w ww.e l s e v i e r . c o m / l o c a t e / b j p
Original
Article
Phytochemical
study
and
anti-inflammatory
and
antioxidant
potential
of
Spondias
mombin
leaves
Bárbara
Cabral
a,
Emerson
M.S.
Siqueira
a,
Mariana
A.O.
Bitencourt
b,
Maíra
C.J.S.
Lima
b,
Ana
K.
Lima
c,
Caroline
F.
Ortmann
d,
Vitor
C.
Chaves
d,
Matheus
F.
Fernandes-Pedrosa
b,
Hugo
A.O.
Rocha
c,
Katia
C.
Scortecci
c,
Flávio
H.
Reginatto
d,
Raquel
B.
Giordani
a,
Silvana
M.
Zucolotto
a,∗ aPharmacognosyLaboratory,DepartmentofPharmacy,UniversidadeFederaldoRioGrandedoNorte,Natal,RN,BrazilbTechnologyandPharmaceuticalBiotechnologyLaboratory,ProgramadePós-graduac¸ãoemCiênciasFarmacêuticas,UniversidadeFederaldoRioGrandedoNorte,Natal,RN,Brazil cNaturalPolymersBiotechnologyLaboratory,DepartmentofBiochemistry,CentrodeBiociências,UniversidadeFederaldoRioGrandedoNorte,Natal,RN,Brazil
dPharmacognosyLaboratory,ProgramadePós-graduac¸ãoemFarmácia,CentrodeCiênciasdaSaúde,UniversidadeFederaldeSantaCatarina,CampusUniversitário–Trindade, Florianópolis,SC,Brazil
a
r
t
i
c
l
e
i
n
f
o
Articlehistory:
Received20October2015
Accepted12January2016
Availableonline15March2016
Keywords: Spondiasmombin
Chemicalmarker
Anti-inflammatoryandantioxidant
a
b
s
t
r
a
c
t
SpondiasmombinL.,Anacardiaceae,isaplantnativeofBrazil,whereitisknownas“cajá”.Inorderto
findapotentialapplicationforthisnativespecies,theanti-inflammatoryandantioxidanteffectswere
investigated.Theanti-inflammatoryactivitywasevaluatedusingtheinvivomodelcarrageenan-induced
peritonitisinmice.Theinvitroantioxidantpotentialaswellthecytotoxicityagainst3T3fibroblastcells
alsowereevaluated.ThroughHighPerformanceLiquidChromatography-diodearraydetectoranalysis,
ananalyticmethodwasdevelopedandvalidated.Itallowedtheidentificationandquantificationof
ellagicacidandchlorogenicacidinhydroethanolicextractofS.mombinleaves.Thisextractshowed
anti-inflammatoryeffectat100,200,300and500mg/kg,however,theethylacetatefraction,at200mg/kg,
showedthehighlightedresults.Ellagicacidandchlorogenicacid(2.5,5and10mg/kg)alsoinhibitedthe
leukocytemigrationtothesiteofinflammation.Theextract,fractionsandcompoundsshowedsignificant
antioxidantpotentialwhenevaluatedindifferentassays.Theresultsshowninthisworksuggestthe
anti-inflammatorypotentialoftheleafextractofS.mombimonperitonitismodelinducedbycarrageenan,
itwasalsoobservedantioxidantpropertiesassociatedwithanabsenceofcytotoxicityincellculture.
Furtherinvivostudiesarerequiredtoconfirmtheanti-inflammatoriesactionofS.mombinanditspossible
anti-inflammatorymechanismsofaction.
©2016PublishedbyElsevierEditoraLtda.onbehalfofSociedadeBrasileiradeFarmacognosia.
Introduction
Theethnopharmacologyassociatedwiththechemicalstudyhas becomeanimportanttoolinbioprospecting.Studieshave associ-atedinformationonthetraditionaluseofmedicinalplantswith phytochemical and pharmacological studies, searching for new drugsandherbalmedicines(Medeirosetal.,2013).Brazilhasoneof theworld’shighestlevelsofbiodiversity,includingseveralplantsof economicinterest(Albuquerqueetal.,2007).TheNorthand North-eastregionsarethosewheremostoftheexistingbiodiversityis concentrated,whichallowsaccesstonumerouskindsofplantsand fruitspecie(Mattiettoetal.,2010).
∗ Correspondingauthor.
E-mail:silvanazucolotto@ufrnet.br(S.M.Zucolotto).
SpondiasmombinL.isaplantbelongingtotheAnacardiaceae family.ThisspeciesisnativeofBrazilandtheirfruitsarecommonly knownas“cajá”.Itoccurswidespreadthroughtropicalregionsin America,AfricaandAsia.InBrazil,itismainlyfoundintheNorth andNortheast(Soares,2005).Thisnativefruithaspotentialuse forprocessingtomakejelly,juice,jamsandicecreammainlyin northeasternBrazilandtheirleavesareusedinfolkmedicinefor thetreatmentofseveraltopicandsystemicdiseaseslike inflam-mationofthemouth and throatand incases ofprostatitis and herpeslabialis(LorenziandMatos,2008).Despitethis therapeu-ticapproach,thechemicalstudiesarescarceandsomephenolic acids,flavonoids,tanninsandtriterpeneshavebeenisolatedfrom
S. mombinleaves so far(Corthout et al., 1991; Fred-Jaiyesimia etal.,2009).Asurveyoftherelevantliteraturerevealedthatthe leavesof S.mombinexhibit antimicrobial,leishmanicide, antivi-ral,antiedematogenic, hypoglycemicand antioxidantproperties
(Corthoutetal.,1994;Fred-Jaiyesimiaetal.,2009;Nworuetal.,
http://dx.doi.org/10.1016/j.bjp.2016.02.002
2011;Silvaetal.,2011,2012).Regardingtheanti-inflammatory effectonlyonereportsupportitspopularuse(Nworuetal.,2011). Thechemicalcharacterizationoftheleafextractfromthisspecies aswellastheidentificationofchemicalmarkersthatcouldbe use-fulinextractstandardizationforapplyingitinherbaldrugswasnot developed.Inaddition,itisimportanttohighlightthattheextract standardizationenableustoguaranteereliableresultsregarding biologicalactivities.
Takentogether,theaimsofthisstudywere:(i)todevelopan analyticmethodologybyHPLC-DADin ordertoquantify chemi-calmarkersinextractfromS.mombinleavesand(ii)toevaluate theiranti-inflammatorypotentialinvivomodel,(iii)toinvestigate theantioxidantpotentialsincetheoxidativestressisan impor-tantaspectassociatedtoinflammationand(iv)andtoevaluatethe cytotoxicityagainst3T3cells.
Materialsandmethods
Phytochemicalprocedures
Plantmaterialandextraction
LeavesofSpondiasmombinL.,Anacardiaceae,werecollectedin DomMarcolinoDantas,RioGrandedoNorte,Brazil(SS-RN◦28′14′′
W35◦27′37′′),onNovember2011.Theplantmaterialwasidentified
byAlandeAraújoRoqueandavoucherspecimenwasdeposited atHerbariumofFederalUniversityofRioGrandedoNorte,Brazil, underthereferencenumber12252.S.mombinleavesweredriedat roomtemperatureandtrituratedmechanically.Theextractfrom
S.mombinleaveswaspreparedbymacerationinethanol:water (70:30,v/v),forsevendays,filteredandlaterlyophilized.Itwas obtainedthehidroethanolicextractfromS.mombinleaves(HE).
InordertocharacterizetheactivecompoundsfromHE,aportion oftheextractwasresuspendedinwaterandsubjectedto liquid-liquidpartitionwithsolventsofincreasingpolarity:hexane(Hex) (3×300ml),dichloromethane(DCM) (3× 300ml),ethylacetate (EtOAc)(3×300ml)andbutanol(ButOH)(3×300ml).Five frac-tionswereobtained(Hex,DCM,EtOAc,ButOH)andanaqueous residualfraction(ARF).Allfractionsweredriedunderreduced pres-sureat45◦C.
Chromatographicprocedures
Theanalyticalstandardswerepurchasedfrom:chlorogenicacid hemihydrate(Sigma–Aldrich®,
≥98%),ellagicacid(Roth®,
≥98%) andisoquercitrin(HwiAnalytikGmbH®
,94.16%).Thesolventsused inextractpreparationwereAnalyticalGradeandinHPLCanalysis wereusedHPLCgradesolvents.Thewaterwaspurifiedwitha Milli-Qsystem(Millipore®,Bedford, USA).All solutionsprepared for
HPLCanalysiswerefilteredthrougha0.45mmmembranebefore use.
Quantitativehigh-performanceliquidchromatographyanalysis
Thequantificationofthephenoliccompoundswasperformedin ahighperformanceliquidchromatography(HPLC)usingaPerkin Elmer200seriesequippedwitha diodearraydetector(DAD),a quaternarypump,adegasseronlineandanautosampler.AllHPLC datawereprocessedusingtheTotalChrom®
Workstationsoftware. Chromatographicanalyzeswereperformedusingareversedphase column(Phenomenex®)C-18(4.6mm
×250mm,5m).An elu-tionsystemwithacetonitrile100%(solventA)andaceticacid1%, adjustedtopH3(solventB)atthefollowgradientflowwasused: 0–30min,alinearchangefromA:B(13:87,v/v)toA:B(15:85,v/v); 30–40min,anisocraticelutionwithA:B(15:85,v/v);40–45min, alinearvariationuptoA:B(20:80,v/v).Theflowratewaskept constantat1ml/min,andthechromatogramswererecordedat 340nm,whereastheUVspectraweremonitoredatwavelength of450–200nm.Thepeakswerecharacterizedbycomparisonof
theirretentiontimesandUVspectrawiththereferencestandards andbyaco-injection(extract+referencestandard).Thereference standardsolutions werepreparedinH2O:methanol(3:2,v/v)at
differentconcentrations: chlorogenicacid– 10, 20,30, 40, and 50g/ml;ellagicacid–20,30,40,50,and60g/ml.TheHEfromS. mombinleaveswasanalyzedat2.5mg/ml.Thereferencestandards wereanalyzedintriplicateandtheaveragepeakareasmeasured.
Validationofanalyticalprocedure
Thevalidationofanalyticalprocedureswasperformedin accor-dancewithICHguidelines(ICH,2005).Thevalidatedparameters werespecificity, linearity,accuracy,precision(repeatabilityand intermediateprecision),limitofquantification(LOQ)andlimitof detection(LOD).
Pharmacologicalassays
Anti-inflammatoryactivity
Animals. Experiments werecarriedout usingmale BALB/cmice (6–8weeksold).Allmicewerehoused, 5–6percageata room temperatureof22±2◦C,anda12h:12hlight/darkcycle,withad libitumaccesstowaterandfood.Groupsofsixanimalswereusedin eachtestgroupandcontrolanimalsreceivedsalineonly.Allinvivo
experimentswereapprovedbytheethicalcommitteeofthe Fed-eralUniversityofRio Grandedo Norte,Brazil(protocolnumber 013/2013)andwerecarriedoutaccordingtothecurrentguidelines forlaboratoryanimalcare.Eachanimalwasusedonlyonce.
Carrageenan-inducedperitonitis. Theinflammationwasinducedin micesbyadministrationof1%carrageenan,intraperitoneally(i.p.). The animals(N=5)werei.p.pretreated with(i)vehicle(saline, 0.1ml/10g)or(ii)dexamethasone(0.5mg/kg)or(iii)HEfromS. mombinleaves(100,200,300or500mg/kg)or(iv)fractionsDCM, EtOAc,ButOHand ARF (200mg/kg) or(v) chlorogenicacidand ellagicacid(2.5,5or10mg/kg).Thirtyminutesaftertreatmentwith thesamples,theanimalsreceivedaninjectionintotheperitoneal cavityof50lof1%carrageenan.After4h,theanimalswere sac-rificedwithanoverdoseofthiopentalat80mg/kg,i.p.,followedby cervicaldislocation.Peritonealexudateswerecollectedwith2ml ofice-coldPBSforabdominallaparoscopy.Theexudateswere cen-trifugedat250×gfor5minat4◦C.Thecellpelletwasdiluted1:10
inTürksolutionandthetotalleukocytenumberwasdetermined inaNeubauerchamber.ThesedosesofHEarebasedonprevious workpublishedinliterature(Nworuetal.,2011)andpilotstudiesin ourlaboratory(datanotshown).Chlorogenicacid(Sigma–Aldrich®,
≥98%)andellagicacid(Roth®,≥98%)usedinexperimentinvivoand invitromodelwerepurchasedcommercially.
Antioxidantactivity. Theantioxidantactivitywasperformedwith HEfromS.mombinleaves,DCM,AcOEt,ButOHandARFfractions andthecompoundsellagicacidandchlorogenicacid. TheDPPH radicalscavenging,Scavengerofsuperoxideion(O2−),Reducing
powerandSequestrationofthehydroxylradical(OH−)activitywas
evaluated.Theextractsandfractionsweretestedat60,125,250and 500g/mlandthecompoundsat5,15,30and60g/ml.
Viabilitycell. The3T3cellsweregrownin75cm2flaskswith9mlof
mediumDulbeccomodifiedEagleculturemedium(DMEM).Then, fortheassaycells,theyweretransferredto96wellplatesandit wasgrownat5×103cell/well.Thesecellsweregrownovernight
inordertoattachin200lmediumat37◦Cand5%CO
2.Afterthat,
20
15
10
5
0
0
HO2C
OH OH
OH
HO
HO
OH
OH HO
HO OH
OH OH
OH OH CH2OH
OH
1
2
3
HO
O O O
O
O
O O
O O
O 5
1
2
3
10 15 20
Time (min)
25 30 35 40
Absorbance (mA
U)
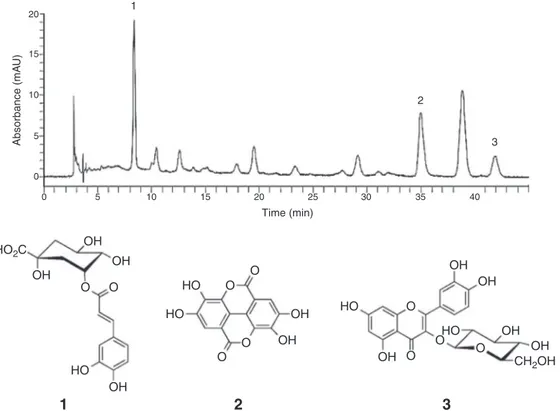
Fig.1. Chromatogramat340nmobtainedbyHPLC-DADofhydroethanolicextractfromSpondiasmombinleaves.Chlorogenicacid(1),ellagicacid(2)andisoquercetrin(3).
wereremovedand cells were washedwith200lPBS(twice), afterthatitwasadded100loffreshmedium.Then,itwasadded 100lofMTTreagent(5mg/ml)(Sigma,M5655)dissolvedinfresh DMEM.Thecellswerethenincubatedfor4hat37◦Cand5%CO
2.
Tosolubilizetheformazancrystalsitwasused100lofethanol whichwasaddedtoeachwellandafter20mintheabsorbancewas readat570nmusingtheELISAreader(BiotecQuant).Asa con-trol,untreatednormalcellswereculturedonlyinthepresenceof DMEM.Thecellproliferation(%)wasexpressedas:[(SampleAbs 570nm×100)/ControlAbs570nm].
Statisticalanalysis
Resultswereexpressedas means±standard device.Statistic analysiswasconductedbyStudent’s t-test or one-wayanalysis ofvariance(ANOVA)followedbytheTukeyposthoctest. Statis-ticalsignificancewasconsideredof95%(p<0.05).Thestatistical programINSTAT,Graph-Pad,SanDiego,CA,wasused.
Resultsanddiscussion
PhytochemicalanalysisofS.mombin
AnalysisbyHPLC-DADthoughUVanalysisofeachpeakshowed thattheHEofS.mombinleavespresentsalargeamountofphenolic andflavonoidderivatives.ThequalitativeanalysisofHEenabled ustoindicatetheoccurrenceofthreephenolicmajorcompounds: chlorogenicacid,ellagicacidandisoquercetinandonenoidentified peakatUV340nm,bytheretentiontime,theUVspectraandby co-injectionoftheHEandreferencestandard(Fig.1).
Intheliteraturethereisnoreportabouttheidentificationof chlorogenicacidandisoquercetininS.mombinspecies,however, ellagicacidhasalreadybeenidentifiedinmethanolextractfrom theleaves(Silvaetal.,2012).Duetothis,ananalyticalmethodology wasdevelopedandvalidatedforthequantificationofchlorogenic acidandellagicacidbyHPLCforHEfromS.mombinleaves.Since thepresenceofflavonoidsandphenolicacidsinspeciesofSpondias
Table1
PhenolicacidscontentinhydroethanolicextractfromSpondiasmombinleaves.a
Species Compound Content/gextract
Spondiasmombin
leaves(2.5mg/ml)
Chlorogenicacid (Rt=8.28min)
12.0mg/gextract
Ellagicacid (Rt=34.21min)
19.4mg/gextract
aExpressedasmg/gofextract±SD(n=3)/Rt=retentiontime.
genusisdescribedinliterature(Corthoutetal.,1992;Satpathyetal.,
2011;Silvaetal.,2012;Engelsetal.,2012),thesecompoundscould
behighlightedaschemicalmarkersforthisgenus.Theflavonoid isoquercetincannotbequantifiedin HEduetounavailabilityof suitableamountoftheirreferencestandard. Themethod devel-opedshowedachromatogramoftheHEfromS.mombinleaves withsymmetricalpeaks(tailingfactor:0.9and0.75,chlorogenic acidandellagicacid,respectively)andappropriatedresolution(2.0 tobothcompounds).
Standard solutionsof chlorogenicacidand ellagicacidwere prepared,ataconcentrationrangefrom10to60g/ml,andthe chromatographic method showeda suitable linear relationship (r2>0.995)forbothstandardsolutions.Thisenabledustomeasure
thecontentofthechlorogenicacidandellagicacidinHE(Table1). Thelimitofquantification(LOQ)andthelimitofdetection(LOD) weredefinedbyrelative standarddeviation(RSD>5%)andbya signal:noiseratioof3:1respectively(Table2).
Table2
Calibration,LODandLOQdataofphenolicacidstandards.
Compound Linearityrange(g/ml) Calibrationequationa Correlationfactor(r2) LODb(g/ml) LOQb(g/ml)
Chlorogenicacid 10–50 y=9727.8x−6129 0.9936 0.625 5.0
Ellagicacid 20–60 y=13976x−28,598 0.9901 1.25 5.0
aSixdatapoint(n=3).
bLOD=limitofdetection;LOQ=limitofquantification.
Table3
Repeatability,intermediateprecisionandaccuracydataofphenolicacidsstandards.
Compound Repeatability Intermediateprecision Recoverya
Concentration(g/ml) R.S.D.(%) Concentration(g/ml) R.S.D.(%) Mean% R.S.D.(%)
Chlorogenicacid 10 1.0 10 1.0 110 1.0
30 2.4 30 0.3
50 0.4 50 1.0
Ellagicacid 20 0.8 20 2.5 102 0.8
30 1.0 30 1.2
50 4.5 50 0.8
aRecoverywasdeterminedbyinjectionofspikedsamples,intriplicate,withreferencestandard.
theliterature(CassandDegani,2001;ICH,2005).Concerning accu-racy,animportantrecoverywasobservedforthestandardsinboth extracts,which wasdeterminedbyspikingsamples(2.5mg/ml) withthestandardsolutionsofellagicacid(50g/ml)and chloro-genicacid(10g/ml)(1:1,v/v)(Table3).
The quantification of individual compounds was performed using an analytical regression curve (r2>0.991). The standards
wereanalyzedintriplicateandtheaverageofthemeasuredpeak areas.Afterobtainingtheequationofthestraightthecompounds werequantifiedaccordingtotheareaunderthecurve.Itwasfound 19.4mg/gofellagicacidand12mg/gofchlorogenicacidintheHEof
S.mombinleaves(Table1).Apreviousreportaboutquantification ofellagicacidinS.mombinshowedacontentof41.56±0.01mg/g inthemethanol:water(80:20,v/v)leavesextract(Silvaetal.,2012) buttheenvironmentalconditionswerenotdescribed.This differ-encecanbeattributedtothesolventusedintheextractionprocess aswellastotheedafoclimaticfeaturesinBraziliannortheast,where phenolicderivativesareparticularlysusceptibleto environmen-talchangesinplantingthebehavior(Araújoetal.,2012).Another possibilityisthebiosynthesisofsecondarymetabolites,sincethe biogenesisofphenolicacids,flavonoidsandtanninsareverysimilar andhavethesameprecursors,whichcangenerateacompensation mechanismfortheproductionofaparticularsecondarymetabolite. Inthisworktheharvestofplantmaterialwascarriedoutin sum-merandinrainforest,thesolventusedforextractionwascomposed ofethanol:water(70:30,v/v).Therearenoreportsintheliterature aboutthequantificationofchlorogenicacidinthespeciesS. mom-binthusithighlightsourresultssinceitwasquantifiedas12mg metabolite/gextract.
Assessmentofanti-inflammatoryactivity
PreviousstudieswithspeciesofSpondiasgenusshowed anti-inflammatoryactivity.AccordingtoliteraturestudieswithSpondias mangiferausingbutanol andethyl acetate fractionsobtainedof ethanolicextractadministeredorallyatdoses75,150,300mg/kg b.w.showedasignificantreduction inpawvolumewhen com-paredwiththerespectivecontrolgroupchallengedbycarrageenan
(Sachanetal.,2011).Itwasalsonotedthestudyofethylacetate
extractofSpondiaspinnatahadanti-inflammatoryeffectsatdoses of 200, 400mg/kg, b.w. (p<0.001) in reducing rat paw edema inducedbycarrageenanassay(Raoetal.,2009).
Additionallywasreportedtheanti-inflammatoryactivityofS. mombinleavesinpawedemamodel(Nworuetal.,2011).Thestudy showedthattreatmentwiththemethanolextractfromS.mombin
leavesat 100, 200and 400mg/kg inhibitedby a dose depend-entwaythepawedemainducedbycarrageenanbesidescausing reductionoftheLPS-inducedbyTNF-␣andinproductionofnitric oxide(Nworuetal.,2011).Basedonthesestudiesandinpopular useofS.mombinininflammatoryprocesses,ourstudyevaluated theinvivoanti-inflammatoryextractpotentialbyacuteperitonitis modelusingcarrageenan-induceddexamethasone(5mg/kg,i.p.)as referencecompound.
Thisstudyaimedtotesttheextracttoanotherinflammation modelinordertoassessotherinflammatoryparameterssuchas leukocytemigration.Thecarrageenan-inducedperitonitisisawell characterizedexperimentalmodelofacuteinflammationwidely usedtotestnewanti-inflammatorytherapies,itenablethe quan-tificationandthecorrelationofcellularmigrationoninflammatory exudate(SedgwickandLees,1986;Paulinoetal.,2008;Bitencourt
etal.,2014;Limaetal.,2014).
Carrageenanisanindirectphlogisticagentthat inrats’ peri-toneum induces neutrophil migration which is dependent of residentmacrophageactivation(Souzaetal.,1988).Furthermore, studiesshowed thatcarrageenan injectioninto theperitoneum ofratsinducestheexpressionofnitricoxidesynthaseand COX-2resultingsoinliberationofalargeamountofnitricoxideand prostaglandins(Tomlinsonetal.,1994;Hatanakaetal.,1999).We havefoundthatintraperitonealinjectionofcarrageenanwasable toinduceaninflammatoryreactionwhichisrevealedbyhighcell migrationtotheperitonealcavity.
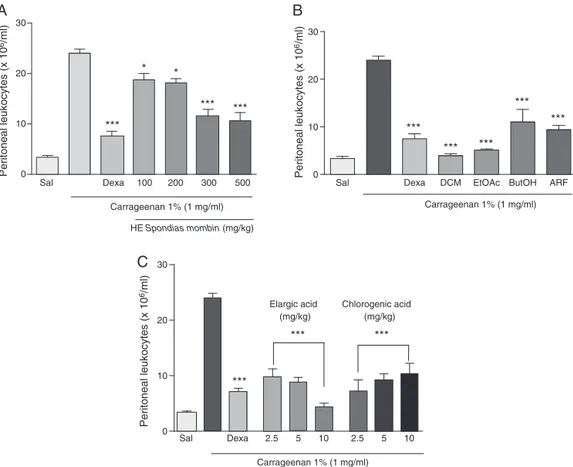
Ourresultsdemonstratedthattheanti-inflammatorypotential of HE of theS.mombin leavesat100, 200, 300 and 500mg/kg reduced thenumber ofleukocytesinfluxtoperitonealcavityof treated animals. In comparison with thecontrol group treated onlywithcarrageenan,theHEat300and500mg/kgshowedthe mostsignificanteffectwithaninhibitionofleukocytemigration of 51.75% and55.54%, respectively (Fig. 2A).Thesigns ofacute inflammationarevasodilatation,edemaandleukocyteinfiltration
(SherwoodandToliver-Kinsky,2004).Thisstudyshowedthatthe
extractofS.mombincouldactinoneoftheinflammation mech-anisminhibitingsignificantly theleukocytemigration tosite of inflammation.
500 300 200 100 Dexa Sal
0 10 20 30
*** ***
***
* *
Carrageenan 1% (1 mg/ml)
HE Spondias mombin (mg/kg)
A
Peritoneal leukocytes (x 10
6/ml)
Peritoneal leukocytes (x 10
6/ml)
Peritoneal leukocytes (x 10
6/ml)
ARF ButOH EtOAc DCM Dexa Sal
0 10 20 30
Carrageenan 1% (1 mg/ml)
***
*** *** ***
***
B
10 5 2.5 10 5 2.5 Dexa Sal
0 10 20 30
Carrageenan 1% (1 mg/ml) Elargic acid
(mg/kg)
Chlorogenic acid (mg/kg)
***
*** ***
C
Fig.2. EffectofhydroethanolicextractoftheSpondiasmombinleavesonleukocytemigrationinducedbycarrageenaninanacuteperitonitismodel.aHEtestedat
concen-trationsof100,200,300and500mg/kg,i.p.bFractions:DCM:dichloromethane,EtOAc:ethylacetate,ButOH:n-butanolandARF:aqueousresidualfractionat200mg/kg,
i.p.cellagicacid,chlorogenicacidtestedatconcentrationsof2.5,5and10mg/kg.Sal:saline.Dexa:dexamethasone(0.5mg/kg,i.p.).Dataareexpressedasmean±standard
deviation.*p<0.05,***p<0.001versusthepositivecontrolgroup(treatedwithcarrageenanonly).N=6.
withseveralpolaritysolventscouldbeconsideredusefulsinceone fraction(EtOAc)showedabetterpotentialincomparisontoHE.
HEand fractions wereanalyzedby HPLC-DAD(340nm)and showthepresenceofphenoliccompounds.Thisisanimportant finding,sincemanyphenolicarerelatedtotheanti-inflammatory activityofvarious plants(Rotelliet al.,2003; Santangeloet al., 2007).InEtOAcfractionwereidentifiedandquantifiedtwo phe-noliccompoundsellagicacidandchlorogenicacid,andthesewere evaluatedyourpotentialanti-inflammatory,inordertoverifythe contributionofthesecompoundsforthebioactivityoftheextract. These compounds were assayedat 2.5, 5 and 10mg/kg, i.p. Nostatisticaldifferencesbetweenthetreatmentgroups(Fig.2C) were observed. It is important to emphasize that the dose of 10mg/kgshowedthebestinhibitionprofiletotheellagicacidand chlorogenicacidthatwasdemonstratingbyinhibitionofleukocyte migrationof82and70%,respectively,tothesiteofinflammation.
Besidestheinhibitionofleukocytemigrationtheellagicacidand chlorogenicacidithasactivityinotherways,asreportedinother studies.
In the literature chlorogenic acid is able to inhibit nitric oxideproduction,inhibittheexpressionofbothCOX-2and pro-inflammatory cytokines (including IL-1 and TNF-␣) (Esposito etal.,2014),aswellasitcanalsoactbyinhibitingthenuclear translocationandactivationofNF-B,amasterregulatorof inflam-mation(Franciscoetal.,2013;Hwangetal.,2014).Also,invivo
studiesshowedthatchlorogenicacidat50and100mg/kg inhib-ited therat pawedema induced by carrageenan (Santoset al., 2006).Inaddition,importantanti-inflammatoryand antinocicep-tiveactivitiesbychlorogenicacidweredemonstratedsince this compoundhastakinggreateractionthansalicylicacid(Yonathan
etal.,2006).
Regardingellagicacid,thiscompoundwasabletoinhibitCOX-2
andNF-B(Marinetal.,2013)andpresentedanti-inflammatory
activity at 10 and 50mg/kg when tested in induced arthritis animalmodel and didnot showanyeffectondisease develop-mentinalowerdose,butinhibitedthepawvolume(p<0.05)with thehigherdose.Inouranalysis,ellagicacidandchlorogenicacid inhibitedaleukocytemigration inacuteperitonitismodel,thus providinganewandadditionalevidenceofitsanti-inflammatory potential.Takentogether,theseresultssuggestthatellagicacidand chlorogenicacidcouldbeinvolvedintheanti-inflammatoryaction observedforHEextract.
Theinhibitionofleukocytemigrationintotheperitoneal cav-itymaybebytwomechanisms:byinhibitingtheproductionof chemotacticsubstancesand/orexpressionofadhesionmolecules, thisbecausetheleukocytesrequirechemotacticsubstancesthat facilitate your migration to theplace of injury (Sherwood and
Toliver-Kinsky,2004).
Insummary,cansaidthattheHEofSmombinhastheabilityto inhibitleukocytemigrationtotheperitonealcavitybyacute peri-tonitismodelandthatthecompoundsellagicacidandchlorogenic acidmaybeinvolvedwiththisactivity.Howeverittakesmore stud-iestojustifytheuseoftheleafextractfromS.mombintotreat inflammatoryproblemsandtoelucidatethemechanismsinvolved inthisaction.
Assessmentofantioxidantactivity
100
A
B
C
D
80 c
a a a a a
d
a a
c b
b
d
b 100
80
60
20 40
0
100
80
60
20 40
0 c
b
60
40
20
0
1.0
a b
b b
b
c b
b 0.8
0.6
0.4
0.2
0.0
Control g
roup DCM
EtO
Ac
ButOH ARF
Ellagic acid
Chlorogenic acid
HE
Spondias mombin
Control g
roup DCM
EtO
Ac
ButOH ARF
Ellagic acid
Chlorogenic acid
HE
Spondias mombin
Control g
roup DCM
EtO
Ac
ButOH ARF
Ellagic acid
Chlorogenic acid
HE
Spondias mombin
Control g
roup DCM
EtO
Ac
ButOH ARF
Ellagic acid
Chlorogenic acid
HE
Spondias mombin
Sca
venging DPPH, %
Sca
venging supero
xide r
adical, %
Sca
venging h
ydro
xyl r
a
dicals
, %
Absorbance (nm)
Fig.3.AntioxidantactivityofhydroethanolicextractoftheSpondiasmombinleaves.XaxiscorrespondstohydroethanolicextractfromS.mombinleaves(HE);fractions:
DCM:dichloromethane,EtOAc:ethylacetate,ButOH:n-butanol,ARF:aqueousresidualfractionandellagicacidandchlorogenicacid.Thegraphsrepresentthedataobtained
forscavengingDPPHradical(controlgroup:gallicacid30mg/ml)a;superoxideradicals(controlgroup:gallicacid30mg/ml)b;reducingpower(controlgroup:ascorbic
acid0.2mg/ml)c;scavenginghydroxylradicals(controlgroup:gallicacid15mg/ml)d;expressedasmean±standarddeviationofpercentageofsequestrationDPPHata
concentrationof60g/mloftheHEandfractionsand30g/mlofchlorogenicacidandellagicacid.a,b,c.Differentlettersmeansignificantdifferencebetweenthecrude
extractfractionsandcompoundsidentified(p≤0.05).
capacity associated with an anti-inflammatory potential are desirablefeaturesforabioactivecompound.Consideringthe anti-inflammatorypotentialdemonstratedbyHE,DCM,EtOAc,ButOH, ARFfractions,andellagicacidandchlorogenicacidcompounds,it isimportanttoevaluatetheirantioxidantpotentials.This inves-tigationwasperformedinseveralapproaches:(i)scavengingfree radicalDPPHassay,(ii)superoxideradicalscavengingactivity,(iii) reducingpower,and(iv)hydroxylradicalscavengingactivity.
IntheDPPHassay(Fig.3)itwasobservedapotentialdonation ofelectronsorH+ionswithvaluesrangingfromscavenging66%
to76%forallsamplestested.TheHE,ButOHandchlorogenicacid wereabletosequester74.53,73.71and91.47%,ofthefree rad-ical,respectively.Intheassayofreducingpowertoevaluatethe reductivecapacityitwasobservedapositiveresultforallsamples analyzed(Fig.3).Furthermore,it wasobservedthat allsamples testedforthehydroxylradicalscavengingactivityshowed seques-trationpercentagehigherthan100%.Theantioxidantcapacityof theleavescanbeassociatedtotheirbioactivecompounds,mainly antioxidantpolyphenols,becauseoftheirabilitytoscavengefree radicals(Mandicetal.,2008).Onthisway,wesuggestthatthe phe-nolicacidspresentintheextractandfractionsofS.mombincould contributefortheextractantioxidantactivity.
Finally,apreliminaryevaluationofthepotentialcytotoxicityof HE,fractions(DCM,AcOEt,ButOH,ARF),andtheellagicacidand chlorogenicacidcompoundswascarriedout.Itwasobservedthat
Table4
Viabilitycelleffectsofthehidroethanolicextract,fractionsandchlorogenicacidand
ellagicacidfromSpondiasmombinleaves.HEandfractionswereassayedat60,125
and250g/mlandcompoundsat5,15and60g/mlon3T3fibroblasts.Resultsare
expressedaspercentageofproliferationmedia±standarddeviationfromtriplicate
assays.
Fibroblasts3T3(24h)
Extractandfractions 60g/ml 125g/ml 500g/ml HE 125±0.0205 129±0.0095 181±0.185 DCM 253±0 128±0.0005 142±0.0025 EtOAc 168±0.0205 204±0.034 128±0.0445 ButOH 141±0.0195 93±0.0005 158±0.048 ARF 100±0.01 108±0.0025 67±0.022
Compounds 5g/ml 15g/ml 60g/ml Chlorogenicacid 122±0.0355 106±0.002 105±0.0025 Ellagicacid 154±0.0535 127±0.0015 181±0.1205 HE=hidroethanolicextract ofSpondiasmombin leaves,DCM=dichloromethane fractionEtOAc=ethylacetatefraction.ButOH=n-butanolfraction.ARF=aqueous residualfraction.
AcOEtandButOH(Table4).Itwasobservedacytotoxiceffectonly atthemaximalconcentration500g/mlfortheresidualfraction (ARF).
Insummary,tothebestofourknowledge,thisisthefirsttime thatisreportedantioxidant/anti-inflammatoryproperty(invitro/in vivo)totheleafextractfromS.mombinalongwith characteriza-tionoftheirchemicalcomposition.Theevidencefoundedinthis work,makesitasuitablecandidateforfurtherconsiderationasan alternativetreatmenttoreduceoxidativestressandinflammation.
Conclusions
Theresultsshowninthisworksuggesttheanti-inflammatory potentialof theleafextract ofS. mombimon peritonitismodel inducedbycarrageenan,Itwasalsoobservedantioxidant prop-ertiesassociatedwithanabsenceofcytotoxicityincellculture. Furthermore,itwasfoundthatchlorogenicacidandellagicacid contributetoactionpharmacologicallyofS.mombin.
Therefore,theseresultsindicatethepotentialoftheleafofS. mombinasasourceofnewanti-inflammatoryherbaldrugand/or molecules,and seemtojustifypartof itsmainpopularuses in traditionalmedicine.Furtherstudiesarerequiredtoconfirmthe anti-inflammatories action of S. mombin and its possible anti-inflammatorymechanismsofaction.
Ethicaldisclosures
Protectionofhumanandanimalsubjects. Theauthorsdeclare thattheproceduresfollowedwereinaccordancewiththe regula-tionsoftherelevantclinicalresearchethicscommitteeandwith thoseoftheCodeofEthicsoftheWorldMedicalAssociation (Dec-larationofHelsinki).
Confidentialityofdata. Theauthorsdeclarethatnopatientdata appearinthisarticle.
Righttoprivacyandinformedconsent. Theauthorsdeclarethat nopatientdataappearinthisarticle.
Authors’contributions
BECVFparticipatedofphytochemicalanalysis,interpretationof dataanddraftedthemanuscript.MMMparticipatedinthe evalua-tionofanti-inflammatoryactivity,acquisitionandinterpretationof data.AHKparticipatedinantioxidantanalysisandrevisedit criti-callythemanuscript.RSparticipatedinitsdesignandcoordination, andhelpedtodraftthemanuscript.
Conflictsofinterest
Theauthorsdeclarenoconflictsofinterest.
Acknowledgments
Theauthorsacknowledgeallparticipantsfortheirvaluabletime andcommitmenttothestudy.WealsothankCNPqforfinancial support(478661/2010-0).TheauthorsarealsogratefultoMScAlan deAraújoRoqueresponsibleforspeciesidentification.
References
Albuquerque,U.P.,Medeiros,P.M.,Almeida,A.L.S.,Monteiro,J.M.,Lins,E.M.F.N.,
Melo,J.G.,Santos,J.P.,2007.Medicinalplantsofthecaatinga(semi-arid)
veg-etationofNEBrazil:aquantitativeapproach.J.Ethnopharmacol.114,325–354.
Araújo,T.A.S.,Castro,V.T.N.A.E.,Amorim,E.L.C.,Albuquerque,U.P.,2012.Habitat
influenceonantioxidantactivityandtanninconcentrationsofCaatingaplants. Pharm.Biol.50,754–759.
Bitencourt,M.A.O.,Lima,M.C.J.S.,Torres-Rêgo,M.,Fernandes,J.M.,Silva-Júnior,
A.A.,Tambourgi,D.V.,Zucolotto,S.M.,Fernandes-Pedrosa,M.,2014.
Neutraliz-ingeffectsofMimosatenuifloraextractsagainstinflammationcausedbyTityus serrulatusscorpionvenom.Biomed.Res.Int.153,890–895.
Cass,Q.B.,Degani,A.L.G.,2001.DesenvolvimentodeMétodosporHPLC:
Fundamen-tos,EstratégiaseValidac¸ão,ed.UFSCar,SãoCarlos,Brasil.
Corthout,J.,Pieters,L.,Claeys,M.,Berghe,D.A.V.,Vlientinck,A.J.,1991.Antiviral
ellagitanninsfromSpondiasmombin.Phytochemistry30,1129–1130.
Corthout,J.,Pieters,L.,Claeys,M.,Berghe,D.A.V.,Vlientinck,A.J.,1992.Antiviral
caffeoylestersfromSpondiasmombin.Phytochemistry31,1979–1981.
Corthout,J.,Pieters,L.,Claeys,M.,Geerts,S.T.,Berghe,D.V.,Vlietinck,A.,1994.
AntibacterialandmolluscicidalphenolicacidsfromSpondiasmombin.Planta Med.5,460–463.
Engels,C.,Gräter,D.,Esquivel,P.,Jiménez,V.M.,Gänzle,M.G.,Schieber,A.,2012.
Characterizationofphenoliccompoundsinjocote(SpondiaspurpureaL.)peels byultrahighperformanceliquidchromatography/electrosprayionizationmass spectrometry.FoodRes.Int.46,557–562.
Esposito,D.,Chen,A.,Grace,M.H.,Komarnytsky,S.,Lila,M.A.,2014.Inhibitoryeffects
ofwildblueberryanthocyaninsandotherflavonoidsonbiomarkersofacuteand chronicinflammationinvitro.J.Agric.FoodChem.29,7022–7028.
Francisco,V.,Costa,G.,Figueirinha,A.C.M.,Pereira,P.,Miguel,N.B.,Celeste,L.M.,
Garcia-Rodriguez,C.,Teresa,C.M.,Teresa,B.M.,2013.Anti-inflammatoryactivity
ofCymbopogoncitratusleavesinfusionviaproteasomeandnuclearfactor-B pathwayinhibition:contributionofchlorogenicacid.J.Ethnopharmacol.148, 126–134.
Fred-Jaiyesimia,A.,Kio,A.,Richard,W.,2009.␣-Amylaseinhibitoryeffectof3
-olean-12-en-3-yl(9Z)-hexadec-9-enoateisolatedfromSpondiasmombinleaf. FoodChem.116,285–288.
Hatanaka,K.,Kawamura,M.,Ogino,K.,Matsuo,S.,Harada,Y.,1999.Expression
andfunctionofcyclooxygenase-2inmesothelialcellsduringlatephaseofrat carrageenaninducedpleurisy.LifeSci.65,161–166.
Hwang,S.J.,Kim,Y.W.,Park,Y.,Lee,H.J.,Kim,K.W.,2014.Anti-inflammatoryeffects
ofchlorogenicacidinlipopolysaccharide-stimulatedRAW264.7cells.Inflamm. Res.63,81–90.
ICH–InternationalConferenceonHarmonization,2005.ValidationofAnalytical
Procedures:TextandMethodology–Q2(R1),London.
Khansari,N.,Shakiba,Y.,Mahmoudi,M.,2009.Chronicinflammationandoxidative
stressasamajorcauseofage-relateddiseasesandcancer.RecentPat.Inflamm. AllergyDrugDiscov.3,73–80.
Lorenzi,H.,Matos,F.J.A.,2008.PlantasmedicinaisnoBrasilnativaseexóticas.Nova
Odessa,SãoPaulo.
Lima,M.C.J.S.,Bitencourt,M.A.O.,Furtado,A.A.,Oliveira-Rocha,H.A.,Oliveira,R.M.,
Silva-Júnior,A.A.,Tabosa,E.E.S.,Tambourgi,D.V.,Zucolotto,S.M.,
Fernandes-Pedrosa,M.F.,2014.Ipomoeaasarifolianeutralizesinflammationinducedby
Tityusserrulatusscorpionvenom.J.Ethnopharmacol.153,890–895.
Mandic,A.L.,Dilas,S.M.,Cetkovic,G.S.,Canadanovic-Brunet,J.M.,Vesna,T.T.,2008.
Polyphenoliccompositionandantioxidantactivitiesofgrapeseedextract.Int. J.FoodProp.11,713–726.
Marin,M.,Maria,G.R.,Rios,J.L.,Recio,M.C.,2013.Intestinalanti-inflammatory
activ-ityofellagicacidintheacuteandchronicdextranesulfatesodiummodelsof micecolitis.J.Ethnopharmacol.150,925–934.
Mattietto,R.A.,Lopes,A.S.,Menezes,H.C.,2010.Caracterizac¸ãofísicaefísico-química
dosfrutosdacajazeira(SpondiasmombinL.)edesuaspolpasobtidaspordois tiposdeextrator.Braz.J.Food.Technol.13,156–164.
Medeiros,P.M.,Ladio,A.H.,Santos,A.M.M., Albuquerque,U.P.,2013. Doesthe
selectionofmedicinalplantsbyBrazilianlocalpopulationssuffertaxonomic influence?J.Ethnopharmacol.146,842–852.
Nworu,C.S.,Akah,P.A.,Okoye,F.B.,Toukam,D.K.,Udeh,J.,Esimone,C.O.,2011.The
leafextractofSpondiasmombinL.displaysananti-inflammatoryeffectand sup-pressesinducibleformationoftumornecrosisfactor-␣andnitricoxide(NO).J. Immunotoxicol.8,10–16.
Paulino,N.,Abreu,S.R.L.,Uto,L.,2008.Anti-inflammatoryeffectsofabioavailable
compound,AstepillinC,inBrazilianprópolis.Eur.J.Pharmacol.587,1–3.
Rao,B.G.,Nath,M.S.,Raju,N.J.,2009.Investigationofanti-inflammatoryactivityof
stemheartwoodofSpondiaspinnata.Int.J.Chem.Sci.7,294–298.
Rotelli,A.E.,Guardia,T.,Juárez,A.O.,De,L.,Pelzer,L.E.,2003.Comparativestudy
offlavonoidsinexperimentalmodels ofinflammation.Pharmacol.Res.48, 601–606.
Sachan,N.K.,Arif,M.,Zaman,K.,Kumar,Y.,2011.Anti-inflammatory,analgesicand
antioxidantpotentialofthestembarkofSpondiasMangiferaWilld.Arch.Biol. Sci.Belgrade2,413–419.
Satpathy,G.,Tyagi,Y.K.,Gupta,R.K.,2011.Preliminaryevaluationofnutraceutical
andtherapeuticpotentialofrawSpondiaspinnataK.,anexoticfruitofIndia. FoodRes.Int.44,2076–2087.
Santangelo,C.,Varì,R.,Scazzocchio,B.,Di,B.R.,Filesi,C.,Masella,R.,2007.
Polyphe-nols, intracellularsignalling and inflammation.Ann. Ist.Super.Sanita 43, 394–405.
Santos,M.D.,Almeida,M.C.,Lopes,N.P.,Souza,G.E.,2006.Evaluationofthe
anti-inflammatory,analgesicandantipyreticactivitiesofthenaturalpolyphenol chlorogenicacid.Biol.Pharm.Bull.29,2236–2240.
Sedgwick,A.D.,Lees,P.,1986.Acomparisonofairpouch,spongeandpleurisymodels
ofacutecarrageenaninflammationintherat.AgentsActions18,439–446.
Sherwood,E.R.,Toliver-Kinsky,T.,2004.Mechanismsoftheinflammatoryresponse.
BestPract.Res.Clin.Anaesthesiol.18,385–405.
Silva,A.R.A.,Morais,S.M.,Marques,M.M.M.,Lima,D.M.,Santos,S.C.C.,Almeida,R.R.,
pheno-liccomponentsoftwoSpondiasspeciesagainstdenguevírus.J.Venom.Anim. ToxinsIncl.Trop.Dis.17,406–413.
Silva,F.V.G.,Silva,S.M.,Silva,G.C.,Mendonc¸a,R.M.N.,Alves,R.E.,Dantas,A.L.,2012.
Bioactivecompoundsandantioxidantactivityinfruitsofcloneandungrafted genotypesofyellowmombintree.FoodSci.Technol.32,685–691.
Soares,E.B.,(Dissertac¸ãodeMestrado)2005.Avaliac¸ãodegenótiposdecajazeira
(SpondiasmombinL.):Caracterizac¸ãofísico-químicadosfrutoserepetibilidade decaracteresmorfoagronômicos.CentrodeCiênciasAgrárias,Universidade Fed-eraldoPiauí.
Souza,G.E.,Cunha,F.Q.,Mello,R.,Ferreira,S.H.,1988.Neutrophilmigrationinduced
byinflammatorystimuliisreducedbymacrophagedepletion.AgentsActions 24,377–380.
Tomlinson,A.,Appleton,I.,Mooregilroy,A.R.,Wills,D.,Mitchell,J.A.,Willoughby,
A.,1994.Cyclo-oxigenaseandnitricoxideisoformsinratcarregeenam-induced
pleurisy.Br.J.Pharmacol.113,693–698.
Yonathan, M.,Asres, K.,Assefa, A., Bucar,F., 2006.In vivo anti-inflammatory