FACULDADE DE CIÊNCIAS
DEPARTAMENTO DE BIOLOGIA VEGETAL
STUDY OF CILIA ASSEMBLY IN TETRAHYMENA AND THE
ROLE OF CYTOSOLIC CHAPERONIN CCT
Ana Cecília Fernandes Seixas
DOUTORAMENTO EM BIOLOGIA
ESPECIALIDADE EM BIOLOGIA MOLECULAR 2008
FACULDADE DE CIÊNCIAS
DEPARTAMENTO DE BIOLOGIA VEGETAL
STUDY OF CILIA ASSEMBLY IN TETRAHYMENA AND THE
ROLE OF CYTOSOLIC CHAPERONIN CCT
Ana Cecília Fernandes Seixas
DOUTORAMENTO EM BIOLOGIA
ESPECIALIDADE EM BIOLOGIA MOLECULAR 2008
Tese orientada pela Professora Doutora Maria Helena Antunes Soares e pelo Professor Doutor Rui Gomes
No cumprimento do disposto no nº 5 do artigo 40º do Regulamento de Estudos Pós-Graduados da Universidade de Lisboa, publicado no Diário da República (II série), nº 153, de 5 de Julho de 2003, e por despacho do Conselho Científico de 01 de Agosto de 2006, foi autorizada a redacção da tese em língua inglesa.
The work presented here and represented in this thesis is the result of several years of dedication and effort, but it would have never become truth if it was not the support, friendship and expertise of many people and institutions that I would like to thank.
To Fundação para a Ciência e a Tecnologia, for funding my research work and specially my PhD fellowship (SFRH/BD/5176/2001).
To Fundação Calouste Gulbenkian, for funding my training stage at Dr Jacek Gaertig laboratory at Athens, USA
To Instituto Gulbenkian da Ciência, I acknowledge all working conditions provided including the exciting scientific environment. In particular to Professor Dr. António Coutinho for his support and incentive.
To my supervisor, Helena Soares, I acknowledge for accepting me in her laboratory and for providing me conditions to develop my work, including financial resources, and for the support given during these years.
To my supervisor Prof. Dr Rui Gomes, for accepting me as his PhD student at Faculdade de Ciências and for his patience and availability in solving the bureaucracies necessaries for admission and realization of the PhD exam.
To my comity thesis members, Christophe Gregoire and António Jacinto, for their valuable discussions about my research work and for sharing openly with me their expertise.
I shall always be grateful to Dr. Jacek Gaertig, at first for giving me the huge opportunity to develop part of my work at his lab, where I learned a lot during the brief period of time I spent at Athens. And for having been since then, an extraordinary mentor, always present, patient and available for discussing the work and ideas. For having challenged me at each scientific discussion to grow up as scientist. Especially, I would like to thank him for encouraging me at the most difficult moments of my PhD and for believing in my potentiality.
To Luís Viseu Melo for his help and contribution in this work, for his patience and support.
To the people in the laboratory, former and present members, Sofia Nolasco, João Gonçalves, Pedro Sanches and Ruben Carvalho for their friendly support and all the motivating discussions; to Miguel Coelho, for his help and contribution in my work; I would like to thank specially to a former member Andreia Feijão, for her genuine empathy and constant support.
I shall never forget Teresa Cruto that has accompanied me in the last months of my bench-work and was always ready to help and supportive. Thanks for having been with me again, at this special moment of my PhD, the writing part, when between laughs and tears, help and presence, your truly friendship was one of the sweetest things of 2007 (Obrigada, Teresa!). To João Coelho, for his friendship and support (not only with PCs!), for encouraging me through the worst moments of the thesis, and for the several great moments shared at the lab
To Claudia Bicho and Joana Duarte, for their friendship and for showing me so many times how life can be simple and making our days in the lab so much easier to take.
To other people in the institute whose sympathy and discussions made my passage in the IGC an enriching experience (scientifically and personally), particularly Irís Caramalho, João Sousa, Ana Mena, Catarina Figueiredo, Beatriz Fernández, Franscisca Fontes, Ana Rita Franca, Claudia Florindo, Thiago Carvalho, Manuel Rebelo…
support and valuable discussions of my work. For being critical and letting me learn with that (and not only scientifically!). Thanks for your patience and encouragement.
To Jacek Gaertig’lab members, specifically, Drashti, Dorota, Kris and Neeraj, for their help at lab during my stay in Athens, for having made my days away of Portugal less hard, sharing with me great moments I shall never forget. Thanks, Dorota for your help at lab, for being a wonderful person with a unique patience and generosity. Thanks Drasthi, my dear friend for always having believed in me and cheering me up, at Athens and now from Athens!
To Isabel Marques, for her precious help with bioinformatic tools and for her friendship.
To Nuno Moreno and Gabriel Martins for their technical help at confocal microscope and for valuable suggestions (Thanks Gabriel, for your constant availability and the fantastic DICs!)
To Joana Monteiro, for being with me in the worst and in the best moments of this period of my life. For her dedication, genuine friendship, patience, wise advices and enriching discussions about my work, and about so many other things, that made me a very lucky person to have met you. Obrigada Joaninha por tudo quanto me deste nestes seis anos que passaram, ajudando me a crescer como cientista e pelo carinho, apoio, pelas ínumeras gargalhadas e idas á Caruagem, pelas aventuras por esta Oeiras que ficará na nossa memória. Obrigada por me fazeres acreditar sempre em mim e me dares força para continuar.
Ao Dr. Fernando Heleno, pela amizade e pela sua enorme paciência e dedicação como médico. Sem a sua ajuda, seu apoio, seus conselhos teria sido muito díficil concluir esta fase. Obrigada por ser a excelente pessoa e profissional que é e me ter ajudado a ultrapassar os piores momentos.
Á minha família que ainda que geograficamene distante sempre torceram e acreditaram em mim e me ajudaram a ultrapasar os momentos mais penosos do percurso, sempre compreensíveis por minha maior “ausência”.
Aos meus amigos, Dina Santos e Nuno Pascoal, Vanda Alves, João Alves e a minha “sobrinha” Maria João, Celeste Carvalho e Claudino Santos. Obrigada pelo carinho, apoio incondicional, e pela compreensão, por me incluirem tão carinhosamente na vossa família. Por acreditarem em mim, “empurando-me” sempre para a frente. Obrigada pelos sábios conselhos.
Aos meus amigos ainda Maria da Luz, Alexandra Almeida, Susana Taveira, Patrícia Lourenço, Hugo Oliveira, Cristina Ribeiro e o carinho de Tatiana, Fernanda Milanezi, Cristina Freitas e Magda Atilano. Por estarem sempre comigo, torcendo por mim, partilhando comigo os bons e maus momentos e relembrando-me que o fim haveria de chegar!
Aos meus pais e ao meu irmão, Rafael, pelo seu amor e apoio constantes, por acreditarem em mim e nas minhas capacidades, por me lembrarem delas quando as esquecia. Por todo o sacrifício e compreensão, sábios conselhos ao longo destes anos. Obrigada por serem a luz que nunca deixa de existir na minha vida.
Resumo
Os cílios são organelos conservados evolutivamente que são requeridos num vasto número de processos celulares tais como locomoção, quimiotaxia, movimento de fluídos e transdução de sinais. Nos últimos anos, um grande número de publicações tem demonstrado o impacto que pequenas alterações no correcto funcionamento dos cílios tem no Homem. Várias doenças humanas que se caracterizam por quadros clínicos complexos, como o síndrome de Bardet-Biedl (BBS) e o síndrome de Meckel-Gruber (MKS) foram relacionadas como directamente causadas por alterações pontuais de componentes do cílio. O número deste tipo de patologias que afectam o Homem, designadas por ciliopatias, tem crescido continuamente. Isto é facilmente compreendido se tivermos em conta que o cílio é uma estrutura bastante mais complexa do que inicialmente se admitia, estimando-se através dos mais recentes estudos proteómicos o envolvimento de aproximadamente 400 proteínas, e várias dessas ainda desconhecidas. A maior parte do conhecimento obtido até à data na área da ciliogénese centra-se sobre o papel das proteínas estruturais do cílio, que participam na montagem da estrutura do axonema ou ligando os diferentes componentes deste. Assim sendo, muitas proteínas encontradas nos cílios, quer seja no espaço ‘citoplasmático’ do cílio como no próprio axonema, tem um papel ainda desconhecido. De igual modo, o processo da ciliogénese ainda é mal conhecido, particularmente entre o passo de ancoragem do corpo basal à superfície da célula e a formação de um cílio completo. Neste trabalho, estudámos o processo da ciliogénese no ciliado eucariota Tetrahymena utilizando microscopia de força atómica (AFM). Para tal, os cílios das células foram removidos, e a diferentes tempos de reciliação foram colhidas amostras de células que sem qualquer tratamento, foram observadas por AFM. Por comparação com os dados já publicados de trabalhos semelhantes mas obtidos por microscopia electrónica, verificou-se que os cílios foram cortados ao nível da região de transição do cílio, ao nível da estrutura fibrosa de padrão estrelado. Os resultados obtidos sugerem que a ciliogénese compreende dois mecanismos que ocorrem sequencialmente, sendo o primeiro deles baseado na adição de material de grandes dimensões (Capítulo II). Este material apareceu associado lateralmente ao cílio em regeneração e parece ser composto por 3 unidades de dimensões, forma e rigidez idênticas. Baseados nas observações feitas, sugerimos que este material é previamente montado no corpo celular ou junto à base do cílio sendo utilizado na montagem do cílio. A utilização destes blocos estruturais é exclusiva do primeiro mecanismo que compreende a ciliogénese, durante o qual é montado o primeiro micrómetro do cílio (tendo um cílio maduro cerca de 7 micrómetros). Aparentemente, uma vez que o cílio possui as estruturas designadas de capacete (“cap”) na sua extremidade distal, a ciliogénese não é mais dependente destas unidades pré-montadas. Supomos que uma vez que o “cap” do cílio exista, a incorporação destas unidades pré-montadas deve ser impedida e o processo de ciliogenése muda de mecanismo. A partir desse momento não observámos mais nenhum material nas paredes dos cílios, sugerindo que a ciliogenése prossegue pela adição de material de dimensões mais discretas, não detectáveis com o AFM.
Uma das classes de proteínas presentes no cílio e das quais pouco se sabe sobre o seu papel no cílio são as chaperones moleculares. Trabalho previamente realizado no laboratório mostrou a presença no cílio maduro e em regeneração de algumas subunidades da chaperonina citosólica CCT. Colocou-se então a hipótese de que as chaperones poderiam manter a conformação funcional nativa dos componentes ciliares, até eles serem introduzidos na estrutura ciliar. Note-se que a incorporação das subunidades de tubulina e diversos componentes ciliares é feita na extremidade distal, pelo que devem ser transportados do local da sua síntese (corpo celular) até à extremidade distal do cilío. De forma a investigarmos o papel das subunidades de CCTα e CCTδ no cílio, foram criadas estirpes de
Tetrahymena deletadas para as respectivas subunidades (Capítulo III, parte A). Os resultados mostram
que ambas as subunidades são essenciais para a sobrevivência da célula. Mais importante, no contexto do nosso objectivo de estudo, notámos que as extremidades distais dos cílios se apresentavam danificadas. A extremidade do cílio encontrava-se bifurcada, ficando uma das partes do cílio na forma de um arco caído para um lado. Quando analisado por microscopia de imunofluorescência com um anticorpo anti-tubulina observámos que a parte em forma de arco era mais estreita que a outra e terminava com duas marcações intensas na forma de círculos. Comparando dados da literatura de microscopia electrónica, interpretámos que se trata da parte do cílio que é composto pelo par central de microtúbulos e as suas respectivas projeções terminando no “cap” central. Note-se que em
Tetrahymena é sabido que o “cap” é composto por duas partes, uma cobrindo e ligando-se
especificamente ao par central, sendo a outra o “cap” dos dupletos de microtúbulos. Para além disso, verificou-se que os cílios das células depletadas em CCTα e CCTδ eram mais curtos que os cílios de células controlo, sugerindo um aumento da taxa de despolimerização dos microtúbulos dos cílios quando estes tem níveis deficientes de CCTα e CCTδ.
No sentido de obter mais dados sobre o papel das subunidades de CCT, introduzimos em células depletadas para CCTα um fragmento de ADN codificando para CCTα-HA, sob o controle de um promotor indutível dependente de cadmio. Conseguimos com esta estirpe e imitar uma situação de “Knockdown” de CCTα que nos confirmou a autenticidade do fénotipo observado para a depleção de CCTα. A depleção parcial trouxe a vantagem de as células poderem assegurar as funções vitais da chaperonina CCT enquanto entidade responsável pela aquisição da estrutura tridimensional correcta. Foi assim possível perceber que a subunidade de CCT tem um papel importante na manutneção da integridade da extremidade do cílio e no controle do comprimento do cílio (Capítulo III, Parte A).
Transversalmente ao nosso interesse em estudar o papel de CCTα e CCTδ no cílio, no decorrer das experiências de recuperação das células depletadas nas subunidades referidas, detectámos a capacidade das células transferirem ADN de um macronúcleo (o macronúcleo “velho”) para outro (o macronúcleo “novo”). Este processo de transferência de ADN para o locus de CCTα e CCTδ respectivamente ocorria quando estirpes heterocarióticas para a deleção do gene eram colocadas em conjugação de modo a obter células de Tetrahymena depletadas de CCTα e CCTδ (Capítulo III, Parte B). Mais, percebemos que as partículas de ouro usadas para bombardear as células provavelmente
actuam como meio de veículo do ADN e por isso são responsáveis pela capacidade das células recuperarem o ADN do macronúcleo “velho” e incorporarem o cromossoma de interesse no “novo” macronúcleo das células exconjugantes. Este resultado constitui o primeiro registo em que este fenómeno pode ocorrer em locus de genes essenciais em Tetrahymena.
No sentido de investigar possíveis defeitos ciliares que pudessem elucidar o papel das subunidades de CCT no cílio, e tirando proveito das células depletadas em CCTδ e CCTα, transformámos estas estirpes com ADN no qual introduzimos respectivamente mutações aleatoriamente e específicas (Capítulo IV e V, respectivamente).
No caso da mutagénese de CCTδ, obtivemos um mutante com um fenótipo complexo, com células de diferentes formas celulares cuja fenótipo se interpretou ser devido a uma diferente dose de allelos contendo a mutação (Capítulo IV). Para além disso, as células mutantes apresentavam uma falta de coordenação total entre os eventos nucleares e corticais que caracterizam a divisão binária em
Tetrahymena. Consequentemente, o mutante denominado EMSD1 apresentava várias hipotéticas
células que se mantiveram unidas numa massa única por incapacidade de completarem a sua citocinése e que sofreram regressão citoplasmática. Curiosamente, esse mutante revelou uma mutação silenciosa num exão e um nível de CCTδ superior ao nível fisiológico, cuja relação deverá ser esclarecida no futuro.
No caso da mutagénese de CCTα, escolhemos reproduzir uma mutação num aminoacido conservado entre CCTα e a proteína BBS6 que se sabe ter evoluido do CCTα e ser uma protéina que participa na ciliogénese. Obtivemos um mutante sensível á temperatura com cílios visivelmente afectados pela mutação introduzida.
No seu conjunto, os resultados apresentados nesta tese apontam para um papel das subunidades de CCTα e CCTδ ao nível da manutenção da integridade da extremidade mais distante do cílio. É razoável sugerir com base nos resultados obtidos que as proteínas CCTα e CCTδ deverão ter um papel activo na estrutura do “cap” do cílio, com consequências na manutenção do equílibrio entre polimerização e despolimerização dos microtúbulos do cílio.
Summary
The architecture of cilia is much more complex than initially thought, being estimated by proteomic studies the participation of ~400 proteins. The precise role of most of them is still unknown, including some proteins known as structural ciliary components. Hence, ciliogenesis event is still poorly described. Therefore, we characterized ciliogenesis in Tetrahymena using atomic force microscopy. The work here presented suggests that ciliogenesis occurs by a two-step mechanism, being the first one based on the assembly of pre-mounted material. The employ of such units is limited to the assembly of the first micrometer of cilia length. Apparently, once the capping-structures are assembled at the distal tip, ciliogenesis does not rely anymore in the pre-mounted units.
One of the classes of proteins found in cilia which role is still unclear is the molecular chaperones. Previously, our laboratory has shown in full-length and regenerating cilia of Tetrahymena the presence of some subunits of the cytosolic chaperonin CCT. It was hypothesized that the chaperones could preserve the native functional conformation of the ciliary components. Thus, we engineered strains of
Tetrahymena completely lacking CCTα and CCTδ subunits to investigate their role in cilia. We show that both CCT subunits are essential for cell survival and that CCT-depleted cells presented splayed cilia tips. In CCTα-KO cells rescued with CCTα-HA transgene, splayed cilia were observed when the expression of the rescuing gene was downregulated correlated with the absence of CCTα in the cilia structure, confirming the specificity of the CCT-KO phenotype.
Looking for ciliary defects that could give more insights concerning CCT role in cilia, we introduced mutations in both CCTα and CCTδ sequences. We obtained a temperature-sensitive CCTα-mutG345E mutant with impaired cytokinesis and abnormal cilia staining using anti-tubulin antibody. A CCTδ mutant containing an exonic silent mutation, and curiously leading to mild overproduction, exhibited cell division defects, and interestingly, as CCTα-G345E mutant, cilia had a dotted staining with anti-tubulin, suggesting an abnormal cilia structure.
Taken together, the results here presented point to a stabiliser role of CCTα and δ subunits, likely at the capping-structure, regulating the dynamic of cilia microtubules.
Content
Chapter I Introduction
1. Structure of cilia ... ..2
1.1 Microtubules... ..4
1.1.1 The doublet and triplet microtubules... ..8
1.2 Outer and inner dynein arms and their role in cilia beating ... 10
1.3 Central apparatus... 11
1.4 Radial spokes... 12
2. Ciliary motility ... 13
3. Basal body ... 14
3.1 Structure of basal body in metazoans and protozoans organisms ... 14
3.2 Morphogenesis of the basal body... 16
4. Transition zone of cilia... 18
5. Tubulin superfamily, the other tubulins ... 21
5.1 γ-Tubulin ... 21
5.2 δ-Tubulin ... 25
5.3 ε-Tubulin ... 26
5.4 η-Tubulin... 27
5.5 ζ-Tubulin ... 28
6. Cilia are dynamic structures ... 29
6.1 Directionality of flagellar assembly ... 29
6.2 Intraflagellar transport... 31
7. Cilia assembly: does the formation of a new cilium always follow the basal body template model?... 38
8. Cilia growth: existence of ciliary precursors in the cell body ... 41
9. Non-structural ciliary proteins... 42
9.1 Microtubule-associated proteins... 42
9.2 Molecular chaperones in cilia... 43
9.3 Microtubule severing proteins... 45
9.4 Ciliary enzymes responsible for post-translational modifications of tubulin in cilia... 47
10. Aims and the biological model... 48
Chapter II
Cilia assembly in Tetrahymena: evidences of a two-step mechanism
1. Abstract ... 69
2. Introduction ... 69
3. Material and methods ... 71
3.1 Strains and culture growth... 71
3.2 Deciliation of cells... 71
3.3 Atomic Force Microscopy (AFM)... 72
4. Results ... 73
4.1 Tetrahymena cell surface immediately after cilia removal presents crater-like structures with a nine-fold arrangement ... 74
4.2 Tetrahymena cilia assembly is made by contribution of prebuilt blocks of material with dimensions similar of 3-fold doublet-like structures ... 76
4.3 Tetrahymena cilia assembly is faster after cap is assembled... 84
5. Discussion ... 85
5.1 Cilia regeneration starts with the contribution of doublet-like pre-mounted units... 85
5.2 Asymmetry of cilia growth... 86
5.3 The pre-mounted units do not seem to be IFT-particles... 87
5.4 Cap assembly precedes the central pair formation ... 88
5.5 Cilia assembly relies in two mechanisms that operate sequentially... 89
6. References ... 92
Chapter III CCTα and CCTδ are essential for survival and maintenance of cilia in Tetrahymena 1. Abstract ... 97
2. Introduction ... 98
3. Material and methods ... 99
3.1 Strains and Culture Growth... 99
3.2 Conjugation of Tetrahymena thermophila ... 101
3.3 Constructs for germline disruption of CCTα or CCTδ gene... 104
3.4 Preparation of total genomic DNA of Tetrahymena thermophila... 105
3.5 Biolistic particle bombardment ... 105
3.6 Germline knockout heterokaryons ... 106
3.8 Construction of new strains to test the germline KO CCTα and CCTδ heterokaryons for
incomplete assortment ... 108
3.9 Rescue transformation of conjugating heterokaryons with CCTα and CCTδ gene ... 109
3.10 Construction of strains with inducible expression of CCTα-TAP and CCTδ-GFP... 109
3.11 Construction of Strains with Inducible Expression of HA-Tagged cDNA CCTα ... 110
3.12 Indirect immunofluorescence microscopy ... 111
3.13 Protein electrophoresis and western blotting... 112
3.14 Deciliation of CCT depleted cells in a small scale... 113
Part A: CCTα and CCTδ subunits are essential for Tetrahymena survival and are required for normal cytokinesis and cilia maintenance 4A. Results ... 113
4A.1 Cells lacking either CCTα or CCTδ do not survive ... 113
4A.2 CCTα null cells loose dynamic microtubules... 118
4A.3 CCTδ null cells present the same phenotype evolution than CCTα-KO cells... 119
4A.4 CCTα and CCTδ depleted cells are unable to reciliate... 124
4A.5 The ciliary signal showed by antibodies against CCT subunits is diminished in the respective CCT depleted cells contrarily to the observed cytoplasmic signal ... 124
4A.6 Cells lacking α- and β-tubulin have a mutant phenotype that is more severe compared to cells lacking CCT subunits ... 125
4A.7 Tubulin null cells also have splayed cilia tips ... 127
4A.8 Knockout cells for both CCTα and δ subunits can be rescued by introduction of the respective wild-type CCT gene ... 131
4A.9 GFP and TAP tagged CCT subunits did not rescue KO cells ... 132
4A.10 HA tagged CCTα fragment inserted in exogenous locus did not rescue KO cells... 133
5A. Discussion ... 142
5A.1 CCTα and CCTδ subunits are required for Tetrahymena survival and cell division ... 142
5A.2 Diverse microtubule types are affected in CCT-depleted cells ... 143
5A.3 CCTα and CCTδ-KO leads to lost of somatic cilia and damaging of cilia tip ... 144
5A.4 Knockdown of CCTα corroborates the importance of CCT subunits at cilia tip ... 147
Part B: Gene transfer occurs between macronuclei of different generations at the CCTα and CCTδ loci 4B. Results ... 148
4B.1 Growing cells among not rescued suppression or incomplete assortment... 148
4B.3 Gene transfer occurs between old and new MAC of exconjugants at loci of CCTδ
and CCTα ... 154
4B.4 Gene transfer occurs exclusively to the new MAC of the exconjugants ... 155
5B. Discussion... 156
5B.1 Gene Transfer Occurs at CCTα and CCTδ loci ... 156
6. References ... 160
Chapter IV Attempts at identification of cilia-specific functional domains of CCTδ by bioinformatics and random mutagenesis 1. Abstract ... 165
2. Introduction ... 166
3. Material and Methods... 167
3.1 Strains and culture growth... 167
3.2 Mutagenesis In Vitro of CCTδ gene fragment with EMS ... 167
3.3 Rescue transformation of CCTδ-KO cells with EMS-mutated CCTδ gene fragment... 168
3.4 Indirect immunofluorescence microscopy ... 169
3.5 Preparation of cDNA from CCTδ mutant ... 169
3.6 Protein Electrophoresis and Western Blotting ... 170
3.7 PubMed Accession Numbers of Protein sequences used in the alignment of CCTδ sequences of different species ... 170
3.8 Prediction of mRNA secondary structure... 171
4. Results ... 171
4.1 Putative Ciliary Motifs are not obvious in CCTδ sequences... 171
4.2 Mutagenesis of DNA in vitro with EMS prior to biolistic transformation of KO cells is functional and efficient... 172
4.3 Characterization of EMSD1 subpopulation cells ... 172
4.4 The different cellular shapes within the EMSD1 mutant population reflect the clonal progression of the phenotype... 175
4.5 EMSD1 cells have defective macronuclear division and migration... 176
4.6 EMSD1 cells have difficulty in assembling macronuclear microtubules but produce native tubulin and do reciliate ... 178
4.7 EMSD1 mutant presents an excessive proliferation of basal bodies and abnormal localization of the oral primordium ... 178
4.9 All cellular shapes of EMSD1 appear to be caused by to the same point mutation ... 181
4.10 The silent mutation detected in cDNA of EMSD1 cells lead to an important modification of mRNA secondary structure ... 183
5. Discussion ... 185
5.1 EMSD1 sub-populations could be a result of a dosage effect of the CCTδ mutated allele... 186
5.2 Sequencing of CCTδ cDNA of EMSD1 cells only revealed a silent substitution... 186
5.3 The silent mutation identified as putatively responsible for the EMSD1 phenotype may affect the mRNA secondary structure and the amount of CCTδ protein in the cell ... 187
5.4 EMSD1 cells experience an excessive proliferation of basal bodies associated with oral apparatus... 188
5.5 Macronuclear migration and division are impaired in EMSD1 mutant ... 189
5.6 Cytokinesis is impaired in EMSD1 mutant ... 190
6. References ... 195
Chapter V A Tetrahymena CCTα G346E mutant has cilia defects and cells multinucleated due to incomplete cytokinesis 1. Abstract ... 197
2. Introduction ... 197
3. Material and methods ... 198
3.1 Strains and culture growth... 198
3.2 Site-directed mutagenesis of CCTα gene fragment using Kunkel method ... 199
3.3 Rescue transformation of CCTα-KO cells with mutated DNA fragment ... 200
3.4 Indirect immunofluorescence microscopy ... 201
3.5 PubMed Accession Numbers of Protein sequences used in the alignment of CCTα and BBS6 sequences of different species... 201
3.6 Analysis of the capacity of cells to form food vacuoles... 203
4. Results ... 203
4.1 G346E mutation in CCTα leads to severe defects in cytokinesis and affects nutrient uptake in Tetrahymena ... 204
4.2 Characterization of the mutant CCTα-mutG346E... 205
4.3 CCTα-mutG346E affects the ciliary localization of CCTα... 208
4.4 CCTα-mutG346E strain is unable to form food vacuoles ... 209
4.5 G346E may induce changes in the secondary structure of the protein apical domain of CCTa... 210
4.6 Suppressor that revert the effect of CCTaG346E mutation was identified in the mutant population ... 211 5. Discussion ... 211 5.1 CCTa G346E strain is a temperature-sensitive mutant with ciliary defects... 211 5.2 Incomplete cytokinesis in CCTa-mutG346E strain is induced by growth at 30ºC ... 212 5.3 CCTa ciliary function: can we learn anything from BBS6 function in vertebrates?... 214 6. References ... 219
Chapter VI
Final Considerations and Perspectives
Final considerations and Perspectives ... 221 References ... 228
Figures index
Chapter I Introduction
Figure 1. Axoneme structure and its major components: schematic diagrams of the cilium axoneme, (A) in cross section illustrating the distinctive “9 + 2” arrangement of microtubules and (B) in length, indicating the different axonemal components ... 3 Figure 2. Microtubule structure... 5 Figure 3. Schematic of interactions between microtubule doublets and other proteins in the
axoneme ... 9 Figure 4. Schematic diagram of transverse sections of flagellar axoneme. The outer and inner
dynein arms (ODA, IDA) and radial spokes (RS) are attached to the outer doublet microtubules... 11 Figure 5. Schematic representation of basal body (A), cross sections through (B) it and cortical
organization of Tetrahymena cells (C)... 16 Figure 6. Scheme of cross sections (1-4) through a transition zone (TZ) and (5) through an
axoneme (Ax)... 20 Figure 7. Schematic drawing of the two models for microtubule nucleation by the γTuRC ... 23 Figure 8. Scheme of the different steps (that probably overlap) involved in the assembly of new
basal body or centriole ... 29 Figure 9. Diagrams showing the cilia structure an the IFT (A), the capping-structures presents
at the tip of the cilia (B) and motor-IFT particles machinery associated with BBS proteins... 32 Figure 10. Schematic diagram illustrating the different post-translational modifications that
proteins α- and β-tubulin may present in their N-terminal and C-terminal... 48 Figure 11. The main structural features of Tetrahymena ... 49
Chapter II
Cilia assembly in Tetrahymena: evidences of a two-step mechanism
Figure 1. Tetrahymena cell surface view in 3D topographic images ... 73 Figure 2. Tetrahymena cell surface view immediately after deciliation ... 75 Figure 3. Tetrahymena cell surface view at ~15 min of reciliation... 77
Figure 4. Cross-section analysis of growing axonemes observed at 15 min of reciliation ... 80 Figure 5. Growing axonemes observed at ~30 min of reciliation ... 81 Figure 6. Growing cilia observed at 45 min and 90 min after deciliation... 82 Figure 7. Cross-section analysis of a cilium with 1 µm of length observed at 45 min of
reciliation ... 84
Chapter III
CCTα and CCTδ are essential for survival and maintenance of cilia in Tetrahymena
Figure 1. Nuclear events in Tetrahymena conjugation... 102 Figure 2. Genetic scheme for creation of homozygous single knockout strains ... 106
Part A: CCTα and CCTδ subunits are essential for Tetrahymena survival and are required for normal cytokinesis and cilia maintenance
Figure 1A. Cytoskeletal organization of wild-type Tetrahymena ... 114 Figure 2A. WT cells and CCT_-KO cells. at 10 h after isolation of heterokaryon pairs ... 115 Figure 3A. Immunofluorescence images of CCTα-KO after 18 h of pair isolation... 117 Figure 4A. Immunofluorescence images of CCTα-KO cells after 22 h and 42 h of pair isolation. 120 Figure 5A. Immunofluorescence images of CCTδ-KO after 18 h of pair isolation ... 122 Figure 6A. Immunofluorescence images of CCTδ-KO cells after 26-30 h of pair isolation ... 123 Figure 7A. Immunofluorescence images of tubulin-KO cells after 18 h of pair isolation ... 126 Figure 8A. Localization of CCT subunits in Tubulin-KO cells after ~18 h of KO... 128 Figure 9A. Tubulin-KO cells after 30 h of pair isolation ... 129 Graph 1A. Percentage of splayed cilia found in CCTδ-KO and tubulin-KO cells... 130 Figure 10A. PCR analysis of strains collected in rescue experiment ... 132 Figure 11A. PCR analysis of the rescued CCTα -HA strain showing its authenticity ... 134 Figure 12A. Western blot analysis of CCTα level present in the rescued CCTα -HA strain... 135 Figure 13A. Rescued CCTα-HA strain characterization by immunofluorescence ... 137 Figure 14A. Analysis by immunofluorescence of the defects found in rescued strain CCTα-HA
grown without cadmium ... 139 Figure 15A. Western blot analysis of protein extract from rescued CCTα-HA cells in the
Part B: Gene transfer occurs between macronuclei of different generations at the CCTα and CCTδ loci
Figure 1B. Analysis of the alleles present in rescued and MT cells... 150 Figure 2B. Schematic diagram of our model for gene transfer occurring in the locus of CCTδ... 158
Supplementary data
Figure S1. Western blot to test specificity of affinity-purified anti-CCTα antibody ... 159 Figure S2. Immunofluorescence obtained in WT strains with secondary antibodies used as
negative control ... 159
Chapter IV
Attempts at identification of cilia-specific functional domains of CCTδ by bioinformatics and random mutagenesis
Figure 1. Tubulin Staining of EMSD1 and WT cells... 174 Figure 2. Percentage of different defined categories found in the progeny of normal-looking
(NL) and elongated (E) cells ... 175 Figure 3. Nuclei patterns and types of mitosis found in EMSD1 sub-populations ... 177 Figure 4. Characterization of the EMSD1 mutant using centrin labelling ... 179 Figure 5. Western Blot of total protein extract of wild-type and EMSD1 cells with anti-CCTδ
antibody... 180 Graph 1. CCTδ protein level in EMSD1 cells... 181 Figure 6. Sequencing of EMSD1 mutation responsible for the phenotype ... 183 Figure 7. Prediction of the secondary structure of the mRNA of wild-type and the detected
silent mutation c.225T>C. using the program RNAfold and the Srna program ... 185
Supplementary data
Figure S1. Multiple sequence alignment of CCTδ sequences in organisms ciliated and non-ciliated... 192 Figure S2. Model of interallelic recombination occurring in EMSD1 cells... 194
Chapter V
A Tetrahymena CCTα G346E mutant has cilia defects and cells multinucleated due to
incomplete cytokinesis
Figure 1. Multiple sequence alignment of BBS6 and CCTα protein sequences ... 204 Figure 2. PCR analysis of the MAC genotype of transformed CCTα-mutG346E strain ... 205 Figure 3. Immunofluorescence images of CCTα-mutG346E strain stained with anti-tubulin
antibody... 206 Figure 4. Immunofluorescence images of CCTα-mutG346E strain stained with anti-centrin
Antibody ... 208 Figure 5. Immunolocalization study of CCTα in the CCTα-mutG346E mutant strain ... 209 Figure 6. CCTα-mutG346E strain exposed to Indian ink to evaluate phagocytosis capacity ... 209 Figure 7. Rasmol representation of the secondary structure of CCTα apical domain in wild-type
and mutant cells (mutation G346E) ... 210
Supplementary data
Figure S1. Multiple sequence alignment of CCTα sequences in organisms ciliated and nonciliated... 215 Figure S2. Multiple sequence alignment of BBS6 and CCTα protein sequences using T-Coffe
Tables index
Chapter III
CCTα and CCTδ are essential for survival and maintenance of cilia in Tetrahymena
Table 1. Strains used in this study and their genotype and phenotype characteristics ... 100
Part A: CCTα and CCTδ subunits are essential for Tetrahymena survival and are required for normal cytokinesis and cilia maintenance
Table 1A. Percentage of CCTα-KO and CCTδ-KO cells able to divide... 116 Table 2A. Length of cilia in rescued CCTα-HA strain ... 141
Part B: Gene transfer occurs between macronuclei of different generations at the CCTα and CCTδ loci
Table 1B. Mating type determination of the MTD and MTA strains... 153 Table 2B. Analysis of paromomycin resistance of the G1 progeny obtained between crosses of
CU427.3 and several MT strains... 155
Chapter IV
Attempts at identification of cilia-specific functional domains of CCTδ by bioinformatics and random mutagenesis
Table 1. Strains used in this study and their respective genotype and phenotype characteristics ... 167 Table 2. Sequence of the primers used to sequence the cDNA of EMSD1 cells ... 170 Table 3. Pubmed accession numbers of CCTδ protein sequence used in a multiple sequence
alignment... 170 Table 4. Frequency of the different nuclei pattern observed in EMSD1 and wild-type population 176
Chapter V
A Tetrahymena CCTα G346E mutant has cilia defects and cells multinucleated due to incomplete cytokinesis
Table 1. Strains used in this study and their respective genotype and phenotype characteristics ... 199 Table 2. Sequence of the primers used to sequence CCTα gene in mutant cells ... 200 Table 3. Pubmed accession numbers of CCTα protein sequence used in a multiple sequence
alignment... 201 Table 4. Pubmed accession numbers of CCTα and BBS6 protein sequences used in a multiple
sequence alignment ... 202 Table 5. Percentage of CCTα-mutG346E strain with incapacity in forming food vacuoles... 210
Abbreviations and symbols % - percentage µ µµ µg - microgram µ µµ µl - microliter µ µµ µm - micrometer µ µµ µM - micromolar Å - angstrom ADP - Adenosine-5’-Diphosphate AFM - Atomic force microscopy Arf - ADP-ribosylation factor
ARS - autonomously replicating species ATP - Adenosine-5’-Triphosphate Ax - axoneme
BBS - Bardet-Biedl Syndrome BSA - Bovine serum albumin
cAMP - Adenosine 3’,5’-cyclic Monophosphate CCT - Chaperonin Containing TCP-1
cDNA - complementary DNA (strand) CK1 - Casein kinase 1
CP - central pair
C-terminal - polypeptidic chain end in which the last aminoacid residue has the carboxyl
function free
cy - Cycloheximide
DIC - Differential interference contrast DMSO - Dimethyl sulfoxide
DNA - Deoxyribonucleic acid DNase - Desoxirribonuclease
dut- - Genetic marker of an E.coli strain that lacks dUTPase activity
E - elongated cell
EB1 - End-binding protein 1
ECL - Enhanced chemiluminiscence EDTA - Ethylenediaminetetraacetic acid
EMS - Ethyl methane sulfonate E-site -exchangeable site
Fig. - Figure g - gram
GAP - GTPase Activating-Protein GDP - Guanosine-5’-diphosphate GFP - Green fluorescent protein GTP - Guanosine-5’-triphosphate h - hour
HC - heavy chain
HEPES - 4-(2-Hydroxyethyl)piperazine-1-ethanesulfonic acid Hh - Hedgehog
HK - heterokaryon Hsp - Heat shock protein IC - intermediate chain IDA - inner dyneins arm IFT - intraflagellar transport Kb - 103 base pairs kDa - 103 Dalton KO - knockout LCs - light chain M - monster cell m/v - mass per volume mA - miliamperes
MAC - polyploid somatic macronucleus MAP - microtubule-associated-protein mg - miligram
MIC - germline diploid micronucleus min - minute
ml - mililiter mm - milimeter mM - milimolar mp - methylpurine
Mt(s) - microtubule(s)
MTOC - microtubule-organizing center Neks - NIMA-related expressed kinases NH4Ac - Ammonium acetate
NL - normal-looking cell nm - nanometer
N-site - non-exchangeable site
N-terminal - polypeptidic chain end which corresponds to the first aminoacid residue that has
the amine function free
ºC - Celsius grade ODA - outer dynein arm
PAGE - PolyAcrylamide Gel Electrophoresis pb - base pair
PBS - Phosphate buffered saline PCR - Polymerase chain reaction PEG - Polyethylene glycol PF - protofilament
pH - negative logarithm of the hydrogenionic concentration PIPES - Piperazine-1,4-bis(2-ethanesulfonic acid)
PKA - cAMP-dependent protein kinase pm - paromomycin
PP2A - protein phosphatase 2A
rATP - riboAdenosine-5’-Triphosphate RNA - Ribonucleic acid
RNAi - interference RNA RNase - Ribonuclease RSP - radial spoke protein RT - room temperature SDS - Sodium dodecyl sulfate
SDS-PAGE - Sodium dodecyl sulphate polyacrylamide gel electrophoresis sec - second
Smo - Smoothened
ssDNA - single stranded DNA TBS - Tris Buffered Saline
TEM - Transmission Electron Microscopy TriC - TCP-1 ring complex
Tris - 2-amino-2-(hydroxymethyl)-1,3-propanediol Triton X-100 - Isso-octyphenoxypolyethoxyethanol TRP - tetratrico peptide repeat
Tween 20 - Polyoxyethylenesorbitan monolaurate TZ - transition zone
U - amount of enzyme that catalyzes the transformation of 1 µmole of substrate/minute ung- - Genetic marker of an E.coli strain that lacks Uracil N-glycosylase activity
UTR - untranslated regions V - Volt
v/v - volume per volume WT - wild-type
xg - unit of gravitational camp used as centrifugal force unit
γTuRC - γ-tubulin ring complex γTuSC - γ-tubulin small complex
Three-letter and single-letter aminoacid code
Ala Alanine (A) Gly Glycine (G) Pro Proline (P)
Arg Arginine (R) His Histidine (H) Ser Serine (S)
Asn Asparagine (N) Ile Isoleucine (I) Thr Threonine (T)
Asp Aspartic acid (D) Leu Leucine (L) Trp Tryptophan (W) Cys Cysteine (C) Lys Lysine (K) Tyr Tyrosine (Y)
Gln Glutamine (Q) Met Methionine (M) Val Valine (V)
CHAPTER I
Introduction
Cilia are cellular projections filled with microtubules (Mts) that function in generation of cell motility and sensation. It is well understood the function of cilia in cell motility in animals, including sperm movement, and the role of cilia in the generation of currents along epithelial cells in lungs and trachea. Moreover, cell biologists knew that almost every cell in the animal body has a single non-motile cilium but the function of this so called “primary cilium” has been a mystery. Some have even considered primary cilia as a useless evolutionary vestige of our single-celled days (Vogel, 2005). Nonetheless, researchers have continuously documented the presence, at least temporarily, of these protruding hair-like organelles on most cells of the body. It was generally accepted that the primary cilium was either a sensory organelle, based on its presence in the nose and eye, or that it is an evolutionary remnant without any function. Only starting in the 90’s, several discoveries were made that revealed a fundamental role of all cilia. The breakthrough came from the discovery of the conserved mechanism of assembly of cilia/flagella (known as intraflagellar transport) in the biflagellate eukaryotic green alga Chlamydomonas that triggered an enormous re-interest in these organelles (Kozminski et al., 1993a). This led to the construction of mutants lacking cilia in the mouse and the discovery that the polycystic kidney disease (Pazour, 2004) is caused most likely because the primary cilium on kidney tubule cells is required as a sensor of the extracellular fluid flow. A flurry of papers has followed documenting the role of cilia in a large number of sensory and developmental functions in animals including mammals (Pazour and Rosenbaum, 2002; Praetorius and Spring, 2005; Bisgrove and Yost, 2006).
Cilia are critically involved in the determination of left-right asymmetry in vertebrates and in normal cardiac development (reviewed in McGrath and Brueckner, 2003), electrolyte balance in the cerebrospinal fluid, and in fertility (reviewed in Bisgrove and Yost, 2006). Cilia also play well documented roles in vision, smell, and mechanosensation (Eley et al., 2005). Non-motile primary cilia can be seen essentially as cellular antennae that sense the different signals present in their environment. Malfunction or malformation of cilia due to genetic mutations that affect the synthesis, transport or the assembly of cilia are a causative factor of number of human disorders, including the polycystic kidney and liver disease, primary ciliary diskinesia, hydrocephalus, retinal degradation Bardet-Biedl syndrome, Alstrom syndrome, Meckel-Gruber syndrome (Pazour, 2004; Afzelius, 2004; Eley et al., 2005; Praetorius and Spring, 2005; Vogel, 2005; Badano et al., 2006; Singla and Reiter, 2006).
Cilia and flagella show a remarkable conservation from protozoa to humans, and they are assigned with two different names mainly to reflect their different beating motions (reviewed in Singla and Reiter, 2006), although they are nearly identical in structure and function. Therefore, we will use the terms “cilium and cilia” in reference to flagella as well.
1. Structure of Cilia
Cilia can be viewed as specialized compartments covered by a membrane that is in continuation with the plasma membrane of the cell. These protruding organelles have a core, called the axoneme, which consists of Mts and many associated proteins. A typical cilium has 9 fused-wall (doublet) Mts arranged into a ring at the periphery (reviewed in Haimo and Rosenbaum, 1981; Bisgrove and Yost, 2006). The mechanism involved in the assembly of these fused-wall Mts is still unclear. Certainly, many proteins must afterwards stabilize the joined Mts since cilia are dynamic organelles with important beating movements, but their characterization is unfinished.
The main focus of my dissertation was to contribute to the understanding of the cilia assembly and how these structures are maintained.
Motile cilia usually also have a pair of singlet Mts in the center. Broadly, cilia are classified in two types, according to their Mt components: i) 9+2 motile cilia; ii) 9+0 primary cilia generally immotile, due to the absence of dynein motor projections; iii) 9+0 nodal cilia, motile cilia (with dynein arm projections on doublet Mts) that form on the embryonic node and are involved in the development of left-right asymmetry (Eley et al., 2005; Singla and Reiter, 2006).
In 9+2 cilia, nine doublets of Mts surround a pair of singlet Mts, whereas 9+0 cilia lack the central pair. While the 9+2 and 9+0 structures are most common, occasionally other arrangements of Mts exist, such as 9+4 in cilia of the notochordal plate of the rabbit embryo, 12+0 and 14+0 axonemes in spermatids of arthropods, and 9+1 axonemes in platyhelmintes (Feistel and Blum, 2006).
The cilium is a complex organelle that contains hundreds of proteins. In pioneering work, using two-dimensional protein electrophoresis, Luck and co-workers (1977) have found that axonemes of Chlamydomonas contain over 250 polypeptides (reviewed in Dutcher, 1995). However, recent proteomic studies indicate that cilia contain at least 450 distinct proteins, (Agrin et al., 2003). Also, Smith and co-workers (2005) have found that cilia are composed of at least 223 proteins in the unicellular eukaryotic organism Tetrahymena thermophila. Some ciliary proteins may have not been identified due to a low abundance, and therefore the
number may be higher. Over half of the proteins that were identified in flagella from Chlamydomonas have mammalian homologues. Thus, the conservation of ciliary structure is associated with conservation of the molecular components of these organelles. Based on the preliminary proteomic analysis of human cilia (Ostrowski et al., 2002), it is expected that the mammalian motile cilium is as complex as cilia and flagella of unicellular organisms. Despite primary cilia (9+0) have not been purified from any source, and their proteome remains uncharacterized, it is likely primary cilia share core components with motile cilia. Indeed, immunodetection studies have located a number of known components of motile cilia inside primary cilia (Pazour, 2004).
The axonemal Mts serve as a scaffold that organizes the hundreds of microtubule-associated proteins. Furthermore, axonemal Mts serve as tracks for molecular motors that move upward and downward into the cilium compartment and transport materials needed for assembly and maintenance of cilia (intraflagellar transport). The Mts extend continuously for the total length of the axoneme that can be 10-50 µm (Afzelius, 2004).
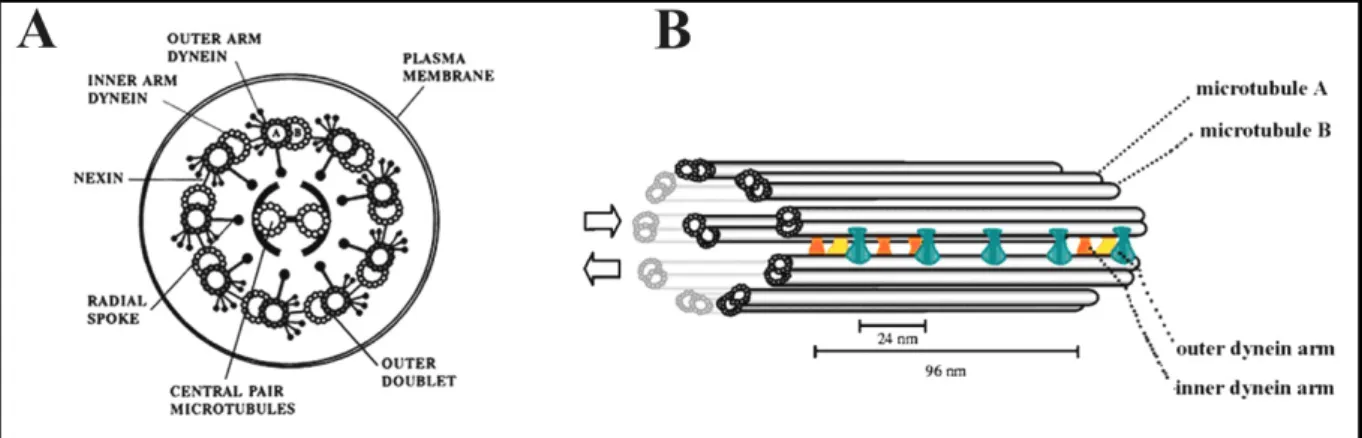
The doublet Mts of motile cilia have arm projections that contain ciliary dynein motors. Each has an outer dynein arm pointing towards the plasma membrane, and an inner dynein arm (Fig. 1) pointing to the center of the axoneme.
Dynein arms are responsible for ciliary bending. Adjacent doublets are connected by flexible structures called “nexin links” that participate in bending by constraining the relative movement of doublets during force generation. Another multiprotein structure named the
Figure 1. Axoneme structure and its major components: schematic diagrams of the cilium axoneme (A) in cross section illustrating the distinctive “9 + 2” arrangement of microtubules and (B) in length, indicating the different axonemal components. A) Nine Mt doublets (each with tubule A and B) surrounding
two central Mt (central pair). The Mts are interconnected by nexin links, radial spokes and dynein arms. B) Cilia beating is originated from the sliding of Mt doublets (double arrow on B). The dynein arms are periodically distributed along the axoneme; outer dynein arms (green) with a 24 nm periodicity and the different isoforms of inner dynein arms (double-headed in yellow and the six single-headed in dark orange) with a 96 nm periodicity. Adapted from Ibañez-Tallon et al., 2003.
radial spoke interconnects A-tubule of each doublet with the center of the axoneme. The motile and highly conserved 9+2 cilia have a central pair made of two singlet Mts, called C1 and C2, which constitute, with their sheath, the central apparatus of the cilium. In the next sections, it will be presented the main characteristics and some relevant data regarding the most important components of the cilia structure and their importance for the proper functioning of the cilium organelle.
1.1 Microtubules
Each one of the nine outer doublet Mts that are present in the axoneme is composed of an A-tubule and a B-tubule (Fig. 1). The A-tubule is a complete Mt, composed of 13 protofilaments, whereas B tubule is an incomplete Mt with 10 protofilaments that is fused to the side of the A-tubule.
The occurrence of doublet Mts is restricted to cilia. In centrioles and basal bodies, a third Mt, named C-tubule, is present giving rise to triplet Mts. Microtubules forming cilia and centrioles/basal bodies are fused-wall Mts and most likely represent more recent evolutionary innovation that were originated from simpler singlet Mts.
To understand the structure and function of ciliary doublets and fused-wall Mts in general, it will be helpful to discuss the properties of more wide-spread singlet Mts first. Examples of singlet Mts include: i) cytoplasmic network Mts that among a large variety of roles, participate in intracellular transport and organization of cellular components; ii) in the axons of neuronal cells; iii) Mts forming the mitotic spindle; iv) central pair Mts inside motile cilia.
The ubiquitous singlet Mts are usually composed of 13 linear protofilaments that form a cylinder. Each protofilament is an array of tubulin dimers connected head-to-tail and can be considered as the basic structural element of a Mt (Fig. 2-A). The tubulin dimers are composed of α- and β-tubulin and the organization of these repeating units in a linear structure give origin to the protofilament.
Moreover, in most commonly observed Mts including the ciliary Mts, the dimers of adjacent protofilaments are arranged in the so called B-lattice configuration (Song and Mandelkow, 1995). In the B-lattice, the dimer positions side-by-side in the adjacent protofilaments are slightly shifted. This results in a lateral arrangement of the dimers in the form of a helical pitch. Furthermore, the B-lattice leads to a discontinuity in the Mt wall (Mandelkow et al., 1986; Erickson and Stoffler, 1996) named the seam. While the lateral contacts between the adjacent protofilaments are mostly homologous (α-α and β-β), at the seam mainly heterologous contacts occur (α-β). Lateral interactions between protofilaments in
one Mt involve the surface containing helix H3 (Fig. 2-B, in red) of the α/β-tubulin dimer which contacts the ML region (Fig. 2-B, in dark purple) of dimers in the adjacent protofilament (Nogales et al., 1999; Inclán and Nogales, 2001). The importance of the seam is still obscure, but could be simply the point where the final closure of a Mt occurs during growth. In the flagellum, the stability or instability of junctions and seams could be modulated by additional proteins. One possible candidate could be a family of protein named tektins, as it will be discussed in a next section.
Nogales and colleagues (1998) have presented the atomic model of the α/β-tubulin heterodimer using electron crystallography. In this model, both α- and β-tubulin have an overall similar 3D structure that can be divided into 3 domains (Fig. 2-C). The N-terminal domain contains the GTP binding site. The intermediate domain is primarily involved in the contact of α- with β-tubulin to form the heterodimer and contains a binding site for the microtubule-stabilizing drug paclitaxel (on β-tubulin). The C-terminal domain is composed of two α-helices which form a ridge on the outside surface of the Mt (Downing, 2000) that functions as a major interface for binding of microtubule-associated-proteins (MAPs) that among other functions regulate the dynamic of Mts. The most extreme, highly acidic, C-terminal tails of both α and β-tubulin have not been resolved in the atomic model, but they are
Figure 2. Microtubule structure. A) Cartoon of a Mt made of 13 protofilaments originated by
head-to-tail arrangement of α/β tubulin heterodimers and creating a hollow cylinder of 25 nm of diameter; α-tubulin in red and β-α-tubulin in green. (+) and (-) define the polarity of the Mt (on the right side of the figure). It is also represented the GTP cap, rich in heterodimers that still have a GTP nucleotide on β-tubulin due to recent addition of those dimers at the fast growing end (the plus end) of the Mt. Adapted from http://www.erin.utoronto.ca/~w3bio315; B) Ribbon diagram obtained by electron crystallography of α-tubulin: polymerization surfaces are colored as follow: +end surface (yellow), -end surface (green), H3 surface (red) and ML surface (purple). Adapted from Inclán and Nogales, 2001; C) Ribbon diagram of α/β tubulin heterodimer: GTP binding sites, namely E- and N-binding sites are identifiable by the presence of GDP and GTP molecules shown in pink respectively on α- and β- tubulin. Taxol is shown in yellow. Adapted from Nogales, 2000.
of critical functional importance as they contribute to the binding site for MAPs and carry most of the conserved post-translational modifications of Mts.
The GTP on α-tubulin cannot be exchanged or hydrolyzed, and for this reason this GTP binding site is named the N-site (from non-exchangeable site). By contrast, the GTP on β-tubulin (in E-site, from exchangeable site) can be exchanged and hydrolyzed (Fig. 2-C). Within the α/β tubulin heterodimer, the minus end of β-tubulin contacts the plus end of α-tubulin, burying its N-site nucleotide (Fig. 2-B) and this is probably why it is not exchanged and hydrolysed. In contrast, polymerization of α/β dimers into protofilaments results in the burial and hydrolysis of the E-site nucleotide as the minus end of α-tubulin interacts with the plus end of β-tubulin (Nogales et al., 1998). While the GTP on β-tubulin plays a major role in the regulation of Mt polymer dynamics, the function of GTP on α-tubulin remains unknown.
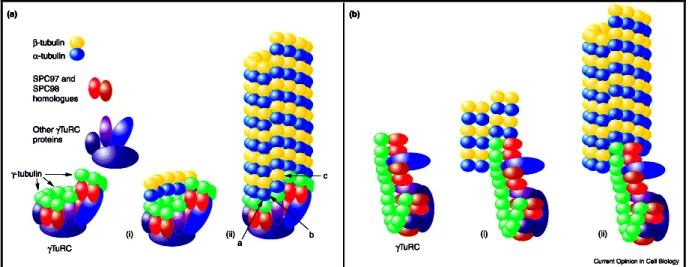
In each protofilament, the α/β-tubulin heterodimers are present in a uniform orientation (see in Fig. 2-A). Consequently the Mts have two structurally distinct ends: a crown of β-tubulin at the so called plus (+) end and a crown of α-tubulin at (-) end (Mitchison, 1993; Nogales et al., 1999). Therefore, Mts are polarized filaments with two different ends. Not surprisingly, the properties of the (+) and (-) ends of the Mts are quite distinct. In an in vitro experiment, both ends of the Mts can be extended by polymerization based on addition of new tubulin dimers. For polymerization to occur, the free dimers must be above a certain concentration that is named the critical concentration. In vitro, polymerization experiments using purified tubulin determined that the critical concentration for the (+) end is lower than the one for the (-) end. Consequently, the two ends have distinct rates of polymerization and depolymerization. The (+) end has a rate of subunit addition approximately three times faster than the (-) end. Thus, the preferred end for Mt growth in vivo is the (+) end. Also, in vivo, minus ends rarely present polymerization (reviewed in Dammermann et al., 2003). Among the different hypotheses presented in the last years of research, the most favored idea is the presence of a minus end capping factor. Indeed, in vivo, the (-) ends of Mts are usually anchored at a so called microtubule-organizing center (MTOC) such as a centrosome or a basal body.
The ability to exchange and hydrolyze GTP on β-tubulin is critical for regulating the dynamic properties of Mts. When the Mt grows, dimers of α/β-tubulin containing GTP in both nucleotide-binding sites are mainly added to the (+) end. The GTP of β-tubulin is rapidly hydrolyzed following addition of subsequent dimers. It appears that the incoming dimer acts as a GTPase-Activating-Protein (GAP) for the dimer that was added previously. Thus, tubulin can be seen as a unique G-protein that self-catalyzes its own GTP hydrolysis via interactions between dimers forming the polymer. As a result of rapid GTP hydrolysis on the E-site, the
(+) end has only a transient “GTP cap” that is made out of newly added tubulin dimers that did not have enough time to hydrolyze GTP (Fig. 2-A), while most of the polymer has GDP on β-tubulin. While the GTP cap is short-lasting, its presence has fundamental consequences for the stability of the (+) end and its ability to serve as a template for polymerization. As long as the rate of addition of new subunits exceeds the rate of GTP hydrolysis inside the Mt, the (+) end is stable or undergoes growth. Once the rate of polymerization drops below the rate of GTP hydrolysis (due for example to a low concentration of dimers), this leads to the loss of GTP cap. The presence of GDP on the E-site at the (+) Mt end dramatically changes the conformation of tubulin dimers (Wang and Nogales, 2005). Specifically, within a dimer with GDP on the E-site, the two monomers (α- and β-tubulin) are tilted. When a GDP dimer is exposed at the (+) end, its own curved conformation leads to destabilization of the (+) Mt end that weakens the lateral interactions between protofilaments, which in turn causes Mt depolymerization. In rapidly frozen depolymerizing ends of Mts, protofilaments appear to peel off at the plus end (Hyman et al., 1995). While GTP hydrolysis on the E-site occurs throughout the polymer shortly after addition of subunits, the fiber remains stable as long as a GTP cap is present at the growing end. This is because the GTP cap acts in a way similar to a zipper, while the energy released by GTP hydrolysis inside the polymer results in tension.
Microtubules show several forms of polymer turnover. For example, singlet cytoplasmic Mts (as well as those assembled in vitro) have ends that alternate between phases of growth and shrinkage, a property known as dynamic instability (Mitchison and Kirschner, 1984). A transition from growth to shortening is called “catastrophe” and a transition from shortening to growth is called “rescue” (Walker et al., 1988). Rescues, i.e., reinitiation of growth, are relatively rare in vitro but occur frequently in vivo suggesting that additional factors, such the association of certain MAPs to the Mt, might regulate this process (Drechsel et al., 1992).
As a consequence of their dynamic instability, the Mts present a remarkable turnover and short half-lives and these features are important for the realization of their cellular functions. While dynamic instability is an intrinsic property of the Mts, in particular the singlet cytoplasmic and spindle Mts, studies in the last years have uncovered cellular factors that regulate Mt turnover in vivo, including catastrophe factors that destabilize Mts (Walczak et al., 1996).
Another behavior displayed by Mts is called treadmilling (Margolis and Wilson, 1981; Rodionov and Borisy, 1997). Treadmilling is produced by addition of tubulin subunits, at the (+) end and removal of subunits, i.e. depolymerization, from the (-) end, leading to a flux of subunits along the polymer. There are several conditions that need to be met for this process
to occur. First, both ends need to be available for subunit addition or removal so that they cannot be “capped” in any way. Second, the concentration of tubulin heterodimers needs to be below the critical concentration for the (-) end but higher than the critical concentration for the Mt (+) end. Microtubule treadmilling was first demonstrated in vitro by Margolis and Wilson (1978) in bovine brain Mts. However, for a long time it was not known whether this phenomenon also occurs in vivo. In cells, Mts (-) ends are often capped, at the MTOC. Therefore, in vivo the fact that the (-) ends of Mts are usually anchored at MTOC such as a basal body probably favours dynamic instability more than the treadmilling of the Mts. However, under certain conditions, Mts are known to undergo rapid treadmilling in vivo. For example, spindle Mts that originate from the poles and are attached at their (+) ends to kinetochores show this behaviour.
1.1.1 The doublet and triplet microtubules
Little is known about the modes of assembly and turnover of fused-wall Mts in cilia and basal-bodies/centrioles. The process of assembly of ciliary Mts is complex, as doublets are formed as extensions of a subset of triplet Mts of the basal body. When new cilia grow, addition of new subunits occurs at the distal end that contains (+) ends of Mts. Following assembly, ciliary Mts have a low turnover rate, based on pulse chase studies. The assembly of triplet Mts occurs in the formation of the structure that is the seed of the basal body, the cartwheel structure (Dippell, 1968). However, the way by which a second Mt (B-tubule) and C-tubule are assembled in contact with the A-tubule is still unclear. It is well-established that the assembly of fusion-wall Mts requires the participation of tubulin proteins identified in the last years of research, the tubulin superfamily (gamma (γ), delta (δ), epsilon (ε), eta (η) and zeta-tubulin (ζ)) (Beisson and Wright, 2003).
One interesting feature of the fusion-Mts is that among the Mts that form their structure, it was found other structural proteins, the tektin proteins (Witman et al., 1972a; 1972b). These proteins were originally characterized as a set of three α-helical proteins highly insoluble, namely tektin A, B and C (Link and Stephens, 1987). Tektins form heteropolymers by coiled coils and have structural similarities to intermediate filament proteins (Norrander et al., 1992). The presence of tektins in doublet Mts is conserved among cilia of diverse organisms. Tektins have also been localized to basal bodies and centrioles and are presumed to be junctional components of the triplet Mts as it happens in the doublet Mts (Link and Stephens, 1987; Stephens and Lemieux, 1998).
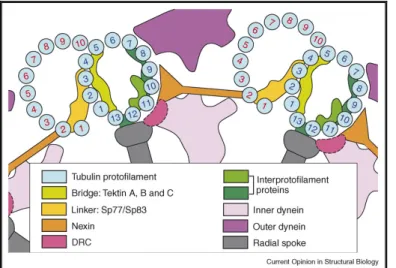
Recently, more insights concerning the composition and the interactions between some of the proteins that form the axoneme and regulate its movements were acquired using cryo-electron tomography (Nicastro et al., 2006; Sui and Downing, 2006). Contrarily to previous suggestions that one of the protofilaments (PFs) of the doublet Mts was formed exclusively by tektin filaments (Nojima et al., 1995), Sui and Downing (2006) have shown that all protofilaments of the doublets are made of tubulin. Using density maps, tektin A, B and C were found in a region of density named by the authors as bridge (Fig. 3) (Sui and Downing, 2006). This density region have a filamentous form, running along PF A3 with projections extending across several adjacent PFs, namely densities with an axial repeat of 8 nm extending from PF A3 to PF A1, and another density domain from PF A3 to PF A4 and A5, with a period of 16 nm (Sui and Downing, 2006; Nicastro et al., 2006). Very likely, the bridge, made of tektin, plays a role in stabilizing PFs providing additional resistance to bending of the doublet. On the other hand, Sui and Downing (2006) observed in their 3D density map a connection every 16 nm between the A- and B-tubules toward the inner region of the doublet. This region was named the linker density. It connects PF A2 to B2 and extends to PF B2 on one side, and to PF A3 on the outside of the A-tubule. The authors hypothesized that the linker provides flexibility to the doublet favoring the twisting of the Mt doublet. Based on immunolabeling studies performed in sea urchin (Hinchliffe and Linck, 1998) and in Chlamydomonas (Ikeda et al., 2003), the most likely components of the linker are the proteins Sp77 (homologue of Rib72 of Chlamydomonas) and Sp83. It is likely that other proteins are associated based on the dimension of the density domain (Sui and Downing, 2006).
Another important component of the doublet Mts that is important to ensure the stability of the axoneme structure is the nexin. This protein has been localized by transmission electron
Figure 3. Scheme of interactions between microtubule doublets and other proteins in the axoneme.
Nexin links connect two adjacent doublet Mts. Note the localization of the bridge composed of tektin A, B and C, close to the linker. These density domains are important for stabilization of protofilaments allowing the flexibility of the doublet necessary to the bending of the axoneme. Adapted from Downing and Sui, 2007.