REGULAR ARTICLE
BASAL MEDIA FORMULATION USING CANAVALIA ENSIFORMIS AS CARBON
AND NITROGEN SOURCE FOR THE GROWTH OF SOME FUNGI SPECIES
Ruth A.O. Gabriel-Ajobiewe*1, B.J. Akinyele2, E.B. Mirrila2
Address*: Dr. Ruth Adefolakemi Gabriel-Ajobiewe, 1 Adekunle Ajasin University,
Akungba-Akoko, Faculty of Science, Department of Microbiology, Ondo State, Nigeria, e-mail: henyinmigabriel@yahoo.com. Phone No: +2348075951165
2 Department of Microbiology, Federal University of Technology, Akure, Nigeria.
ABSTRACT
Keywords: fungi, nutrients, minerals, Canavalia ensiformis, Basal media.
INTRODUCTION
Jack bean (Canavalia ensiformis, L) is an annual underutilized leguminous semi-erect, drought resistant plant originated from tropical South American bearing long pods containing white seeds (Stephens, 1994). Jack beans have been reported to be rich in protein and could serve as potential protein source, and also has a high potential as protein replacer (Seena et
al., 2006). Raw unprocessed seed of Jack bean contains about 300g crude protein and 600g
carbohydrates kg-1 dry matter (Udedibie, 1990). Although it is low in methionine, it is relatively high in lysine (Stephens, 1994). The knowledge of the source of nutrients that encourage the growth of microorganisms is a useful determinant factor in considering the availability of the enzyme present in the microorganisms which can be industrially useful.
Fungi are a group of eukaryotic spore bearing, achlorophyllous organisms that generally reproduce asexually and sexually. Some are agent of diseases in plants and animals (parasitic), while some are saprophytic. Fungi are regarded as General Manager in Nutrients recycling department of nature (Khalid et al., 2006). Fungi due to their competitive saprophytic ability expressed by fast mycelia growth, spores production, presence of efficient and extensive systems of powerful enzymes are able to utilize complex polysaccharides and protein as their carbon and nitrogen sources (Wubah, 1999).
Pelczar et al., (1993) defined a culture medium simply as any material in which
MATERIAL AND METHODS
Preparation of Raw Carbon and Nitrogen Source
The jack bean (Canavalia ensiformis) seeds used were obtained from International Institute of Tropical Agriculture (IITA), Ibadan, Nigeria. The clean healthy seeds were soaked with distilled water for two days to remove the seed coat and rinsed in changes of distilled water. The dehulled seeds were dried at 50oC for 48 hours in the drying cabinet and
pulverized into fine powder with the Marlex portable stainless grinder. The materials were stored in sterile transparent polythene bags, tightened and kept at -20oC for further usage.
Biochemical Analyses
The proximate parameters were determined according to AOAC (2005) while minerals was analysed using Atomic Absorption spectrophotometer (AAS). Data were analyzed according to the analysis of variance (ANOVA) procedures and means separated using Duncan new Multiple Range Test.
Preparation of Growth Media
Eight different growth media were used, six of the broth were compounded by replacing either the carbon or nitrogen source in the basal medium formulated as modified by
Olutiola et al (2000), while the other two were Potato Dextrose Broth (PDB) (positive
control) and basal medium (negative control).
Medium A – Standard/Positive Control was prepared according to manufacturer’s specification.
Medium B – Basal medium is an enrichment culture medium used to test the ability of the microorganisms to degrade and utilize the carbon and nitrogen source (Canavalia ensiformis, L) as substrates for production of biomass. It contained 0.25grams - peptone, 0.26grams – KH2PO4, 1gram – NaNO3, 0.025grams – MgSO4.7H2O, 0.5 grams – Na2HPO4, 5.0grams –
sucrose; into 250millilitres distilled water.
Medium D (as carbon source) – Has all the reagents compounded for the basal medium but the carbon source (sucrose) was replaced with (5.0grams) Jack beans powder.
Medium E - All the reagents remains has in the basal medium except the replacement of both the nitrogen source with Jack beans and carbon source with glucose.
Medium F – Formulation was done according to medium E with change in the carbon source as maltose.
Medium G – Formulation was done according to medium E with change in the carbon source as lactose.
Medium H – Formulation was done according to medium E with change in the carbon source as galactose.
Test Organisms
The test organisms, isolated from the fermenting jack beans using Malt extract agar, were identified using standard microbiological methods (Onions et al., 1995). The fungi isolates were Aspergillus flavus, Aspergillus niger, Neurospora crassa, Meria coniospora, Mucor mucedo and Rhizopus oryzae.
Inocula were drawn from pure cultures of the isolates before 72th hour of growth aseptically to ensure that growth was still high at logarithmic phase when it would have uniform physiological characteristics (Nwachukwu and Akpata, 2003).
Determination of Fungal Biomass
The biomass produced in each broth medium was determined by dry weight measurement applying the method of Nwanze et al (2005). The weight of biomass of the culture were determined by drying and weighing using a pre-weighed filter paper under aseptic condition with the Mettler balance (PM400).
Physicochemical Analysis
RESULTS AND DISCUSSION
Observation of the result of proximate and mineral analysis carried out on Canavalia ensiformis shows it contains appreciable amounts of nutrients required for the good growth of fungi. This can be seen in biomass yield of six fungi in the formulated media.
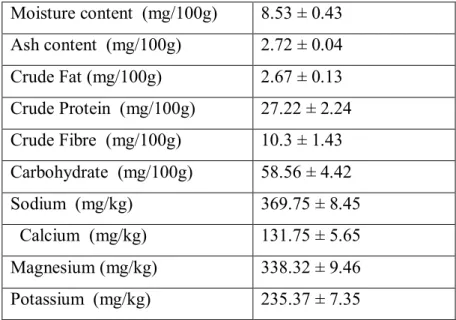
Table 1 Proximate Composition of Raw dehulled Jackbeans (Canavalia ensiformis L.)
Table 1 shows the moisture content of the sample (Canavalia ensiformis), as well as the ash content, percentage fat, percentage protein, crude fibre content and carbohydrate which were given as 8.53, 2.72, 2.67, 10.3, 27.22 and 58.56g/100g respectively. Although moisture (water) is required by all organisms for their life processes and fungi in particular require water for extracellular digestion of nutrients. The moisture content of the sample has negligible or no effect on the growth of the fungi tested because they were grown in solution (broth). However, water is important for the cultivation of fungi on solid substrate (Olutiola
et al., 2000; and Silva et al., 2005).
The ash content of the sample (jack beans) was recorded as 2.72g/100g; this shows that jack beans have a low percentage ash. The crude fibre is a representation of the organic matter in the sample. It gives an estimate of the proteins, lipids (fats), carbohydrate and the nucleic acid component of the sample. From table 1, the percentage crude fibre composition and fat composition are (10.3 and 2.67g/100g) respectively. Although there is a relationship between lipids content of media and fungal growth according to Nwanze et al., (2005) which
Moisture content (mg/100g) 8.53 ± 0.43 Ash content (mg/100g) 2.72 ± 0.04 Crude Fat (mg/100g) 2.67 ± 0.13 Crude Protein (mg/100g) 27.22 ± 2.24 Crude Fibre (mg/100g) 10.3 ± 1.43 Carbohydrate (mg/100g) 58.56 ± 4.42
Sodium (mg/kg) 369.75 ± 8.45
reported that lipids had significant effects on the mycelia wet and dry weight of Lentinus
squarrosulus (Mont) singer and Psathyrella atroumbonata Pegler in submerged liquid
culture. The carbon to nitrogen ratio (C/N) has been said to influence rapidly with a high degree of efficiency the rate of assimilation of nitrogen into microbial biomass (Zibilske,
1999; Narasimha et al., 2006). Also represented on table 1, is the percentage nitrogen
content of Jack beans (Canavalia ensiformis) which was given as an index of the percentage protein content which is 27.22g/100g. This may be referred as a contributory factor to the massive growth of organisms cultured in the media with jack beans as the raw carbon source. Another observation from table 1 is the concentration of minerals (both macro and micro elements) in the raw carbon/nitrogen substrate which must have contributed to the growth of some of the fungi in the media. The raw carbon/nitrogen source contained the tested minerals in concentrations as shown below sodium: 369.75mg/Kg, calcium 131.75 mg/Kg, magnesium 338.32 mg/Kg, and potassium 235.37 mg/Kg.
Legend: Potato Dextrose Broth (PDB). d1 – d5 represents day 1 to day 5.
Growth of the Selected Fungi on the Formulated Media and the Controls
Maximum growth of Aspergillus flavus (figure 4) was observed within 120 hours of inoculation of spores into sample formulated with jack beans as the carbon source and NaNO3
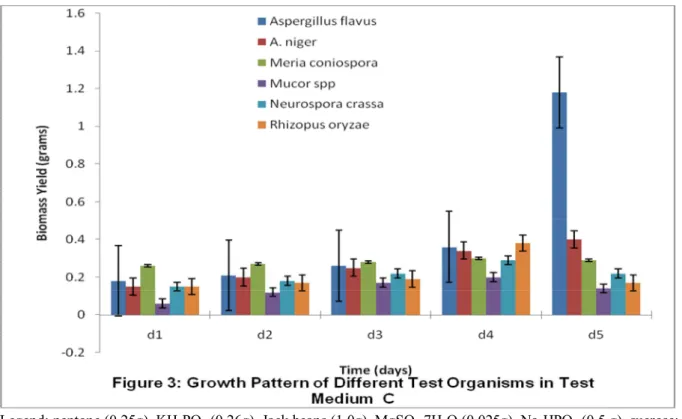
Canavalia ensiformis as nitrogen source and sucrose as carbon source (medium C that is figure 3) and sample formulated with Canavalia ensiformis as nitrogen source and lactose as carbon source (medium G/ figure 7) with maximum biomass yields of 1.148, 1.137 and 1.01grams respectively. Minimum biomass yield was recorded in the sample with nitrogen source as Canavalia ensiformis and carbon source as galactose (medium H) as 0.653grams. Although, medium B (basal formula) and the standard were expected to have supported the growth of the fungus within few days because their carbon sources are easily digestible than that of the raw carbon source maximum growth was still recorded in medium 4 after five days. This is an indication that natural substances contain a lot of complex substances such as vitamins and minerals (table 1) that are essential for good growth. This is in line with the findings of Akinyele and Adetuyi (2005) and Silva et al (2005), which shows that agricultural waste materials supports the good growth of fungi.
Legend: peptone (0.25g), KH2PO4 (0.26g), NaNO3 (1.0g), MgSO4.7H2O (0.025g), Na2HPO4 (0.5 g), sucrose;
Legend: peptone (0.25g), KH2PO4 (0.26g), Jack beans (1.0g), MgSO4.7H2O (0.025g), Na2HPO4 (0.5 g), sucrose;
(5.0g) & distilled water (250.0ml). d1 – d5 represents day 1 to day 5.
Legend: peptone (0.25g), KH2PO4 (0.26g), NaNO3 (1.0g), MgSO4.7H2O (0.025g), Na2HPO4 (0.5 g), Jack beans
(5.0g) & distilled water (250.0ml). d1 – d5 represents day 1 to day 5.
The maximum growth of Meria coniospora was recorded as 0.455grams in figure 4, relative to 0.07 g in the standard (figure 1) on the third day of both medium. The change on the fifth day as a growth 0.382g was recorded as the maximum in the standard (figure 1) and the growth in figure 4 declined to 0.268grams. This may lead to the inference that Meria
coniospora easily digests raw carbon sources than refined and that raw carbon sources
contains the essential vitamins and minerals (table 1) is capable of supporting the rapid growth of Meria coniospora within 72hours. The media in which jack beans was used as nitrogen sources had their maximum growth of the fungus recorded as 0.334g in figure 5 on the day five. This could be as a result of the fungus being able to digest glucose (a monosaccharide) faster than maltose, lactose, sucrose etc (which are disaccharides). In addition, the presence of glucose in a medium represses the synthesis of enzymes needed for the utilization of other carbohydrate substrates in the medium (Ruijter and Visser, 1997; Suto and Tomita, 2001).
As observed, the maximum biomass growth of 0.536grams was recorded for the Mucor sp with the medium D, relative to 0.442g in the standard, 0.079g in basal medium and 0.149g as the highest recorded in the media with nitrogen sources as sample Canavalia
some fungi grow profusely in organic substances than inorganic. The medium D has the same growth pattern as the standard medium, that is, a gradual increase in biomass, while media C and F shows an initial increase followed by a decrease to day 5.
The maximum growth of Neurospora crassa was recorded as 1.052 grams on the fifth day while the standard had a growth of 0.238grams on the same days. Rather, the maximum growth in the standard was recorded on the third day as 0.481grams and later declined. This may be caused by maximum utilization of the nutrients by the fungi which led to the shortage of nutrient after days three in the standard. Optimal nutrient medium should provide not simply adequate growth but the best possible growth in order to allow molds grow without restriction and express all phenophytes (Meletiadis et al., 2001).
The highest biomass of Rhizopus oryzae was recorded in medium D with a mass of 0.486gm and not only thus the medium has the highest growth for the fungus but shows the best support for the fungus which is evident in its growth from day 3 to 5 when compared with that of the standard medium. Medium E shows good support for the growth of fungus up to day 4 with a increase biomass which start decreasing from day 5.
Legend: peptone (0.25g), KH2PO4 (0.26g), Jack beans (1.0g), MgSO4.7H2O (0.025g), Na2HPO4 (0.5 g), glucose
Legend: peptone (0.25g), KH2PO4 (0.26g), Jack beans (1.0g), MgSO4.7H2O (0.025g), Na2HPO4 (0.5 g), maltose;
(5.0g) & distilled water (250.0ml). d1 – d5 represents day 1 to day 5.
Comparison of Growth on the Formulated Media
Of all the medium formulated with Jack beans whether as a source of carbon or nitrogen, medium D where Jack beans act as the source of carbon gave the best support for the growth of all the fungi used as test organism when compared to the standard medium. Even though, the standard medium shows a great support for the growth of all the fungi but not as good as those of medium D. The basal medium shows a continuous growth support for A. flavus and M. coniospora, while its support for the other fungi diminished after the fourth day. The replacement of the nitrogen source with an organic source in medium C shows a better growth support for all the fungi except Mucor sp, R. oryzae and N. crassa which are
Mucoraceae and Sordariaceae when compared with the basal medium (B). The substitution
support for all the fungi until day 4, while there was a growth regression for the others except
the Aspergilli. Medium H that has galactose as its carbon source while Jack beans still
remains the nitrogen source displays a progressive growth support for all the fungi with the exception of Meria coniospora on the fifth day. This change in carbon source results was in line with the finding of Akinyosoye and Akinyanju (1989).
Although other sources of carbon (such as sucrose, maltose, lactose, galactose and glucose) were expected to have supported the growth of the tested organisms (fungi) better than the raw carbon sources (jack beans), because their carbon sources are easily digestible than that of the raw carbon source, minimum biomass yield was recorded more on these refined carbon sources than that of the raw carbon sources (figures 1-8). This is an indication that natural substances such as vitamins and mineral (table 1) are essential for good growth.
Legend: peptone (0.25g), KH2PO4 (0.26g), Jack beans (1.0g), MgSO4.7H2O (0.025g), Na2HPO4 (0.5 g), lactose;
Legend: peptone (0.25g), KH2PO4 (0.26g), Jack beans (1.0g), MgSO4.7H2O (0.025g), Na2HPO4 (0.5 g),
galactose; (5.0g) & distilled water (250.0ml). d1 – d5 represents day 1 to day 5.
Generally, it can be drawn from this work that when organic substances are used as carbon or nitrogen sources for the cultivation of some species of fungi, the growth is found to be about the same or sometimes better in the formulated media relative to that of the standard as reported by Adesemoye and Adedire (2005).
CONCLUSION
In addition, the study has revealed that this raw carbon substrate contains the minerals and nutrients that can meet the nutritional requirements of the fungi, thus it can be utilized as an alternative material in the preparation of culture media for the in vitro cultivation of fungi for teaching and research purposes. Further research are still needed in the application of modern tools and methods to the study of fungal physiology as this will assist in the use of manipulation of waste materials into forms that can be useful.
Also, in solving the problem of shortage of culture media for laboratory practical, the results of this research will go a long way in ameliorating this problem and also assist in conserving the much foreign exchange for other developmental projects.
RECOMMENDATION
The scarcity of synthetic culture media in the local market due to its high price and the difficulties encountered in obtaining foreign exchange for the importation have led to the on-going research being abandoned by microbiologists an students of Microbiology (Alonge,1992).
However, with the help of this work, the problem can be minimized by making use of locally sourced alternative food materials such as jack beans (Canavalia ensiformis).In this study, all the organisms showed good growth in the medium/sample formulated with jack beans as the carbon source. Therefore, it is evident that this alternative medium could be a better substrate for the culturing of fungi.
REFERENCES
ADESEMOYE, A. O. AND ADEDIRE, C. O. 2005. Use of cereals as Basal Medium for the Formulation of Alternative Culture Media for Fungi. In World Journal of Microbiology and Biotechnology, vol. 3, 2005, no. 21, p. 329-336.
AKINYELE, B. J. AND ADETUYI, F. C. 2005. Effects of Agrowastes pH and Temperature Variation on the Growth of Volvariella volvacea. In African Journal of Biotechnology vol. 4, 2005, no. 12, p. 1390-1395.
A.O.A.C. 2005, In Official methods of analysis, 17th edition. Association of Official
Analytical Chemists. Gaithersburg, Washington D. C. A.O.A.C. press, U.S.A.
KHALID, M. YANG, W., KISHWAR, N., RAJPUT AND ARIJO, A. G. 2006. Study of Cellulosic soil fungi and two nova species and new medium. In Journal of Zhejiang University Science B., vol. 7, 2006, no. 6, p. 459 - 466.
MELETIADIS, J., JACQUES, F.G., MEIS, M. MOUTON, J. W. AND VERWEIJ, P. E. 2001. Analysis of Growth Characteristics of Filamentous Fungi in Different Nutrient Media. In Journal of Clinical Microbiology. vol. 39, 2001, no. 2, p. 478-484.
NARASIMHA, G., SRIDEVI, A., BUDDOLLA, V., SUBHOSH, C.M. AND RAJASEEKHAR, R.B. 2006. Nutrient Effect on production of cellulolytic enzymes by Aspergillus niger. In African Journal of Biotechnology, vol. 5, 2006, no. 5, p. 472-476. NWACHUKWU, S.C.U AND AKPATA, T.V.I. 2003. Principles of Quantitative Microbiology. University of Lagos Press, Lagos, Nigeria. p. 30-37. ISBN 978-017-636-5. NWANZE, P.J., KHAN, A.V., AMEH, J.B. AND UMOH, V.J. 2005. The effect of media, oil type, and rate on the mycelia wet and dry weights of Lentinus squarrosulus (Mont.) singer
and Psathyrella atroumbonata pegler in submerged liquid culture. In African Journal of
Biotechnology. vol. 4, 2005, no. 3, p. 326-331.
OLUTIOLA, P.O, FAMUREWA, O. AND SANNTAG, H.G. 2000. An introduction to General Microbiology. Heidelberger Venargsansault und Duckier Gambit. Germany. p. 46-69.
ONIONS A. H. S., ALLSOPP D AND EGGINS H. O. W 1995. Smith’s introduction to
industrial mycology. 8th ed., The pitman press, bath. p. 15 - 64.
PELCZAR, M.J., CHAN, E.C.S. AND KRIEG, N.R. 1993. Microbiology. 5th edition Tata
McGraw-Hill, New Delhi, India: p. 917.
RUIJTER, B.R. AND VISSER, J. 1997. Carbon repression in Aspergillus niger. In Journal of Biotechnology, vol. 5, 1997, no. 5, p. 472-476.
SEENA. S., SRIDHAR. K. R., ARUN. A. B. AND YOUNG. C. C. 2006. Effect of roasting and pressure-cooking on nutritional and protein quality of seeds of mangrove legume
Canavalia cathartica southwest coast of India. In of Food composition and Analysis, vol. 19,
2006, p. 284 – 293.
STEPHENS, J.M. 1994. Bean Jack (Canavalia ensiformis). Bean, sword (Canavalia gladiata). Institute of food and Agriculture Sciences University of Florida, Gainesville FL 32611. p. 4.
SUTO, M. AND TOMITA, F. 2001. Induction and catabolic repression Mechanisms of cellulose in fungi. In: Nutrient effect on production of cellulolytic enzymes by Aspergillus niger. In African Journal of Biotechnology, vol. 5, 2001, no. 5, p. 472 - 476.
UDEDIBIE. A. B. I. 1990. Nutritional evaluation of Jackbeans (Canavalia ensiformis) for the Nigeria Poutry industry. In Ambio,vol. 19, 1990, no. 8, p. 361 – 365.
VALHIDI, H., KBARFARD, F. AND NAMJOYAN F. 2004. Effect of condition on growth and antifungal activity of Mycena leptocephala. In African Journal of Biotechnology, vol. 3, 2004, no. 11, p. 606-609.
WUBAH, A.D. 1999. An Excerpt on Fungal Nutrition.
http://www.towson.edu~wubahmycology/Nutrition class.htm.