Lindman (Marsileaceae) in the presence of hexavalent chromium
Kieling-Rubio, MA.
a,b, Droste, A.
c* and Windisch, PG.
aaPrograma de Pós-graduação em Botânica, Universidade Federal do Rio Grande do Sul – UFRGS,
Av. Bento Gonçalves, 9500, CEP 91501-970, Porto Alegre, RS, Brazil
bPrograma de Pós-graduação em Biologia, Universidade do Vale do Rio dos Sinos – Unisinos,
Av. Unisinos, 950, CEP 93022-000, São Leopoldo, RS, Brazil
cPrograma de Pós-graduação em Qualidade Ambiental, Universidade Feevale, Rod. RS-239, 2755,
CEP 93352-000, Novo Hamburgo, RS, Brazil *e-mail: annette@feevale.br
Received February 21, 2010 – Accepted April 29, 2010 – Distributed December 31, 2010 (With 1 figure)
Abstract
Regnellidium diphyllum Lindman is a heterosporous fern, growing in aquatic environments and surrounding wetlands, which is assumed to be threatened by increasing water pollution and disappearance of its natural habitats. Among contaminants, hexavalent chromium - Cr(VI) - is known to be present in effluents from some leather tanning factories. Megaspore germination tests were performed using Meyer’s solution, at concentrations 0 (control), 0.1, 0.5, 1, 5, 10, 15, 20, 30, 50, and 80 mg.L-1, from a standard solution of Titrisol® 1000 mg.L-1. The primary development of apomictic
sporophytes was studied using solutions containing 0.025 to 4.8 mg.L-1 of Cr(VI). The experiments were conducted in
a growth chamber at 24 ± 1 °C and for a 12-hour photoperiod under fluorescent lights, providing a nominal irradiance of 77 µmol.m-2/s. Significant differences in megaspore germination, with subsequent sporophytic development, were
verified from 0.5 mg.L-1 Cr(VI) concentration onwards. Growth of primary root and primary and secondary leaves was
significantly reduced at 3.2 mg.L-1 Cr(VI) concentration or higher. Considering the pollution from Cr(VI) in some areas
of R. diphyllum natural occurrence, these data indicate that low reproductive rates and disappearance of populations are likely to occur in these situations.
Keywords: heavy metals, ferns, aquatic macrophytes, pollution.
Germinação e desenvolvimento esporofítico de
Regnellidium diphyllum
Lindman (Marsileaceae) na presença de cromo hexavalente
Resumo
Regnellidium diphyllum Lindman é uma filicínea heterosporada que se desenvolve em ambientes aquáticos e áreas úmidas circundantes, sendo considerada ameaçada pelo aumento da poluição e desaparecimento dos seus hábitats naturais. Entre os contaminantes, o cromo hexavalente - Cr(VI) - é conhecido por estar presente nos efluentes de algumas indústrias de curtimento de couro. Testes de germinação foram realizados em meio líquido de Meyer, com concentrações de 0 (controle); 0,1; 0,5; 1; 5; 10; 15; 20; 30; 50; e 80 mg.L-1 de Cr(VI), a partir de uma solução padrão
de Titrisol® a 1000 mg.L-1. O desenvolvimento primário dos esporófitos apomíticos foi analisado em meios contendo de
0,025 a 4,8 mg.L-1 de Cr(VI). Os experimentos foram conduzidos em câmara de crescimento a 24 ± 1 °C, fotoperíodo
de 12 horas com lâmpadas fluorescentes fornecendo irradiância nominal de 77 µmol.m-2/s. Diferenças significativas na
germinação dos megásporos e seu subsequente desenvolvimento foram verificadas a partir da concentração 0,5 mg.L-1
de Cr(VI). O crescimento da raiz primária e das folhas primárias e secundárias foi significativamente reduzido na concentração 3,2 mg.L-1 de Cr(VI) ou superior. Considerando a poluição proveniente por Cr(VI) em algumas áreas de
ocorrência natural de R. diphyllum, esses dados indicam que as baixas taxas de reprodução e mesmo o desaparecimento das populações podem ser esperadas nessas situações.
1949; Alonso-Paz and Bassagoda, 2002). It grows in shallow waters or surrounding mud areas subjected to periodic flooding. In water, rhizomes grow attached to the mud and leaf laminae develop above the water surface. In Rio Grande do Sul, Brazil’s southernmost state, it occurs mainly in the southern, southeastern and central-western lowlands of the state. This species has been assigned a vulnerable status on the list of endangered species in the state of Rio Grande do Sul, Brazil (SEMA, 2010).
As to its occurrence in the Sinos River Watershed, southern Brazil, there are records from 1935 and a single record from 2000. Voucher specimens are deposited at the Herbarium Anchieta (PACA), Universidade do Vale do Rio dos Sinos (PACA 2574, PACA 75252, PACA 89142) and at the Herbarium ICN, Instituto de Biociências, Universidade Federal do Rio Grande do Sul (ICN 15211). Sampling efforts conducted in recent years revealed several populations in other watersheds, but not in the Sinos River Watershed. It is worth noting that the Sinos River Watershed was also the habitat of another representative of the Marsileaceae, Pilularia americana A. Braun, which is currently considered to be extinct in the state (SEMA, 2010), being known all over Brazil based on this single collection (Esteio, leg. A. Schultz, 1955, ICN 1224). Several attempts to recollect this species in its original habitat were unsuccessful, and alterations in habitat conditions may have well accounted for its disappearance.
Considering that natural environments are exposed to intense and continuous alterations and pollution due to human activities and that little is known about the sensitivity of Regnellidium diphyllum to contaminants (Wunder et al., 2009; Cassanego et al., 2010), megaspore germination and initial development of sporophytes in the presence of chromium were investigatedin vitro in order to establish the species’ tolerance limits to this contaminant, as well as to evaluate biological responses during the initial stages of the life cycle.
2. Material and Methods
Mature sporocarps of Regnellidium diphyllum were obtained from different plants in the municipality of Viamão (30° 05’ 00’’ S and 50° 47’ 00’’ W, Rio Grande do Sul State, Brazil) and kept at room temperature (about 25 °C). Voucher specimens were deposited at the Herbarium Anchieta (PACA) at Universidade do Vale do Rio dos Sinos, São Leopoldo, Brazil.
After washing in tap water, sporocarps were rinsed in 70% ethanol solution, kept for 10 minutes in 7% sodium hypochlorite solution, then washed in sterile distilled water and dried on sterile filter paper at room temperature. Sporocarps were mechanically cracked to liberate the spores. Megaspores were manually isolated from microspores under a stereomicroscope. Megaspores from sporocarps of different plants were mixed to compose the sample. Taking into account the common occurrence of apogamy in megagametophytes of Regnellidium diphyllum (Mahlberg and Baldwin, 1975), only megaspores were used in order
1. Introduction
The intense use of products that contain heavy metals increases pollution levels in various ecosystems, causing adverse effects and altering patterns of natural biogeochemical cycles. Therefore, heavy metals have received special attention since they are not biodegraded or biotransformed, remaining as persistent contaminants in ecosystems and food chains (Shanker et al., 2005).
Chromium (Cr) is widely distributed in nature, being the seventh most abundant metal in the Earth’s crust (Panda and Choudhury, 2005). It occurs in several oxidation states, ranging from Cr(III) to Cr(VI), trivalent (III) and hexavalent (VI) chromium being the most stable and common states in the environment (Chandra and Kulshreshtha, 2004). Cr(VI) is considered the most toxic form of chromium, usually associated with oxygen as chromate (CrO42-) or
dichromate (Cr2O72-) oxyanions (Shanker et al., 2005). Due
to their negative charge, both chromate and dichromate are rarely adsorbed by organic particles, being highly soluble in water. Cr(III) has lower mobility and toxicity and is found binding to organic matter in soil and aquatic environments (Becquer et al., 2003).
Contamination of soil and water due to the use of Cr(VI) in industrial activities has become a serious concern over the past two decades. Nriagu and Pacyna (1988) estimated that around 480 to 1,300 thousand tons of chromium are being added to soil each year, resulting mainly from the metallurgical and leather industry, fossil fuel combustion, and sewage sludge. The impact of this widespread contamination poses significant ecological risks (Roos, 1995), since Cr is considered to be a mutagenic, carcinogenic and teratogenic agent (Mitteregger-Junior et al., 2006). The biotoxicity of Cr(VI) is related to its ability to cross biological membranes, its strong oxidising capacity and its interference with the processes of respiration and photosynthesis (Shanker et al., 2005).
Chromium is found in all phases of the environment, including air, water and soil (Shanker et al., 2005). In Brazil, soil chromium content, found naturally in different types of soil, ranges from 9.6 to 75 mg.kg-1 (Fadigas et al.,
2002). According to the Brazilian National Environmental Council (CONAMA), the current regulatory limit for Cr in water is 0.05 mg.L-1 (MMA, 1986).
Although chromium is not known to play any direct role in metabolic process, it is absorbed by plants, being detrimental to their growth and development (Shanker et al., 2005). The phytotoxicity of Cr generates an oxidative stress that leads to peroxidation of lipids. Consequently, severe damage to cell membranes and degradation of photosynthetic pigments and enzymes result in inhibition of germination and decreased plant growth (Poschenrieder et al., 1991; Panda and Choudhury, 2005), which may have a negative impact on the establishment of sensitive species.
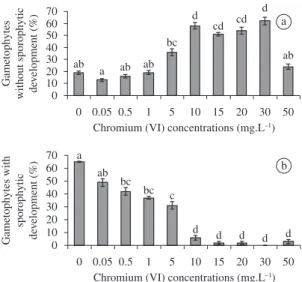
in sporophyte formation in the treatments from 0.5 mg.L-1
Cr(VI) concentration onwards (Figure 1b). Only the lowest Cr(VI) concentration tested (0.05 mg.L-1) did not differ
significantly from the control. From 10 mg.L-1 Cr(VI)
concentration onwards, only 6 to 0% of the gametophytes formed sporophytes.
The length of the primary root of sporophytes was affected negatively by Cr(VI) after seven and 28 days in culture (Table 1). The development of primary and secondary leaves were also negatively affected by the presence of Cr(VI) in both recorded times. In treatments at 3.2 and 4.8 mg.L-1 Cr(VI) concentrations, the secondary leaf was
not formed after seven days in culture. After 28 days, small and morphologically anomalous leaves could be observed only in a reduced number of individuals at 4.8 mg.L-1
Cr(VI) concentration (Table 1). Plantlets exposed during 28 days to 3.2 and 4.8 mg.L-1 Cr(VI) concentrations showed
visible symptoms of leaf chlorosis and necrosis, as well as necrotic areas in the roots.
4. Discussion
Chromium is considered to be toxic to plants and does not play any role in plant metabolism (Dixit et al., 2002). Germination is the first physiological process affected by Cr(VI). Hydrolysis of proteins and starch provides amino acids and sugars essential for germination. Cr can decrease the production of α- and β-amylase, inhibiting germination and reducing the sugar content necessary for the development of embryo axes. On the other hand, to obtain uniform cultures, thus avoiding the mixture of
sexually and apomictically formed sporophytes. As culture medium, Meyer’s solution (Meyer et al., 1955) was prepared at different concentrations of Cr(VI) in the form of potassium dichromate (K2Cr2O7). The pH was adjusted with NaOH to 6.0 before autoclaving and K2Cr2O7 was added to the medium after autoclaving. Two experiments were conducted to evaluate megaspore germination and sporophyte formation. In Experiment I, megaspores germination was evaluated at 0 (control), 0.1, 0.5, 1, 5, 10, 15, 20, 30, and 50 mg.L-1 K
2Cr2O7. Twenty-five
megaspores were placed in each glass vial (4.5 × 10 cm) with 25 mL of the solution, with four repetitions for each treatment. In Experiment II, sporophytic development was evaluated at 0 (control), 0.025, 0.05, 0.1, 0.2, 0.4, 0.8, 1.6, 3.2, and 4.8 mg.L-1 K
2Cr2O7. Fifteen megaspores were
placed in each glass vial with 20 mL of the solution, with five repetitions for each treatment. The culture vials for both experiments were maintained in a growth chamber at 24 ± 1 °C, under artificial light with nominal irradiance of 77 µmol.m-2/sand a 12-hour photoperiod.
In Experiment I, observations were made once a week for a total of 21 days. Every seven days, three germinated individuals were taken from each repetition. Megaspores showing at least one globular green structure with a crown of rhizoids were considered germinated. After 21 days, total percentage germination was calculated for each treatment.
In Experiment II, 10 individuals were eliminated from each glass vial at the end of seven days, thus maintaining five individuals in each repetition. The length of the primary root and the length of the primary and secondary leaves were measured for each individual after seven and 28 days in a laminar flow chamber.
Data on megaspore germination and sporophyte formation were then converted into percentages. Data were tested for normality and homogeneity using the Kolmogorov-Smirnov test and Levene’s test, and ANOVA was applied to the data meeting the normality and homogeneity assumption. Differences between means were verified using Tukey’s test, with α = 0.05. The Kruskal-Wallis test was applied to data that did not conform to normal distribution, and differences between means were verified using Dunn’s test, with α = 0.05 (Zar, 1999). The analyses were conducted using the SPSS 12.0 software.
3. Results
The different Cr(VI) concentrations tested in Experiment I adversely affected megaspore germination. Germinated megaspores were classified in two types; those showing sporophytic structures and those without any further development. Cr(VI) concentration interfered with the capacity to develop sporophytes. From 10 mg.L-1 onwards,
the percentage of germinated megaspores that did not form sporophytes was higher than the percentage obtained for megaspores exposed to lower Cr(VI) concentrations (Figure 1a). A significant negative influence was observed
a
b
Figure 1. Germination of Regnellidium diphyllum Lindman
chlorophyll-b and carotenoid concentrations are related to growth inhibition and could be observed in plants such as Lemna minor L., Pistia stratiotes L. (Bassi et al., 1990), and Taxithelium nepalense (Schwägr.) Broth. (Panda and Choudhury, 2005).
Considering the negative impact of Cr(VI) on megaspore germination and initial sporophytic development of Regnellidium diphyllum even at low concentrations, the contamination of aquatic environments by industrial effluents could be regarded as a threat to the establishment and preservation of populations of the currently vulnerable R. diphyllum.
Acknowledgements – The authors are grateful to the Universidade
do Vale do Rio dos Sinos for the use of laboratories and for the scholarship granted to the first author; to the Universidade Feevale, to the Universidade Federal do Rio Grande do Sul (UFRGS), and to the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) for the support and research grants.
References
ALONSO-PAZ, E. and BASSAGODA, MJ., 2002. Revisión de las Marsileaceae del Uruguay y primera cita de Pilularia americana A.
Braun. Comunicaciones Botânicas - Museo Nacional de Historia Natural y Antropologia, vol. 125, no. 6, p. 18.
BASSI, M., CORRADI, MG. and FAVALI, MA., 1990. Effect of chromium (VI) on two fresh water plants, Lemna minor and Pistia stratiotes: II. Biochemical and physiological observations. Cytobios, vol. 62, p. 101-109.
BECQUER, T., QUANTIN, C., SICOT, M. and BOUDOT, JP., 2003. Chromium availability in ultramafic soils from New Caledonia.
Science of the Total Environment, vol. 301, p. 251-261.
CASSANEGO, MBB., DROSTE, A. and WINDISCH, PG., 2010. Effects of 2,4-D on the germination of megaspores and initial protease activity can be enhanced by the presence of Cr,
which also inhibits germination (Zeid, 2001).
The growth reduction of sporophytic structures, such as primary root, primary leaf and first secondary leaf, reported in this study for Regnellidium diphyllum was also verified in other species of aquatic macrophytes. For Myriophyllum spicatum L., maximum growth was obtained at Cr(VI) concentrations up to 50 µg.L-1 and concentrations higher
than 1 mg.L-1 caused a linear reduction in plant weight
and length (Guillizzoni et al., 1984).
Nicholset al. (2000) verified a significant growth reduction in the fern Salvinia minima Baker cultivatedin vitrowith Hoagland solution at Cr(IV) concentrations of 1 mg.L-1 and higher. Cr(VI) was shown to have a cumulative
effect on the fern, and growth reduction could be related to a decreased nutrient uptake. Sela et al. (1989) showed that the presence of Cr(VI) can lead to a decrease in Ca, Mg and K uptake. Similar influence of Cr on the uptake of mineral nutrients was reported for P, Na, N, Fe, and Cu (Moral et al., 1995, 1996).
In the present study, white and brown leaves, as well as root browning, could be observed in sporophytes exposed to 0.2 mg.L-1 Cr(VI) concentration and higher.
Similar observations were made concerning the aquatic macrophyte Limnanthemum cristatum (Roxb.) Griseb. exposed to a 1 mg.L-1 Cr concentration, which showed
reduced length and lack of colour or brown colour of roots, in addition to significant morphological changes (Garg et al.,1994).
Symptoms of chlorosis in young leaves and the associated reduction in chlorophyll content can be attributed to Cr accumulation in plants (McGrath, 1985; Panda and Patra, 1997; Panda and Dash, 1999; Nichols et al., 2000; Panda, 2003; Panda et al., 2003). Ultrastructural modifications in chloroplasts with significant reduction in chlorophyll-a,
Table 1. Influence of different Chromium(VI) concentrations on the growth of Regnellidium diphyllum Lindman. Mean length of primary root and primary and secondary leaves after 7 and 28 days in culture (mean ± standard deviation). Means with different letters in the same column are significantly different by Dunn’s test (P = probability; H = statistics of the Kruskal-Wallis test; d.f. = degrees of freedom).
Chromium (mg.L-¹)
Primary root (mm) Primary leaf (mm) Secondary leaf (mm) 7 days 28 days 7 days 28 days 7 days 28 days
0.0 5.72 ± 1.30a 6.80 ± 0.72a 8.00 ± 0.86a 10.12 ± 0.50a 0.86 ± 0.43a 9.04 ± 0.75a
0.025 5.04 ± 1.17a 6.10 ± 2.69ab 8.36 ± 1.66a 6.42 ± 2.20ab 0.54 ± 0.34a 9.20 ± 0.65a
0.05 2.02 ± 0.67cd 2.46 ± 0.40cde 4.32 ± 0.44b 4.84 ± 1.45bc 0.12 ± 0.22a 6.04 ± 0.93ab
0.1 4.24 ± 1.20abc 5.10 ± 1.11abc 7.60 ± 1.46a 6.60 ± 2.66ab 0.08 ± 0.13a 8.80 ± 2.35a
0.2 2.06 ± 0.77cd 2.48 ± 1.23cde 4.82 ± 2.02b 4.40 ± 2.98bcd 0.16 ± 0.22a 6.28 ± 1.28ab
0.4 2.49 ± 1.40bcd 3.14 ± 1.53cde 8.72 ± 0.44a 8.16 ± 0.97ab 0.83 ± 0.63a 7.00 ± 1.05a
0.8 4.86 ± 1.47ab 5.46 ± 1.16abc 8.72 ± 0.44a 10.40 ± 0.60a 0.85 ± 0.54a 9.00 ± 1.24a
1.6 3.96 ± 2.12abc 3.72 ± 3.00bcd 8.18 ± 1.68a 8.40 ± 3.57ab 0.80 ± 0.76a 8.64 ± 3.38a
3.2 0.15 ± 0.16d 0.0e 0.62 ± 0.84c 0.0d 0.0b 0.0d
4.8 0.38 ± 0.55d 0.56 ± 0.78d 1.18 ± 1.81c 1.64 ± 2.48cd 0.0b 2.48 ± 4.08cd
H = 37.752 d.f. = 9, 24 p < 0.001
H = 37.016 d.f. = 9, 24 p < 0.001
H = 36.703 d.f. = 9, 24 p < 0.001
H = 36.571 d.f. = 9, 24 p < 0.001
H = 31.89 d.f. = 9, 24
p < 0.001
NICHOLS, PB., COUCH, JD. and AL-HAMDANI, SH., 2000. Selected physiological responses of Salvinia minima to different
chromium concentrations. Aquatic Botany, vol. 68, p. 313-319.
NRIAGU, JO. and PACYNA, JM., 1988. Quantitative assessment of worldwide contamination of air, waters and soils with trace metal. Nature, vol. 333, p. 134-139.
PANDA, SK. and CHOUDHURY, S., 2005. Chromium stress in plants. Brazilian Journal of Plant Physiology, vol. 17, no. 1, p. 95-102.
PANDA, SK. and DASH, M., 1999. Regulation of senescence by Cr(VI) ions in excide wheat leaves. Journal of the Nature and Botanical Society, vol. 53, p. 35-37.
PANDA, SK. and PATRA, HK., 1997. Physiology of chromium toxicity in plants – A review. Plant Physiology and Biochemistry, vol. 24, no. 1, p. 10-17.
PANDA, SK., 2003. Heavy metal phytotoxicity induced oxidative stress in Taxithelium sp. Current Science, vol. 84, p. 631-633.
PANDA, SK., CHOUDHURY, I. and KHAN, MH., 2003. Heavy metals induce lipid peroxidation and affects antioxidants in wheat leaves. Biologia Plantarum, vol. 46, p. 289-294.
POSCHENRIEDER, C., VAZQUEZ, MD., BONET, A. and BARCELO, J., 1991. Chromium III iron interaction in iron sufficient and iron deficient bean plants. II ultrastructural aspects.
Journal of Plant Nutrition, vol. 14, p. 415-428.
ROOS, MC., 1995. Charting tropical plant diversity: Europe´s
contribution and potential. Working document European Science Foundation/Linnean Society/Rijksherbarium Hortus Botanicus/ Systematics Association workshop “Systematics Agenda 2000: the challenge for Europe”, Leiden.
SCHULTZ, AR., 1949. Contribuições ao conhecimento de
Regnellidium diphyllum Lindman. Lilloa, vol. 17, p. 139-144.
Secretaria do Meio Ambiente - SEMA. Espécies da flora ameaçadas de extinção do Rio Grande do Sul. Porto Alegre: Secretaria do Meio Ambiente. Available from: <http://www.sema.rs.gov.br/sema/ html/pdf/especies-ameacadas.pdf>. Access in: 05 jan. 2010.
SELA, M., GARTY, J. and TEL-OR, E., 1989. The accumulation and the effect of heavy metals on the water fern, Azolla filiculoides. New Phytologist, vol. 112, p. 7-12.
SHANKER, AK., CERVANTES, C., LOZA-TAVERA, H. and AVUDAINAYAGAM, S., 2005. Chromium toxicity in plants.
Environment International, vol. 31, p. 739-753.
WUNDER, DA., DROSTE, A. and WINDISCH, PG., 2009. Megaspore germination and initial development of Regnellidium diphyllum Lindman (Pteridophyta, Marsileaceae) sporophytes
in the presence of cadmium. Revista Brasileira de Botânica, v. 31, p. 177-181.
ZAR, JH., 1999. Biostatistical analyses. 4th ed. Upper Saddle
River: Prentice-Hall, 931 p.
ZEID, IM., 2001. Responses of Phaseolus vulgaris to chromium and cobalt treatment. Biologia Plantarum, vol. 44, p. 111-115. development of Regnellidium diphyllum Lindman (Monilophyta,
Marsileaceae). Brazilian Journal of Biology, vol. 70, no. 2, p. 361-366.
CHANDRA, P. and KULSHRESHTHA, K., 2004. Chromium accumulation and toxicity in aquatic vascular plants. The Botanical Review, vol. 70, no. 3, p. 313-327.
DIXIT, V., PANDEY, V. and SHYAM, R., 2002. Chromium ions inactivate electron transport and enhance superoxide generation in vivo in pea (Pisum sativum L. cv: Azad) root mitochondria. Plant Cell & Environment, vol. 25, p. 687-693.
FADIGAS, FS., AMARAL-SOBRINHO, NMB., MAZUR, N., ANJOS, LHC. and FREIXO, AA., 2002. Concentrações naturais de metais pesados em algumas classes de solos brasileiros.
Bragantia, vol. 61, no. 2, p. 151-159.
GARG, P., CHANDRA, P. and DEVI, S., 1994. Chromium(VI) induced morphological changes in Limnanthemum cristatum
Griseb.: A possible bioindicator. Phytomorphology, vol. 44,
no. 3-4, p. 201-206.
GUILLIZZONI, P., ADAMS, MS. and MCGAFFEY, N., 1984. The effect of chromium on growth and photosynthesis of a submerged macrophyte, Miriophyllum spicatum. In RASMUSSEN.
Ecotoxicology: Proceedings of the third Oikos Conference. Stockholm: Ecological Bulletins, p. 90-96.
MAHLBERG, PG. and BALDWIN, M., 1975. Experimental studies on megaspore viability, parthenogenesis and sporophyte formation in Marsilea, Pilularia and Regnellidium. Botanical Gazette, vol. 136, no. 3, p. 269-273.
McGRATH, SP., 1985. Chromium and nickel. InALLOWAY, BJ.
Heavy metals in soil. London: Chapman and Hall, p. 139-167.
MEYER, BS., ANDERSON, DB. and SWANSON, CA., 1955.
Laboratory plant physiology. Princeton: Van Nostrand, 168 p.
Ministério do Meio Ambiente - MMA, 1986. Resolução CONAMA no. 20, de 18 de junho de 1986. Dispõe sobre a classificação das águas e estabelece os limites máximos das substâncias potencialmente prejudiciais. Diário Oficial da União, Poder
Executivo, Brasília, DF, 30 jul. 1986. Seção II, p. 8.
MITTEREGGER-JUNIOR, H., FERRAZ-DIAS, J., LÚCIA-YONEMA, M., ARENZON, A. and PEGAS-HENRIQUES, JA., 2006. Avaliação das atividades tóxicas e mutagênicas da água e do sedimento do arroio Estância Velha, região coureiro-calçadista, utilizando Allium cepa.Journal of the Brazilian Society of Ecotoxicology, vol. 2, no. 1, p. 147-151.
MORAL, R., GOMEZ, I., PEDRENO, JN. and MATAIX, J., 1996. Absorption of Cr and effects on micronutrient content in tomato plant (Lycopersicum esculentum M). Agrochimica, vol. 40, p. 132-138.