r e v b r a s r e u m a t o l . 2016;56(5):458–463
ww w . r e u m a t o l o g i a . c o m . b r
REVISTA
BRASILEIRA
DE
REUMATOLOGIA
Case
report
Rituximab
for
the
therapy
of
systemic
sclerosis:
a
series
of
10
cases
in
a
single
center
Verônica
Silva
Vilela
a,∗,
Giselle
Baptista
Maretti
a,
Lívia
Marques
da
Silva
Gama
b,
Claudia
Henrique
da
Costa
b,
Rogério
Lopes
Rufino
b,
Roger
A.
Levy
aaDisciplinadeReumatologia,FaculdadedeCiênciasMédicas,UniversidadedoEstadodoRiodeJaneiro(UERJ),RiodeJaneiro,RJ,Brazil
bDisciplinadePneumologia,FaculdadedeCiênciasMédicas,UniversidadedoEstadodoRiodeJaneiro(UERJ),RIodeJaneiro,RJ,Brazil
a
r
t
i
c
l
e
i
n
f
o
Articlehistory:
Received26August2015 Accepted4April2016 Availableonline22July2016
Keywords: Systemicsclerosis Rituximab Pulmonaryfibrosis ModifiedRodnanskinscore
a
b
s
t
r
a
c
t
Systemicsclerosis(SSc)isachronicautoimmunediseasewithahighmorbidityand mortal-ity.Althoughcyclophosphamideiseffectiveforsevereandrefractorycases,thereisdemand fornewtreatments.ThebiologicaltreatmentwithB-celldepletionwithrituximab(RTX)has demonstratedefficacyforthisdemandinopen-labelstudies.
Objective:Thisstudywasconductedwiththeaimtoretrospectivelyevaluateallpatients whousedRTXforthetreatmentofSScinourcenter.
Patientsandmethods:WeretrospectivelyevaluatedmedicalrecordsofallpatientswithSSc whousedRTXtotreatthisdiseasefromJanuary2009toJanuary2015.Systemic,cutaneous, andpulmonaryinvolvementdataandlaboratoryresultsbeforeandsixmonthsafterthe firstinfusionofRTXwerecollected.
Results:Tenpatientsreceivedtreatmentduringthestudyperiodandwereincludedinthis series.Allpatientshadadiffuseformofthedisease.Fivepatientssufferedfromanearly (durationofdiseaseshorterorequaltofouryears),rapidlyprogressivedisease,andanother fivereceivedRTXatlatestagesofthedisease.Inbothgroupsofpatients,stabilizationofthe pulmonarypicturewasobserved,withafallintheskinscoreinthosepatientswithearly formsofthedisease.
Discussion:Similartofindingsinpreviousstudies,RTXwaseffectiveintreatingearlyand rapidlyprogressiveformsofSSc.Wealsofoundthatpatientswithlong-termillnessmay benefitfromthetreatment.
©2016PublishedbyElsevierEditoraLtda.ThisisanopenaccessarticleundertheCC BY-NC-NDlicense(http://creativecommons.org/licenses/by-nc-nd/4.0/).
∗ Correspondingauthor.
E-mail:veronicavilelavs@yahoo.com.br(V.S.Vilela).
http://dx.doi.org/10.1016/j.rbre.2016.06.003
Tratamento
da
esclerose
sistêmica
com
rituximabe:
uma
série
de
10
casos
em
centro
único
Palavras-chave: Esclerosesistêmica Rituximabe Fibrosepulmonar
Escorecutâneomodificado deRodnan
r
e
s
u
m
o
A esclerosesistêmica (ES)é umadoenc¸aautoimune crônica dealtamorbimortalidade. Aindaqueaciclofosfamidasejaeficaz,paracasosgraveserefratárioshádemandapara novostratamentos.Aterapiabiológicacomdeplec¸ãodecélulasBcomrituximabe(RTX) demonstroueficáciaparataldemandaemestudosabertos.
Objetivo: AvaliarretrospectivamentetodosospacientesquefizeramusodoRTXpara trata-mentodeESemnossocentro.
Pacientesemétodos: Foramavaliadosretrospectivamentetodososprontuáriosdepacientes comESquefizeramusodeRTXparatratamentodaESdejaneirode2009ajaneirode2015. Dadosdeacometimentosistêmico,cutâneo,pulmonarelaboratoriaisanteseseismeses apósaprimeirainfusãodeRTXforamcoletados.
Resultados: Dezpacientesreceberamotratamentonoperíododeestudoeforamincluídos napresentesériedecasos.Todosospacientestinhamaformadifusadadoenc¸a.Cinco pacientestinhamformasiniciais(tempodedoenc¸amenorouigualaquatroanos)e rapida-menteprogressivadadoenc¸aecincoreceberamoRTXemfasestardiasdadoenc¸a.Houve estabilizac¸ãodoquadropulmonaremambososgruposdepacientesereduc¸ãonoescore cutâneonospacientescomformasiniciaisdadoenc¸a.
Discussão: Similaraoencontradoemestudosprévios,oRTXfoieficaznotratamentode for-masiniciaiserapidamenteprogressivasdaES.Verificamostambémbenefícioempacientes comlongadurac¸ãodadoenc¸a.
©2016PublicadoporElsevierEditoraLtda.Este ´eumartigoOpenAccesssobuma licenc¸aCCBY-NC-ND(http://creativecommons.org/licenses/by-nc-nd/4.0/).
Introduction
Systemicsclerosis (SSc) isachronic systemicautoimmune disease characterized by fibrosis,generalized vasculopathy andautoimmunity.Thevasculopathyischaracterizedby Ray-naud’sphenomenon and pulmonaryarterial hypertension. Fibrosisoccursmainlyintheskin,causingsclerodactylyand skinthickening;inthelungs,SSCproducesaprogressiveand irreversibleinterstitiallungdisease.1,2
Pulmonaryfibrosisremainstheleadingcauseofdeathin SSc.Notwithstanding thattheconventional treatmentwith cyclophosphamidehasbeenshowntobeeffective,therewere no decreases in5- and 10-year mortality and moresevere formsofthedisease maynotrespondtothis treatment.3–5
Itwas alsoreported animprovement inskin involvement, assessedbythemodifiedRodnanskinscore(mRSS);however, thereisatendencyforrecurrenceoftheconditionwhenthe therapyisdiscontinued.5Cyclophosphamideisahighly-toxic
drugandshallnotbeusedaboveacumulativedoseof18g. Thus,newtherapiesareinorderforseriousformsofSSc, espe-ciallyinpatientswith extensivecutaneousand pulmonary involvement.
Biological therapy has revolutionized the treatment of other autoimmune diseases such as rheumatoid arthritis and,morerecently,thistherapeuticstrategyhasbeenproven effectiveinpatientswithsystemiclupuserythematosus.In rheumatoidarthritis, the biologicaltherapy, including anti-TNF agents, depletion ofB-cells and anti-IL-6 agents, was effectiveincontrollingdiseaseactivityandindecreasingthe radiographicprogression.6 In patientswithGranulomatosis
withPolyangiitis,thetherapywithB-celldepletionwiththe useofrituximab(RTX)waseffectiveininducingdisease remis-sion,withfewersideeffectsversusconventionaltherapywith cyclophosphamide.7 Among immunobiological agents, RTX
hasbeen the mostwidely useddruginother autoimmune diseases,inadditiontorheumatoidarthritis.
ThepossibilityoftheuseofRTXinthetreatmentofsevere formsofSScwasconsideredafterevidencethatBlymphocytes performapathogenicroleinthisdisease.ActiveB lympho-cyteswereisolatedfromlungandskinbiopsiesfrompatients withSSc.8,9Lafyatisetal.demonstratedareductionin
cuta-neousBcellinfiltratesinSScpatientstreatedwithRTX.10
In the face of biological evidence of effectiveness and considering the need for a systemic therapy superior to cyclophosphamide,theuseofRTXwastestedinopen-label seriesofpatientswithsevereandearlyformsofSSc.Smith etal.prospectivelystudiedeightpatientswithlessthantwo years duration ofthe diffuseformofSSctreated withtwo infusionsof1gofRTX.11,12Therewasareductioninthe
mod-ifiedRodnanskinscoreandindiseaseactivityscores,andno progressionofdiffuseinterstitialpneumonitiswasobserved. Boselloetal.studiedtwoopen-labelseriesofpatientswith a diffuse disease with less than three years duration and achieved similar results.13,14 InGiuggioli et al. seriesof 10
cases,theseauthorsachievedasignificantreductionofmRSS inmostpatients.15Inaproof-of-conceptstudy,RTXwas
supe-rior to conventional treatment in eight patients.16 In the
Scleroderma Trials and Research (EUSTAR)database, ritux-imabwassuperiortoconventionaltreatment.17Currently,an
460
rev bras reumatol.2016;56(5):458–463Basedonthisevidence,RTXseemstobeanalternativefor thetreatmentofsevereformsofSSc.Itislikelythatdatafrom theRECOVERstudywillbeusefulforfutureguidanceonthis therapy.Thisstudywasconductedinordertoretrospectively evaluateaseriesofpatientswithdiffuseformsandwithsevere cutaneousand/ororganinvolvementtreatedwithRTXinour centersince2009to-date.PatientswhoreceivedRTXwith indi-cationduetotheseverity oftheimpairment,regardlessof diseaseduration,werestudied.
Patients
and
methods
AllmedicalrecordsofSScpatientswhohadbeentreatedwith RTXwithitsfirstinfusionfromJanuary2009toJanuary2015 were retrospectively reviewed. In this study, patients were includedirrespectiveofindicationofRTXandoftheformof thedisease.Patientswithotherconcomitantsystemic autoim-mune diseaseswere excluded. In ourseries, the outcomes evaluatedweresafety andefficacy ofRTXinthetreatment ofSSc.Thetreatmentprotocolwasasfollows:RTX1gIV fol-lowedbyasecondinfusionafter15days.Dataobtainedbefore andsixmonthsaftertheinfusionofRTXwerereviewed.
Demographic, clinical, laboratory and immunological (includingpositivity,titleandpatternofantinuclear antibod-ies [ANA]) data were collected, as well as the presence of specificantibodiesagainstSSc.
ThereasonforanindicationofRTX,prioruseof immuno-suppressants, and cumulative dose of cyclophosphamide wereevaluated.Laboratorydata(erythrocytesedimentation rate–ESR),andskinactivityandpulmonaryfunctionfindings wererecordedbeforeandsixmonthsaftertheinfusionofRTX. AsaroutineoftheInfusionCenterintheRheumatology outpa-tientclinic,patientsareobservedfordrugsafetyparameters. Wecheckedtheoccurrenceofanyinfusionreaction(allergic, orofanyothertype,duringinfusion)and/orinfectionofany origin.
Regarding theefficacy ofthedrug, the parameters eval-uated were mRSS and pulmonary function assessed by pulmonaryfunctiontests(PFTs)beforeandaftertheinfusion ofRTX.Cutaneousactivity was assessedusing mRSSin17 bodyareas;the testwas performedbeforeand sixmonths afterRTX, always bythe same female examiner. Although mRSS has been performed in a non-blind way, the eval-uation was carried out with an interval of 6 months and theexaminerdidnothaveaccess tothe previousresult. A goodintra-examinerreproducibilityofmRSS,whenapplied byanexperiencedexaminer,wasdemonstrated;however,the inter-examinerreproducibilityhasnotbeenproven.19Inthis
context,we consider mRSSas a possiblyreliable test only whenappliedbyanexperiencedexaminer.Pulmonary activ-itywasevaluatedbeforeandaftertheinfusionofRTXthrough PFTs andhigh-resolutioncomputed tomography(HRCT).In PFTs,forcedvitalcapacity (FVC)and carbonmonoxide dif-fusingcapacity(DLCO)were evaluated beforeand afterthe
infusionofRTX.HRCTreportswerereviewedbeforeandsix monthsafteradministrationofRTXwiththeuseofa qual-itativescore. Thefollowing ratingscores were assigned:1. Normal;2. Thepresenceof aground glass pattern;3. The presenceofagroundglasspatternandbronchiectasis;4.The
presenceofagroundglasspattern,bronchiectasisand bron-chiolectasis; 5.Thepresenceofconsolidationareas;6.The presenceofhoneycombingareas,orofpulmonaryfibrosis.20
Thestabilizationoflungfunction,inaccordancewithother studieswhichevaluatedthetreatmentofpulmonaryfibrosis associatedwithSSc,wasconsideredasasatisfactoryresponse totreatment.5,17 TheprotocolwasapprovedbytheResearch
EthicsCommitteeofourhospital.
Statisticalanalysis
Fortheanalysisofcontinuousvariables(withnormal distri-bution)ofFVCandmRSSvalues,theStudentttestwasused. Weconsideredasstatisticallysignificantpvalues<0.05.
Results
Tenpatients,allofthemwithdiffusedisease(ninefemaleand onemale)receivedRTXforthetreatmentofsevere manifesta-tionsofSScduringthestudyperiod.Table1summarizesthe clinicalandimmunologicalprofile,theindicationofRTXand laboratory,skinandlungactivitydatabeforeandsixmonths after the treatment with RTX. The mean age was 38.3±
12years;diseaseduration6.6±4.3years,andmean cumula-tivedoseofcyclophosphamide12.9±5.2g.Allpatientswere in concomitant use ofan immunosuppressiveagent; eight patientswerebeingmedicatedwithazathioprine2mg/kg/day andtwousedmycophenolatemofetil2g/day.Nopatientwas beingtreatedwithcorticosteroids.Regardingthesafety end-point,nopatienthadanadverseeventwithin6monthsafter theuseofRTX.
r
e
v
b
r
a
s
r
e
u
m
a
t
o
l
.
2
0
1
6;
5
6(5)
:458–463
461
Patientnumber Disease
duration
RTXindication Cumulative
doseofCTX
ESRbeforeand afterRTX
FVC%before andafterRTX
DLCO%before
andafterRTX
HRCTbefore andafterRTX
mRSSbefore andafterRTX
ANAandantibodies
1.1.F,39years 3years Pulmonarydisease
refractorytoCTX
12g 65/25 71/70 NA 4/4 21/14 Nucleolar1:640+aScl70
2.2.F,39years 3years Pulmonaryandskindisease
andrefractorytoMMF
0g Previous useof MMF
16/20 85/84 NA 4/4 14/4 Nucleolar1:320+aScl70
3.3.F,23years,brown 4years Pulmonaryandcutaneous
diseaserefractorytoCTX
12g 15/5 90/90 NA 4/4 18/14 Nucleolar1:640+aScl70
4.4.F,56years, Caucasian
4years Pulmonaryandcutaneous
diseaserefractorytoCTX
12g 5/5 58/61 60/60 6/6 51/28 Nucleolar1:160
5.5.F,50years, Caucasian
3years Worseningof10pointsin mRSSaftermaximumdose ofCTX
12g 5/5 77/77 NA 3/3 18/16 1:160finelystippled
6.6.F,38years,AA 13years Pulmonaryfunction
worsening
12g 45/10 40/40 6/NA 6/6 18/6 Nucleolar1:320+aScl-70
7.7.F,62years, Caucasian
10years Pulmonaryfunction
worsening
12g 30/5 37/39 28/30 6/6 14/6 Nucleolar1:320+aScl-70
8.8.M,51years, Caucasian
13years Pulmonaryfunction
worsening
18g 10/10 71/71 37/40 6/6 7/4 Nucleolar1:160
9.9.F,34years,brown 5years Pulmonaryfunction
worsening
12g 15/10 66/65 NA 6/6 10/4 Nucleolar1:160+aScl-70
10.10.F,37years, Caucasian11.
6years Significantcutaneous
worsening
12g 47/20 52/56 66/66 4/4 40/32 Nucleolar1:640+aScl-70
462
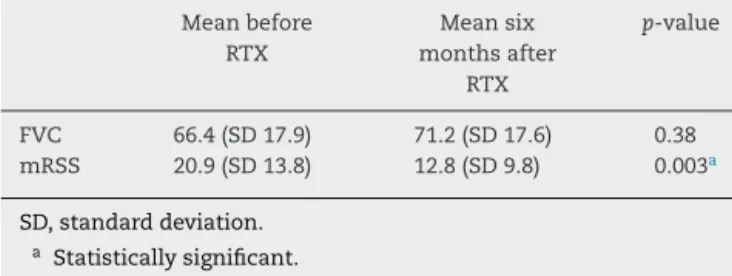
rev bras reumatol.2016;56(5):458–463Table2–FVC(forcedvitalcapacity)andmRSS(modified
Rodnanskinscore)valuesbeforeandsixmonthsafter
therituximabinfusion.
Meanbefore RTX
Meansix monthsafter
RTX
p-value
FVC 66.4(SD17.9) 71.2(SD17.6) 0.38
mRSS 20.9(SD13.8) 12.8(SD9.8) 0.003a
SD,standarddeviation. a Statisticallysignificant.
hermyopathy,witharecoveryofstrengthandnormalization ofmuscleenzymes.Allpatientsshowedstabilizationoftheir lungdiseasesixmonthsafterRTXinfusion,undertheprotocol describedinthestudy(Table1).
Astopatientsinthesecondgroup(patients6–10),RTXwas indicatedduetoasharpworseningoftheirpulmonaryorskin condition, aftera lapseofmorethan four years ofillness. ThesepatientshadanimmunologicalprofileofANAwitha nucleolarpattern,andalsowiththepresenceofanti-Scl-70in fourpatientswho,despitethis,showedaprotractedevolution ofthedisease,seeminglywithapredominantly irreversible fibrosis(HRCTscore=6inpatients6–9).ThevaluesofFVC% andDLCO%weresharplyreducedinpatients6,7,9and10.The
patient8showedahistoryofquickdecreaseinFVC%(from80 to71%)inthelastyear,evenwhenhewastaking mycopheno-latemofetil,andwithacumulativedoseofcyclophosphamide of18g.mRSSwasabove14inonlytwopatients,indicatingalso aloweractivityofskindisease.Butthesepatientsresponded welltotreatment,withimprovementinmRSSandreportsof improvedexerciseability.Therewasnoincreaseinthevalues ofFVC%andDLCO%,but allpatientsreportedimprovement
indyspnea;andonefemalepatientdiscontinuedtheuseof supplementaloxygen.
Considering the whole group of patients, a statistically significantimprovementinmRSSwasnoted.Therewasno significantimprovementinFVC, buta stabilizationoflung functiondidoccur.Table2summarizesFVCandmRSSvalues beforeandaftertreatmentinall10patients.
Discussion
Ourresultssuggest that, inagreementwithprevious stud-ies,RTXisasafeandeffectivetherapyforthetreatmentof severeformsofSSc.WefoundnoimprovementinFVC,DLCOor
HRCT;however,thestabilizationofthepatients’lungdisease didoccur.Asignificant improvementwasobserved inskin condition,accordingtotheassessmentbymRSS.Considering thatallpatients hadbeen medicatedwithhigh cumulative doses ofcyclophosphamide,RTXwas analternative tothe continuationoftreatment.Therewerenoinfusionreactions orinfectionswithin6monthsafterthefirstinfusion.Inthis context,thesafetyoftreatmentwithRTXinpatientswithSSc isoneofthemainresultsofourstudy.
Thefirstgroupofpatientsinourstudyhasaprofilesimilar topatientsinthestudiesofSmithetal.,Boselloetal.,Daoussis etal.,andGiuggiolietal.11–15Theseinitialstudiesevaluated
seriesconsistingof10–20patientsinanopen-labelstrategy.
Thesewerepatientswithshort-termdiseaseandwithrapidly progressiveforms,characterizedbyanextensiveskin involve-ment(mRSS>14),withanincipientpulmonarydisease,and refractorytoconventionaltreatmentwithcyclophosphamide. Smith et al. also reported a sustained safety and efficacy aftertwoyearsoffollow-up.12Also,accordingtothe
litera-ture, ourgroupofpatientswiththis profileshowed agood responsetotreatmentwithRTX,withasignificantdecrease of mRSS in most patients and with stabilization of lung functioninallfemalepatients.Itispossiblethatinthis pop-ulation ofpatients withearly formsofthe disease,RTXis beneficial inthe longterminaroleofadiseasemodifying agent.
Giuggiolietal.reportedimprovementinother manifesta-tionsofSSc,suchasleucomelanodermaandcalcinosis.15We
alsofoundthatoneofourfemalepatientsshowed improve-mentinherleucomelanodermaandanotherpatientimproved her myopathy. This result, although described in isolated cases,isnoteworthysincethesemanifestationsaretypically refractorytoalltherapeuticarsenal.Whilenotconstituting alife-threateningorseriousorgandamagerisk,these man-ifestationsgenerate ahugeestheticandfunctional impact, affectingthequalityoflifeofpatients.
In a review, McQueen and Solanki commented that althoughthepackageinsertofRTXdoesnotindicateitsuse inthetreatmentofSSc,theresultsofopen-labelstudiesare encouraging.21 Theauthors arguethat, giventhisevidence,
it may not be appropriate to wait for the results of con-trolledstudiestoindicatethetreatmentwithRTXinpatients exhibiting amore severeprofile.In ourstudy, weincluded patientswithamoreseverediseaseandinahigh-mortality risk.Accordingtothereview,wespeculatedthatthemortality inourpatientswouldbereducedwiththeintroductionofRTX, in anticipation ofthe publication ofprospectivecontrolled studies.
MorerecentlytheresultsoftheEUSTARdatabase,which retrospectivelyassessedSScpatientstreatedwithrituximab, have been published.17 A greater number of patients (63
cyclophosphamide.Thisoutcome,coupledwiththeevidence fromtheEUSTARdatabaseandfromtheDaoussis’ proof-of-conceptstudy,suggeststhattheimprovementshouldnotbe attributedto the naturalhistory ofthe disease,but tothe pharmacologicaltreatment.Thisquestionshallbeanswered with the results of the RECOVER trial, that prospectively includesagroupoftreatmentwithrituximabandacontrol group.18
However,ourstudyhasitslimitations.Ithasaretrospective design,withthepossibilityofdataloss.Thereisalsothelackof acontrolgroup,andwecouldnotguaranteethatthe improve-mentwouldhaveoccurred,eveninuntreatedpatients.Itwas notpossibletoperformavolumetricquantitativeassessment ofHRCT changes.Thefollow-up periodwas alsorelatively short;thus,itwasnotpossibletoassesswhethertherewould bebetterresultsinalongerterm.
FurtherstudieswiththeinhibitionofBcellsasatherapyfor SScatearlierstagesofthediseaseandwithpreciseindications arenecessaryandcanchangethecourseofthisdisease,with itshighmorbidityandmortality.
Conflicts
of
interest
Theauthorsdeclarenoconflictsofinterest.
r
e
f
e
r
e
n
c
e
s
1. vandenHogenF,KhannaD,FransenJ,JohnsonSR,BaronM, TyndallA,etal.2013Classificationcriteriaforsystemic sclerosis.ArthritisRheum.2013;65,2737047.
2. GabrielliA,AvvedimentoEV,KriegT.Mechanismsofdisease: escleroderma.NEnglJMed.2009;360:1989–2003.
3. ElhaiM,MeuneC,AvouacJ,KahanA,AllanoreY.Trendsin mortalityinpatientswithsystemicsclerosisover40years:a systematicreviewandmeta-analysisofcohortstudies. Rheumatology.2012;51:1017–26.
4. TashkinD,ElashoffR,ClemmentsPJ,GoldinJ,RothMD,Furst DE.Cyclophosphamideversusplaceboinsclerodermalung disease.NEnglJMed.2006;354:2655–66.
5. BérezneD,ValeyreD,RanqueB,GuillevinL,MouthonL. Interstitiallungdiseaseassociatedwithsystemicsclerosis. Whatistheevidenceforefficacyofcyclophosphamide?Ann NYAcadSci.2007;1110:271–84.
6. EmeryP,FleischmannR,Filipowicz-SosnowskaA,
SchechtmannJ,SzczepanskiL,RacewiczAJ,etal.Theefficacy andsafetyofrituximabinpatientswithactiverheumatoid arthritisdespitemetothrexatetherapy:resultsofaphaseIIB, randomized,double-blind,placebo-controlleddoseranging study.ArhtritisRheum.2006;54:1390–400.
7.StoneJH,MerkelPA,SpieraR,SeoP,LangfordCA,Hoffman GS,etal.RituximabversuscyclophosphamideforANCA associatedvasculitis.NEnglJMed.2010;363:221–32.
8.LafyatisR,O’HaraC,Feghali-BostwickCA,MattesonEB.Bcell infiltrationissystemicsclerosis-associatedinterstitiallung disease.ArthritisRheum.2007;56:3167–8.
9.FleischmajerR,PerlishJS,WestWP.Ultrastructureof cutaneouscellularinfiltratesinscleroderma.ArchDermatol. 1977;113:1661–6.
10.LafyatisR,KissinE,YorkM,FarinaG,VigerK,FritzlerMJ,etal. Bcelldepletionwithrituximabinpatientswithdiffuse cutaneoussystemicsclerosis.ArthritisRheum. 2009;60:578–83.
11.SmithVP,VanPraetJT,VandoorenBR,VanderCruyssenB, NaeyartJM,DecumanS,etal.Rituximabindiffusecutaneous systemicsclerosis:anopen-labelclinicalandhistopathologic study.AnnRheumDis.2010;69:193–7.
12.SmithVP,PietteY,VanPraetJT,DecumanS,DeschepperE, ElewautD,etal.Twoyearsresultsofanopenpilotstudyofa 2-treatmentcoursewithrituximabinpatientswithearly systemicsclerosiswithdiffusecutaneousinvolvement.Ann RheumDis.2013;40:52–7.
13.BoselloS,DeSantisM,LamaG,SpanoC,AngelucciC,Tolusso B,etal.Bcelldepletionindiffuseprogressivesystemic sclerosis:safety,skinsocremodificationandIL-6modulation inanuptothirty-sixmonthsfollow-upopenlabeltrial. ArthritisResTher.2010;12:1–10.R54.
14.BoselloS,DeLucaG,RuccoM,BerardiG,FalcioneM,Danza FM,etal.Long-termefficacyofBcelldepletiontherapyon lungandskininvolvementindiffusesystemicsclerosis. SeminRheumArhtritis.2015;44:428–36.
15.GiuggioliD,LummetiF,ColaciM,FallahiP,AntoneliA,FerriC, etal.Rituximabinthetreatmentofpatientswithsystemic sclerosis.Ourexperienceandreviewoftheliterature. AutoimmunRev.2015;14:1072–8.
16.DaoussisD,LiossisSN,TsamandasAC,KalogeropoulouC, KazantziA,SirinianC,etal.Experiencewithrituximabin scleroderma:resultsfroma1-year,proof-of-principlestudy. Rheumatology.2010;49:271–80.
17.JordanS,DistlerJHW,MauerB,HoscherD,VanLaarJM, AllanoreY,etal.Effectsandsafetyofrituximabinsystemic sclerosis:anannalsfromtheEuropeanSclerodermaTrialand Research(EUSTAR)group.AnnRheumDis.2015;74:1188–94.
18.AvouacJ.Rituximabinsystemicsclerosis.Availablefrom:
www.clinicaltrials.gov,ClinicalTrialsNCT01748084. 19.ClementsP,LachenbruchPA,SeiboldJR,WhiteB,WeinerR,
MartinR,etal.Interandintraobserveroftotalskinscore (modifiedRodnanTSS)insystemicsclerosis.JRheum. 1995;22:1281–5.
20.IchikadoK,SugaM,SakamotoN,HaraS,KakugawaT, TsubamotoM,etal.Predictionofprognosisforacute respiratorydistresssyndromewiththinsectionCT: validationin44cases.Radiology.2006;238:321–9.