1413-8670/© 2012 Elsevier Editora Ltda. All rights reserved.
www.elsevier.com/locate/bjid
The Brazilian Journal of
INFECTIOUS DISEASES
A R T I C L E I N F O
Article history: Received 17 July 2011 Accepted 5 August 2011
Keywords:
Urinary tract infections Anti-bacterial agents Drug resistance, bacterial Fluoroquinolones Escherichia coli
A B S T R A C T
Background: Optimal empirical therapy of urinary tract infection requires accurate knowledge of local susceptibility patterns, which may vary with organism and patient characteristics.
Methods: Among 9,798 consecutive, non-duplicate, community-source urine isolates from ambulatory patients ≥ 13 years old, from clinical laboratory and an academic medical center in Curitiba, Brazil (May 1st to December 1st, 2009), susceptibility data for ampicillin, nitrofurantoin, trimethoprim-sulfamethoxazole, gentamicin, fluoroquinolones, and ceftriaxone/cefotaxime were compared with organism and patient gender and age.
Results: The female-to-male ratio decreased with age, from 28.1 (among 20-29 year-olds) to 3.3 (among > 80 year-olds). Overall, susceptibility prevalence varied widely by drug class, from unacceptably low levels (53.5% and 61.1%: ampicillin and trimethoprim-sulfamethoxazole) to acceptable but suboptimal levels (81.2% to 91.7%: fluoroquinolones, ceftriaxone, nitrofurantoin, and gentamicin). E. coli isolates exhibited higher susceptibility rates than other isolates, from 3-4% higher (fluoroquinolones, gentamicin) to ≥ 30% (nitrofurantoin, ceftriaxone). Males exhibited lower susceptibility rates than females. Within each gender, susceptibility declined with increasing age. For females, only nitrofurantoin and gentamicin were suitable for empirical therapy (≥ 80% susceptibility) across all age cohorts; fluoroquinolones were suitable only through age 60, and ceftriaxone only through age 80. For males, only gentamicin yielded ≥ 80% susceptibility in any age cohort.
Conclusion: Few suitable empirical treatment options for community-source urinary tract infection were identified for women aged over 60 years or males of any age. Empirical therapy recommendations must consider the patient’s demographic characteristics. Site-specific, age and gender-stratified susceptibility surveillance involving all uropathogens is needed.
© 2012 Elsevier Editora Ltda. All rights reserved.
Original Article
Sex, drugs, bugs, and age: rational selection of empirical
therapy for outpatient urinary tract infection in an era of
extensive antimicrobial resistance
Jaime L. Rocha
a, Felipe Francisco Tuon
b*, James R. Johnson
caDivision of Microbiology, Frischmann Aisengart/DASA Medicina Diagnóstica and VITA Curitiba Hospital, Curitiba, PR, Brazil bDivision of Infectious and Parasitic Diseases, Hospital Universitário Evangélico de Curitiba, Curitiba, PR, Brazil
cVeterans Affairs Medical Center and University of Minnesota, Minneapolis, MN, USA
* Corresponding author at: Division of Infectious and Parasitic Diseases, Hospital Universitário Evangélico de Curitiba, Alameda Augusto Stellfeld 1908, 3º andar - SCIH - Bigorrilho, Curitiba, RS, 80730-150, Brazil
Introduction
Symptomatic urinary tract infections (UTIs) are among the commonest bacterial infections, resulting in millions of physician visits (mostly for acute cystitis) and hundreds of thousands of hospital admissions (mostly for acute pyelonephritis) annually in the United States.1 A variety of
antimicrobial regimens, with varying efficacy, tolerability, and cost, can be used to treat UTIs. Therapy usually is selected before culture and susceptibility test results are available. Thus, expert panels have developed evidence-based guidelines for empirical treatment of UTI, which requires the availability of high-quality data from surveillance studies regarding a major challenge in UTI therapy: antimicrobial resistance among uropathogens.
The previous (1999) guidelines from the Infectious Diseases Society of America (IDSA) suggested fluoroquinolones (FQs) as an appropriate first-line therapy for uncomplicated acute cystitis or pyelonephritis in women if the local prevalence of resistance to trimethoprim-sulfamethoxazole (TMP-SMX) exceeds 10-20%.2 However, the emergence of FQ resistance
among uropathogens may jeopardize the empirical use of this drug class. For example, a 2005-2006 study from London found 9.3% FQ resistance among E. coli urinary isolates, with a higher percentage among male and elderly patients.3 Indeed,
the susceptibility of community-source uropathogens to most commonly prescribed antimicrobials is decreasing globally.4-6
Although the IDSA guidelines have been updated to address recent antimicrobial resistance trends,7 they still may not
be applicable to developing countries, since few relevant resistance surveillance studies are available,8 and the existing
ones include relatively few isolates and/or focus on hospital-source uropathogens.9
Because of the paucity of data regarding antimicrobial resistance among community-source uropathogens in Brazilian cities, and increasingly frequent clinical failures (unpublished data, Rocha JL and Tuon FF), we assessed the susceptibility to relevant antimicrobials among 9,798 consecutive urine isolates from ambulatory patients in Curitiba, Brazil (2009). We then analyzed these data to identify suitable empirical therapy options for cystitis and more complicated presentations of UTI, both overall and specifically in relation to patient gender and age.
Methods
Setting
The study site, Curitiba, is the capital city of the Brazilian state of Paraná. As the seventh largest city in Brazil (1.8 million inhabitants), Curitiba is the largest economic and cultural center in southern Brazil and is home to four medical schools. The university hospital included in this study is a tertiary center with 700 beds. The private laboratory included in this study is the main private clinical laboratory in Curitiba, having 35 outpatient units distributed widely across the city, which send specimens for analysis to the central laboratory.
Samples and subjects
From May 1st to December 1st, 2009, all community-source
urine isolates from patients presenting to outpatient clinics at the university hospital or recovered by the private laboratory were considered for possible study inclusion. Isolates from urine cultures that yielded ≥ 105 CFU/mL underwent automated
susceptibility testing (Vitek 2®) and were eligible for the study.
Exclusion criteria included fungi, mixed cultures, multiple specimens from a given patient (in which case only the first sample per patient was used), age less than 13 years, and hospital inpatient status at the time of specimen collection.
Susceptibility breakpoints were as specified by the Clinical and Laboratory Standard Institute.10 Intermediate
susceptibility was analyzed as resistant. Results are presented for 6 drugs (classes): ampicillin, nitrofurantoin, FQs, TMP-SMX, gentamicin, and extended-spectrum cephalosporins (ESCs). Ciprofloxacin and levofloxacin were analyzed as a single drug class (i.e., FQs), as were ceftriaxone and cefotaxime (i.e., ESCs). Susceptibility of Enterococcus spp. to gentamicin was assessed only with respect to high concentration testing (for synergy). Intrinsic resistance to ampicillin was assumed for
Klebsiella spp., Pseudomonas spp., and Enterobacter spp.; to ESCs for Pseudomonas spp. and Enterococcus spp.; to TMP-SMX for
Pseudomonas spp.; and to nitrofurantoin for Proteus spp and
Pseudomonas spp.
Data were analyzed using the free software R, version 2.11 (The R Foundation for Statistical Computing). Graphics were created using GraphPad Prisma 4.0 (GraphPad, La Jolla – USA). Clinical data included age and gender. Aggregate susceptibility data were reported as percentages with 95% confidence intervals. Susceptibility values < 80% were regarded as indicating inadequate activity of the corresponding drug class for use as empirical therapy of UTI. Continuous data were expressed as mean ± standard deviation (SD) or median with ranges. Dichotomous variables were compared using a χ2 test.
The significance threshold was set at 0.05.
Results
Study population
Between May 1st and December 1st, 2009, the 36 participating
clinical laboratories in Curitiba, Brazil processed 67,650 outpatient urine cultures, of which 12,567 (18.6%) were positive. Of the positive cultures, 2,769 (22%) had ≥ 1 exclusion criteria, leaving 9,798 non-duplicate urine isolates and the corresponding source patients as the study population.
the female-to-male ratio was highest (at 28.1) in the second-youngest cohort (age 21-30 years), then declined progressively with increasing age to a minimum of 3.3 in the age > 81 years cohort (Fig. 1). Thus, age significantly influenced the gender mix within the population.
Enterobacter spp. and Pseudomonas spp. (Table 1). These 7 organisms accounted for 91.8% of overall pathogens.
Susceptibility results
The susceptibility of the 9,798 study isolates to the 6 studied antimicrobial agents varied widely by drug class, ranging from unacceptably low values (for empirical therapy) of 53.5% and 61.1% for ampicillin and TMP-SMX, respectively, to acceptable but still suboptimal values of 81.2% to 91.7% for FQs, ESCs, nitrofurantoin, and gentamicin (Table 2). Notably, the corresponding values for the 6,480 E. coli isolates were 1% to 11% higher, in each drug class, than the total values, evidence that
E. coli overestimated the susceptibility of the total uropathogen pool. Differences in susceptibility prevalence between E. coli
and non-E. coli were substantially greater, ranging from 3-4% (for FQs and gentamicin) to ≥ 30% (for nitrofurantoin and ESCs). Three drug classes, i.e., nitrofurantoin, FQs, and ESCs, exhibited ≥ 80% susceptibility for E. coli but < 80% susceptibility for non-E. coli (Table 2), evidence that their acceptability for empirical therapy would depend on the likelihood that the urine organism was E. coli – which, as noted above, varied with gender, whereas the gender mix varied with age.
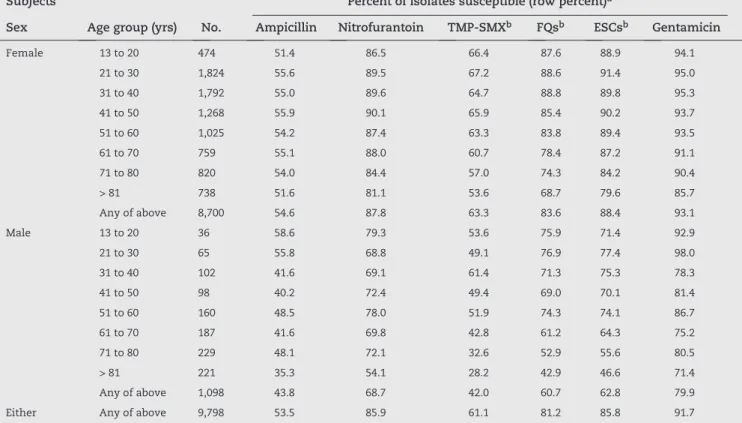
In view of these relationships, stratification of susceptibility results by gender and age revealed marked heterogeneity among subgroups (Table 3). Overall, females exhibited higher susceptibility values for all six drug classes than males. Moreover, within each gender, susceptibility to a given drug declined progressively with increasing age, in some instances after an initial increase (Table 3).
Consequently, for females, only nitrofurantoin and gentamicin provided adequate activity for empirical therapy (i.e., ≥ 80% susceptibility) across all age cohorts, whereas FQs provided adequate activity through age 60 but not thereafter, and ESCs did so through age 80 but not thereafter. For males, the situation was even worse, with only gentamicin being suitable for empirical therapy in any of the age cohorts, and only in 5 of the 8 cohorts (Table 3).
Fig. 1 - Age distribution by sex among 9,798 ambulatory patients with a positive urine culture (Curitiba, Brazil, 2009). The female:male ratio (no. of females/no. of males) within each age cohort is expressed as a decimal value.
Organisms
In turn, gender significantly influenced the distribution of urine organisms (Table 1). Although E. coli, Enterococcus spp., and Klebsiella spp. were the three most prevalent pathogens among both females and males, E. coli was significantly more prevalent among females, whereas Enterococcus and Klebsiella
were significantly more prevalent among males. Moreover, whereas for females the 4th and 5th most prevalent pathogens
were Proteus spp. and S. saprophyticus, for males they were
Number of isolates (column %)
Organism Total
(n = 9,798)
Female (n = 8,700)
Male (n = 1,098)
p-valuea female vs. male
Escherichia coli 6,480 (66.1) 6,015 (69.1) 465 (42.3) < 0.001
Enterococcus spp. 793 (8.1) 626 (7.2) 167 (15.2) < 0.001
Klebsiella spp. 579 (5.9) 473 (5.4) 106 (9.7) < 0.001
Staphylococcussaprophyticus 381 (3.9) 372 (4.3) 9 (0.8) < 0.001
Proteusmirabilis 379 (3.9) 330 (3.8) 49 (4.5)
Enterobacter spp. 266 (2.7) 193 (2.2) 73 (6.6) < 0.001
Pseudomonasaeruginosa 117 (1.2) 48 (0.6) 69 (6.3) < 0.001
Othersb 803 (8.2) 643 (7.4) 160 (14.6) < 0.001
ap-values (as calculated using the c2 test) are shown where p < 0.05; bother organisms, in descending order of overall prevalence (8.2% of total),
included: coagulase-negative Staphylococcus (3.7%), Citrobacter spp. (1.2%), Staphylococcusaureus (1.1%).
Table 1 - Distribution of urinary organisms by patient sex among 9,798 community-source urine isolates from Curitiba, Brazil (2009)
Number of cases
Male Female
13-20 21-30 32-40 41-50 51-60 61-70 71-80 > 80 2500
2000
1500
1000
500
Susceptible, no. (column %) p-valuea
Antimicrobialb Total
(n = 9,798)
E. coli
(n = 6,480)
Others (n = 3,318)
E. coli
vs. total
E. coli
vs. others
Ampicillin 5,242 (53.5) 3,622 (55.9) 1,620 (48.8) 0.003 < 0.001
Nitrofurantoin 8,416 (85.9) 6,221 (96.0) 2,195 (66.2) < 0.001 < 0.001
TMP-SMXb 5,987 (61.1) 4,264 (65.8) 1,723 (51.9) < 0.001 < 0.001
FQsb 7,956 (81.2) 5,327 (82.2) 2,629 (79.2) < 0.001
ESCsb 8,407 (85.8) 6,273 (96.8) 2,134 (64.3) < 0.001 < 0.001
Gentamicin 8,985 (91.7) 6,033 (93.1) 2,952 (89.0) 0.001 < 0.001
ap-values (by Fishers exact test) are shown where p < 0.05; bTMP-SMX, trimethoprim-sulfamethoxazole; FQs, fluoroquinolones (ciprofloxacin
and levofloxacin); ESCs, extended-spectrum cephalosoporins (ceftriaxone and cefotaxime).
Subjects Percent of isolates susceptible (row percent)a
Sex Age group (yrs) No. Ampicillin Nitrofurantoin TMP-SMXb FQsb ESCsb Gentamicin
Female 13 to 20 474 51.4 86.5 66.4 87.6 88.9 94.1
21 to 30 1,824 55.6 89.5 67.2 88.6 91.4 95.0
31 to 40 1,792 55.0 89.6 64.7 88.8 89.8 95.3
41 to 50 1,268 55.9 90.1 65.9 85.4 90.2 93.7
51 to 60 1,025 54.2 87.4 63.3 83.8 89.4 93.5
61 to 70 759 55.1 88.0 60.7 78.4 87.2 91.1
71 to 80 820 54.0 84.4 57.0 74.3 84.2 90.4
> 81 738 51.6 81.1 53.6 68.7 79.6 85.7
Any of above 8,700 54.6 87.8 63.3 83.6 88.4 93.1
Male 13 to 20 36 58.6 79.3 53.6 75.9 71.4 92.9
21 to 30 65 55.8 68.8 49.1 76.9 77.4 98.0
31 to 40 102 41.6 69.1 61.4 71.3 75.3 78.3
41 to 50 98 40.2 72.4 49.4 69.0 70.1 81.4
51 to 60 160 48.5 78.0 51.9 74.3 74.1 86.7
61 to 70 187 41.6 69.8 42.8 61.2 64.3 75.2
71 to 80 229 48.1 72.1 32.6 52.9 55.6 80.5
> 81 221 35.3 54.1 28.2 42.9 46.6 71.4
Any of above 1,098 43.8 68.7 42.0 60.7 62.8 79.9
Either Any of above 9,798 53.5 85.9 61.1 81.2 85.8 91.7
aShaded cells have values < 80%, implying unsuitability for empirical therapy for cystitis; bTMP-SMX, trimethoprim-sulfamethoxazole; FQs,
fluoroquinolones (ciprofloxacin and levofloxacin); ESCs, extended-spectrum cephalosoporins (ceftriaxone and cefotaxime). Table 2 - Susceptibility of 9,798 community-source urinary isolates to six antimicrobial classes
Table 3 - Antimicrobial susceptibility of 9,798 community-source urinary isolates according to patient age and sex subgroup
Discussion
To guide appropriate selection of empirical antimicrobial therapy for acute outpatient UTI in a large Latin American city and to identify simple demographic and microbial predictors of resistance that might be more broadly generalizable, we analyzed susceptibility data from 9,798 consecutive positive urine cultures from ambulatory patients in Curitiba, Brazil
evident, particularly for complicated infections. Fourth,
E. coli, although the single most common organism overall, overestimated aggregate susceptibility results, particularly for males and older women. These findings, which reflect today’s reality in a developing country setting and may foretell future trends in industrialized countries, demonstrate the importance of tailoring empirical treatment regimens to the patients’ demographic profile. This also indicate a pressing need to develop new antimicrobials, to devise innovative strategies for using current agents that will extend their utility and address existing resistance, and to closely monitor emerging resistance trends.
The overall susceptibility data suggested that four drug classes, i.e. nitrofurantoin, FQs, ESCs, and gentamicin, should be acceptable in our community as empirical therapy, at least for cystitis (given the 80% susceptibility criterion for acceptability, notwithstanding the undesirability of using parenteral agents for cystitis), since they were active against from 81.2% (FQs) to 91.7% (gentamicin) of all urine isolates. However, this projection proved overly optimistic after stratification of the susceptibility results by age and gender, which identified many patient subgroups with few or no suitable oral antimicrobial options, particularly for complicated UTI. Specifically, the only drugs that exhibited ≥ 80% susceptibility among women aged 60 to 80 years were nitrofurantoin, ESCs, and gentamicin, and among women older than 80, nitrofurantoin and gentamicin. In individual patients the suitability of even these agents might be further limited by patient-specific factors, such as renal dysfunction, hypersensitivity, concomitant nephrotoxic drugs, systemic or complicated infection, and desire for an all-oral regimen. Thus, in our setting many female patients and their providers are in a tenuous position regarding the availability of suitable empirical antimicrobial therapy for community-acquired UTI.
The situation was even worse among males, for whom the only agent exhibiting ≥ 80% aggregate susceptibility in any age cohort was gentamicin, and even that drug was acceptable in only 5 of 8 cohorts. This poses a considerable therapeutic challenge with no obvious solutions. Options might include 1) administration of an initial dose of a broad-spectrum parenteral agent (e.g., a carbapenem or piperacillin-tazobactam), 2) use of alternative oral agents, e.g., fosfomycin and amoxicillin-clavulanate (providing these are sufficiently active against local uropathogens, which requires confirmation), and 3) combination therapy, e.g., with amoxicillin (for enterococci) plus a Gram-negative agent. Regardless of the empirical regimen, urine culture clearly should be done for all males with suspected UTI in our setting, to guide rational selection of an alternative regimen in case the initial empirical regimen proves inadequate. Additionally, performance of direct susceptibility testing on the initial urine specimen may warrant consideration, since this can provide susceptibility results within 24h, avoiding the delays associated with standardized testing of isolated colonies.11
Our findings suggest that host age and sex are critical determinants of aggregate susceptibility prevalence, which are relied on by expert panels in devising empirical antimicrobial therapy recommendations for UTI. Fortunately, age and gender, unlike most other resistance-associated
epidemiological variables (e.g., prior antimicrobial exposure, recent health care contact, underlying complicating conditions, international travel, past culture results, etc.),12,13 are readily
available at the point of care and to the clinical microbiology laboratory. This feature recommends their use in compiling stratified aggregate susceptibility results, which would allow informed selection of host-specific empirical treatment regimens for UTI, in contrast to conventional “one size fits all” approaches that consider only overall susceptibility data, and perhaps only for E. coli.1 Our data indicate that clinical
investigators and laboratories, in settings where the local prevalence suggests no overall resistance problem, should consider performing stratified analysis similar to those shown here, to screen for unrecognized more-resistant subgroups.
In that regard, we found that aggregate susceptibility data for E. coli significantly overestimated the combined uropathogen results by 1% to 11% per drug, and overestimated the non-E. coli results by even more, i.e., 3% to > 30% per drug. Indeed, stratification by age and gender showed that E. coli
was a suitable indicator organism for overall uropathogen susceptibility only among females < 60 years old. In contrast, for women aged over 60 and males, the overall
E. coli susceptibility results overestimated the combined uropathogen susceptibility results by up to 50%.
The encountered susceptibility results lower among males than females was likely due in part to men having intrinsically more-resistant organisms (e.g., Enterococcus, Enterobacter, and Pseudomonas), and to women having intrinsically more-susceptible organisms (e.g., E. coli and S. saprophyticus). The observed trends toward increasing resistance with age may be due to older patients possibly having had more cumulative exposure to antimicrobials for UTI or other infections, which would tend to select both for more resistant species and for resistant variants of a given species.
Of note, despite the E. coli data underestimating the overall prevalence of resistance, they nonetheless indicated higher resistance levels than observed in previous studies of community-source urinary E. coli. For example, in contrast to our approximately 18% prevalence of FQs resistance in E. coli, the European ECO SENS study, which involved 2,478 E. coli urinary isolates from 252 community health care centers in 17 countries (early 2000s), found approximately 2% ciprofloxacin resistance.14,15 Likewise, a Greek study of
1,936 E. coli uncomplicated cystitis isolates (January, 2005 to March, 2006) found < 5% ciprofloxacin resistance,16 whereas, as
noted above, a London study from 2005-2006 showed 9.3% FQs resistance among E. coli from the local community (including some hospitalized patients). Whether our markedly higher resistance prevalence reflects differences in geography, time period, patient selection, or other factors is unknown.
Notably, our E. coli resistance results were actually comparable to those documented previously among hospitalized patients, including in regions known for FQ resistance. For example, the Spanish EARSS study, with 7,098 invasive E. coli isolates from 32 Spanish hospitals (2001-2003), reported resistance prevalence values for ciprofloxacin (19.3%) and TMP/SMX (32.6%)17 similar to those observed among our
1,961 E. coli urinary isolates from hospitalized patients (1997 and 2000) found FQ resistance prevalence values similar to ours (17.5-18.9%).9 This suggests that over the past decade
in our region of Latin America the prevalence of FQs resistance among outpatient E. coli isolates has risen to levels previously associated with hospital isolates.
Limitations of the study warrant attention. First, potentially relevant variables such as clinical manifestations, severity of illness, comorbid or predisposing conditions, UTI history, and prior antibiotic exposure were not analyzed. Second, as in most surveillance studies, selection bias is possible regarding which patients had urine cultures performed. Accordingly, our findings might represent a “worst-case” scenario, if cultures were biased toward patients in whom resistance was anticipated based on persistent or recurrent infection, prior antimicrobial use, complicating conditions, etc. Third, the clinical significance for UTI therapy of in vitro resistance is uncertain for most drug classes. Although TMP-SMX resistance clearly predicts treatment failure in cystitis or pyelonephritis,18 for other
drugs this remains poorly defined. Fourth, we analyzed ente-rococcal susceptibility to gentamicin using synergy-based criteria, which likely overestimated the actual suitability of gentamicin for empirical therapy. This would tend to strengthen our conclusion regarding limited treatment options, especially for males, among whom the enterococcal infections were concentrated. As such, our conclusions may actually understate the gravity of the situation. Study strengths include the large and recent study population; inclusion of multiple widely distributed clinics within the study community, which provided a quasi-population-based analysis; and inclusion of all qualifying urine cultures, which permitted analysis by age and gender and made the results relevant to the entire community of patients with UTI.
In conclusion, this large surveillance study identified serious problems with antimicrobial resistance among recent (2009) community-source urinary organisms in a large southern Brazilian city. Stratification by gender and age identified a dearth of suitable empirical treatment options for women over 60 years and males of any age, and E. coli proved to be an unsuitable indicator organism for uropathogen susceptibility except among females ≤ 60 years old. Our findings challenge the notion that no guideline-suggested antimicrobial regimen, especially based on aggregate E. coli susceptibility data, can fit all. Since gender and age are readily available demographics, we propose that age and gender-stratified surveillance studies that include all uropathogens (not just E. coli) should be done in each setting and, ideally, repeated at intervals, since resistance is a moving target. Additionally, development of new antimicrobials, prudent and creative use of available agents, and improved methods for prediction and rapid detection of resistance are clearly needed.
Acknowledgements
We are in debt to all Frischmann Aisengart Laboratory microbiologists. We also thank Flaviane Peccin for her invaluable statistical advice.
Conflict of interests
Jaime L. Rocha received grants from Sanofi Pasteur and Pfizer. Felipe Francisco Tuon received grants from Pfizer and Merck. James R. Johnson received research grants or contracts from Merck and Rochester Medical. This material is based in part upon work supported by Office of Research and Development, Medical Research Service, Department of Veterans Affairs (J.R.J.).
R E F E R E N C E S
1. Foxman B. Epidemiology of urinary tract infections: incidence, morbidity, and economic costs. Am J Med. 2002;113:5S-13S.
2. Warren JW, Abrutyn E, Hebel JR, et al. Guidelines for antimicrobial treatment of uncomplicated acute bacterial cystitis and acute pyelonephritis in women. Infectious Diseases Society of America (IDSA). Clin Infect Dis. 1999;29:745-58.
3. Bean DC, Krahe D, Wareham DW. Antimicrobial resistance in community and nosocomial Escherichia coli urinary tract isolates, London 2005-2006. Ann Clin Microbiol Antimicrob. 2008;7:13.
4. Astal Z, Sharif FA, Abdallah SA, et al. Multiresistant Escherichia coli isolated from women with community-acquired urinary tract infections in the Gaza Strip. J Chemother. 2002;14:637-8.
5. Kurutepe S, Surucuoglu S, Sezgin C, et al. Increasing antimicrobial resistance in Escherichia coli isolates from community-acquired urinary tract infections during 1998-2003 in Manisa, Turkey. Jpn J Infect Dis. 2005;58:159-61. 6. McIsaac WJ, Mazzulli T, Permaul J, et al. Community-acquired
antibiotic resistance in urinary isolates from adult women in Canada. Can J Infect Dis Med Microbiol. 2006;17:337-40. 7. Gupta K, Hooton TM, Naber KG, et al. International clinical
practice guidelines for the treatment of acute uncomplicated cystitis and pyelonephritis in women: A 2010 update by the Infectious Diseases Society of America and the European Society for Microbiology and Infectious Diseases. Clin Infect Dis. 2011;52:e103-e120.
8. Kiffer CR, Mendes C, Oplustil CP, et al. Antibiotic Resistance and Trend of Urinary Pathogens in General Outpatients from a Major Urban City. Int Braz J Urol. 2007;33:42-8.
9. Gales AC, Sader HS, Jones RN. Urinary tract infection trends in Latin American hospitals: report from the SENTRY antimicrobial surveillance program (1997-2000). Diagn Microbiol Infect Dis. 2002;44:289-99.
10. National Committee for Clinical Laboratory Standards. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically. Approved standard M7-A4. Wayne: National Committee for Clinical Laboratory Standards; 2009.
11. Johnson JR, Tiu FS, Stamm WE. Direct antimicrobial susceptibility testing for acute urinary tract infections in women. J Clin Microbiol. 1995;33:2316-23.
12. Laupland KB, Ross T, Pitout JD, et al. Community-onset urinary tract infections: a population-based assessment. Infection. 2007;35:150-3.
14. Gupta K, Hooton TM, Stamm WE. Increasing antimicrobial resistance and the management of uncomplicated community-acquired urinary tract infections. Ann Intern Med. 2001;135:41-50.
15. Kahlmeter G. Prevalence and antimicrobial susceptibility of pathogens in uncomplicated cystitis in Europe. The ECO. SENS study. Int J Antimicrob Agents. 2003;22 Suppl 2:49-52. 16. Katsarolis I, Poulakou G, Athanasia S, et al. Acute
uncomplicated cystitis: from surveillance data to a rationale for empirical treatment. Int J Antimicrob Agents. 2010;35:62-7.
17. Oteo J, Lazaro E, de Abajo FJ, et al. Antimicrobial-resistant invasive Escherichia coli, Spain. Emerg Infect Dis. 2005;11:546-53.