estado do meu lado em todos os momentos. Posso, sem dúvida, afirmar que sem ela não teria chegado onde cheguei. É o meu pilar e, sobretudo, o meu porto seguro! E, claro, ao meu cunhado por sempre ter estado presente e disponível para o que eu precisasse.
À tia Céu e ao tio Betinho por terem acompanhado de perto esta importante etapa e por terem acreditado em mim. É devido a este apoio que se tornam muito especiais na minha vida. À Leonor pela amizade e disponibilidade para estar comigo ano após ano, dia após dia. Foi, é e sempre será uma importante ajuda a ultrapassar as dificuldades. Mas essencialmente quero agradecer por todos os sorrisos e gargalhadas que me arrancou, mesmo quando nada me faria sorrir. São amizades como esta que ficam para a vida!
À Sandrine e à Mélanie por todo o apoio e por estarem sempre presentes, nos momentos bons e menos bons.
Aos meus colegas de laboratório pelo excelente ambiente de trabalho que me proporcionaram e por me terem ajudado quando precisei.
1. Phenolic characterization of red grapes and wine
Phenolic compounds are responsible for red wine quality, namely for their appearance, flavour and fragrance. Phenols are benzene compounds, having one or more hydroxyl groups associated directly with the ring structure. The phenolic compounds found in wine are flavonoids and/or non-flavonoids (Cabrita et al., 2003; Jackson, 2008; Riberéau-Gayon et al., 2006).
The most common flavonoids in red wine are flavonols, catechins (flavan-3-ols) and anthocyanins. They can appear free or associated to other flavonoids, sugars or non-flavonoids. They constitute more than 85% of the phenolic content in red wine, about 1000 mg/L (Jackson, 2008).
Quercetin, kaempferol and myricetin are examples of flavonols. In wine, these flavonols may be copigmented with anthocyanins. On the other hand, catechin, epicatechin, gallocatechin, procyanidins and proanthocyanidins are examples of flavan-3-ols (Jackson, 2008).
Anthocyanins appear as glycosides in grapes and are derived from anthocyanidins (flavonoid component – aglycone) by adding sugars, typically glucose. The substitution occurs at C3 and if in the diglycosides, at positions C3 and C5. Each anthocyanin may be further substituted (acylated) at the sugar moiety with acetic acid, p-coumaric acid or caffeic acid (Jackson, 2008; Monagas and Bartolomé, 2009) (Figure 1.1). They can be divided into five classes: cyanidin, delphinidin, malvidin, peonidin, and petunidin (Jackson, 2008). Anthocyanins are responsible for red wines colour (Jackson, 2008; de Villiers et al., 2011) and in young red wines, anthocyanins typically occur as a dynamic equilibrium among five molecular states: four free forms and one bonded to sulphur dioxide. The most predominant anthocyanin in grapes is malvidin, which is the reddest anthocyanin. So, it contributes more to the colour of red wines (Jackson, 2008).
Figure 1.1 - Structure of anthocyanins (adapted from Monagas and Bartolomé, 2009).
Non-flavonoid compounds include benzoic acids and hydroxycinamic acids (Figure 1.2). Benzoic acids include vanillic acid, gallic acid and hydrolysable tannins as examples. Ferulic acid, caffeic acid and p-coumaric acid are hydroxycinamic acids (Jackson, 2008; Riberéau-Gayon et al., 2006). They appear essentially associated to tartaric acid, however they can be associated to sugars, alcohols or other organic acids. They can negatively influence wine with production of volatile phenols (Jackson, 2008).
Figure 1.2 – Structure of (1) benzoic and (2) cinnamic acids (Riberéau-Gayon et al., 2006).
2. Wine quality
Wine occupies an important position among foods. Its quality depends on grape variety and its management in the vineyard, fermentation technique and all the process of winemaking and aging (Simon, 2001). In addition to all these factors, it is extremely important to pay attention to spoilage microorganisms during winemaking and bottling. These microorganisms can produce compounds that contribute to olfactory defects in wine, named
off-flavours (Lisanti et al., 2008). At a certain concentration, the sensorial defects are clearly perceptible and it would be wrong to believe that they could add complexity to wines. So, it is an aim to oenology to find out how to avoid such problems and their consequences (Riberéau-Gayon et al., 2006).
Brettanomyces has been a concern as a spoilage microorganism due to the production of volatile phenols like 4-ethylphenol, 4-ethylguaiacol and 4-ethylcatechol (Chatonnet et al., 1995; Chatonnet and Viala, 1997). This yeast causes serious economic losses in wine industry (Fugelsang and Edwards, 2007), so it is one of the most feared by winemakers (Loureiro and Malfeito-Ferreira, 2006; Renouf et al., 2006). It is necessary to understand how winemaking and aging processes affect the concentration of both volatile phenols and develop procedures for rapid wine analysis in the laboratory and in winery (Monje et al., 2002).
3. Characterization of Brettanomyces/ Dekkera
Brettanomyces spp. is the non-spore forming of genus Dekkera, although the domination Brettanomyces is the most usual in literature (Fugelsang and Edwards, 2007; Wedral et al., 2010). Five species belong to genus Brettanomyces/ Dekkera: Brettanomyces naardenensis, Brettanomyces anomalus, Brettanomyces custersianus, Brettanomyces bruxellensis and Brettanomyces nanus (Kurtzman et al., 2011).
The presence of Brettanomyces is described in many different locals and products, namely in wines, beers, winery equipment, tequila and ciders. This happens because they can survive for long periods of time and they can initiate their growth in products already finished and stored (Loureiro and Malfeito-Ferreira, 2006).
Brettanomyces bruxellensis is the yeast that most influences the amount of volatile phenols in wine and can be found on the surfaces of grapes or in oak barrels used for fermentation and wine aging (Rayne and Eggers, 2008; Wedral et al., 2010). These yeasts develop essentially after alcoholic and malolactic fermentations (Fariña et al., 2007; Riberéau-Gayon et al., 2006). Its presence is noticed only in the long term and even after wine bottling due to its slow growth. B. bruxellensis has an oval or elliptical form (Figure 1.3) and it reproduces assexually by budding (Fugelsang and Edwards, 2007; Wedral et al., 2010).
Figure 1.3 – B. bruxellensis (Wedral et al., 2010).
The presence of these yeasts has been studied because they have the capacity of Custer effect. So they can inhibit the alcoholic fermentation under anaerobic conditions and thus they produce high levels of acetic acid (Fugelsang and Edwards, 2007; Henschke and Curtin, 2007; Van Dijken and Scheffers, 1986; Vigentini et al., 2008).
4. Volatile phenols production
Volatile phenols like 4-ethylphenol and 4-ethylguaiacol in wine have been a concern in oenology (Cabrita et al., 2012). Firstly, the presence of these phenols in wine was associated to lactic acid bacteria but now it is known that under oenological conditions these bacteria only produce very small quantities of vinylphenols. Other yeasts like Saccharomyces cerevisiae, Torulaspora spp., Zygosaccharomyces spp. and Pichia spp. are capable to produce 4-vinylphenol but can’t reduce it to 4-ethylphenol (Chatonnet et al., 1993, 1995).
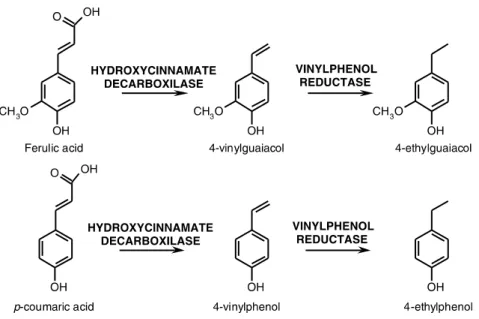
These phenols affect wine quality and are responsible for a sensorial defect described as “Brett” character (Romano et al., 2009). Brettanomyces/Dekkera produces 4-ethylphenol and 4-ethylguaiacol from hydroxycinnamic acids (Cabrita et al., 2012; Fariña et al., 2007; Flamini and Traldi, 2010). p-Coumaric acid and ferulic acid are converted into 4-vinylphenol and 4-vinylguaiacol respectively by the hydroxycinnamate decarboxilase. Then they are reduced to 4-ethylphenol and 4-ethylguaiacol by vinylphenol reductase (Suárez et al., 2007) (Figure 1.4).
Figure 1.4 -Production of ethylphenols from their precursors (Suárez et al., 2007).
The critical characteristic of these compounds is that their sensory thresholds are usually lower than the habitual concentration ranges in wines, so their undesirable contribution to aroma is significant (Riberéau-Gayon et al., 2006). In the literature, the odour threshold limits in wine are 440 μg/L for 4-ethylphenol and 33 μg/L for 4-ethylguaiacol (Flamini and Traldi, 2010). The “Brett” character of wines becomes too evident to be acceptable when the total concentration of 4-ethylphenol and 4-ethylguaiacol is higher than 620 μg/L (Chatonnet et al., 2003). The ratio of 4-ethylphenol and 4-ethylguaiacol typically are 8:1 (Chatonnet et al., 1992).
The aroma of 4-ethylphenol has been associated in red wine to “horsy”, “medicinal”, “leather” and “barnyard”. 4-Ethylguaiacol in red wine has a smoky, stable, animal and spicy aroma (Chatonnet et al., 1992; Riberéau-Gayon et al., 2006). Mousy odour can be associated to spoilage by Brettanomyces due to its production of tetrahydropyridines from lysine and ethanol (Heresztyn, 1986).
The production of volatile phenols in wine by Brettanomyces is proportional to the size of the population of these yeasts and depends on the presence of their precursors (Gerbeaux et al., 2000).
Another off-flavour compound produced by Brettanomyces is 4-ethylcatechol associated to medicinal aroma. Its precursor is caffeic acid (Loureiro and Malfeito-Ferreira, 2006). This compound, compared to the other both volatile phenols, seems to have a less effect on sensorial quality of red wines (Nicolini et al., 2007). This volatile phenol has not
been studied as much as the other phenols because there is the necessity to derivatize to be detected by gas chromatography (Loureiro and Malfeito-Ferreira, 2006).
5. The presence of Brettanomyces in wine
In the 50s and 60s, Brettanomyces spp. were isolated from wine in Italy, France and South Africa but they are characterized by its contribution to the wine only in 80s and 90s (Henschke et al., 2007).
Brettanomyces/ Dekkera grow up during wine aging in oak barrels (Fugelsang and Edwards, 2007). Oak barrels improve wine sensorial characteristics but, on the other hand, they are porous and difficult to clean and sterilize. Under these conditions, a favourable environment is created for growth and survival of these microorganisms and they can be transferred to wines. Besides, these yeasts have the ability to adapt in poor nutrients environments and with high levels of ethanol. So they can remain in winery equipment (Suárez et al., 2007).
Red wines have low acidity, high polyphenol content and are usually stored for long periods in oak barrels so they are particularly susceptible to Brettanomyces influence (Wedral et al., 2010; Suárez et al., 2007).
Furthermore, besides the flavour changes, wines affected with Brettanomyces may exhibit an unattractive colour. Glycosidic activities or formation of vinylphenolic pyranoanthocyanins may be the cause (Oelofse et al., 2008).
5.1. Growth and control of Brettanomyces during winemaking
Brettanomyces can be found at any stage in winemaking but it is most linked to aging in oak barrels, malolactic fermentation and slow or stuck alcoholic fermentations due to its high levels of ethanol and low sugar tolerances (Dias et al., 2003; Renouf et al., 2006). In red wine, these yeasts can tolerate 14.0-14.5% of ethanol (Loureiro and Malfeito-Ferreira, 2006).
As these yeasts are found mostly on damaged grapes and on winery equipment, it is very important an effective disinfection in order to prevent spoilage (Wedral et al., 2010).
Oak barrels surface is very porous, so yeasts deep in the barrel are difficult to eliminate. When oak barrels are contaminated with Brettanomyces they cannot be effectively sterilized (Suárez et al., 2007). In order to prevent the activity and the spoilage of these yeasts in wine there are measures that can be applied. These measures include the use of inhibitory or lethal agents like chemical treatments. Clarification or filtration can be also used to reduce
or eliminate possible spoilage microorganisms (Malfeito-Ferreira, 2010). Unfortunately, these processes are undesirable in high quality wines despite of their capacity to reduce levels of Brettanomyces (Arvik and Henick-Kling, 2002). The most common cleaning and disinfection treatments are warm water, sulphur dioxide and ozone. However, the efficiency of these treatments is limited due to oak barrels porosity (Laureano et al., 2004).
In oak barrels, the oxygen spreads in a continuous way until values of 30 mg/L/year -(Ribéreau-Gayon et al., 2006) and it contributes to growth of Brettanomyces (Malfeito-Ferreira et al., 2001; du Toit et al., 2006).
A control factor in fermenting a wine is the use of a starter culture, which can decrease indigenous fermentation and consequently decreasing the opportunity for Brettanomyces to grow (Vigentini et al., 2008).
Two active toxins against Brettanomyces were reported (Comitini et al., 2004) and they are produced by Pichia anomala and Kluyveromyces wickerhamii. Both toxins show a fungicidal effect against Brettanomyces in wine (Suárez et al., 2007).
6. Chemical treatments
6.1. Sulphur dioxide
Adequate levels of sulphur dioxide should be maintained during fermentation in order to inhibit the growth of these yeasts (Riberéau-Gayon et al., 2006; Wedral et al., 2010). Free sulphur dioxide should be above 30 mg/L for the entire process of winemaking (Barata et al., 2008). A problem associated to the use of sulphur dioxide is the maximum legal limit that is allowed depending on the type of wine in European Union (Loureiro and Malfeito-Ferreira, 2003). The additions should be monitored by measuring the sulphite after treatments. However, high additions of sulphur dioxide before fermentation process can increase the production of acetaldehyde by fermentative yeasts.
In order to prevent the proliferation of Brettanomyces, the levels of molecular sulphur dioxide should be at 1 mg/L (Barata et al., 2008). Brettanomyces has the ability to be in a viable-but-not-culturable (VNC) state after the addition of sulphur dioxide, that is why these yeasts may be present in wine after the depletion of free sulphur dioxide (Umiker and Edwards, 2007).
6.2. Dimethyl dicarbonate (DMDC)
Dimethyl dicarbonate can inhibit the activity of Brettanomyces and other yeasts that may cause damages in wine by initial bottle fermentation. This chemical agent can be used to treat wines that have residual sugar or were not filtered (Oelofse et al., 2008). In Europe, DMDC has a maximum limit of 200 mg/L (Anonymous, 2006). Dimethyl dicarbonate must be used routinely with sulphur dioxide during wine bottling or storing (Fugelsang and Edwards, 2007). The effect of DMDC has been studied in many microorganisms such as yeasts. Its effectiveness depends on the strain and wine characteristics (Costa et al., 2008).
7. Detection of Brettanomyces in wine
The presence of Brettanomyces should be monitored periodically when wine is stored because only one viable cell in a bottle may cause damages to the product (Barata et al., 2008).
Brettanomyces can be detected directly by classic microbiological methods like yeast cultures or morphological features (Wedral et al., 2010). On the other hand, these yeasts can be detected indirectly by analysis of 4-ethylphenol and 4-ethylguaiacol (Suárez et al., 2007; Wedral et al., 2010). Gas chromatography mass spectrometry can identify both compounds with high sensitivity and when it is coupled with sensorial analysis performed by a trained taster could be a great tool in quality control (Suárez et al., 2007). Volatile phenols can be detected even when yeast cultures show no cells or minimal quantity of them. When these metabolites are detected it may be too late to do something (Suárez et al., 2007; Tofalo et al., 2012; Wedral et al., 2010).
This yeast is difficult to isolate due to its slow growth rate. In addition, Brettanomyces has the ability to stay in a VNC or dormant state in wine for a long time, meaning cells cannot be cultured by classic methods (Arvik and Henick-Kling, 2002).
Couto et al. (2005) developed a selective enrichment liquid medium to differentially promote the growth and activity of Brettanomyces. After the inoculation with wine, the medium is monitored visually and by various olfactive analyses. So, spoiled wines will acquire visible turbidity in the medium and will produce volatile phenols that can be clearly identified by smelling. The method is simple to execute in a winery routine analysis.
8. Fining agents
Vinification and aging processes require attention to possible chemical or microbiological spoilage. These contaminations often result in the appearance of off-odours that can badly contribute to sensorial quality of wines. However, even when special attention is paid during winemaking, problems could happen so there are necessary remedial treatments to solve them (Lisanti et al., 2008). The knowledge of the impact of fining agents in red wines chemical characteristics is important for fining optimization and therefore to improve red wine quality (Cosme et al., 2007; Cosme et al., 2009; Gonçalves and Jordão, 2009).
8.1. Polysaccharide source
8.1.1. Carboxymethylcellulose (CMC)
Carboxymethylcellulose is a polysaccharide (Riberéau-Gayon et al., 2006) obtained by chemical transformation of cellulose extracted from wood and it also known as cellulose gum, sodium CMC and sodium salt of the carboxymethyl ether of cellulose (International Œnological Codex, 2012). CMC is constituted by cellulose substituted by carboxymethyl organic acid frequently in its sodium form (Figure 1.5) (Gerbaud et al., 2010).
CMC is characterized by its degree of etherification of its alcohol groups: degree of substitution (DS) and also by its average number of glucopyranose units per polymer molecule: degree of polymerization (DP). The last one determines the viscosity of CMC and the higher the molecular weight of the CMC the higher the viscosity (Riberéau-Gayon et al., 2006).
Carboxymethylcellulose is soluble in hot water and it is necessary a complete dissolutions before adding to wine (Gerbaud et al., 2009; Tusseau, 2009). However, its water solubility varies with its degree of substitution and polymerization (Riberéau-Gayon et al.,
2006). This polysaccharide inhibits tartaric precipitation through a “protective colloid” effect (International Œnological Codex, 2012; Riberéau-Gayon et al., 2006). CMC effectiveness varies according to the presence of protective colloids in wine, so it depends on wine matrix (Riberéau-Gayon et al., 2006).
CMC’s can be found on the market in the powder form and in the aqueous form with a concentration above of 3.5 % of CMC (International Œnological Codex, 2012; Riberéau-Gayon et al., 2006).
CMC can be useful in winemaking due to its capacity to promote solubilisation of proteins (Feddersen and Thorp, 1993). In fact, CMC effectiveness is due to its capacity of reducing the growth rate of crystals (Riberéau-Gayon et al., 2006).
The use of CMC in winemaking was authorized in 2008 by the OIV (International Organization de la Vigne et du Vin), considering the opinion of the “Food Safety”, in white and in sparkling wines (Bosso et al., 2010; Bowyer et al., 2010). The restrict use of these substance was changed by European Union with Commission Regulation (EC) No. 606/2009 that approved the addition of CMC to all type of wines in order to guarantee tartaric stability (Bowyer et al., 2010). Commission Regulation (EC) No. 606/2009 also determines that the allowed maximum doses of CMC is 100 mg/L.
Studies carried out by Bowyer et al. (2010) showed that CMC doesn’t make any chemical modifications in wine comparatively to mannoproteins. There are also no alterations in wine pH when compared to metatartaric acid. Other authors showed that CMC have some economic advantages. Additionally, it is an inert product easy to use (Crachereau et al., 2001; Wucherpfennig et al., 1987; Wucherpfennig et al., 1988).
Guise et al. (2014) tested the impact of CMC’s with different degrees of substitution and molecular weight on some characteristics of white wines, namely phenolic compounds, chromatic and sensory characteristics. They compared the effectiveness of CMC’s with other oenological additives like arabic gum and mannoproteins. This study showed that all CMC’s stabilized the wines and some arabic gums and mannoproteins don’t. All oenological additives tested do not change significantly phenolic compounds and wine sensorial characteristics.
8.1.2. Chitin
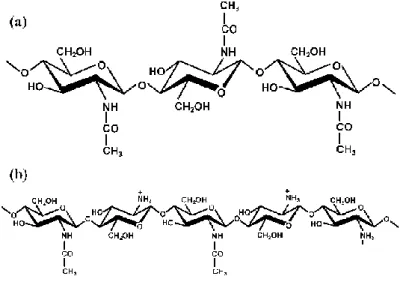
Chitin is one of the most abundant natural biodegradable polymers (Kumar, 2000; Quintela et al., 2012; Vårum and Smidsrød, 2006). Chitin is a linear polysaccharide
constituted by (1-4)-β-linked 2-acetamido-2-deoxy-β-D-glucopyranose units (Figure 1.6a), which is insoluble in aqueous solvents (International Œnological Codex, 2012; Kajiwara and Miyamoto, 2005; Vårum and Smidsrød, 2006; Stephen and Churms, 2006). The amino groups in chitin are highly acetylated (Vårum and Smidsrød, 2006).
Chitin can be found in the exoskeleton of crustaceans and shrimp shells, insects and fungi. Besides food industry, it can also have pharmaceutical and biomedical applications (Thanou and Junginger, 2005).
Vicenzi et al. (2005) studied chitin on a laboratory scale in white wine and concluded that was a reduction in wine protein content of less than 29%. Effects on wine quality were not known because this study doesn’t include a chemical or a sensorial analysis of wine aroma compounds after wine treatment with chitin. Another study performed by Quintela et al. (2012) showed that chitin, at a concentration of 50 g/hL, removed 18% of ochratoxin A (OTA) from red wine, without affecting significantly the wine quality parameters.
8.1.3. Chitosan
Chitosan is a linear polysaccharide composed by (1-4)-β-linked 2-amino-2-deoxy-D-glucose units (Figure 1.6b), obtained by deacetylation of chitin by hydrolysis under alkaline or acidic conditions (International Œnological Codex, 2012; Kajiwara and Miyamoto, 2005; Vårum and Smidsrød, 2006; Yui and Ogawa, 2005). The chemical reactivity of chitosan is higher than chitin due to the presence of free primary amino groups in chitosan, dispersed regularly in its chain (Yui and Ogawa, 2005).
It has been shown that chitosan has a selective effect on Brettanomyces and can cause a delay in its latent phase in cultures with Saccharomyces cerevisiae (Gómez-Rivas et al., 2004).
Chitosan presented a high affinity to phenolic compounds in white wines, specifically to cinnamic acids. Therefore, this polysaccharide can be comparable to the conventional adsorbents. On the other hand, chitin showed a lower adsorbing capacity to reduce phenolic compounds content (Spagna et al., 1996).
Quintela et al. (2012) studied the effect of chitosan in ochratoxin A (OTA) removal from red wine. Chitosan had the best performance by removing 67% of OTA from wine. However, it affected wine quality (pH, colour parameters and polyphenol concentration).
8.1.4. Arabic Gum
Arabic gum (also known as acacia gum) is obtained from the natural exudation of branches of certain trees in the acacia species (International Œnological Codex, 2012; Riberéau-Gayon et al., 2006; Thevenet, 2010). It is constituted by a polysaccharide rich in arabinose and galactose and a small protein fraction: (1-3)-linked β-D-galactopyranose units with various side chains of D-galactopyranose, arabinofuranose, D-glucuronic acid and L-rhamnopyranose residues (International Œnological Codex, 2012; Lindblad and Albertsson, 2005). All molecules are partially neutralised with sodium, potassium, calcium and magnesium salts (Thevenet, 2010).
This oenological product must be used in clear wines ready for bottling and it can stabilize turbidity (Riberéau-Gayon et al., 2006). It has a stabilizing power against precipitation of colouring substances, iron or copper instabilities (International Œnological Codex, 2012). So, the use of arabic gum is a preventive treatment for colloid precipitations such as iron and copper casses due to its similar chemical composition to the natural colloidal substances of wine. Arabic gum can inhibit normal transformations in certain aged wines related with colloidal phenomena. The doses usually used are 5 – 20 g/hL (Riberéau-Gayon et al., 2006; Thevenet, 2010).
8.1.5. Alginic Acid
Alginic acid is a colloidal polysaccharide, which is extracted from brown algae within a long and slow process (Helgerud et al., 2010; International Œnological Codex, 2012;
Riberéau-Gayon et al., 2006). It is a polymer constituted by β-D-mannuronic acid (Helgerud et al., 2010; International Œnological Codex, 2012).
This colloidal polysaccharide is negatively charged even at low pH and can precipitate positively charged proteins (Cabello-Pasini et al., 2005).
Cabello-Pasini et al. (2005) carried out a study in order to evaluate the capacity of some polysaccharides namely alginic acid to flocculate and precipitate proteins from wine. They concluded that the adsorption capacity of alginic acid was maximum at protein levels lower than 50 mg/L. The results of this study indicate that alginic acid has a good wine stabilization capacity without wine tannin contents modification, because this polysaccharide cannot adsorb tannins.
8.1.6. Microcrystalline cellulose
Cellulose is a polysaccharide, which consists in a linear chain of (1-4)-linked β-D-glucose units. (Riberéau-Gayon et al., 2006).
Microcrystalline cellulose is purified natural cellulose (International Œnological Codex, 2012; Tuason et al. 2010), treated with hydrochloric acid in order to hydrolyse the amorphous regions and leave the less reactive crystalline regions as fine crystals (Avicel MCC Product Description, 1982; Battista and Smith, 1962). The viscosity of the dispersions is heat and pH independent (Avicel MCC Product Description, 1982).
This product is found in white, tasteless and odourless powder form and can be used to increasing the fermentability of grape juices (International Œnological Codex, 2012).
Microcrystalline cellulose has been widely used in food, cosmetic and medical industries (El-Sakhawy and Hassan, 2007).
8.1.7. Locust bean gum
Locust bean gum is a hydrocolloid that occurs naturally, composed by galactomannan polysaccharides. The active macromolecular component is a linear mannan chain (Doublier and Launay, 1981), having smooth regions of unsubstituted or sparingly substituted (1-4)-linked β-D-mannopyranose residues and hairy regions that are substituted with α-D-galactopyranose residues (Figure 1.7) (Rees et al., 1982).
Figure 1.7 – Structure of locust bean gum (Dionísio and Grenha, 2012).
This polysaccharide is used in food industry, because it forms aqueous solutions that are pH independent and heat and salts resistant (Dea and Morrison, 1975; Doublier and Launay, 1981).
8.1.8. Gellan gum
Gellan is a linear anionic extracellular heteropolysaccharide originated from Sphingomonas elodea (Chandrasekaran et al., 1988; Valli and Clark, 2010). The native gellan (produced by the bacteria) has a repeating unit of glucose, rhamose and glucuronic acid units (Crescenzi and Dentini, 1988; Valli and Clark, 2010) (Figura 1.8) with L-glycerate and O-acetyl groups attached as additional substituents compared to the commercial one (Kuo et al., 1986; Mazen et al., 1999; Rinaudo and Milas, 2000). The classification of low- and high-acyl gellan gum is made by the presence or absence of acyl groups and it has an effect on its functional properties (Valli and Clark, 2010).
Figure 1.8 – Structure of repeating unit of gellan (Burchard, 2005)
Gellan gum has a wide variety of applications in biotechnology and food industry due to its ability to give a transparent gel which is unaffected by pH or heat (Chandrasekaran et al., 1988). Deacylated gellan is the mostly used form, which can be applied with gelatine or arabic gum (Nishinari et al., 1999).
8.1.9. Pectin
Pectins are a group of complex heteropolysaccharides with linear chains composed by galacturonic acid units with alfa 1-4 linkages occasionally interrupted by rhamnose residues. A significant amount of these galacturonic acid residues are esterified as methyl esters. The homogalacturonan regions (galacturonic regions) are mentioned as “smooth” regions and the rhamnose-rich zones are the “hairy” regions having neutral sugars side chains (Lopes da Silva and Rao, 2006; Morris et al., 2002; Thakur et al., 1997).
These biopolymers can be found in the cell walls of several plants and in the middle lamella with the highest concentration of pectins (Lopes da Silva and Rao, 2006; Thakur et al., 1997).
Commercially pectins are used in food industry due to their gelling and stabilizing capacities (Leroux et al., 2003; Lopes da Silva and Rao, 2006; Ralet et al., 2003). Nevertheless, not all pectins have the same gelling capacity because it depends on the molecular size, esterification and branching (Thakur et al., 1997).
8.2. Protein source 8.2.1. Isinglass
Isinglass is an unprocessed product obtained from swim bladder of certain fish such as sturgeon. It consists in collagen fibers and can be found in the market in sheets form, in strips which have the appearance of dry parchment, in vermiculated or powder form (International Œnological Codex, 2012; Riberéau-Gayon et al., 2006). This product has been used since 18th century (Riberéau-Gayon et al., 2006).
This fining agent becomes opaque in cold water and can be dissolved in hot water acidified with tartaric acid. It forms a colourless jelly after cooling with a 30-50 parts of hot water (International Œnological Codex, 2012).
Isinglass is manly used to clarify rosé and white wines (International Œnological Codex, 2012) and the normal doses in white wines ranges between 1.25 and 2.5 g/hL (Riberéau-Gayon et al., 2006).
Cosme et al. (2007) studied nineteen commercial protein fining agents in order to characterize them and evaluate the influence on some phenolic characteristics on red wine: two potassium caseinates, four egg albumins, seven gelatines, two caseins and four isinglasses from different companies. The isinglasses induced a minor decrease in total monomeric
anthocyanin content (less than 5%) when compared with the other fining agents studied. Isinglass with bands at MW > 94.0, 94.0-43.0 and at 20.1 kDa had a greater effect on the total phenolic compounds than isinglass with bands at MW < 20.1 kDa due to removing a significant amount of non-flavonoid compounds. The worst results for limpidity were obtained with isinglass with bands at MW < 20.1 kDa. Another study carried out by Gonçalves and Jordão (2009) evaluates the impact of different commercial fining agents like isinglass and egg albumin and their concentration on antioxidant activity and proanthocyanidin fraction content (monomeric, oligomeric and polymeric) of a red wine from grape cv. Baga. The monomeric flavonols were notably depleted by egg albumin and isinglass. At high concentrations, these fining agents decrease the oligomeric fraction. Both fining agents induce a reduction of antioxidant activity of red wine.
A study performed by Cosme et al. (2009) compares the effects of oenological protein fining agents on the structural characteristics of proanthocyanidins, on the three main tannin fractions (monomeric, oligomeric and polymeric flavan-3-ols) and on colour and pigments of red wine. They used one egg albumin, two isinglasses, one potassium caseinate, one casein and three gelatins. Fish swim bladder isinglass decreased the polymeric and monomeric tannin fractions more than fish skin isinglass.
8.2.2. Egg albumin
Egg albumin is composed by various protein fractions being ovalbumin the main fraction (Riberéau-Gayon et al., 2006). This fining agent is presented in solid form, white to golden yellow flakes or powder, obtained by desiccating fresh egg white, or in sterilized liquid form (International Œnological Codex, 2012; Riberéau-Gayon et al., 2006). It is not completely soluble in water but it is soluble in alkaline solutions (International Œnological Codex, 2012) so sodium carbonate can be added to facilitate the process of dissolution (Riberéau-Gayon et al., 2006). Heating the preparation to facilitate dissolution is inadvisable because egg albumin may be desnaturated by heat (Riberéau-Gayon et al., 2006).
Egg albumin is the first protein that was used as fining agent and now it is universally known for its qualities in red wines (Riberéau-Gayon et al., 2006). It is also used for clarifying wines (International Œnological Codex, 2012). This protein softs wines that have high tannin content and consequently excessive astringency. The effects of oenological protein fining agents on the structural characteristics of proanthocyanidins was carried out by Cosme et al. (2009), on the three main tannin fractions (monomeric, oligomeric and
polymeric flavan-3-ols) and on colour and pigments of red wine. Egg albumin showed a higher affinity for oligomeric and polymeric proanthocyanidins. The application of egg albumin also affected the colour hue. Egg albumin is not recommended for white wines and musts and should be used with caution on light red wines (Riberéau-Gayon et al., 2006).
8.2.3. Gelatines
Gelatines are obtained by the partial hydrolysis of collagen from animal skins, bones and connective tissue (International Œnological Codex, 2012; Riberéau-Gayon et al., 2006; Stevens, 2010). Gelatines are positively charged proteins at wine pH (Castellari et al., 2001).
Gelatine has the capacity of lowering high molecular weight tannin concentration, so it can reduce turbidity and astringency in red wine. Besides, this protein can improve the colour of wines (Stevens, 2010).
Sims et al. (1995) studied the effects of pre- and post-fermentation gelatine addition and concluded that gelatine had a little effect on the colour, phenols or sensory characteristics of red wine. Cosme et al. (2007) studied the effect of seven gelatines with different structural characteristics on some red wine phenolic compounds. The results obtained showed that gelatine (MW < 14.4 kDa) with lower molecular weight decreased more the monomeric anthocyanins and removed significantly more total phenolic compounds than the gelatines with MW < 43.0 kDa and MW > 43.0 kDa; the gelatine with MW < 43.0 kDa reduced mainly the non-flavonoid compounds and the gelatine with MW < 14.4 kDa reduced more the flavonoids. Castellari et al. (2001) carried out a study with some fining agents namely gelatine in order to remove OTA from red wine and they concluded that gelatine is able to remove this fungal toxin up to 30% from wine.
8.2.4. Mannoproteins
Mannoproteins are extracted from Saccharomyces cerevisiae cell walls by enzymatic methods using β-glucosidase exo-1,3 EC 3.2.1.58 for glucans digestion, or by physico-chemical methods like heat treatment of yeast cell walls at high temperatures and sterilization system with citrate buffer at pH 7 (International Œnological Codex, 2012).
Mannoproteins are glycoproteins regularly highly glycosylated (Klis et al., 2002). They have different structures depending on many factors such as molecular weight, degree and type of glycosylation and load size (International Œnological Codex, 2012). The degree
of glycosylation is variable; some mannoproteins can contain 90% of sugars like mannose (Ribéreau-Gayon et al., 2006).
The main functions of yeast mannoproteins are the improvement of malolactic bacteria growth (Guilloux-Benatier et al., 1995; Rosi et al., 2000); adsorb ochratoxin A (Batista et al., 2004); prevent haze (Waters et al., 2005); improve sensory characteristics like reducing astringency, increment wine roundness and sweetness (Escot et al., 2001; Guadalupe et al., 2007, 2010; Vidal et al., 2004); promote flocculation and yeast autolyses in sparkling wines (Nunez et al., 2006) and interact with phenolic compounds (Escot et al., 2001; Poncet-Legrand et al., 2007; Riou et al., 2002; Vasserot et al., 1997).
Caridi (2006) concluded that yeast mannoproteins might bring chemical and sensorial benefits, so they can improve wine quality. Ribeiro et al. (2014) also performed a study in order to evaluate the effectiveness of different commercial mannoproteins on protein stabilization and their effect on phenolic compounds, chromatic and sensorial characteristics of white wine. Commercial mannoproteins containing higher mannose to glucose ratio are more efficient in white wine proteins stabilization. An advantage in using manoproteins is maintaining or improving the wine sensorial characteristics.
8.2.5. Potassium caseinate
Potassium caseinate is obtained by dissolving casein in aqueous potassium hydroxide and then spray drying the solution (International Œnological Codex, 2012; Weber et al., 2009). Casein is a heteroprotein containing phosphorus and it is obtained by coagulating skimmed milk (International Œnological Codex, 2012; Riberéau-Gayon et al., 2006). Casein is insoluble at wine pH, so potassium caseinate is more used due to its higher solubility (Weber et al., 2009).
Potassium caseinate is a white powder and it yields a colloidal solution in water (International Œnological Codex, 2012).
This fining agent is very effective for polyphenols and this is why it is used for minimize white wines browning (Cosme et al., 2011). A comparative fining trial on a red wine was performed by Gambuti et al. (2012), and a decrease in monomeric anthocyanins was observed when higher concentrations of gelatine and potassium caseinate were used. A significant decrease in colour intensity, total phenolic compounds and tannins were also observed in wines treated with potassium caseinate.
8.3. Mineral source 8.3.1. Bentonite
Bentonites are hydrated aluminium silicates that belong to the montmorillonite group. These fining agents have exchangeable cations like Ca2+, Mg2+ or Na+ (International Œnological Codex, 2012; Riberéau-Gayon et al., 2006) that influence their physicochemical properties (Riberéau-Gayon et al., 2006).
Montmorillonite is structured in flakes, which are organized in a regular pattern. Every flake is constituted by two rows of tetrahedral chained together and between them there are a series of octahedral structures. A negative charge is created on the surfaces between the flakes due to the difference in charges with the rows of tetrahedra containing Si4+, creating a gap (Figure 1.9). The cations are adsorbed into these gaps as well as water molecules responsible for the swelling capacities of bentonites (Riberéau-Gayon et al., 2006).
Figure 1.9 – Structure of montmorillonite (Riberéau-Gayon et al., 2006).
Bentonites are useful in grape musts and wines clarification and protein stabilization. Their effectiveness in clarification varies with wine composition and with bentonite type. They also can fix coloured matter (International Œnological Codex, 2012). When bentonite is added to wines, natural proteins are eliminated and it will be protected from copper and protein casse. But not all the bentonites can be used for treating wines because some of them can give wines off-flavours or don't have enough adsorbent and clarifying capacities (Riberéau-Gayon et al., 2006).
Bentonite can be used for treating wines with high levels of unstable colloidal colouring matter. Bentonite is negatively charged and tannins, some polysaccharides, proteins
and anthocyanins in the flavylium form are positively charged, so bentonite can eliminate these matters and stabilize wines, although this process implies colour loss (Riberéau-Gayon et al., 2006).
There are two types of bentonites, according to the exchangeable cations present, which vary with geographical origin: bentonites from Germany or North Africa where calcium is the major exchangeable cation and bentonites from United States which mainly contain sodium (International Œnological Codex, 2012; Riberéau-Gayon et al., 2006).
Sodium bentonites swell and absorb readily (International Œnological Codex, 2012) so they are the most effective in treating wines comparatively to calcium bentonites (Riberéau-Gayon et al., 2006). In fact, the flakes are more spaced than those of calcium bentonite. Calcium bentonite has lower swelling and is less absorbent than sodium bentonites (International Œnological Codex, 2012).
Bentonite could also interact with aromatic compounds (Moio et al., 2004), reducing the wine volatile molecules, resulting in aroma and flavour loss (Lambri et al., 2010).
8.4. Synthetic source
8.4.1. Polyvinylpolypyrrolidone (PVPP)
Polyvinylpolypyrrolidone is an insoluble polymer made by polymerization of vinylpyrrolidone in alkaline medium (Figure 1.10) (International Œnological Codex, 2012; Riberéau-Gayon et al., 2006). The adsorption of PVPP depends on the rate of polymerization (International Œnological Codex, 2012).
Figure 1.10 – Structure of PVPP (Magalhães et al., 2010).
This fining agent has high affinity for polyphenols with low molecular weight and can fix them in wine (International Œnological Codex, 2012; Riberéau-Gayon et al., 2006). In white wines, PVPP helps minimizing the tendency to browning. It eliminates oxidizable cinnamic acids, phenols and also quinones (Riberéau-Gayon et al., 2006).
PVPP are used in excessively tannic red wines in order to reduce astringency. However, it has less effect on anthocyanins than on tannins (Riberéau-Gayon et al., 2006).
In France, this product can be used at maximum doses of 80 g/hL. Doses of 20-30 g/hL are enough to prevent browning and have no negative sensorial effects (Riberéau-Gayon et al., 2006).
Some wineries use PVPP to treat wines containing 4-ethylphenol and 4-ethylguaiacol and the recommended doses are between 0.06 and 0.48 g/L (Suárez et al., 2007). Lisanti et al. (2009) carried out a study in order to evaluate the efficacy of PVPP in decreasing the levels of 4-ethylphenol and 4-ethylguaiacol in red wines. They concluded that PVPP could be useful in treating red wines with low concentrations of these volatile phenols. However, the treatment with PVPP affects negatively wine colour and phenolic compounds content. Castillo-Sánchez et al. (2007) evaluated, over three harvests, a few properties of Vinhão red wines with and without the use of different fining agents (PVPP, gelatine, egg albumin and casein). Fined wines have a decrease in colour, especially wines treated with PVPP. The use of PVPP also resulted in lower anthocyanin content.
8.5. Other sources
8.5.1. Activated carbon
Carbon is original from plant (typically wood) and can be subjected to activation processes at a high temperature or at a lower temperature in the presence of an acid in order to increase their adsorption properties. It can appear in the form of very fine black powder or granulated (International Œnological Codex, 2012).
Absorbent carbon has been used for several years to eliminate unpleasant odours. The results depend on either the quality of the carbon and the type of defect, but all wines treated with it lose their fruity aromas and freshness (Riberéau-Gayon et al., 2006). So, the absorption by carbon is not selective (International Œnological Codex, 2012). There is necessary to stir the wine for several days in order to improve the treatment effectiveness (Riberéau-Gayon et al., 2006).
Activated carbons remove colloids from solutions and in wine they interact with colouring matter, proteins and tannins (Riberéau-Gayon et al., 2006). It can also be used to reduce levels of Ochratoxin A (International Œnological Codex, 2012; Castellari et al. 2001). Activated carbons adsorbed high amount of OTA without removing total polyphenols but
coloured polyphenols were removed from red wine. The results show that activated carbon and potassium caseinate have the best performance in removing amount of OTA from red wine but this fining agent affected the red wine characteristics (Castellari et al. 2001). Some wineries also use this adsorbent to treat wines contaminated with Brettanomyces and the recommended doses are between 0.015 and 0.24 g/L for minimal concentrations and between 0.12 and 0.96 g/L for more concentrated off-odours (Suárez et al., 2007).
8.5.2. Shellac
Shellac is a natural polymer produced by lac insects, Luccifer lacca, which grows on trees in China, Thailand and India (Limmatvapirat et al., 2004; Pearnchob et al., 2003; Soradech et al., 2012).
A mixture of esters constitutes shellac: a hard resin and soft resin of polyesters and single esters which have hydroxyl and carboxyl groups (Figure 1.11).
Figure 1.11 – Structure of shellac. (a) Polyesters; (b) single esters (adapted from Limmatvapirat et al., 2004)
This biopolymer is used in food industry for spoilage protection and extending the shelf life of some products due to its good ability of forming films. It has been used in pharmaceutical industry too (Limmatvapirat et al., 2004; Soradech et al., 2012).
9. Aim of the study
Volatile phenols like 4-ethylphenol and 4-ethylguaiacol in red wines have been a concern to the industry because they affect adversely the quality of wines as they are responsible for a sensorial defect described as “animal odor” or “Brett” character. Preventive or curative measures are important for the wine industry in order to deal with this frequent and persistent problem. In this work a screening of potential curative treatments for removing 4-ethylphenol and 4-ethylguaiacol from red wine was performed and their effect on the physicochemical wine characteristics, namely wine colour parameters, chromatic characteristics, total phenolic compounds, flavonoids and non-flavonoids. In order to access the effect of the fining agents used in the 4-ethylphenol and 4-ethylguaicol levels a simple method based on liquid-liquid extraction and quantification by gas chromatography-mass spectrometry was optimized and validated These results could provide important information to the wine industry to select treatments based on fining agents to remove 4-ethylphenol and 4-ethylguaiacol, in order to improve wine quality.
REFERENCES
Anonymous (2006). Commission Regulation (EC) No 643/2006 of 27 April 2006. Official Journal of the European Union, 28.4.2006, L115/6–L115/9.
Arvik, T., Henick-Kling, T. (2002). Brettanomyces bruxellensis occurrence, growth, and effect on wine flavor. In Proceedings from the 31st Annual New York Wine Industry Workshop, Geneva, New York.
Avicel, MCC Product Description (1982), Bulletin 934, FMC Corporation.
Barata, A., Caldeira, J., Botelheiro, R., Pagliara, D., Malfeito-Ferreira, M., Loureiro, V. (2008). Survival patterns of Dekkera bruxellensis in wines and inhibitory effect of sulphur dioxide. International Journal of Food Microbiology, 121 (2), 201-207.
Batista, A. S., Horii, J., Calori-Domingues, M. A., Micotti da Glória, E., Salgado, J. M., Vizioli, M. R. (2004). The capacity of manno-oligosaccharides, thermolysed yeast and active yeast to attenuate aflatoxicosis. World Journal of Microbiology and Biotechnology, 20, 475–481.
Battista, O. A., Smith, P. A. (1962). Microcrystalline Cellulose: the oldest polymer finds new industrial uses. Industrial and Engeneering Chemistry, 54, 20-29.
Bosso, A., Salmaso, D., Faveri, E., Guaitai, M., Franceschi, D. (2010). The use of carboxymethylcellulose for the tartaric stabilization of white wines in comparison with other oenological additives. Ciência Técnica Vitivinícola, 49 (2), 95-99.
Bowyer, P., Gouty, C., Moine, V., Marsh, R., Battaglene, T. (2010). CMC a new potassium bitartrate stabilisation tool. The Australian & New Zealand Grapegrower & Winemaker, 558, 65-68.
Burchard, W. (2005). Light Scattering from Polysaccharides. In Polysaccharides: Structural Diversity and Functional Versatility. 2nd edition. New York: Marcel Dekker. ISBN: 0-8247-5480-8.
Cabello-Pasini, A., Victoria-Cota, N., Macias-Carranza, V., Hernandez-Garibay, E., Muñiz-Salazar, R. (2005). Clarification of wines using polysaccharides extracted from seaweeds. American Journal of Enology and Viticulture, 56, 52-59.
Cabrita, M. J., Laureano, O., Ricardo-da-Silva, J. (2003). Os compostos fenólicos das uvas e dos Vinhos. In I Seminário Internacional de Vitivinicultura. Instituto Superior de Agronomia, Universidade Técnica de Lisboa.
Cabrita, M. J, Palma, V., Patão, R., Costa Freitas, A. M. (2012). The conversion of hydroxycinnamic acids into volatile phenols (in a synthetic media and in red wine) by Dekkera bruxellensis.Ciência e Tecnologia dos Alimentos,32, 1-6.
Caridi, A. (2006). Enological functions of parietal yeast mannoproteins. Antonie van Leeuwenhoek Journal, 89, 417-422.
Castellari, M., Versari, A., Fabiani, A., Parpinello, G. P., Galassi, S. (2001). Removal of ochratoxin A in red wines by means of adsorption treatments with commercial fining agents. Journal of Agriculture and Food Chemestry, 49, 3917-3921.
Castillo-Sánchez, J. X., García-Falcón, M. S., Garrido, J., Martínez-Carballo, E., Martins-Dias, L. R., Mejuto, X. C. (2007). Phenolic compounds and colour stability of Vinhão wines: influence of wine-making protocol and fining agents. Food Chemistry, 106, 18-26.
Chandrasekaran, R., Millane, R. P., Arnott, S., Atkins, E. D. T. (1988). The crystal structure of gellan. Carbohydrate Research, 175, 1.
Chatonnet, P., Boidron, J. N., Dubourdieu, D. (2003). Influence des conditions d’élevage et de sulfitage des vins rouges en barriques sur leur teneur en acide acètique et en èthyl-phènols. Journal International des Sciences de la Vigne et du Vin, 27, 277-298.
Chatonnet, P., Dubordieu, D., Boidron, J. (1995). The influence of Brettanomyces/Dekkera sp. and lactic acid bacteria on the ethylphenol content of red wines. American Journal of Enology and Viticulture, 46, 463-468.
Chatonnet, P., Dubourdieu, D., Boidron, J., Lavigne, V. (1993). Synthesis of volatile phenols by Saccharomyces cerevisiae in wines. Journal of Science Food and Agriculture, 62, 101–202.
Chatonnet, P., Dubourdieu, D., Boidron, J., Pons, M. (1992). The origin of ethylphenols in wines. Journal of the Science of Food and Agriculture, 60, 165–178.
Chatonnet, P., Viala, C. D. (1997). Influence of polyphenolic components of red wines on the microbial synthesis of volatile phenols. American Jorurnal of Enology and Viticulture, 48, 443 - 448.
Comitini, F., Ingeniis De, J., Pepe, L., Mannazzu, I., Ciani, M. (2004). Pichia anomala and Kluyveromyces wickerhamii killer toxins as new tools against Dekkera/Brettanomyces spoilage yeasts. FEMS Microbiology Letters, 238, 235–240.
Cosme, F., Capão, I., Filipe-Ribeiro, L., Bennett, R. N., Mendes-Faia, A. (2011). Evaluating potential alternatives to potassium caseinate for white wine fining: effects on physicochemical and sensory characteristics. LWT – Food Science and Technology, 46, 382-387.
Cosme, F., Ricardo-da-Silva, J. M., Laureano, O. (2007). Protein fining agents: characterization and red wine fining assays. Italian Journal of Food Science, 19, 39-56.
Cosme, F., Ricardo-da-Silva, J. M., Laureano, O. (2009). Effect of various proteins on different molecular weight proanthocyanidin fractions of red wine during wine fining. American Journal of Enology and Viticulture, 60, 74-81.
Costa, A., Barata, A., Malfeito-Ferreira, M., Loureiro, V. (2008). Evaluation of the inhibitory effect of dimethyl dicarbonate (DMDC) against wine microorganisms. Food Microbiology, 25, 422-427.
Couto, J. A., Barbosa, A., Hogg, T. (2005). A simple cultural method for the presumptive detection of the yeasts Brettanomyces/Dekkera in wines. Letters in Applied Microbiology, 41, 505-510.
Crachereau, J. C., Gabas, N., Blouin, J., Hébrard, B., Maujean, A. (2001). Stabilisation tartrique des vins par la carboxyméthylcellulose. Bulletin OIV, 841-842.
Crescenzi, V., Dentini, M. (1988). Solution conformation of the polysaccharide gelan. In Gums and Stabilizers for the Food Industry. Oxford: IRL Press.
De Villiers, A., Cabooter, D., Lynen, F., Desmet, G., Sandra, P. (2011). High-efficiency high performance liquid chromatographic analysis of red wine anthocyanins. Journal of Chromatography A, 1218(29), 4660-4670. ISSN 0021-9673.