O
r i g i n a la
rt i c l e3 1 7 Arq Bras Oftalmol. 2017;80(5):317-20 http://dx.doi.org/10.5935/0004-2749.20170077
Corneal endothelial cell density and pterygium: a cross-sectional study
Contagem de células endoteliais e pterígio: um estudo transversal
Hugo CoelHo CarvalHo SouSa1, ludmila NaSCimeNto PiNto Silva1, PatriCk FreNSel tzelikiS1,2
Submitted for publication: February 15, 2017 Accepted for publication: May 2, 2017
1 Cornea and External Disease Department, Hospital de Base do Distrito Federal, Brasilia, DF, Brazil. 2 Hospital Oftalmológico de Brasília, Brasília, DF, Brazil.
Funding: No specific financial support was available for this study.
Disclosure of potential conflicts of interest: None of the authors have any potential conflict of interest to disclose.
Corresponding author: Patrick Frensel de Moraes Tzelikis, SQN 203, Bloco K, Apart 502 - Brasília, DF - 70833-110 - Brazil - E-mail: tzelikis@gmail.com
Approved by the following research ethics committee: Hospital Oftalmológico de Brasília (#17/ 2015).
ABSTRACT
Purpose: To investigate the effects of pterygium on corneal endothelial cell density in patients with unilateral pterygium.
Methods: We performed a cross-sectional analysis of data from patients with unilateral pterygium who were selected from September 1, 2015 to July 31, 2016 at Hospital de Base do Distrito Federal to assess the corneal endothelial cell density, coefficient of variation in the cell area, hexagonality, and corneal pa chymetric results. In all patients, noncontact specular microscopy was per-formed in both eyes and a minimum endothelial cell count of 75 cells/mm2 was required for inclusion in the study. The contralateral eye served as the control.
Results: Sixty-one patients were included in the study. Twenty-nine (47.5%) patients were men and 32 (52.5%) were women (mean age, 50.84 ± 13.8). The percentage of pterygium that invaded the cornea ranged from 4.87% to 24.59% (median, 9.70% ± 4.99%). The mean corneal endothelial cell density (cells/mm) was lower in the pterygium eyes than in the controls (2451.83 ± 284.96 vs. 2549.95 ± 268.94, respectively; p=0.04). No differences in the mean coefficients of variation of cell size, hexagonality, and corneal pachymetric results were observed between the patients and controls. The Pearson correlation test showed a significant negative linear relationship between pterygium invasion and endothelial cell density [p<0.001, n=61, r=-0.553 (95% CI, -0.34 to -0.73)].
Conclusion: Compared with the contralateral eyes, those of patients with unilateral pterygium were associated with a decrease in corneal endothelial cell density.
Keywords: Pterygium; Corneal endothelial cell; Endothelium, corneal; Microsco-py/methods
RESUMO
Objetivo: Investigar os efeitos do pterígio na densidade de células endoteliais cor-neanas em pacientes com pterígio unilateral.
Métodos: Foi realizado um estudo do tipo transversal envolvendo pacientes com pterígio unilateral selecionados entre 1 de setembro de 2015 a 31 de julho de 2016 no Hospital de Base do Distrito Federal para avaliar a densidade de células endoteliais corneanas, coeficiente de variação da área celular, hexagonalidade, e paquimetria corneana. Em todos os pacientes foram realizadas microscopias especulares de não-contato em ambos os olhos, sendo necessário obter uma contagem endotelial mínima de 75 células/mm2 para que o paciente fosse incluído no estudo. O olho contralateral funcionou como grupo controle.
Resultados: Um total de 61 pacientes foram incluídos no estudo. Vinte e nove (47,5%) eram homens e 32 (52,5%) mulheres. A média de idade era de 50,84 ±13,8. O percentual de invasão do pterígio na córnea variou entre 4,87% a 24,59%, com uma mediana de 9,70% ± 4,99%. A media de densidade de células endoteliais corneanas foi menor nos olhos com pterígio quando comparados ao grupo controle (2451,83 ± 284,96 vs 2549,95 ± 268,94; p=0,04). Não foram encontradas diferenças entre os casos e controles em relação à média do coeficiente de variação da área celular, hexagonalidade, e paquimetria. Teste de correlação de Pearson mostrou uma relação linear negativa entre a invasão do pterígio e a densidade de células endoteliais corneanas [p<0,001, n=61, r=-0,553 (95% CI -0,34 a -0,73)].
Conclusão: Em pacientes com pterígio unilateral, o olho com pterígio está asso ciado a uma menor densidade de células endoteliais corneanas quando comparado ao olho contralateral.
Descritores: Pterígio; Células endoteliais da córnea; Endotélio corneano; Micros co-pia/métodos
InTRoduCTIon
Pterygium is a wing-shaped ocular surface lesion described as an invasion of the bulbar conjunctiva onto the cornea(1). From the histopathological point of view, pterygium is a hyperplastic, cen tripetally directed growth of modified limbal epithelial cells accompanied by BL dissolution, epithelial mesenchymal transi-tion, and activation of fibroblastic stroma associated with inflam-mation, neovascularization, and matrix remodeling, all of which are mediated through the combined actions of cytokines, growth factors, and matrix metalloproteinases(1,2).
The most often-cited factors suggested to have a role in the pathogenesis of pterygium are genetic factors, proinflammatory cytokines, and ultra-violet (UV) light(1-3). UV irradiation in the cornea has been shown to induce mutations in TP53 tumor suppressor genes in limbal basal cells and to upregulate many cytokines, angio-genic, and fibrogenic growth factors, such as interleukin (IL)-1, IL-6,
IL-8, and tumor necrosis factor-α production(3,4). In addition, the ex-pression of proteases, such as matrix metalloproteinases (MMPs), that degrade basement membrane and BL have been found to be elevated in the leading edges of pterygia(5). These proteases re-leased by pterygium cells facilitate invasion by degrading basement membrane components, and dissolving BL and adjacent stromal matrix(5,6).
Co r n e a le n d o t h e l i a lC e l ld e n s i t ya n dp t e ry g i u m: aC r o s s-s e C t i o n a ls t u d y
3 1 8 Arq Bras Oftalmol. 2017;80(5):317-20
METHodS
STUDY DESIGN
A cross-sectional study was conducted between September 1, 2015 and July 31, 2016 in accordance with the ethical principles of the Declaration of Helsinki and the principles of current Good Clinical Practices. The study protocol was approved by the local Institutional Review Board. All patients provided written informed consent. The study was designed by all the authors. The last author ensured the completeness and accuracy of the data and analyses of the study.
STUDY POPULATION
Patients were enrolled at only one site in Brasilia, DF, Brazil. Eligi-ble patients were ≥18 years of age with unilateral pterygium and a good-quality endothelial cell count image assessed by noncontact specular microscopy. The contralateral eye of each patient served as a control. A comprehensive ophthalmic examination, including best corrected visual acuity, slit-lamp examination, Goldmann applana-tion tonometry, and fundus examinaapplana-tion, was performed for all parti-cipants. The mean corneal power (KM) and corneal astigmatism (AST) in the 3.00-mm central zone were also evaluated for each patient by using a Zeiss Atlas 995 corneal topographer. The exclusion criteria were previous ocular surgery, trauma, uveitis, contact lens use in either eye, central cell count of <1800 cells/mm2, keratitis, glaucoma or intraocular pressure of >21 mmHg, and diabetes mellitus.
Color images of each eye with pterygium were acquired by using a slit-lamp-mounted camera phone (iPhone 5S; Apple, Inc.). The corneal surface area covered by the pterygium was estimated using the measuring tool of AutoCAD® software (version 19.1, Auto-desk Inc., CA, USA) (Figure 1).
Endothelial cell count was measured at the central cornea using a noncontact specular microscope at a resolution of 640 x 480 pixels (ROBO; Konan Storage System KSS 300; Konan Medical, Hyogo, Japan). Three endothelial measurements were obtained for each patient, and the average was taken as the mean. All measurements were made by one person at a single clinical site. To ensure relia-bility in assessing the corneal endothelium, a cell count ≥75 cells measured from the central cornea should be obtained to allow detection of a 2% difference between groups of data(8).
STATISTICAL ANALYSIS
We calculated the sample size from pilot study observations and accounted for a potential dropout rate of 10%. The sample size was calculated from the observed diference and pooled standard dife-rence (SD) from a previous study that assessed human corneal ECD with a sensitivity capable of detecting a 10% reduction(7,8). A power of 80% and conidence level of 95% yielded a sample size of ≥22 per arm. The primary null hypothesis was no between-group diference in the endothelial cell count. The alternative hypothesis was that the endothelial cell count would be lower in the pterygium group than in the contralateral eye group (without pterygium). Given the sample of ≥60 patients, the study had a power of almost 90% to show the primary end point. The normal distribution of each continuous va-riable was assessed by performing the Shapiro-Wilk test. We used Student’s t-test for two independent samples to compare the ECD. Quantitative data were described as the mean ± standard deviation (range). A two-sided p value of <0.05 was considered as indicating statistical signiicance. The Pearson correlation test and regression analysis method were used to study the association between the percentage of pterygium invasion of the cornea and a decrease in the ECD. All data analyses were performed by using the SPSS statistical program (version 17.0; SPSS, Inc, Chicago, Illinois, USA).
RESuLTS
From September 2015 through July 2016, 73 patients were enrol-led in the study. Eleven of these patients were ineligible at baseline (5 had previous ocular surgery, three had contact lens use, two had diabetes mellitus, one had glaucoma, and one had a central cell count of <1800 cells/mm2). After exclusions, a total of 122 eyes of 61 patients were eligible for the study. Twenty-nine (47.5%) were men and 32 (52.5%) were women. The mean age of the patients was 50.84 years (range, 25-77 years; SD, ± 13.8 years). All patients had unilateral pterygium. The percentage of pterygium that invaded the cornea ranged from 4.87% to 24.59%, with a median of 9.70% ± 4.99%.
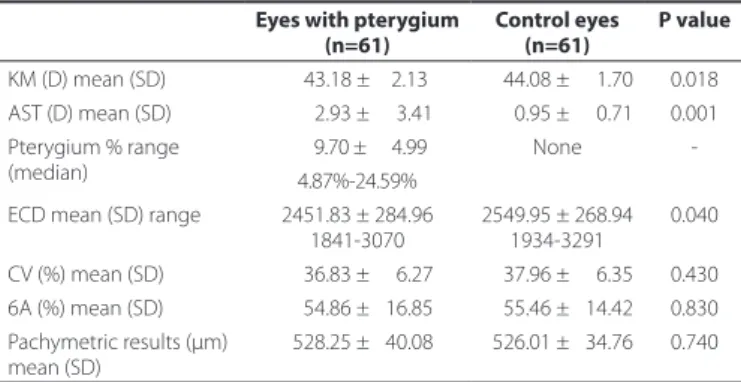
Table 1 compares the outcome measures between the patients and controls. The mean corneal ECD (cells/mm) was lower in the
Figure 1. Photograph showing estimation of the pterygium surface on the cornea. The actual surface area as calculated by software (AutoCAD® software, version 19.1, Autodesk Inc., CA, USA) is shown in blue.
Table 1. Basic demographic data of the 61 patients with unilateral pterygium
Eyes with pterygium Control eyes P value
(n=61) (n=61)
KM (D) mean (SD) 0043.18 ±002.13 0044.08 ± 001.70 0.018 AST (D) mean (SD) 0002.93 ± 003.41 0000.95 ± 000.71 0.001 Pterygium % range
(median)
0009.70 ± 004.99 None -4.87%-24.59%
ECD mean (SD) range 2451.83 ± 284.96 1841-3070
2549.95 ± 268.94 1934-3291
0.040
CV (%) mean (SD) 0036.83 ± 006.27 0037.96 ± 006.35 0.430 6A (%) mean (SD) 0054.86 ± 016.85 0055.46 ± 014.42 0.830 Pachymetric results (μm)
mean (SD)
0528.25 ± 040.08 0526.01 ± 034.76 0.740
So u S a HCC, e t a l.
3 1 9
Arq Bras Oftalmol. 2017;80(5):317-20
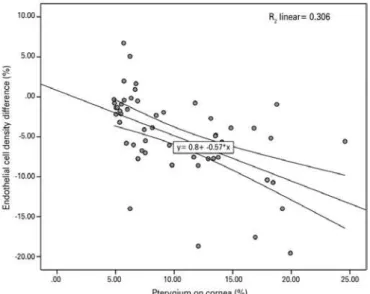
pterygium eyes (cases) than in the controls (2451.83 ± 284.96 vs. 2549.95 ± 268.94; p=0.04). No diferences were observed between cases and controls with regard to the mean CV of cell size, hexagona-lity, and central corneal thickness. The diference in the corneal ECD between eyes with pterygium and control eyes ranged from +6.72 to -19.56, with a median of -5.18%. The Pearson correlation test showed a signiicant negative linear relationship between pterygium invasion and ECD [p<0.001, n=61, r=-0.553 (95% CI, -0.34 to -0.73)] (Figure 2).
dISCuSSIon
This is the second study to evaluate the association between pterygium and the corneal ECD. Our cross-sectional study involving 61 patients demonstrated a signiicant decrease in ECD related to pterygium involvement in the cornea. We also found a negative cor-relation between the corneal ECD and the percentage of pterygium invasion of the cornea.
Despite advances in understanding of its complex pathogene-sis, the origin of pterygia is still not clearly understood. Common his-tological characteristics include an invasive front of the pterygium epithelium that suddenly transitions into corneal epithelium at the advancing edge. At the junction between the normal cornea and pterygium, the stroma has been frequently characterized by feeder blood vessels that preceded the fibroblastic stroma and intravas-cular inflammation(9). Different studies have provided information indicating the presence of inflammatory cells in pterygia. Several cy tokines, such as basic fibroblast growth factor, tumor necrosis factor, platelet-derived growth factor and abundant levels of enzymes, such as MMPs, collagenases, and gelatinases, have also been loca-lized in pterygium cells(9,10).
For that reason, it is possible that all of these chronic inflamma-tory components and angiogenesis act in concert to degrade BL, a characteristic feature of pterygia, and all of the other tissue structu-ral components. Therefore, it is not unreasonable to propose that pterygium invasion may induce even deeper changes in the cornea at the level of the endothelium and Descemet membrane, which could explain the lower corneal ECD in the eyes with pterygium than in the controlateral eye (control group) found in our study.
Evidence that the presence of pterygia is associated with deep corneal changes at the level of the endothelium and Descemet membrane was irst described by Mootha et al.(11). They found deep
corneal marks in long-standing nasal pterygium in seven elderly pa-tients. The authors at that time hypothesized a lower ECD in eyes with pterygium. Recently, in a retrospective comparative study performed by Hsu et al.(7), an analysis of 90 patients with unilateral pterygium revealed a signiicant between-group diference, with a decrease in the corneal ECD in eyes with pterygium involvement. In our study, the corneal ECD was also negatively associated with pterygium invasion. These indings reinforce the suggestion that pterygium invasion exerts possible deep layer changes in the cornea.
Different corneal measurements could have a direct impact on the analysis of ECD values at the central corneal surface(12). The cor-neal parameter that has most commonly been assessed for its efect on ECD is corneal thickness. According to the literature, a lower ECD value would be expected in thinner corneas. In our study, there was no statistically significant difference in pachymetric results between eyes with pterygium and control eyes. Besides corneal thickness, ECD also has been reported to be lower in steeper corneas. In the present study, steeper corneas were observed in the control group than in the pterygium group (44.08 D in the control group vs. 43.18 D in the pterygium group, p=0.018). It could be argued that the effects of these corneal measurements in the current study were not relevant to the ECD observed.
Studies have been conducted to assess corneal astigmatism in-duced by pterygium(13,14). In another cross-sectional study in 2008, Mohammad-Salih et al.(13) studied the relationship between ptery-gium size and corneal astigmatism in eyes with unilateral primary pterygium. As in our study, the authors observed that the mean va-lue of corneal astigmatism was significantly higher in the pterygium group than in the control group (p<0.001). Induced astigmatism is in most cases characterized as “with-the-rule astigmatism” resulting from localized flattening of the cornea caused by the contractile effect exerted by the pterygium.
Our study had some limitations. First, this was a cross-sectional stu dy and there was no follow-up, so we cannot document the long-term endothelial changes that may become evident over the long term. Second, we were able to identify associations but could not determine cause and efect; therefore, we have not provided an ex-planation of our indings. Third, the efect of the pterygium thickness on corneal endothelium was not considered because this type of measure is very diicult to perform.
In summary, this cross-sectional study showed that pterygium was associated with a decrease in corneal ECD. It is important that further longitudinal studies be conducted to conirm this association between these two variables.
REFEREnCES
1. Di Girolamo N, Chui J, Coroneo MT, Wakeield D. Pathogenesis of pterygia: role of cytokines, growth factors, and matrix metalloproteinases. Prog Retin Eye Res. 2004; 23(2):195-228.
2. Chui J, Di Girolamo N, Wakeield D, Coroneo MT. The pathogenesis of pterygium: current concepts and their therapeutic implications. Ocul Surf. 2008;6(1):24-43. 3. Threlfall TJ, English DR. Sun exposure and pterygium of the eye: a dose-response curve.
Am J Ophthalmol. 1999;128(3):280-7.
4. Kennedy M, Kim KH, Harten B, Brown J, Planck S, Meshul C, et al. Ultraviolet irradiation induces the production of multiple cytokines by human corneal cells. Invest Ophthal-mol Vis Sci. 1997;38(12):2483-91.
5. Dushku N, John MK, Schultz GS, Reid TW. Pterygia pathogenesis. Corneal invasion by matrix metalloproteinase expressing altered limbal epitelial basal cells. Arch Oph thalmol. 2001;119(5):695-706.
6. Di Girolamo N, McCluskey P, Lloyd A, Coroneo MT, Wakeield D. Expression of MMPs and TIMPs in human pterygia and cultured pterygium epithelium cells. Invest Oph-thalmol Vis Sci. 2000;41(3):671-9.
7. Hsu MY, Lee HN, Liang CY, Wei LC, Wang CY, Lin KH, et al. Pterygium is related to a decrease in corneal endothelial cell density. Cornea. 2014;33(7):712-5.
8. Doughty MJ, Müller A, Zaman ML. Assessment of the reliability of human corneal endothelial cell-density estimates using a noncontact specular microscope. Cornea. 2000;19(2):148-58.
Co r n e a le n d o t h e l i a lC e l ld e n s i t ya n dp t e ry g i u m: aC r o s s-s e C t i o n a ls t u d y
3 2 0 Arq Bras Oftalmol. 2017;80(5):317-20
9. Chui J, Coroneo MT, Tat LT, Crouch R, Wakeield D, Di Girolamo N. Ophthalmic pterygium. A stem cell disorder with premalignant features. Am J Pathol. 2011;178(2):817-27. 10. Kria I, Amemiya T. Immunohistochemical localization of basic ibroblast growth factor,
platelet derived growth factor, transforming growth factor-B and tumor necrosis factor-a in the pterygium. Acta Histochem. 1996;98(2):195-201.
11. Mootha VV, Pingree M, Jaramillo J. Pterygia with deep corneal changes. Cornea. 2004; 23(6):635-8.
12. Müller A, Craig JP, Grupcheva CN, Mc Ghee. The efects of corneal parameters on the assessment of endotelial cell density in the elderly eye. Br J Ophthalmol. 2004; 88(3):325-30.
13. Mohammad-Salih PA, Sharif AF. Analysis of pterygium size and induced corneal as-tigmatism. Cornea. 2008;27(4):434-8.
14. Han SB, Jeon HS, Kim M, Lee SJ, Yang HK, Hwang JM, et al. Quantiication of astigmatism induced by pterygium using automated image analysis. Cornea. 2016;35(3):370-6.