JAL 8041
Synthesis and characterization of lanthanide trifluoroacetate
complexes with 3-picoline-N-oxide
L. B. Zinner, C. A. Alves de C a r v a l h o a n d K. Zinner
Instituto de Quimica, Universidade de $6~o Paulo, C.P. 20.780, CEP 01498, Sdo Paulo SP (Brazil)
A b s t r a c t
Complexes with composition Ln(F3C--COO)3-2(3-picNO).xH20 were synthesized. Ac- cording to X-ray powder patterns and IR data five series of isomorphous compounds were detected: L a N d ; Sm-Eu; Gd>Tm, Y; Ho, Er, Tb; Dy, Yb, Lu. They behave as non- electrolytes in methanol. IR spectra show a shift of rNO to lower frequencies, as compared with the free ligand, as a result of coordination through the oxygen. The bands attributed to the anions indicate two modes of coordination. The neodymium spectrum at 77 K shows that the central ion is not involved in a cubic site. Electrostatic bonds between Nd a+ and ligands were evidenced by the nephelauxetic, Sinha's parameters and covalent factor from the room temperature spectrum. Using the electronic emission spectrum, at 77 K, it was possible to conclude that europium ions present two different symmetry sites, since a splitting of the 5Do-~ ~F9 band was observed. The complex species are probably polymeric or at least dimeric, and europium ions are involved in two different sites, but with the same C3~ symmetry.
1. I n t r o d u c t i o n
L a n t h a n i d e t r i f l u o r o a c e t a t e c o m p l e x e s with 3 - p i c o l i n e - N - o x i d e (3-picNO) as ligand a n d o f c o m p o s i t i o n L n ( F 3 C - C O O ) 8 - 2 ( 3 - p i c N O ) . x H 2 0 ) (the w a t e r c o n t e n t is c l o s e t o unity, v a r y i n g f r o m 0 . 7 5 to 1.33) w e r e p r e p a r e d a n d c h a r a c t e r i z e d .
2. E x p e r i m e n t a l d e t a i l s
A m i x t u r e o f t h e h y d r a t e d l a n t h a n i d e t r i f l u o r o a c e t a t e s a n d t h e ligand ( m o l a r ratio, 1:2) w a s g e n t l y w a r m e d a n d t h e c o m p l e x e s w e r e p r e c i p i t a t e d b y a d d i t i o n o f d r y ethyl e t h e r u n d e r v i g o r o u s stirring with a g l a s s rod. T h e c o m p o u n d s w e r e d e c a n t e d , w a s h e d with e t h e r a n d dried i n v a c u o o v e r a n h y d r o u s c a l c i u m chloride.
L a n t h a n i d e i o n c o n t e n t w a s d e t e r m i n e d b y E D T A c o m p l e x o m e t r i c titration. C a r b o n , h y d r o g e n a n d n i t r o g e n w e r e m e a s u r e d b y t h e u s u a l m i c r o a n a l y t i c a l p r o c e d u r e s .
C o n d u c t a n c e m e a s u r e m e n t s w e r e p e r f o r m e d at 2 5 . 0 0 ± 0.02 °C u s i n g a p o i n t e r g a l v a n o m e t e r , a r e s i s t a n c e b o x a n d a L e e d s a n d N o r t h r u p cell
( K c = 0 . 1 0 7 08 cm-1). IR spectra were recorded in a Perkin-Elmer 1750 s p e c t r o p h o t o m e t e r using KBr pellets or Nujol mulls b e t w e e n KBr windows. X-ray p o w d e r patterns were obtained on a Rigaku RU-200B instrument using Cu Ka radiation. The absorption spectra of the neodymium c o m p o u n d were obtained on a Cary 2300 spectrophotometer. The fluorescence spectrum of the europium complex was recorded on an MPF-4 P e r k i n - E l m e r spectro- fluorometer with 394 n m excitation. Thermogravimetric (TG) studies employed Perkin-Elmer TGA-7 and DSC-7 systems using c a . 2 mg samples and several
heating rates.
3. R e s u l t s a n d d i s c u s s i o n
Analytical results are in accordance with the p r o p o s e d composition Ln(FsC-COO)3.2(3-picNO) "xH20 (Table 1). According to TG analysis water is eliminated in the 4 0 - 1 3 0 °C range. Differential scanning calorimetry curves for the lighter lanthanides reveal m o r e than one peak in this range. The water content varied b u t was generally close to unity. Detailed studies related to the decomposition of the complexes are under investigation. The non- hygroscopic c o m p l e x e s are only sufficiently soluble in methanol where con- ductance data (Am = 60 ~ - 1 cm 2 mol-1) are indicative of a non-electrolyte behaviour [1].
X-my patterns, that in several cases indicate p o o r crystaUinity, can be arranged, considering also IR data (Table 1), in five series as follows: (a) lanthanum-neodymium; (b) samarium-europium; (c) gadolinium, terbium, yttrium; (d) holmium, erbium, terbium; (e) dysprosium, ytterbium, lutetium. All IR spectra exhibit water bands [2]. The u (NO) stretching bands of the c o m p o u n d s cannot be clearly distinguished from the corresponding fl- CH (ligand) and us(CFs), u=(CFa) (trifluoroacetate) frequencies, as they occur in the same region ( 1 2 1 0 - 1 1 3 0 c m -1) [3-5]. However, the general aspect of the bands exhibits a tendency towards lower frequencies, indicative of a ~ N O ) shift as c o m p a r e d with the free ligand (1269 cm -1) and interpreted as a ligand coordination to the Ln 3 + ions through the oxygen atom of the NO group. Further evidence is provided b y the shift of the 7-CH band ( 8 7 7 - 7 5 5 c m - I ) towards higher frequencies [6].
©
o
,a ¢'¢j
o 0
g
0
0
0
¢,J
I
0 , . ~
8~
i
, ' a
d
0
m o d e of sodium trifluoroacetate [5, 7, 15, 16]. However, as the distinction b e t w e e n the different trifluoroacetate coordination m o d e s is not unambiguously clear, a m o n o d e n t a t e coordination is not to be totally excluded [ 17]. Detailed X-ray single-crystal diffraction studies are clearly necessary in order to establish definitely a correlation b e t w e e n stretching frequency shifts and structural parameters.
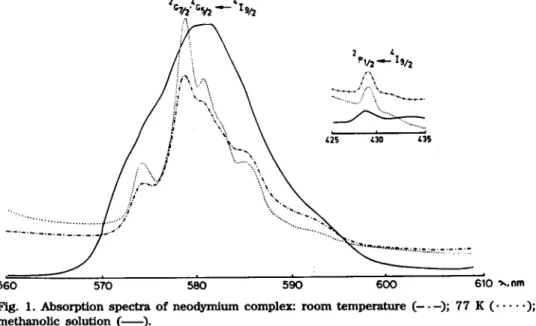
Figure 1 shows the absorption spectra of the neodymium c o m p o u n d in the 2G7/2, 4G5/2 ~ 419/2 and 2P1/2 *- 419/2 transition regions. The n u m b e r of bands of the 77 K spectrum is indicative of an involvement of Nd 8+ in a non-cubic symmetry. The spectroscopic parameters ~ - - 0 . 9 8 9 , bI/2 -- 0.0735 [18] and 5 - - 1 . 0 9 [19] indicate an essentially electrostatic bond b e t w e e n Nd s+ and the ligands. The spectrum in methanol solution is different from the room temperature solid state spectrum owing to modifications around the central ion.
The 77 K emission spectrum of the europium c o m p o u n d is shown in Fig. 2. More than three or five lines are observed in the 5 D o ~ S F ~ and 5Do-~ ~F2 transition regions respectively, suggesting different coordination m o d e s around the metal centre, since all f - f transitions have a maximum of 2 J + 1 c o m p o n e n t s [20]. The 5 D o - ~ F 9 hypersensitive transition is the m o s t intense, indicating low symmetry sites without inversion centres. As b o t h the 5D o and ~Fo levels do not resolve under crystal field effects, the 5Do ~ ~Fo transition can p r e s e n t multiple lines o n l y w h e n differently coordinated europium(Ill') species are present [21, 22]. The fact that they are resolved permits u s to conclude that the complex species are possibly polymeric or, at least, dimeric, containing bridge trifluoroacetates, europium ions being involved in two different sites. The extent o f the polymerization m a y b e also
PI/2 .-,- IS/2
/ ~ - .. / \
!; ~. :~ ".. ....
/
/
'\
.... \
,.
,,o
,,,
~ . ~
l/
h ".,
"....
.9"
"% "~.~
.
560 570 580 590 600 610 ~.,nm
F ~ . ] . A b s o r p t i o n spectra o f neodymitLm c o m p l e x : r o o m temperatmre (--.--); 77 K ( . . . );
$ 7
O o ~ F 2
5Oo-JF,
5 7
Fig. 2. Emission
5DO~ F4
d o °m
spectrum of the europium compound at liquid nitrogen temperature.
responsible for the different amount o f water detected. The presence of the SD0--*~F o band is suggestive of C., Cs or C~v symmetries [23]. On the basis of the n u m b e r and intensity of bands we suggest europium ions involved in different sites but with the same C3~ symmetry [24].
Acknowledgments
C ~ , ~ C . is deeply indebted to Conselho Nacional de Desenvolvimento Cientflico e Tecnol6gico (CNPq) and to C o o r d e n a ~ o de Aperfei~aomento de Pessoal do Ensino Superior (CAPES) for a Ph.D. fellowship and financial support.
References
1 W. J. Geary, Coord. Chem. Rev., 7 (1971) 81.
2 K. Nakamoto, Infrared Spectra of Inorganic and Coordination Compounds, v~rfley, New
York, 1978, p. 226.
3 A. R. Katritzky, J. A. T. Beard and N. A. Coats, J. Chem. Soc., (1959) 3680.
4 J. A. Faniran and K. S. Patel, Spectrochirr~ Acta A, 32 (1976) 1351.
5 K. O. Christe and D. Naumann, Spectrochim. Acta ,4, 29 (1973) 2017.
6 S. Kida, J. V. Qu~gliano, J. A. Walmsley and S. Y. Tyree, Spectrochim. Acta, 19 (1963)
189.
7 C. D. Garner and B. Hughes, Adv. Inorg. Chem~ Radiochem., 17 (1975) 1.
8 V. Ya. Kavun, V. I. Sergienko, V. I. Kostin, B. N. Chernyshov and N. I. Steblevskaya, Russ.
J. Inorg. Chem., 30 (1985) 190.
9 M. Singh and S. N. Misra, J. Inorg. NucL Chem., 40 (1978) 1939.
10 V. Ya. Kavun, B. N. Chernyshov and N. S. Vasilyuk, Russ. J. Inorg. Chem., 31 (1986)
358.
11 O. Velasquez, K. W. Bagnall, H. H. Alvarez, I. Berrio and L. Montalvo, Rev. Colomb. Quire.,
12 S. P. Bone, D. B. Sowerby and R. D. Verma, J. Chem. Soc., Dalton Trans., 11 (1978) 1544.
13 E. E. Castellano, G. Oliva, L. B. Zinner and G. Vicentini, Inorg. Chim. Acta, 110 (1985)
77.
14 L. B. Zinner, W. Oliveira, J. L. Fejfar, E. E. Castellano and J. Zukerman-Schpector, Eur.
J. Solid State Inorg. Chem., 28 (1991) 283.
15 G. B. Deacon and R. J. Phillips, Coord. Chem. Rev., 33 (1980) 327.
16 B. S. Manhas and A. K. Trikha, J. I n d i a n Chem. Soc., 59 (1982) 315.
17 S. N. Misra and M. Singh, Synth. React. Inorg. Met.-Org. Chem., 11 (1981) 157.
18 D. E. Henrie and G. R. Choppin, J. Chem. Phys., 49 (1968) 477.
19 S. P. Sinha, Spectrochim~ Acta~ 22 (1966) 57.
20 G. Vicentini and A. B. Nascimento, An. Acad. Bras. Ci~nc., 58 (1986) 199.
21 J. C. Biinzli and A. Giorgetti, Inorg. Chim. Acta, 110 (1985) 225.
22 V. Balzaxd, N. Sabbatini and F. Scandola, Chem. Rev., 86 (1986) 319.
23 P. Porcher and C. Caro, Semin. Chim. Etat Solide, 5 (1972) 141.