Preparation and Characterization of Starch Grafted with Toluene
Poly(propylene oxide) Diisocyanate
D.C. Dragunski, A. Pawlicka*
Instituto de Química de São Carlos, Universidade de São Paulo, 13560-970 São Carlos - SP, Brazil
Received: November 11, 2000; Revised: April 20, 2001
Amylopectin-rich starch samples (Amidex 4001 Corn Products Brasil Ingredientes Industriais Ltda.) were grafted with polyethers with the purpose of obtaining new materials for application as solid polymeric electrolytes. Grafting reaction was performed by the addition of starch dissolved in DMSO to toluene poly(propylene oxide) diisocyanate (Resibras) dissolved in the same solvent. This reaction produced a film with good mechanical properties. The film samples were characterized by
13
C-NMR, FTIR, DSC, X-Ray and SEM. The FTIR spectrum shows a sharp NH band and a very small urethane band. The 13C-NMR spectrum revealed a peak at 20 ppm, that can be attributed to the CH3 of the polyether chain, and two small peaks at 117 and 140 ppm, attributed to the aromatic
ring. The X-ray diffractograms also indicated that after the grafting reaction, the samples of amylopectin-rich starch are more amorphous. Moreover, the glass transition temperature (Tg) dropped from 50 °C to -11 °C. These results indicate formation of grafted products and the low Tg of the samples suggests that polyether-grafted starch is a good candidate to obtain solid polymeric electrolytes.
Keywords: starch, grafting, solid polymeric electrolytes
1. Introduction
The development of new solid materials for application as electrolytes allows for the creation of modern energy generation and storage systems. Among these materials, solid polymeric electrolytes, usually elastomers containing lithium salts, offer a promising alternative to replace the liquid electrolytes and inorganic crystals used in batteries, sensors and electrochromic devices1.
Several types of solid electrolytes are being investi-gated. The most studied of these is poly(ethylene oxide) (PEO), which has lithium salt dissolving properties2. How-ever, its tendency to crystallization reduces its ionic con-ductivity at room temperature, limiting its possible application as a solid polymeric electrolyte3. In order to take advantage of the excellent solubilization properties of these salts and simultaneously reduce the system’s crystal-linity, PEO can be modified by Al2O3 addition, resulting in
blends4, co-polymerizations5 or grafting reactions with other polymers containing metals such as Si or totally
organic polymers5,7,8.The last one include natural polymers such as polysaccharides, the principal ones being cellu-lose9, quitosane10 and starch11.
Some polysaccharides and their derivatives are proc-essable in the form of films, e.g., starch, hydroxyethyl cellulose (HEC). Thus, the films obtained from this poly-mer have good mechanical properties, besides being eco-nomically viable materials owing to both their easy availability in nature and their renewability12.
Similarly to HEC and hydroxypropyl cellulose (HPC)13, starch can be modified by grafting reactions. The introduction of ramifications in the polymer chain can produce marked differences in its chemical and physico-chemical behavior. Grafted products can be obtained by different chemical processes (radical or ionic), through reactions of substitution or addition14.
The main objective of this work, therefore, is to discuss the preparation and characterization of starch films grafted with toluene poly(propylene oxide) diisocyanate for poten-tial application as solid polymeric electrolytes.
*e-mail: agnieszka@iqsc.sc.usp.br
2. Experimental
The amylopectin-rich starch samples (Amidex 4001 Corn Products Brasil Ingredientes Industriais Ltda.) were characterized by infrared spectroscopy (FTIR), nuclear magnetic resonance (1H-NMR), X-ray diffraction (XRD), differential scanning calorimetry (DSC) and the film sam-ples visualized by scanning electron microscopy (SEM).
The FTIR spectra of the samples of dry amylopectin, pressed into pellets with KBr (proportion 1:100 in weight), were obtained with a BOMEM model MB-102 spectrome-ter.
The 1H-NMR spectra of the starch samples (3% w/v
solution in deutered DMSO) were obtained using a BRUCKER AC200 (200 MHz) spectrometer.
The CP/MAS 13C-NMR spectra were obtained with a Varian Unity 400 spectrometer (100,58 MHz) (Embrapa -São Carlos, SP). The references used were tetramethylsi-lane and hexamethylbenzene as secondary reference (132,3 ppm).
The differential scanning calorimetry (DSC) analyses were performed using a SHIMADZU DSC-50 equipment, in a dynamic nitrogen atmosphere (20 mL/min flow) and a heating rate of 10 °C/min.
The X-ray analyses were obtained with an Carl Zeiss Jena URD-6 diffractometer with CuK irradiation (λ = 1540 Å), and the micrographs were obtained with a ZEISS-LEICA 400 scanning electron microscope.
The toluene poly(propylene oxide) diisocyanate (Resi-bras) was characterized by FTIR and 1H-NMR, using the same procedure described above for amylopectin.
The grafting reactions were performed by mixing an amylopectin solution (0,05 g)/DMSO(5 mL) with a solu-tion of diisocyanate (0,78 g)/DMSO(10 mL) in a glove box under dry N2 atmosphere. The films were molded on a
teflon plate and, left to rest for 48 hours, oven dried at 50 °C under vacuum, and then characterized by FTIR, NMR, X-ray and SEM.
3. Results and Discussions
Figure 1 presents the infrared spectra of amylopectin-rich starch and toluene poly(propylene oxide) diisocyanate. The wide band observed at 3348 cm-1 can be attributed to the O-H stretching of the amylopectin and its width was ascribed to the formation of inter and intramolecular hydro-gen bonds. The bands at 2935 and 2887 cm-1 were attrib-uted to the asymmetric stretching of C-H, while the band at 1656 cm-1 was ascribed to adsorbed water and the bands at 1421 and at 1357 cm-1 to the angular deformation of C-H. The C-O ether bond shows stretching at 1156 cm-1 while the C-O alcohol bond shows stretching at 1015 cm-1. Sev-eral main diisocyanate absorption bands were found, as follows: the band at 2260 cm-1 represents stretching of the NCO group; the low intensity bands at 1600 and 3400 cm-1
were probably caused by vibrations of the NH bonds; the band at 1726 cm-1 represents stretching of the C=O group; the one at 1100 cm-1 was caused by stretching of the C-O group; the band at 2870 cm-1 represents stretching of the C-H group; the one at 2970 cm-1 was caused by stretching of the C-H (CH3) group; the band at 1370 cm-1 represents
deformation of the C-H bond; and finally, the bands at 1537 and 1450 cm-1 probably represent a three-substituted ben-zene ring15.
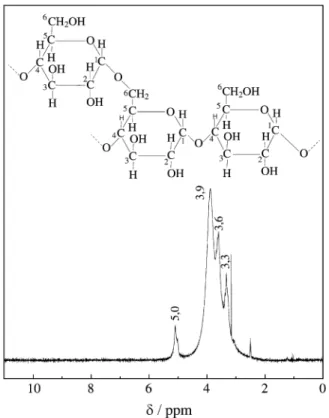
Figure 2 shows the 1H-NMR amylopectin spectrum while Fig. 3 presents that of the diisocyanate. Characteristic amylopectin peaks were found at 3.3 ppm, which was attributed to the hydrogens linked to the C6 carbon of
CH2-O and CH2-OH; at 3.6 ppm, attributed to the
hydro-gens linked to the C1 and C4 carbons of CH-O, at 3.9 ppm,
attributed to the hydrogens linked to the C2, C3 and C5
carbons of CH-OH; and at 5.0 ppm, attributed to the hydro-gens of the R-OH hydroxyl groups. This peak at 5.0 ppm is slightly larger because of the presence of hydrogen bonds. Characteristic diisocyanate peaks were found at 1.05 ppm, which was attributed to the CH3 of the
poly(pro-pylene oxide) chain; at 1.99 ppm, ascribed to the toluene’s CH3; at 2.21 ppm, attributed to the CH2 of the
poly(propyl-ene oxide) chain of CH2-C(=O)N; and at 3.45 ppm,
as-cribed to CH2 and CH linked to oxygen. This peak is wider
due to the very close chemical shift of these two hydrogens. Additional diisocyanate peaks were also present at 4.2 ppm, referring to CH linked to the CH-O-CH of the poly(propylene oxide) terminal group; at 5.0 ppm, for the hydrogen of the NH urethane bond; and at 7.2 ppm, which was attributed to the hydrogens of three-substituted ben-zene ring. The peak at 2.5 ppm was attributed to the DMSO hydrogens15.
(Fig. 4) shoved that the band attributed to the NCO group (2260 cm-1) disappeared and that a marked narrowing of another band at 3422 cm-1 occurred, which may be attrib-uted to the formation of new NH bonds. In addition, a new urethane band was observed at approximately 1730 cm-1.
Figure 5 shows the 13C RMN CP/MAS spectrum of amylopectin and grafted amylopectin. The peak at 64 ppm can be attributed to C6 carbon; the one at 74 to the C2, C3,
C4 and C5 carbons; and the peak at 103 ppm to the C1
carbon. The grafted amylopectin showed peaks appearing at 20 ppm, attributed to the CH3 of the poly(propylene
oxide) chain; at 67 ppm, produced by the CH2-O; at 117
and at 140 ppm, which refer to the aromatic ring; and at 160 ppm, attributed to the urethane bond. A decrease was also observed in the intensity of the C6 peak, as well as a
separation and displacement of the peaks attributed to the C2, C3, C4 and C5 carbons. These results suggest the
forma-tion of grafted amylopectin15.
The X-ray diffraction analysis (Fig. 6) also revealed a change in the structure of the amylopectin in relation to the amylopectin grafted with diisocyanate. The diffractograms of the amylopectin used for grafting presented three distinct peaks for the Bragg angles of 15, 18 and 23 degrees. This result is characteristic of cereal starch crystals, in this case corn; these crystals are known as A type16. The charac-teristic amylopectin peaks disappeared after the grafting reaction, indicating a predominantly amorphous state. This Figure 2.1H-NMR spectrum of the amylopectin-rich starch.
Figure 3. 1H-NMR spectrum of toluene poly(propylene oxide) diisocy-anate.
Figure 4. FTIR of the amylopectin-rich stanch grafted with toluene poly(propylene oxide) diisocyanate.
result appears to corroborates the results obtained by scan-ning electronic microscopy. The amylopectin micrographs (Fig. 7) show a granular structure, with average grain diameter ranging from 7 to 15 µm. The films of grafted amylopectin appear without grains, although there are some lighter domains (Fig. 8), which may be due to the larger concentration of film in the sample.
Figure 9 shows the DSC curves of the pure and grafted amylopectins. It can be observed, that the glass transition temperature (Tg) for pure amylopectin is about 50 °C (usual for this kind of amylopectin sample17,18) and after grafting reaction this Tg value drop to -11 °C. This lower than room temperature Tg in grafted samples is a highly relevant factor for the potential application of this material as solid electrolytes, since low Tg allows for greater chain mobility and, hence, improve solvation and ion conduction.
4. Conclusion
Amylopectin-rich starch was characterized and submit-ted to a grafting reaction with toluene poly(propylene ox-ide) diisocyanate with the purpose of producing a new starting material for the preparation of solid polymeric
electrolytes. After the grafting reaction, the FTIR analysis revealed appearance of band attributed to the NH bond and the disappearance of diisocyanate NCO bands. The RMN spectra showed new peaks, which were attributed to the poly(propylene oxide) methyl groups, and to aromatic tolu-ene ring peaks indicative of the occurrence of a grafting reaction. The SEM micrographs of pure and grafted amy-lopectin samples showed that the characteristic crystalline granules of starch disappear after the grafting reaction. Thermal analysis reveal also that the Tg values of the pure samples drop from 50 °C to -11 °C after grafting reaction. These results show that this kind of material is a good potential candidate for solid polymeric electrolyte applica-tions.
Acknowledgments
The authors thank FAPESP for the financial support of this research work. The contributions of starch from Corn Products Ingredientes Industriais Ltda. and of diisocyanate from Resibras are also gratefully acknowledged. Special Figure 6. X-ray diffractograms of pure and grafted amylopectin-rich
stanch.
Figure 7. SEM micrograph of amylopectin-rich stanch grains deposited on carbon films (1000x).
Figure 8. SEM micrograph of amylopectin-rich stanch grafted with toluene poly(propylene oxide) diisocyanate (1000x).
thanks are due to Prof. Dr. A. Aprigio S. Curvelo for his highly relevant scientific discussions.
References
1. Regiani, A.M.; Pawlicka, A.; Curvelo, A.A.S.; Gand-ini, A.; Le Nest, J.F. Polímeros: Ciência e Tecnologia, n. 3, p. 45, 1999.
2. Armand, M.B.; Chabagno, J.M.; Duclot, M.J. Fast Ion Transport in Solids, eds. Vashishta, P; Mundy, J.N.; Shenoy, G. K. Amsterdam, North-Holland, p. 131, 1979.
3. Carvalho, L.M.; Guégan, P.; Cheradame, H.; Gomes, A.S. European Polymer Journal, v. 36, p. 401, 2000. 4. Tambelli, C.E.; Donoso, J.P.; Magon, C.J.; Ângelo, A.C.D.; Florentino, A.O.; Saeki, M.J. Solid State Ion-ics, v. 136, p. 243, 2000.
5. Zoppi, R.A.; Fonseca, C.M.N.P.; De Paoli, M.A.; Nunes, S.P. Solid State Ionics, v. 91, p. 123, 1996. 6. Zoppi, R.A.; Fonseca, C.M.N.P.; De Paoli, M.A.;
Nunes, S.P. Acta Polymer., v. 48, p. 193, 1997. 7. Munro, B.; Krämer, S.; Zapp, P.; Krug, H.; Schmidt,
H. SPIE, v. 3136, p. 470, 1997.
8. Dahmouche, K.; Atik, M.; Mello, N.C.; Bonagamba, T.; Panepucci, H.; Judeinstein, P.; Aegerter, M.A. Solar Energy Materials and Solar Cells, v. 54, p. 1, 1998.
9. Schoenenberger, C.; Le Nest, J.F.; Gandini, A. Elec-trochimica Acta, v. 40, p. 2281, 1995.
10. Velazquez M.P; Le Nest, J.F.; Gandini, A. Electro-chimica Acta, v. 43, p. 1275, 1998.
11. Galliard, T. Starch: Properties and potential, Critical Reports on applied chemistry, Galliard, T., ed. John Wiley & Sons, v. 13, p. 1, 1987.
12. Lorcks, J. Polymer Degradation and Stabability, v. 59, p. 245, 1998.
13. Regiani, A M.; Tambelli, C.E.; Pawlicka, A; Curvelo, A.A.S.; Gandini, A.; Lenest, J.F.; Donoso, J.P. Poly-mer International, v. 49, p. 960, 2000.
14. Athawale, V.D.; Lele, V. Carbohydrate Polymers, v. 41, p. 407, 2000.
15. Silverstein, R.M.; Bassler, C.G.; Morril, T.C. Identi-ficação Espectrométrica de Compostos Orgânicos, 5º edição, ed. Guanabara Koogan S. A., Rio de Janeiro, 1991.
16. van Soest, J.J.G.; Vliegenthart, F.G.J. TIBTECH, v. 15, p. 208, 1997.
17. Rindlav, A.; Hulleman, S.H.D.; Gatenholm, P. Car-bohydrate Polymers, v. 34, p. 25, 1997.
18. Song, Y.; Jane, J. Carbohydrate Polymers, v. 41, p.365, 2000.