r e v b r a s r e u m a t o l . 2015;55(2):146–158
w w w . r e u m a t o l o g i a . c o m . b r
REVISTA
BRASILEIRA
DE
REUMATOLOGIA
Review
article
Infliximab,
methotrexate
and
their
combination
for
the
treatment
of
rheumatoid
arthritis:
a
systematic
review
and
meta-analysis
夽
Juliana
de
Oliveira
Costa
a,∗,
Lívia
Lovato
Pires
de
Lemos
b,
Marina
Amaral
de
Ávila
Machado
a,
Alessandra
Maciel
Almeida
c,
Adriana
Maria
Kakehasi
d,
Vânia
de
Eloísa
Araújo
e,
Mariângela
Leal
Cherchiglia
f,
Eli
Iola
Gurgel
Andrade
f,
Francisco
de
Assis
Acurcio
caPost-GraduatePrograminPublicHealth,SchoolofMedicine,UniversidadeFederaldeMinasGerais,BeloHorizonte,MG,Brazil
bPost-GraduatePrograminMedicationsandPharmaceuticalAssistance,SchoolofPharmacy,UniversidadeFederaldeMinasGerais,
BeloHorizonte,MG,Brazil
cDepartmentofSocialPharmacy,SchoolofPharmacy,FaculdadedeFarmácia,UniversidadeFederaldeBeloHorizonte,BeloHorizonte,
MG,Brazila
dDepartmentofLocomotorSystem,SchoolofMedicine,UniversidadeFederaldeMinasGerais,BeloHorizonte,MG,Brazil
eEvidence-BasedMedicine/PGMIT,UniversidadeFederaldeSãoPaulo,SãoPaulo,SP,Brazil
fDepartmentofPreventiveandSocialMedicine,SchoolofMedicine,UniversidadeFederaldeMinasGerais,BeloHorizonte,MG,Brazil
a
r
t
i
c
l
e
i
n
f
o
Articlehistory:
Received23October2013 Accepted6October2014 Availableonline7January2015
Keywords:
Infliximab Systematicreview Meta-analysis Rheumatoidarthritis Efficacy
a
b
s
t
r
a
c
t
We performed a systematic review to evaluate the efficacy and safety of inflix-imab+methotrexate(IFX+MTX)regimensversusMTXaloneorincombinationwithother disease-modifyinganti-rheumaticdrugs(DMARDs).Wesearchedthroughmajordatabases, thegreyliteratureanddidamanualsearch.Twoindependentreviewersconductedthe selec-tion,dataextractionandanalysisofthequalityofthestudies.Meta-analysiswasconducted usingReviewManager®5.1software.Ninetrialswereincluded.ThemeanmodifiedJadad
scorewas4.4,butonlyonestudyshowedlowriskofbias.IFX+MTXregimenpresented betterresponsesinclinicaloutcomesofACRandDAS28byupto54weeks,andof radio-graphicprogressionbyupto104weeks.Withdrawalsduetolackofefficacywaslowerinthe IFX+MTXgroup.Nosignificantdifferenceinadverseeventswasobserved.TheIFX+MTX combinationismoreeffectivethantreatmentwithMTXaloneorDMARDscombination.This regimenpresentedgoodtolerabilityinpatientspreviouslytreatedwithDMARDs,nottreated withMTXorwithinsufficientresponsestoMTX.TheefficacyofIFX+MTXisnotedprimarily duringinitialperiodsoftreatment.HighdosesofIFXwereaseffectiveasthestandarddose, butwithpossiblehigherriskofseriousinfections.Therefore,weadviseclinicianstousethe standarddoseofIFX3mg/kgevery8weeks.
©2014ElsevierEditoraLtda.Allrightsreserved.
夽
Institution:SchoolofMedicine,UFMG,BeloHorizonte,MG,Brazil.
∗ Correspondingauthor.
http://dx.doi.org/10.1016/j.rbre.2014.10.009
rev bras reumatol.2015;55(2):146–158
147
Infliximabe,
metotrexato
e
sua
combinac¸ão
no
tratamento
da
artrite
reumatoide:
revisão
sistemática
e
metanálise
Palavras-chave:
Infliximabe Revisãosistemática Metanálise Artritereumatoide Eficácia
r
e
s
u
m
o
Foifeitaumarevisãosistemáticaparaavaliaraeficáciaeaseguranc¸adoesquema inflix-imabe+metotrexato(IFX+MTX)versusMTXisoladamenteouemcombinac¸ãocomoutros medicamentosmodificadoresdocurso dadoenc¸a(MMCD).Pesquisou-senasprincipais bases de dadoseletrônicas e naliteratura cinzenta e fez-seuma busca manual. Dois revisoresindependentesfizeramaselec¸ão,extrac¸ãodedadoseanálisedaqualidadedos estudos.AmetanálisefoifeitacomosoftwareReviewManager®5.1.Incluíram-senove estudos.OescoremédionaescaladeJadadmodificadafoide4,4,massomenteumestudo mostroubaixoriscodeviés.OesquemaIFX+MTXapresentoumelhoresrespostasnos desfechosclínicosdoescoreACRedoDAS28poraté54semanasenaprogressão radio-gráficaporaté104semanas.Osabandonosdecorrentesdafaltadeeficáciaforammenores nogrupoIFX+MTX.Nãofoiobservadadiferenc¸aestatisticamentesignificantenoseventos adversos.Acombinac¸ãoIFX+MTXémaiseficazdoqueotratamentocomMTXisolado ouemcombinac¸ãocomMMCD.Esseesquemaapresentouboatolerabilidadeempacientes previamentetratadoscomMMCD,nãotratadoscomMTXoucomrespostasinsuficientes aoMTX.AeficáciadoregimeIFX+MTXéobservadaprincipalmenteduranteosperíodos iniciaisdotratamento.AltasdosesdeIFXforamtãoeficazesquantoadosepadrão,mas comapossibilidadede“um”maiorriscodeinfecc¸õesgraves.Recomenda-se,portanto,que osmédicosutilizemadosepadrãodeIFXde3mg/kgacadaoitosemanas.
©2014ElsevierEditoraLtda.Todososdireitosreservados.
Introduction
Rheumatoidarthritis(RA)isanautoimmunedisease charac-terisedbyperipheral,symmetricpolyarthritiswithpotential forjointdeformitythatcancausefunctionaldisability, prema-turemortalityandreducedqualityoflife.Itisestimatedthat 0.3–1.0%ofthepopulationworldwideisaffectedbyRA,which ismostfrequentlyobservedindevelopingcountriesand in women.1
ThetreatmentofRApatientscombineseducational, pre-ventiveandnon-pharmacologicalinterventionswith pharma-cologicaltreatmentandsurgicalprocedures.First-linetherapy includes the early use of a synthetic disease-modifying anti-rheumaticdrug(DMARD),suchasmethotrexate(MTX), which is the drug of choice.2 However, only 20–40% MTX
monotherapy-treated patients show a satisfactory clinical response.3 Drug combinations are avalid strategy in
non-responsive patients, which may include the addition of anothersyntheticDMARDor thebiologicalDMARDagents, suchastumournecrosisfactor␣blockers(anti-TNF␣).4
Inflix-imab(IFX)isachimericmonoclonalantibody(murine)ofthe anti-TNF␣classthatrepresentsapproximately40%of
biolog-icalagentprescriptions.5,6
Second-linetreatmentstrategiesshowsimilarratesof suc-cessandthechoiceamongthem isbasedprimarilyonthe presence or absence of a poor prognosis and in the dis-easeactivity.4,7Thebenefitsofaddingsulfasalazine(SSZ)and
hydroxychloroquine(HCQ),8–11leflunomide12orcyclosporin13
to MTX therapy have been demonstrated. The IFX+MTX
combination has been assessed in numerous systematic
reviews14–19 but theircontrolgroups includedonlyplacebo
or MTX treatment. Key issues, including the effect of dis-easeduration,doseandpatientprofile,werenotsufficiently addressedinmostofthesereviews.
Withthissystematicreviewandmeta-analysisweaimedto assesstheefficacyandsafetyofIFX+MTXcomparedtoMTXin monotherapyorincombinationwithothersyntheticDMARDs consideringtreatment-relevantclinicaloutcomes.
Methods
A systematic review with meta-analysis was performed
accordingtotheCochraneHandbookforSystematicReviews ofInterventions.Theresultswerereportedaccordingtothe “PreferredReportingItemsforSystematicReviewsandMeta Analyses: ThePRISMA statement”.20 Thisreviewis partof
anotherprojectentitled“Evaluationoftheeffectivenessand safetyofbiologicalagentsadalimumab,etanercept,infliximab andrituximabusedinthetreatmentofrheumatoidarthritis, psoriaticarthritisandankylosingspondylitis,BrazilandMinas Gerais”,whichwasperformedbytheResearchGroupon Phar-macoepidemiologyandResearchGrouponHealthEconomics attheUFMG.
Searchstrategy
148
rev bras reumatol.2015;55(2):146–158referencesofall includedstudies and previouslypublished systematicreviews.Wealsosearchedthegreyliteratureinthe AnnalsoftheAmericanCollegeofRheumatology(ACR,2011, 2012), the European League Against Rheumatism (EULAR, 2010–2012)meetingsandthethesisanddissertationdatabases oftheCoordinationfortheImprovementofHigherEducation Personnel,Brazilian DigitalLibraryofTheses and Disserta-tions,theDigitalLibraryofThesesandDissertationsofUSP andtheProQuestDissertationandThesesDatabase.Ongoing RandomisedClinicalTrials(RCT)weresurveyedinthe Interna-tionalClinicalTrialsRegistryPlatformSearchPortal,Brazilian RegistryofClinicalTrialsandatclinicaltrials.gov.
Eligibilitycriteria
WeincludedphaseIIIRCTsthatevaluatedRApatients diag-nosed accordingto theAmerican CollegeofRheumatology (ACR)criteria198721regardlessofdiseaseduration.We
con-sidered eligible studies comparing IFX+MTX versus MTX as a monotherapy or in combination with other synthetic DMARDs.Theminimalfollow-upperiodwastwomonths.
Thefollowingexclusioncriteriawereapplied:studiesthat werenotperformedexclusivelyonRApatients;changes in therapyovertime;drug-conversionstudies;pilotstudies; edi-torials/reviews/letters/comments; and studies published in languagesotherthanPortuguese,SpanishorEnglish.
Studyselection
Two reviewersindependentlyevaluated thetitles,abstracts andfulltextofallidentifiedstudiestoassesstheireligibility. Athirdreviewerresolveddisagreements.
Assessmentofmethodologicalqualityandriskofbias
MethodologicalqualitywasassessedusingthemodifiedJadad scale,22whichevaluatesrandomisation,blindingandlossto
follow-upusingsevendichotomousquestionsthatareworth onepointeach.Studieswithinadequaterandomisationlose onepoint.Thefinalscorerangesfrom0to6:0–2indicatea low-qualitystudy,3or4indicatesadequatequality,and5or 6indicateshighquality.Theriskofbiaswasassessedusing theCochraneCollaborationtool,23whichconsiderssix
dimen-sions:randomsequencegeneration,allocationconcealment, blindingofparticipantsandpersonnel,blindingofoutcome assessment,incompleteoutcomedataandselectivereporting ofoutcomes.Thestudywasclassifiedashavinglowriskofbias whenallcriteriawerereportedandwereadequate,ahighrisk ofbiaswhenatleastoneofthecriteriawasinadequate,and anuncertainriskofbiaswhenoneormoreitemswerenot reported.Theinter-rater reliabilitywasmeasuredusingthe KappastatisticaccordingtoLandisandKock24andcalculated
usingSPSS®17.0software.Inter-raterreliabilitywas substan-tialforthemodifiedJadadscale(0.70±DP0.73)andmoderate fortheriskofbiasassessment(0.55±0.78).
Datacollection
Two independentreviewers collecteddata onstudy design, methodologicalquality, riskofbias,patientprofile,efficacy
and safety outcomes using an electronic form that was designedinExcel® 2007.Aconsensusresolvedall disagree-ments.
The primary outcome was the measurement of ACR20,
which is defined as 20% improvement in swollen joints and joint painincombination witha 20%improvementin three of five criteria: patient’s global assessment of pain; patient’s global assessment ofdisease activity; physician’s globalassessmentofdiseaseactivity;patient’sassessmentof physicalfunction;andC-reactiveproteinlevels.25Secondary
outcomesincludedtheACR50andACR70,clinicalremission (definedasaDAS28[DiseaseActivity Score28]<2.6), radio-graphicdata,losstofollow-upandadverseevents.
Statisticalanalysis
Arandomeffectsmodelwasusedforallmeta-analysesdue to the clinical heterogeneity of the included studies. Rela-tive risk (RR) was used as a measure of treatment effect, and it was calculated using the Mantel–Haenszel method forbinary data. Meandifferences andthe inverse variance methodwereusedforcontinuousdata.Confidenceintervals of95%werepresentedforbothmeasures.Heterogeneitywas assessedusingthe2testforheterogeneityandtheI2index
andwasconsideredstatisticallysignificantwhenpvaluewas lowerthan0.10andI2valuehigherthan40%.26Weidentified
thesourceofheterogeneitythroughasensitivityanalysis,in whichstudieswereremovedfromthemeta-analysisoneby onetoinvestigatepossiblecausesrelatedtopatientsandstudy characteristics.Subgroupanalysiswasalsoperformedto eval-uatetheeffectsofpre-treatment,studylength,dosageandthe IFXadministrationregimen.Inthemeta-analysisweincluded resultsfromthelongestavailablefollow-up,unlessindicated otherwise. We usedReview Manager® softwareversion 5.1 forstatisticaltests(Copenhagen:TheCochraneCollaboration, 2011).
Results
Weidentified5782articles,ofwhich249wereconsideredfor fullreadingand74articleswereselected.Atotalof11articles, representingmultiplepublicationsofninestudies,evaluated IFXandmettheinclusioncriteriaforthisreview(Fig.1).We foundonecompletedphaseIIIclinicaltrialwithunpublished results(Table1,onlineresource).Wehavenotfoundany the-sisordissertations.Abstractsfoundintheannalsofmeetings hadtheirrespectivefullarticlescollectedbymanualsearch. Thecharacteristicsofthenineincludedtrialsarepresented in Table 1. The studies were published between 1998 and 2012,andthefollow-upperiodsrangedfrom14to104weeks. Patientsprofileincludedindividualspreviouslytreatedwith DMARDs,nottreatedwithMTXorthosethathadinsufficient responsestoMTX.
Most studies defined active RA by the presence of six or more swollen joints and six or more tender joints in combination with the additional criteria of morning stiff-ness,C-reactiveproteinlevelsanderythrocytesedimentation rates. ASPIRE27 and ATTEST28 defined active RA as 10 or
rev bras reumatol.2015;55(2):146–158
149
Records identified throught
database searching: 5782 Manual search: 9
Excluded duplicates: 92
(2648) excluded based on:
Records sereened on basis of abstract: 3051
Records screened on basis of full text: 249
Total records included: 74 Other biologics: 63 publications Infliximabe: 11 publications, 9 RCTs
(175) excluded based on: Records screened on basis
of title: 5699
(1912) excluded based on: Records identified throught
systematic literature search: 5791
PubMed: 3620
Lilacs: 98
Embase: 1577
Central:487
Type of study: 1123
Type of participant: 283
Type of intervention: 135
Outcome not evaluated: 1107
Type of study: 2256
Type of participant: 79
Type of intervention: 83
Outcome not evaluated: 384
Type of study: 96
Type of participant: 02
Type of intervention: 13
Outcome not evaluated: 24
Not available: 40
150
r
e
v
b
r
a
s
r
e
u
m
a
t
o
l
.
2
0
1
5;
5
5(2)
:146–158
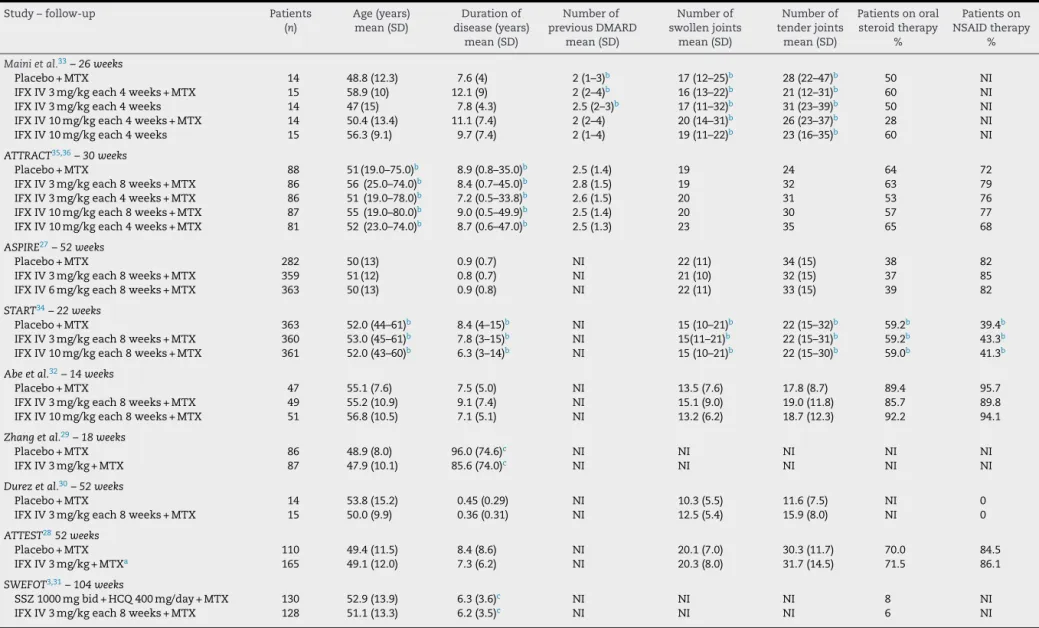
Table1–Populationbaselinecharacteristicsoftheincludedstudies.
Study–follow-up Patients Age(years) Durationof Numberof Numberof Numberof Patientsonoral Patientson
(n) mean(SD) disease(years) previousDMARD swollenjoints tenderjoints steroidtherapy NSAIDtherapy
mean(SD) mean(SD) mean(SD) mean(SD) % %
Mainietal.33–26weeks
Placebo+MTX 14 48.8(12.3) 7.6(4) 2(1–3)b 17(12–25)b 28(22–47)b 50 NI
IFXIV3mg/kgeach4weeks+MTX 15 58.9(10) 12.1(9) 2(2–4)b 16(13–22)b 21(12–31)b 60 NI
IFXIV3mg/kgeach4weeks 14 47(15) 7.8(4.3) 2.5(2–3)b 17(11–32)b 31(23–39)b 50 NI
IFXIV10mg/kgeach4weeks+MTX 14 50.4(13.4) 11.1(7.4) 2(2–4) 20(14–31)b 26(23–37)b 28 NI
IFXIV10mg/kgeach4weeks 15 56.3(9.1) 9.7(7.4) 2(1–4) 19(11–22)b 23(16–35)b 60 NI
ATTRACT35,36–30weeks
Placebo+MTX 88 51(19.0–75.0)b 8.9(0.8–35.0)b 2.5(1.4) 19 24 64 72
IFXIV3mg/kgeach8weeks+MTX 86 56(25.0–74.0)b 8.4(0.7–45.0)b 2.8(1.5) 19 32 63 79
IFXIV3mg/kgeach4weeks+MTX 86 51(19.0–78.0)b 7.2(0.5–33.8)b 2.6(1.5) 20 31 53 76
IFXIV10mg/kgeach8weeks+MTX 87 55(19.0–80.0)b 9.0(0.5–49.9)b 2.5(1.4) 20 30 57 77
IFXIV10mg/kgeach4weeks+MTX 81 52(23.0–74.0)b 8.7(0.6–47.0)b 2.5(1.3) 23 35 65 68
ASPIRE27–52weeks
Placebo+MTX 282 50(13) 0.9(0.7) NI 22(11) 34(15) 38 82
IFXIV3mg/kgeach8weeks+MTX 359 51(12) 0.8(0.7) NI 21(10) 32(15) 37 85
IFXIV6mg/kgeach8weeks+MTX 363 50(13) 0.9(0.8) NI 22(11) 33(15) 39 82
START34–22weeks
Placebo+MTX 363 52.0(44–61)b 8.4(4–15)b NI 15(10–21)b 22(15–32)b 59.2b 39.4b
IFXIV3mg/kgeach8weeks+MTX 360 53.0(45–61)b 7.8(3–15)b NI 15(11–21)b 22(15–31)b 59.2b 43.3b
IFXIV10mg/kgeach8weeks+MTX 361 52.0(43–60)b 6.3(3–14)b NI 15(10–21)b 22(15–30)b 59.0b 41.3b
Abeetal.32–14weeks
Placebo+MTX 47 55.1(7.6) 7.5(5.0) NI 13.5(7.6) 17.8(8.7) 89.4 95.7
IFXIV3mg/kgeach8weeks+MTX 49 55.2(10.9) 9.1(7.4) NI 15.1(9.0) 19.0(11.8) 85.7 89.8
IFXIV10mg/kgeach8weeks+MTX 51 56.8(10.5) 7.1(5.1) NI 13.2(6.2) 18.7(12.3) 92.2 94.1
Zhangetal.29–18weeks
Placebo+MTX 86 48.9(8.0) 96.0(74.6)c NI NI NI NI NI
IFXIV3mg/kg+MTX 87 47.9(10.1) 85.6(74.0)c NI NI NI NI NI
Durezetal.30–52weeks
Placebo+MTX 14 53.8(15.2) 0.45(0.29) NI 10.3(5.5) 11.6(7.5) NI 0
IFXIV3mg/kgeach8weeks+MTX 15 50.0(9.9) 0.36(0.31) NI 12.5(5.4) 15.9(8.0) NI 0
ATTEST2852weeks
Placebo+MTX 110 49.4(11.5) 8.4(8.6) NI 20.1(7.0) 30.3(11.7) 70.0 84.5
IFXIV3mg/kg+MTXa 165 49.1(12.0) 7.3(6.2) NI 20.3(8.0) 31.7(14.5) 71.5 86.1
SWEFOT3,31–104weeks
SSZ1000mgbid+HCQ400mg/day+MTX 130 52.9(13.9) 6.3(3.6)c NI NI NI 8 NI
IFXIV3mg/kgeach8weeks+MTX 128 51.1(13.3) 6.2(3.5)c NI NI NI 6 NI
NSAID,nonsteroidalanti-inflammatorydrugs;IFX,infliximab;HCQ,hydroxychloroquine;MTX,methotrexate;SSZ,sulfasalazine;DMARD,disease-modifyinganti-rheumaticdrug;IV,intravenously;
SD,standarddeviation;BID,twiceaday;NI,notinformed.
a Infliximabatdays1,15,43,85andeach56days.
b Median.
rev bras reumatol.2015;55(2):146–158
151
definedactiveRAasthreeormoreswollenandeightormore tenderjointsinadditiontofurthercriteria.Non-steroidal anti-inflammatorydrugs(NSAIDs)atstabledosesandlowdosesof oralglucocorticoid(≤10mg/dayprednisolone)wereallowedin alltrialsexceptinDurezetal.30Thisstudy30andSWEFOT31
evaluated onlypatients with initialRA, definedas disease duration ofless than 12 months. TheASPIRE27 and Durez
etal.30studiesevaluatedMTX-naivepatients,buttheother
studiesevaluatedpatientswithinsufficientresponsestoMTX. ThemeanweeklydoseofMTXwas7.2±2.0mginAbeetal.,32
7.5mginMainietal.33andrangedfrom7.5to20mginDurez
etal.30andZhang29studies.MTXdoserangedfrom15to20mg
intheotherstudies.3,27,28,31,34–36
Methodologicalqualityandriskofbias
Nine trials were classified as randomised,but onlytwo of these studies reported the methods of randomisation.27,35
The Jadad scale score was generally good (ranging from moderatetohigh).Thepharmaceuticalindustry fundedsix studies.27,28,30,31,34,35 Weidentifiedapotentialsourceofbias
inthreetrials,30,31,33andonlyonestudy35wasclassified as
lowriskofbias(Table2).
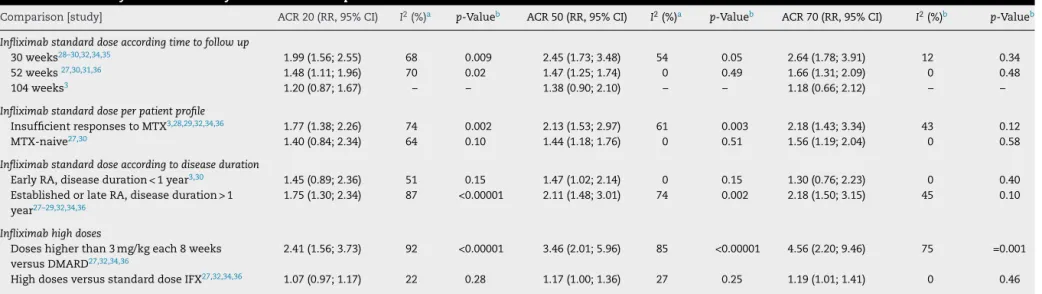
ACRresponse
Infliximabstandarddose(3mg/kgeveryeightweeks)per follow-upperiod
Eleven studies were included in this analysis. Six ofthem included 1470 patients and presented results of up to 30 weeksoffollow-up,28–30,32,34,35fourstudieswith1086patients
presented results of 52 weeks27,30,31,36 and one study of
258patientspresentedresultsof104 weeks.3 Patients who
received combination therapy with IFX showed a better ACR response than patients treated with MTX alone or in combinationwith DMARDsuntil30 and52 weeksof treat-ment.However,nosignificantdifferencebetweengroupswas observedafter104weeksoffollow-up.Theheterogeneityof ACR20and ACR50wassignificant and moderateforthe 30 weeksoffollow-upstudies(Table3).Heterogeneityat52weeks offollow-up was assessedusing the stratification between MTX-naivepatientsandpatientswithinsufficientresponses toMTX(seeSection“Heterogeneityassessmentandsubgroup analysis”).
Infliximabstandarddoseperpatientprofile
The patient’s previous treatments, regardless of follow-up period, revealed that the IFX+MTX combination achieved betterresultsthan syntheticDMARDsinpatientswho had previouslyfailedtoMTXtreatment,comparedtoMTX-naïve patients(Table3).
Infliximabstandarddoseaccordingtodiseaseduration
CombinationtherapywithIFXshowedbetterACRresponses thanMTXaloneorincombinationwithsyntheticDMARDsin patientswithestablishedRA.Thisresultwasnotobservedin patientswithearlyRA(Table3).
High-doseinfliximab
Patients that used IFX regimens with doses higher than 3mg/kgorinshorterintervalsshowedbetterACRresponses thanpatientstreatedwithMTXaloneorincombination. Meta-analysis ofhigh-dose IFX versus standard dose of 3mg/kg everyeightweeksdidnotshowdifferenceinACR20andACR50 outcomeswithin54weeksoffollow-up.Despitebeing statis-ticallysignificant,differenceinACR70responsewasboarder linefavouringIFXhigh-doses(Table3).
Clinicalremission
Twostudiesassessedclinicalremissionat2828,34and54weeks
offollow-up27,30 inpatientsusingthestandarddoseofIFX.
Meta-analysisfavouredIFX+MTXcombinationinall follow-up periodsand intheoverall analysis,withnostatistically significantheterogeneityobserved.TheRRupto28weeksof treatmentwas2.57([1.44;4.60];I2=30%;p=0.23).TheRRat52 weekswas1.48([1.07;2.05];I2=0%;p=0.62),andtheoverall analysis,whichassessed1569patients,producedanRR=1.92 ([1.35;2.74];I2=49%;p=0.12).
Radiographicprogression
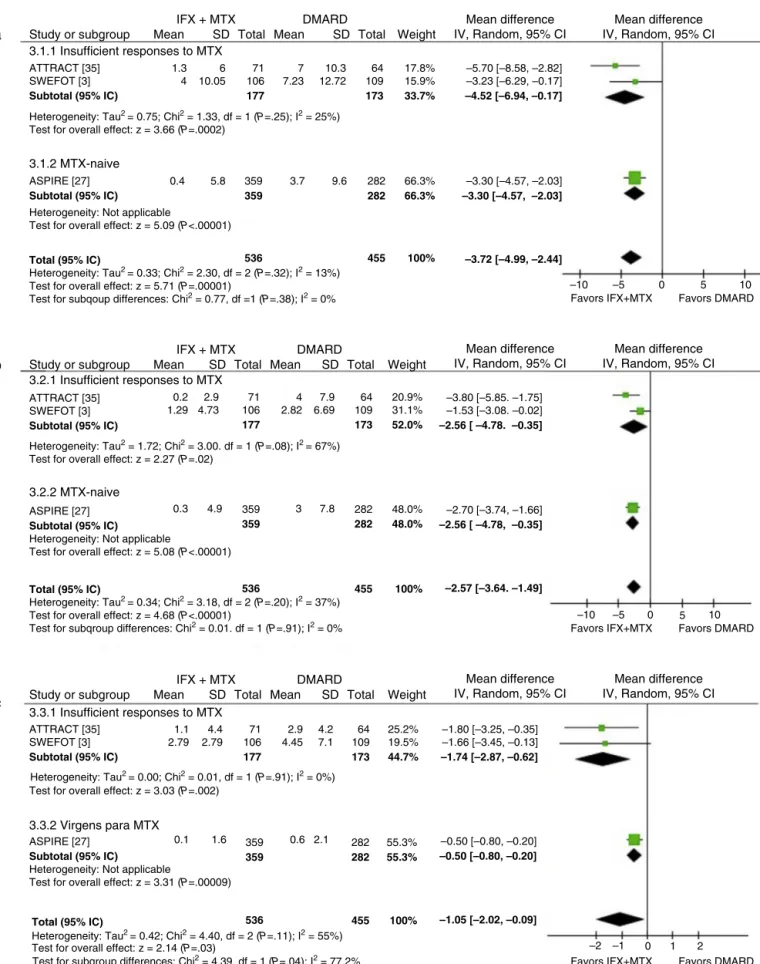
Ameta-analysisofthreestudies3,27,36revealedlower
radio-graphic progression in patients who were treated with IFX+MTX at a standard dose than in patients who were treatedwithothersyntheticDMARDsat52and104weeks. Sta-tisticallysignificantdifferenceswereobservedinpatientswith aninsufficientresponsetoMTXandtreatment-naivepatients (Fig.2).
Withdrawals
A meta-analysis of five studies,3,27,28,32,36 comprising 1474
patients, revealed a lower risk of withdrawals due to
lack of efficacy in the group treated with IFX+MTX
(RR=0.33[0.17;0.63];I2=45%;p=0.12).Meta-analysisofeight studies,3,27–30,32,34,36 whichassessed2399 patients, revealed
nodifferencebetweengroupsinwithdrawalsduetoadverse events(RR=1.59[0.96,2.65];I2=40%;p=0.11).Afterremovalof SWEFOT3study,inwhichthecontrolgroupincludedpatients
whoreceivedHCQandSSZincombinationwithMTX,the over-allriskofwithdrawalsduetoadverseeventswashigherinthe IFX+MTXgroup(RR=2.05[1.33;3.16];I2=0%;p=0.43). Anal-ysisofwithdrawalsduetoadverseeventsbypatientprofile revealedanincreasedriskforMTX-naivepatients(RR=3.01 [1.49;6.06];I2=0%;p=0.97)butnotinpatientswithan insuffi-cientresponsetoMTX.Regimenswithhigh-doseIFXwereas safeasthestandardregimenregardingdiscontinuationdue toadverseevents(RR=1.12[0.80;1.57]).
Safety
152
rev bras reumatol.2015;55(2):146–158IFX + MTX Study or subgroup
a
b
c
3.1.2 MTX-naive
Total (95% IC)
Test for overall effect: z = 5.71 (P=.00001)
Test for subqoup differences: Chi2 = 0.77, df =1 (P=.38); I2 = 0%
Total (95% IC)
Test for overall effect: z = 4.68 (P<.00001)
Test for subqroup differences: Chi2 = 0.01. df = 1 (P=.91); I2 = 0%
Test for subqroup differences: Chi2 = 4.39, df = 1 (P=.04); I2 = 77.2%
Total (95% IC)
Test for overall effect: z = 2.14 (P=.03)
3.1.1 Insufficient responses to MTX
3.2.1 Insufficient responses to MTX
3.2.2 MTX-naive
3.3.2 Virgens para MTX
ATTRACT [35] SWEFOT [3]
Subtotal (95% IC)
ATTRACT [35] SWEFOT [3]
Subtotal (95% IC)
ATTRACT [35] SWEFOT [3]
Subtotal (95% IC)
1.3 4 0.2 1.29 0.3 1.1 2.79
0.1 1.6 0.6 2.1
4.4 2.79 71 106 177 64 109 173 25.2% 19.5% 44.7%
–1.80 [–3.25, –0.35] –1.66 [–3.45, –0.13]
–1.74 [–2.87, –0.62]
2.9 4.45
4.2 7.1
4.9 3 7.8
2.9 4.73 71 106 177 359 359 359 359 282 282 55.3% 55.3%
–0.50 [–0.80, –0.20]
–0.50 [–0.80, –0.20]
282 282 48.0% 48.0% 64 109 173 20.9% 31.1% 52.0%
–3.80 [–5.85. –1.75] –1.53 [–3.08. –0.02]
–2.56 [ –4.78. –0.35]
–2.70 [–3.74, –1.66]
–2.56 [ –4.78, –0.35]
4 2.82
7.9 6.69 ASPIRE [27]
Subtotal (95% IC)
Heterogeneity: Not applicable
Test for overall effect: z = 5.09 (P<.00001)
ASPIRE [27]
Subtotal (95% IC)
Heterogeneity: Not applicable
Test for overall effect: z = 5.08 (P<.00001)
ASPIRE [27]
Subtotal (95% IC)
Heterogeneity: Not applicable
Test for overall effect: z = 3.31 (P=.00009)
0.4 5.8 3.7 9.6
–10 –5 0 5 10
–10
–1
–2 0 1 2
–5 0 5 10
Favors DMARD Favors IFX+MTX Favors DMARD Favors IFX+MTX Favors DMARD Favors IFX+MTX 282 282 66.3% 66.3% 359 359 536 536 455 100% 6 10.05
Heterogeneity: Tau2 = 0.75; Chi2 = 1.33, df = 1 (P=.25); I2 = 25%)
Heterogeneity: Tau2 = 0.33; Chi2 = 2.30, df = 2 (P=.32); I2 = 13%)
Heterogeneity: Tau2 = 0.34; Chi2 = 3.18, df = 2 (
P=.20); I2 = 37%)
Heterogeneity: Tau2 = 0.00; Chi2 = 0.01, df = 1 (
P=.91); I2 = 0%)
Heterogeneity: Tau2 = 0.42; Chi2 = 4.40, df = 2 (P=.11); I2 = 55%)
Test for overall effect: z = 3.66 (P=.0002)
Heterogeneity: Tau2 = 1.72; Chi2 = 3.00. df = 1 (P=.08); I2 = 67%) Test for overall effect: z = 2.27 (P=.02)
Test for overall effect: z = 3.03 (P=.002) 71 106 177 7 7.23 10.3 12.72 64 109 173 17.8% 15.9% 33.7%
–5.70 [–8.58, –2.82] –3.23 [–6.29, –0.17]
–4.52 [–6.94, –0.17]
–3.30 [–4.57, –2.03]
–3.30 [–4.57, –2.03]
–3.72 [–4.99, –2.44]
455 100% –2.57 [–3.64. –1.49]
536 455 100% –1.05 [–2.02, –0.09]
Mean SD Total Mean SD Total Weight
DMARD Mean difference
IV, Random, 95% CI
Mean difference IV, Random, 95% CI
Mean difference IV, Random, 95% CI
Mean difference IV, Random, 95% CI IFX + MTX
Study or subgroup Mean SD Total Mean SD Total Weight DMARD
Mean difference IV, Random, 95% CI
Mean difference IV, Random, 95% CI IFX + MTX
Study or subgroup Mean SD Total Mean SD Total Weight DMARD
3.3.1 Insufficient responses to MTX
Fig.2–Meta-analysisofradiologicalprogressionaccordingtotheVanderHeijdemodifiedSharpscore2a–TotalScore;2b–
r
e
v
b
r
a
s
r
e
u
m
a
t
o
l
.
2
0
1
5;
5
5(2)
:146–158
153
Table2–Within-studyqualityandriskofbiasassessment.
Study ModifiedJadadscale Riskofbias Randomisation Appropriate
randomisation method
Inappropriate randomisation method
Concealment Appropriate Concealment
method
Lossesto follow-up
ITT analysis
Score Sequence generation
Allocation concealment
Blindingofparticipants andpersonnel
Blindingof outcome assessment
Incomplete outcomedata
Selective outcome reporting
Overallrisk ofbias
ATTRACT35,36 1 1 0 1 1 1 1 6 L L L L L L L
Mainietal.33 1 0 0 1 1 1 1 5 U L L L L H H
ASPIRE27 1 1 0 1 1 1 0 5 L L L L U L U
START34 1 0 0 1 1 1 1 5 U U L L L L U
ATTEST28 1 0 0 1 1 1 1 5 U U L L L L U
Zhang29 1 0 0 1 0 1 1 4 U U U U L U U
SWEFOT3,31 1 1 0 0 0 1 1 4 U H H H L L H
Abeetal.32 1 0 0 0 0 1 1 3 U U U U L L U
Durezetal.30 1 0 0 0 0 1 1 3 U U H L L L H
U,unclearriskofbias;L,lowriskofbias;H,highriskofbias.
Table3–Meta-analysisoftheefficacyofinfliximabcomparedtocontrol.
Comparison[study] ACR20(RR,95%CI) I2(%)a p-Valueb ACR50(RR,95%CI) I2(%)a p-Valueb ACR70(RR,95%CI) I2(%)b p-Valueb
Infliximabstandarddoseaccordingtimetofollowup
30weeks28–30,32,34,35 1.99(1.56;2.55) 68 0.009 2.45(1.73;3.48) 54 0.05 2.64(1.78;3.91) 12 0.34
52weeks27,30,31,36 1.48(1.11;1.96) 70 0.02 1.47(1.25;1.74) 0 0.49 1.66(1.31;2.09) 0 0.48
104weeks3 1.20(0.87;1.67) – – 1.38(0.90;2.10) – – 1.18(0.66;2.12) – –
Infliximabstandarddoseperpatientprofile
InsufficientresponsestoMTX3,28,29,32,34,36 1.77(1.38;2.26) 74 0.002 2.13(1.53;2.97) 61 0.003 2.18(1.43;3.34) 43 0.12
MTX-naive27,30 1.40(0.84;2.34) 64 0.10 1.44(1.18;1.76) 0 0.51 1.56(1.19;2.04) 0 0.58
Infliximabstandarddoseaccordingtodiseaseduration
EarlyRA,diseaseduration<1year3,30 1.45(0.89;2.36) 51 0.15 1.47(1.02;2.14) 0 0.15 1.30(0.76;2.23) 0 0.40
EstablishedorlateRA,diseaseduration>1
year27–29,32,34,36
1.75(1.30;2.34) 87 <0.00001 2.11(1.48;3.01) 74 0.002 2.18(1.50;3.15) 45 0.10
Infliximabhighdoses
Doseshigherthan3mg/kgeach8weeks
versusDMARD27,32,34,36
2.41(1.56;3.73) 92 <0.00001 3.46(2.01;5.96) 85 <0.00001 4.56(2.20;9.46) 75 =0.001
HighdosesversusstandarddoseIFX27,32,34,36 1.07(0.97;1.17) 22 0.28 1.17(1.00;1.36) 27 0.25 1.19(1.01;1.41) 0 0.46
CI,confidenceinterval;DMARD,disease-modifyinganti-rheumaticdrug;IFX,infliximab;RA,rheumatoidarthritis;RR,relativerisk;–,notestimable.
a I2>40%indicateheterogeneitybetweenstudies.
b p-Value<0.10of
154
rev bras reumatol.2015;55(2):146–158showedmoderateheterogeneity.Subgroupanalysisrevealed thatMTX-naive patientswho receivedIFX+MTX had more seriousinfectionsthantheMTXgroup(2.80[1.14;6.84]).Still, thisresultwasobtainedfromasinglestudy27(Table4).
Regimenswithhigh-doseIFXwereassafeasthestandard regimenregardingseriousadverseevents(1.15[0.77;1.71])and seriousinfections(1.84[0.71,4.79]).However,the heterogene-ityofseriousinfectionswasmoderateandsignificant(68%; 0.04),thestudyASPIRE27beingthesourceofthis
heterogene-ity.Theriskofseriousinfectionsbecamehigherinpatients whoreceivedhigh-doseIFXthanpatientswho receivedthe standard IFX dose when the ASPIRE study was excluded, andnosignificantheterogeneitywasobserved(RR=3.07[1.42; 6.64]).
Heterogeneityassessmentandsubgroupanalysis
TheheterogeneityofACR20andACR50wassignificantand moderate for the studies with up to 30 weeks of follow-up(Table3).Heterogeneitybecamenon-significant andthe results remained favourable to IFX+MTX (1.74 [1.32; 2.29]) whentheATTRACT35andSTART34studieswereexcludedfrom
theACR20meta-analysisof30weeks.Heterogeneitybecame non-significantandtheresultsstillfavouredthegroup receiv-ingIFX+MTX(1.74[1.32;2.29])whentheSTARTstudy34was
excludedfromtheACR50meta-analysisof30weeks.No rea-sonableexplanationforthesourceofheterogeneitycouldbe established.ThecauseofheterogeneityofACRanalysisat52 weekswasthe differenceinpatient profilewithrespectto previousexperience withDMARs. Therefore, weconducted asubgroupanalysis, inwhichACR20showed nostatistical significancebetweenthe groupswhoreceivedIFX+MTXor DMARDsin patients with aninsufficient responseto MTX (1.72[0.92;3.22])andinMTX-naivepatients(RR=1.40[0.84; 2.34]).For ACR50,IFX+MTXregimenwas superiorin MTX-naivepatients(1.44[1.18;1.76])butnotinpatientswhohad an insufficient response to MTX (1.72 [0.98; 3.00]). In con-trast,IFX+MTXwasstatisticallysuperiortoDMARDtherapy inMTX-naivepatients(1.56[1.19;2.04])andinpatientswith aninsufficientresponsetoMTX (2.20[1.06; 4.56])inACR70 meta-analysis.
Discussion
Thissystematicreviewandmeta-analysisincludednine ran-domisedcontrolledclinicaltrialsandoneongoingstudy,and showedsuperiorresultsofefficacyasevaluatedbyACRand DAS28,and ofradiographic progressionforIFX+MTX com-pared to MTX monotherapy or in combination with other DMARDs,regardlessofdiseaseduration,doseandpatient pro-file.
TheefficacyoftheIFX+MTXregimenwasassessedfrom14 weeksoftreatment32to104weeks3inpatientswithan
insuf-ficientresponsetoMTXandinMTX-naivepatients.IFX+MTX use beganearly afterRAdiagnosis30,31 or after10 years of
diseaseduration32,35,36onaverage.Thetherapeuticregimen
wasvariableandincludedtheadministrationof3or10mg/kg IFXeveryfouroreightweeks33,35,36or6mg/kgIFXeveryeight
weeks.27Thecontrolgroupincludedplacebo+MTXora
com-binationofsyntheticDMARDs.
These differences affected the sizeand direction ofthe effect, which favoured IFX+MTX especially during shorter periods of follow-up in patients with established RA and aninsufficientresponsetoMTX.These resultssupportthe treatment withsyntheticDMARDs,reservingtheIFX+MTX regimenforcasesofafailureofthefirst-lineregimen.This approachiscorroboratedbyDuPanetal.,37whoperformed
asystematicreviewthatspecificallyevaluatedpatientswith earlyRAandrecommendedtheuseofIFX+MTXforcaseswith rapidradiographicprogression,insufficientresponsetoMTX or other clinical and biological signs ofaggressive disease, sincethereisnorobustevidencethatsupportstheefficacy, safetyandcostoftheearlyuseofIFX.
Theresultsofefficacyandsafetyalsoencouragetheuseof therecommendedstandardregimenof3mg/kgIFXatweeks0, 2and6andtheneveryeightweeksincombinationwithMTX, insteadofincreaseddoseorshorterintervalsofIFX adminis-tration,asdescribedinindividualstudies.4,7
ClinicalremissionfavouredtheuseofIFX+MTXafter24 and 54 weeksof treatmentand the ACR responseafter 52 weeks. However, the high heterogeneity ofthe analysisof ACR20andACR50forupto30weeksoftreatmentand the lackofeffectofACR20at52 weeksinthesubgroup analy-sisbypastDMARDexposurewerenotable.Wiensetal.18also
reportedinconsistentresultsintheACR20and50responses after30weeks,andconsideredthehighnumberofpatients whoachievedthetherapeuticresponseinthetestand con-trolgroupsontheSTART34studyasapossibleexplanation.
The ACR20 response may be more sensitive to the use of sometreatmentsbecauseitislessstrict.However,differences betweentreatments becamemoreevidentwithmorestrict ACR responses because in this case these responses were directlyrelatedtotheefficacyofthebiologicalagentuse.
Furthermore, only the SWEFOT study3,31 assessed
effi-cacy outcomes forup to 104 weeksof follow-up, and this study reportednodifferenceintheACRresponsesbetween IFX+MTX and DMARD combination groups. This affected also the meta-analysis ofour study, because when it was excludedtheresultofefficacyoutcomeswasfavourablefor theIFX+MTX.Incontrast,thepreventionofradiographic pro-gressionusingIFX+MTXwasconfirmed,despitethecontrol group(i.e.,withDMARDcombinationorMTXmonotherapy).
Withdrawalsduetoadverseeventswereespeciallyaffected by the exclusion ofthe SWEFOT study.3,31 With the
exclu-sionofthisstudytheanalysisbecamestatisticallysignificant favouring IFX+MTX. Thisresult wasexpected because the additionofahighernumberofdrugstoatherapeuticregimen increasestheprobabilityofadverseeventsanddecreasesthe differencesbetweenstrategies.Othersystematicreviewsthat didnotincludethisstudyalsoreportedagreaterlossto follow-upasaresultofadverseeventsintheIFX+MTXgroup.16,18
Considering withdraw due to adverse events according to patients’ past experience with DMARDs,the results ofour meta-analysis showed higher risk of lossin the IFX+MTX group forMTX-naive patients,but notforpatientswithan insufficient response toMTX, as reported byChen et al.15
rev bras reumatol.2015;55(2):146–158
155
Table4–Safetymeta-analysisofstandarddoseIFX3mg/kgeach8weeks.
Outcome Numberof
evaluatedstudies [study]
Patients(n) RR(IC95%) I2a p-Valueb
Infections 4 658 1.04(0.73;1.47) 66% 0.03
InsufficientresponsestoMTX 33,28,32 629 1.21(0.75;1.98) 57% 0.10
MTX-naïvepatients 130 29 0.81(0.61;1.06) – –
Seriousinfections 6 2128 1.19(0.48;2.93) 56% 0.08
InsufficientresponsestoMTX 43,28,34,36 1428 0.83(0.33;2.06) 31% 0.24
MTX-naïvepatients 227,30 700 2.80(1.14;6.84) – –
Seriousadverseevents 8 2397 1.02(0.79;1.33) 0% 0.57
InsufficientresponsestoMTX 63,28,29,32,34,36 1697 0.86(0.61;1.21) 0% 0.57
MTX-naïvepatients 227,30 700 1.32(0.87;1.98) 0% 0.63
Tumours 7 2369 1.87(0.42;8.35) 0% 0.92
InsufficientresponsestoMTX 63,28,29,32,34,35 1698 1.87(0.42;8.35) 0% 0.92
MTX-naïvepatients 127 671 – – –
Tuberculose 5 1928 4.12(0.47;36.07) 0% 0.78
InsufficientresponsestoMTX 428,29,32,34 1265 2.97(0.12;71.81) – –
MTX-naïvepatients 127 663 5.48(0.28;105.67) – –
Death 6 2052 1.05(0.20;5.42) 0% 0.55
InsufficientresponsestoMTX 43,28,32,34 1352 2.47(0.26;23.61) 0% 0.86
MTX-naïvepatients 227,30 700 0.40(0.04;4.38) – –
Infusionreactions 3 1042 2.21(1.63;2.99) 72% 0.03
InsufficientresponsestoMTX 228,32 371 1.52(1.02;2.26) 0% 0.40
MTX-naïvepatients 127 671 3.16(1.98;5.03) – –
CI,confidenceintervals;RR,relativerisk;MTX,methotrexate;–,notestimable.
a I2>40%indicateheterogeneitybetweenstudies.
b p-Value<0.10of2indicatesheterogeneitybetweenstudies.
Nodifferences insafety were observedinthis or previ-oussystematicreviews,15,18,19butotherevidencesourceshave
reportedthat the use ofanti-TNF␣ increasespatients’ risk
ofdevelopingtuberculosis(TB)andotherinfections.38–40 An
evaluationofthebiologicalproductsdatabaseoftheSpanish SocietyofRheumatologyrevealedincidenceratesof1113per 100,000in2001,whichwassignificantlyhigherthannational rates.38Ameta-analysisofsafetyfromobservationalstudies40
relatedanincreasedriskofinfectionsofapproximately40% forRApatients whowere treatedwithanti-TNF␣ (RR=1.37
[1.18;1.60]).TheseriskssupporttheuseofTBscreeningforall patientswho mightreceiveanti-TNF␣treatment,andthese
patientsshouldbefollowedusingnewtestsforthesignals andsymptomsofinfections,especiallyduringthefirstyearof treatment.7,41
Regarding the comparison of high-dose IFX and the
standarddoseof3mg/kgevery8weeks,wedidnotfind signifi-cantdifferencesinACRoutcomesafter54weeksoffollow-up. Therefore,the lowerdoseof3mg/kgIFX everyeightweeks wasaseffectiveastheotherdosesinisolatedstudies32,34,35
andinthemeta-analysisofthisreview.However,theresults ofthemeta-analysisonseriousinfectionsshowedsignificant heterogeneity.TheASPIRE27study,whichincludedMTX-naive
patients, was excluded, and the risk ofinfections became higherwithhighdosesofIFX+MTX,showingthatMTX-naive patients may be moresusceptible toinfections. Therefore, the group that received high doses of IFX had high infec-tionratescomparedtothestandard doseIFXgroup.These resultswereconsistentwithAaltonenetal.19andAlonso-Ruiz
etal.16whoreportednodifferencesinefficacybetweenhigh
andstandarddosesofIFX.However,nodifferenceinsafety between the doseregimens was described,19 although this
study didnotanalyseheterogeneityorperformasubgroup analysisbypatientDMARDexposure.AnIFXinduction reg-imen using 10mg/kgisnotindicated bythemanufacturer, anditisdiscouragedbytheresultsofclinicaltrialsandthis systematic review,since it provides no additionalbenefits. Besides,thisstrategycanincreasetheriskofinfections com-paredtoplacebo+MTXgroup.34,35
OneRCTthatwasnotincludedinoursystematicreview becauseofthedoseescalationanalysedtheincreaseinIFX dosefrom3to5mg/kgevery8weeksandrevealedno addi-tionalbenefittotheprimaryoutcome(DAS28after28weeks) or secondary outcomes (e.g., number of swollen and ten-derjoints,C-reactiveproteinanderythrocytesedimentation rate)within52weeks.Furthermore,anincreaseinthe inci-dence of adverse events was observed, but these adverse events did not include serious adverse events or serious infections.42
Ollendorfetal.43alsodemonstratedthatpatientsusingIFX
increasedthedose morefrequently overshorterperiodsof timethanpatientsusingetanerceptandadalimumab(32.1%, 8.5% and 4.7%,respectively). Consequently, the cost ofIFX treatment was approximately 30% higher than other anti-TNF␣agents.TheseresultssuggestthatincreasingIFXdose
156
rev bras reumatol.2015;55(2):146–158Limitations
A random effects model was used, and the results ofthe subgroupanalyseslostpartofitsinferencevalueduetothe clinical heterogeneity of trials in the evaluated outcomes, patientprofile,periodoffollow-up,doseandadministration period. However, the results were consistent with the lit-erature, andalthoughnotentirely reflectiveofreality,they demonstratethe directionoftheeffect. Other limitation is that onlythreeofthe published RCTswere notfunded by thepharmaceuticalindustry.Studiesfundedbythe pharma-ceuticalindustryaremorelikelytoreportfavouringoutcomes totheirproducts45andtherefore,theresultsofthesestudies
shouldbeinterpretedcautiously.Becauseofthat,the assess-mentofriskofbiasbecamemorepertinent,andonlyonestudy inthisreviewwasclassifiedasalow-riskofbiasinallassessed domains.Also,wecouldnotassessthepublicationbiasofthe outcomesbecausetherecommendednumberofclinicaltrials toperformthisanalysiswithrobustnessis10studies. How-ever, weconductedthemanualsearch and searchthrough thegreyliteraturetominimisethis effect.Evidence onthe IFX+MTXregimenversusMTXiswellestablished,butother typesofcomparisonmustbeexplored.Onlyonepublished study3,31 assessedthe regimenINF+MTX versus a DMARD
combination.Becauseofthat,thissystematicreviewcouldnot establishthebeststrategyforpatientsinwhomfirst-line ther-apyfails.ClinicaltrialsthatcomparetheefficacyofIFX+MTX tocombinationsofDMARDsarerequiredtocoverthis knowl-edgegap.
Implicationsforclinicalpractice
TheuseofIFX+MTXpromotedradiographicandclinical ben-efitsthat were less significant in early RApatients, which suggeststhatearlytreatmentwithanysyntheticDMARDis moreimportantthanearlytreatmentusingabiologicalagent. ThecombinationofIFX+MTXmaybeabetterstrategyforthe preventionofradiographicprogressionthanMTXalone.The choicebetweenINF+MTXovercombinationDMARDsshould considertheirabilitytoreducefunctionallossovertime(i.e., radiographicprogression)balancedwiththesmallclinical dif-ferencesthatareobservedoverlongperiodsoffollow-up,and thehighercostofanti-TNF␣treatment.
Conclusions
IFX+MTX therapeutic regimens showed better results of clinical efficacy evaluated by ACR and DAS28 than MTX monotherapy or combined DMARDs, regardless of disease duration,doseandpatientpastexperiencewithDMARD.The efficacy of IFX+MTX was more evident in shorter periods offollow-up,patientswithestablishedRAandpatientswith insufficientresponsetoMTX.Radiographicprogressionwas avertedinlongerfollow-upperiods.Thelowestdose,3mg/kg IFXeveryeightweeks,wasaseffectiveastheotherIFXdoses. ThedataonsafetysuggestthatincreasesinIFXdoseswere relatedtoanincreasedincidenceofinfections,andtherefore shouldnotbeused.
Funding
National Counsel of Technological and Scientific Develop-ment R$ 95.567,16 (ConselhoNacional de Desenvolvimento Científico e Tecnológico – CNPq) (Public notice MS-SCTIE-DECIT/CNPqno.69/201;Caseno.564778/2010-9).
Conflicts
of
interest
Theauthorsdeclarenoconflictsofinterest.
Acknowledgments
The authors thank the Pharmacoepidemiology Research
GroupoftheUFMG,especiallyMarianaMichelBarbosaand FelipeFerré,fortheircontributionstotheexecutionofthis study.
Appendix
A.
Supplementary
data
Supplementary material associated with this article can be found in the online version available at doi:10.1016/ j.rbre.2014.10.009.
r
e
f
e
r
e
n
c
e
s
1.AlamanosY,DrososAA.Epidemiologyofadultrheumatoid arthritis.AutoimmunRev.2005;4(3):130–6.
2.PincusT,YaziciY,SokkaT,AletahaD,SmolenJS.
Methotrexateasthe“anchordrug”forthetreatmentofearly rheumatoidarthritis.ClinExpRheumatol.2003;215Suppl. 31:S179–85.
3.VanVollenhovenRF,GeborekP,ForslindK,AlbertssonK, ErnestamS,PeterssonIF,etal.Conventionalcombination treatmentversusbiologicaltreatmentin
methotrexate-refractoryearlyrheumatoidarthritis:2year follow-upoftherandomised,non-blinded,parallel-group Swefottrial.Lancet.2012;379(9827):1712–20.
4.SmolenJS,LandewéR,BreedveldFC,DougadosM,EmeryP, Gaujoux-VialaC,etal.Eularrecommendationsforthe managementofrheumatoidarthritiswithsyntheticand biologicaldisease-modifyingantirheumaticdrugs.Ann RheumDis.2010;69(6):964–75.
5.MachadoJ,MoncadaJC,PinedaR.Profileofuseofantitumor necrosisfactorinColombianpatients.Biomedica.
2011;31(2):250–7.
6.TittonDC,SilveiraIG,Louzada-JuniorP,HayataAL,Carvalho HM,RanzaR,etal.Brazilianbiologicregistry:BiobadaBrasil implementationprocessandpreliminaryresults.RevBras Reumatol.2011;51(2):152–60.
7.SinghJA,FurstDE,BharatA,CurtisJR,KavanaughAF,Kremer JM,etal.2012updateofthe2008AmericanCollegeof Rheumatologyrecommendationsfortheuseof
disease-modifyingantirheumaticdrugsandbiologicagents inthetreatmentofrheumatoidarthritis.ArthritisCareRes (Hoboken).2012;64(5):625–39.
rev bras reumatol.2015;55(2):146–158
157
oracombinationofallthreemedications.NEnglJMed. 1996;334(20):1287–91.
9. CalgüneriM,PayS,Calis¸kanerZ,Apras¸S,KirazS,ErtenliI, etal.Combinationtherapyversusmonotherapyforthe treatmentofpatientswithrheumatoidarthritis.ClinExp Rheumatol.1999;17(6):699–704.
10.O’DellJR,LeffR,PaulsenG,HaireC,MallekJ,EckhoffPJ,etal. Treatmentofrheumatoidarthritiswithmethotrexateand hydroxychloroquine,methotrexateandsulfasalazine,ora combinationofthethreemedications:resultsofatwo-year, randomized,double-blind,placebo-controlledtrial.Arthritis Rheum.2002;46(5):1164–70.
11.MöttönenT,HannonenP,Leirisalo-RepoM,NissiläM, KautiainenH,KorpelaM,etal.Comparisonofcombination therapywithsingle-drugtherapyinearlyrheumatoid arthritis:arandomisedtrial.Lancet.1999;353(9164):1568–73.
12.KremerJM,GenoveseMC,CannonGW,CaldwellJR,CushJJ, FurstDE,etal.Concomitantleflunomidetherapyinpatients withactiverheumatoidarthritisdespitestabledosesof methotrexate.Arandomized,double-blind,
placebo-controlledtrial.AnnInternMed.2002;137(9):726–33.
13.TugwellP,PincusT,YocumD,SteinM,GluckO,KraagG,etal. Combinationtherapywithcyclosporineandmethotrexatein severerheumatoidarthritis.TheMethotrexate-Cyclosporine CombinationStudyGroup.NEnglJMed.1995;333(3):137–41.
14.BlumenauerBTB,JuddM,WellsGA,BurlsA,CranneyA, HochbergMC,etal.Infliximabforthetreatmentof rheumatoidarthritis.CochraneDatabaseSystRev.2002. Availablein:http://www.mrw.interscience.wiley.com/ cochrane/clsysrev/articles/CD003785/frame.html
15.ChenYF,JobanputraP,BartonP,JowettS,BryanS,ClarkW, etal.Asystematicreviewoftheeffectivenessofadalimumab, etanerceptandinfliximabforthetreatmentofrheumatoid arthritisinadultsandaneconomicevaluationoftheir cost-effectiveness.HealthTechnolAssess.2006;10(42):1–229.
16.Alonso-RuizA,PijoanJI,AnsuateguiE,UrkaregiA,Calabozo M,QuintanaA.Tumornecrosisfactoralphadrugsin rheumatoidarthritis:systematicreviewandmetaanalysisof efficacyandsafety.BMCMusculoskeletDisord.2008;9:52.
17.ZintzarasE,DahabrehIJ,GiannouliS,VoulgarelisM, MoutsopoulosHM.Infliximabandmethotrexateinthe treatmentofrheumatoidarthritis:asystematicreviewand meta-analysisofdosageregimens.ClinTher.
2008;30(11):1939–55.
18.WiensA,CorrerCJ,VensonR,GrochockiMC,OtukiMF, PontaroloR.Ameta-analysisoftheefficacyandsafetyof usinginfliximabforthetreatmentofrheumatoidarthritis. ClinRheumatol.2009;28(12):1365–73.
19.AaltonenKJ,VirkkiLM,MalmivaaraA,KonttinenYT,
NordströmDC,BlomM.Systematicreviewandmeta-analysis oftheefficacyandsafetyofexistingTNFblockingagentsin treatmentofrheumatoidarthritis.PLOSONE.
2012;7(1):e30275.
20.LiberatiA,AltmanDG,TetzlaffJ,MulrowC,GøtzschePC, IoannidisJP,etal.ThePrismastatementforreporting systematicreviewsandmeta-analysesofstudiesthat evaluatehealthcareinterventions:explanationand elaboration.BMJ.2009;339:b2700.
21.ArnettFC,EdworthySM,BlochDA,McShaneDJ,FriesJF, CooperNS,etal.TheAmericanRheumatismAssociation 1987revisedcriteriafortheclassificationofrheumatoid arthritis.ArthritisRheum.1988;31(3):315–24.
22.WoodroffeR,YaoGL,MeadsC,BaylissS,ReadyA,RafteryJ, etal.Clinicalandcost-effectivenessofnewer
immunosuppressiveregimensinrenaltransplantation:a systematicreviewandmodellingstudy.HealthTechnol Assess.2005;9(21):1–179.
23.HigginsJPTAD,SterneJAC.Assessingriskofbiasinincluded studies.In:HigginsJPT,AltmanDG,SterneJAC,editors.
Cochranehandbookforsystematicreviewsofinterventions version5.1.0.2012[chapter8].[accessedMarch2011].
24.LandisJR,KochGG.Themeasurementofobserveragreement forcategoricaldata.Biometrics.1977;33(1):159–74.
25.FelsonDT,AndersonJJ,BoersM,BombardierC,FurstD, GoldsmithC,etal.American-college-of-rheumatology preliminarydefinitionofimprovementin
rheumatoid-arthritis.ArthritisRheum.1995;38(6):727–35.
26.DeeksJJHJ,AltmanDG.Analysingdataandundertaking meta-analyses.In:HigginsJPT,GreensS,editors.Cochrane handbookforsystematicreviewsofinterventionsversion 5.1.0.2012[chap.9].Availablein:
www.cochrane-handbook.org[accessedMarch2011]. 27.ClairEWS,VanderHeijdeD,SmolenJS,MainiRN,BathonJM,
EmeryP,etal.Combinationofinfliximabandmethotrexate therapyforearlyrheumatoidarthritis–arandomized, controlledtrial.ArthritisRheum.2004;50(11):3432–43.
28.SchiffM,KeisermanM,CoddingC,SongcharoenS,BermanA, NayiagerS,etal.Efficacyandsafetyofabataceptorinfliximab vsplaceboinATTEST:aphaseIII,multi-centre,randomised, double-blind,placebo-controlledstudyinpatientswith rheumatoidarthritisandaninadequateresponseto methotrexate.AnnRheumDis.2008;67(8):1096–103.
29.ZhangF-C.Infliximabversusplaceboinrheumatoidarthritis patientsreceivingconcomitantmethotrexate:apreliminary studyfromChina.AplarJRheumatol.2006;9(2):127–30.
30.DurezP,MalghemJ,NzeusseuToukapA,DepresseuxG, LauwerysBR,WesthovensR,etal.Treatmentofearly rheumatoidarthritis:arandomizedmagneticresonance imagingstudycomparingtheeffectsofmethotrexatealone, methotrexateincombinationwithinfliximab,and
methotrexateincombinationwithintravenouspulse methylprednisolone.ArthritisRheum.2007;56(12):3919–27.
31.VanVollenhovenRF,ErnestamS,GeborekP,PeterssonIF, CosterL,WaltbrandE,etal.Additionofinfliximabcompared withadditionofsulfasalazineandhydroxychloroquineto methotrexateinpatientswithearlyrheumatoidarthritis (Swefottrial):1-yearresultsofarandomisedtrial.Lancet. 2009;374(9688):459–66.
32.AbeT,TakeuchiT,MiyasakaN,HashimotoH,KondoH, IchikawaY,etal.Amulticenter,double-blind,randomized, placebocontrolledtrialofinfliximabcombinedwithlowdose methotrexateinJapanesepatientswithrheumatoidarthritis. JRheumatol.2006;33(1):37–44.
33.MainiRN,BreedveldFC,KaldenJR,SmolenJS,DavisD, MacfarlaneJD,etal.Therapeuticefficacyofmultiple intravenousinfusionsofanti-tumornecrosisfactoralpha monoclonalantibodycombinedwithlow-doseweekly methotrexateinrheumatoidarthritis.ArthritisRheum. 1998;41(9):1552–63.
34.WesthovensR,YocumD,HanJ,BermanA,StrusbergI, GeusensP,etal.Thesafetyofinfliximab,combinedwith backgroundtreatments,amongpatientswithrheumatoid arthritisandvariouscomorbidities:alarge,randomized, placebo-controlledtrial.ArthritisRheum.2006;54(4): 1075–86.
35.MainiR,StClairEW,BreedveldF,FurstD,KaldenJ,Weisman M,etal.Infliximab(chimericanti-tumournecrosisfactor alphamonoclonalantibody)versusplaceboinrheumatoid arthritispatientsreceivingconcomitantmethotrexate:a randomisedphaseIIItrial.Lancet.1999;354(9194):1932–9.
36.LipskyPE,VanderHeijdeDM,StClairEW,FurstDE,Breedveld FC,KaldenJR,etal.Infliximabandmethotrexateinthe treatmentofrheumatoidarthritis.Anti-TumorNecrosis FactorTrialinRheumatoidArthritiswithConcomitant TherapyStudyGroup.NEnglJMed.2000;343(22):1594–602.
158
rev bras reumatol.2015;55(2):146–15838.Gómez-ReinoJJ,CarmonaL,ValverdeVR,MolaEM,Montero MD,GroupB.Treatmentofrheumatoidarthritiswithtumor necrosisfactorinhibitorsmaypredisposetosignificant increaseintuberculosisrisk:amulticenter
active-surveillancereport.ArthritisRheum.2003;48(8):2122–7.
39.DixonWG,SymmonsDPM,LuntM,WatsonKD,HyrichKL, SilmanAJ,etal.Seriousinfectionfollowinganti-tumor necrosisfactoralphatherapyinpatientswithrheumatoid arthritis–lessonsfrominterpretingdatafromobservational studies.ArthritisRheum.2007;56(9):2896–904.
40.BernatskyS,HabelY,RahmeE.Observationalstudiesof infectionsinrheumatoidarthritis:ametaanalysisoftumor necrosisfactorantagonists.JRheumatol.2010;37(5):928–31.
41.MotaLMHd,CruzBA,BrenolCV,PereiraIA,Rezende-Fronza LS,BertoloMB,etal.Consenso2012daSociedadeBrasileira deReumatologiaparaotratamentodaartritereumatoide– 2012BrazilianSocietyofRheumatologyConsensusforthe
treatmentofrheumatoidarthritis.RevBrasReumatol. 2012;52(2):152–74.
42.PavelkaK,JarosovaK,SuchyD,SenoltL,ChroustK,DusekL, etal.Increasingtheinfliximabdoseinrheumatoidarthritis patients:arandomised,doubleblindstudyfailedtoconfirm itsefficacy.AnnRheumDis.2009;68(8):1285–9.
43.OllendorfDA,KlingmanD,HazardE,RayS.Differencesin annualmedicationcostsandratesofdosageincrease betweentumornecrosisfactor-antagonisttherapiesfor rheumatoidarthritisinamanagedcarepopulation.ClinTher. 2009;31(4):825–35.
44.VanVollenhovenRF.Howtodoseinfliximabinrheumatoid arthritis:newdataonaseriousissue.AnnRheumDis. 2009;68(8):1237–9.