DOI 10.1007/s00044-017-2022-7
RESEARCH
ORIGINAL RESEARCH
Antibacterial effect on mature bio
fi
lms of oral streptococci and
antioxidant activity of 3
β
,6
β
,16
β
-trihydroxylup-20(29)-ene from
Combretum leprosum
Francisco Flávio Vasconcelos Evaristo1,2●Mayron Alves de Vasconcelos1,3●
Francisco Vassiliepe Sousa Arruda1,2●Anna Luísa Pereira1●Alexandre Lopes Andrade1●
Daniel Barroso de Alencar4●Mariana Ferreira do Nascimento5●
Alexandre Holanda Sampaio4●Silvana Saker-Sampaio4●Paulo Nogueira Bandeira5●
Hélcio Silva dos Santos5●Edson Holanda Teixeira1
Received: 3 May 2017 / Accepted: 31 July 2017 / Published online: 17 August 2017 © Springer Science+Business Media, LLC 2017
Abstract This study aimed to evaluate the antioxidant effect and antibacterial activity of the triterpene 3β,6β,16β -trihydroxylup-20(29)-ene (CLF1) isolated fromCombretum leprosum leaves on mature biofilms from Streptococcus mutansandS. parasanguinis, both involved in dental caries. In order to determine the bacterial death time curve pro-moted by CLF1, minimum inhibitory concentration and minimum bactericidal concentration were also assessed. The susceptibility of mature biofilms from both species to
CLF1 was investigated through total biomass quantification
and enumeration of biofilm-entrapped colony forming units.
Moreover, the effect of CLF1 on S. mutansbiofilm
archi-tecture was investigated by scanning electron microscopy. Additionally, the antioxidant properties of CLF1 were assessed by scavenging of 1,1-Diphenyl-2-picryl-hydrazyl
(DPPH) free radical, ferrous ion chelating assay, ferric-reducing antioxidant power, and β-carotene bleaching assay. The minimum inhibitory concentration and minimum bactericidal concentration of CLF1 for both species were 3.9 and 15.6μg/mL, respectively, and no bacterial growth was detected at 24 h treatment. Moreover, CLF1 reduced the biomass and viability of biofilms from both species.
Electron micrographs ofS. mutansbiofilms confirmed such
results and showed damages on bacterial surface. Regarding antioxidant activity, CLF1 showed efficient antioxidant
ability in scavenging DPPH radical and inhibiting β-carotene oxidation. In summary, CLF1 should be con-sidered as an effective molecule against infections caused by S. mutans and S. parasanguinis biofilms, as well as a
natural antioxidant.
* Edson Holanda Teixeira edson@ufc.br
Francisco Flávio Vasconcelos Evaristo ffvebio@gmail.com
Mayron Alves de Vasconcelos mayronvasconcelos@gmail.com Francisco Vassiliepe Sousa Arruda vassiliepe@gmail.com
Anna Luísa Pereira annaluisa.pe@gmail.com Alexandre Lopes Andrade alexandre.andrade@uece.br Daniel Barroso de Alencar daniellpesca@gmail.com Mariana Ferreira do Nascimento maryanna_lindda@hotmail.com Alexandre Holanda Sampaio alexholandasampaio@gmail.com
Silvana Saker-Sampaio sakersil@gmail.com Paulo Nogueira Bandeira bandeirapn@yahoo.com.br Hélcio Silva dos Santos helciodossantos@gmail.com
1 Laboratório Integrado de Biomoléculas, Departamento de
Patologia e Medicina Legal, Universidade Federal do Ceará, Fortaleza, CE, Brazil
2 Curso de Odontologia, Centro Universitário INTA, Sobral, CE,
Brazil
3 Departamento de Ciências Biológicas, Universidade do Estado do
Rio Grande do Norte, Mossoró, RN, Brazil
4 Departamento de Engenharia de Pesca, Universidade Federal do
Ceará, Fortaleza, CE, Brazil
5 Centro de Ciências Exatas e Tecnologia, Universidade Estadual
Keywords Combretum leprosum●Antibacterial●Mature
biofilm●Antioxidant
Introduction
The oral cavity houses a system of complex microorgan-isms with over 700 bacterial species colonizing the surface of the teeth, tongue, and oral mucosa (Achtman and Wagner 2008; Koo et al. 2013). Bacteria considered as “primary colonizers”adhere to the dental enamel with the assistance of afilm of glycoproteins that recovers the teeth and
syn-thesize a matrix of extracellular polymer substances (EPS), which together with cells act as substrates for the adhesion of new bacterial species and consequently the formation of oral biofilms, also called dental plaques (Lamont et al.
1991; Davey and O’Toole G 2000; Forssten et al. 2010; Kolenbrander et al.2010; Marsh et al.2011). The irregular distribution of microorganisms in the oral cavity can trigger a large number of chronic infectious diseases such as caries, gingivitis, and periodontitis (Herrero et al.2016).
Dental caries is an infectious, chronic, multifactorial disease that hasStreptococcus mutans as the main etiolo-gical agent (Kt et al.2013). The involvement ofS. mutans with the production of lactic acid from some carbohydrates commonly used as energy sources results in decrease in environmental pH, thus producing demineralization of the bone tissue and consequently the formation of dental caries (Almaz et al.2017).
In addition to dental caries, inflammatory processes
generated by bacteria can stimulate the synthesis of
proin-flammatory cytokines such as interferon gamma (IFN-γ), interleukin-1βamong others, which will activate or enhance specific processes of inflammation and immune response.
Nevertheless, some of these molecules participate in the synthesis of free radicals (Maeda and Akaike1998; Closa and Folch-Puy2004).
Free radicals are atoms or molecules that exhibit a reduction in the number of their electrons, very unstable, becoming highly reactive, and possessing the ability to combine with several cellular molecules. In the case of organisms, their excess has detrimental effects on the integrity of cell membranes, tissue and membrane proteins, carbohydrates and DNA molecules (Pham-Huy et al.2008). Thus, free radicals are counteracted by antioxidant agents produced by the body or obtained by the ingestion of antioxidant substances (Smina et al.2011).
Chemical compounds such as chlorhexidine andfluoride
are effective againstS. mutansand consequently have been used for the treatment and prevention of dental caries. They have been found in a wide variety of oral hygiene products such as mouthwashes and toothpastes (Varoni et al.2012;
Rabe et al. 2015; Marinho et al. 2016). However, the excessive use of these compounds may induce the appear-ance of side effects. Fluorides can induce abdominal pains and stains on the teeth (Wong et al. 2010), and chlorhex-idine can result in tooth discoloration (Tredwin et al.2005). According to Jeon et al. (2011), therapies that present antibacterial activity against the etiological agents of dental caries are highly targeted because they are capable of pre-venting and treating the disease.
Several products of plant origin are mentioned as pro-mising agents in the treatment and prevention of dental caries, given their ability to inhibit the biofilm formation by
microorganisms directly related to dental caries (Hu et al. 2006; Asokan et al.2008; Agarwal and Nagesh2011; Jeon et al.2011).
Combretum leprosum is a shrub popularly known as “mufumbo” or “cipoaba”. Such plant is a Combretaceae widely found in the North and Northeast Brazil. Com-bretaceae consists of ~600 species, being the genus Com-bretumthe most important with about 370 species, and the genus Terminaliawith ~200 species (McGaw et al. 2001; Katerere et al. 2003). It is commonly used as expectorant, sedative, healing and hemorrhagic, as well as an anti-microbial agent (Lira et al.2002; Nunes et al.2009).
In 1993, Facundo and colleagues isolated and character-ized the pentacyclic triterpene 3β, 6β, 16β-trihydroxylup-20 (29)-ene (CLF1) from the ethanolic extract ofC. leprosum leaves (Facundo et al.1993). Since then several effects have been attributed to CLF1 as antinociceptive, anti-infl
amma-tory, and anticancer (Pietrovski et al.2006; Longhi-Balbinot et al.2012; Viau et al.2014). Previously, we have demon-strated that CLF1 was able in inhibiting the planktonic growth and biofilm formation of S. mutans and S. para-sanguinis(Evaristo et al.2014). Here, we are contributing to elucidate the antibacterial effect of CLF1 on mature biofilms
of such strains, as well as shedding light on the mechanism of action of CLF1 on bacterial cells. Lastly, the antioxidant potential of CLF1 was also exploited.
Materials and methods
Plant material
FreshC. leprosumleaves were collected in August 2013 in Salgado dos Machados, Sobral, Ceará, Brazil (3°46'00.1“S 40°20'08.0“W). After collection, the material was properly identified by Dr. Elnatan Bezerra de Souza (a plant
Isolation and structural characterization of CLF1
The obtention of CLF1 followed the same steps as pre-viously described by Evaristo et al. (2014). The structural characterization was determined by nuclear magnetic reso-nance (NMR) spectroscopy of 1H and 13C uni and
two-dimensional and infrared, and compared with literature data. The molecular structure is shown in Fig.1.
Bacterial strains
The assays to determine the antimicrobial potential of CLF1 were carried out onStreptococcus mutans(UA 159) ATCC 700610 andS. parasanguinisATCC 903. Both are Gram-positive biofilm producing strains widely related to dental
caries.
Cultivation of microorganisms
Bacterial growth started from a stock culture in brain heart infusion (BHI) broth with 20% glycerol and kept at−80 °C. Then, an aliquot was seeded in Petri dishes containing BHI agar and incubated for 24 h at 37 °C with 5% CO2.
After-wards, isolated colonies were removed and inoculated into 5 mL of fresh BHI broth, and incubated under the same conditions for 18 h. Subsequently, bacterial suspensions were centrifuged (3200g, 4 °C for 5 min), and suspended in fresh BHI with 2% sucrose for assays involving biofilm
formation and without sucrose for tests with planktonic cells. Immediately before starting assays, bacterial suspensions were adjusted to 2×106cells/mL by monitoring absorbance at 620 nm by a spectrophotometer (Amersham Biosciences).
Antibacterial activity of CLF1
CLF1 was solubilized in sterile distilled water with 8% dimethyl sulfoxide (DMSO) to a final concentration of
125μg/mL. Afterwards, the solution was submitted to ultrasonic bath for 3 min and serially diluted in BHI broth to concentrations ranging from 62.5 to 1.95μg/mL. CLF1 was then distributed in 96-well polystyrene plates together with 100μL of bacterial suspension at 2×106cells/mL to afinal
volume of 200μL. The plates were then incubated for 24 h at 37 °C with 5% CO2. The evaluation of antibacterial
activity was performed by assessing optical density at 620 nm (OD620) by a microplate reader (Amersham
bios-ciences). The lowest concentration of CLF1 able in inhi-biting visual bacterial growth was considered as MIC (Minimum Inhibitory Concentration). For the determination of MBC (Minimum Bactericidal Concentration), samples consisting of 10μL of each well in which no visible growth was detected were inoculated in Petri dishes containing BHI agar, and subsequently monitored for growth at 24 h.
Bacterial death time curve
Both bacteria were grown in BHI broth as previously reported. Afterwards, 125μL of bacterial suspension at 2× 106 cells/mL were mixed with CLF1 at concentrations of
62.5 and 31.25μg/mL, and distributed in 96-well poly-styrene plates. The plates were then incubated at 37 °C with 5% CO2. Following this step, 20μL of the content of each
well were removed at specific time intervals (0, 2, 4, 6, 8,
10, 12, and 24 h), serially diluted, plated in BHI agar and grown for 48 h at 37 °C with 5% CO2. The number of
colony forming units (CFU) was determined and the results were presented as log CFU/mL.
Effect of CLF1 on preformed biofilms
The assays were carried out in 96-well polystyrene plates as described by Stepanovic et al. (2000), with some mod-ifications. Briefly, CLF1 was solubilized in sterile distilled
water containing 8% dimethyl sulfoxide (DMSO) to afinal
concentration of 1 mg/mL. Subsequently, the compound was submitted to ultrasonic bath for 3 min and serially diluted to concentrations ranging from 500 to 15.6μg/mL. In order to evaluate the action of CLF1 on preformed
bio-films, each well of the polystyrene plate wasfilled with 200
μL of bacteria at the concentration of 2×106cells/mL and incubated at 37 °C with 5% CO2for 24 h. Afterwards, the
supernatant was removed and replaced with 100μL of sterile BHI supplemented with 2% sucrose together with 100μL of CLF1 at different concentrations for further 24 h under the same growth conditions.
Activity of CLF1 on biomass
The crystal violet staining method was chosen for this purpose. After 24 h incubation, the plates were washed with
Fig. 1Structure of 3β,6β,16β-trihydroxylup-20(29)-ene extracted
distilled water to remove weakly adhered biomass. After drying, the wells werefilled with 200μL of 95% methanol for 15 min tofix biofilms. Then, 200μL of 1% crystal violet was then added and kept for 5 min at 25 °C. Thereafter, excess dye was removed and the plates washed with dis-tilled water. The remaining dye was solubilized with 33% acetic acid and then the biomass was quantified by
mea-suring the optical density at 590 nm (OD590) by a
micro-plate reader (SpectraMax i3 Multi-Mode).
Effect of CLF1 on viability of cells in preformed biofilms
The viability of biofilm-entrapped cells of preformed bio-films treated with CLF1 was assessed by determining the
number of CFU. First, the content of wells was discarded and each well was washed three times with distilled water to remove not adhered cells. Subsequently, the wells were
filled with 200μL of sterile distilled water and submitted to ultrasonic bath (Cristófoli/EQM-CF) for 10 min. Then, decimal dilutions were performed and plated on BHI agar. Afterwards, the plates were incubated for 24 h at 37 °C. Then, the number of CFU was expressed as log CFU/mL.
Effect of CLF1 on bacterial cell morphology
The assays were performed in 24-well polystyrene plates following the same methodology used in the assays with preformed biofilms. Briefly,S. mutansbiofilms were grown
in the presence of CLF1 at 500μg/mL. The cells in sus-pension were then discarded and the wells washed with sterile water. Afterwards, the biofilms were dehydrated with
ethyl alcohol (70% for 10 min, 95% for 10 min, and 100% for 20 min). Subsequently, the plates were packed in a desiccator at room temperature and only the bottoms of the plates containing biofilms were removed. Subsequently,
biofilms were coated with gold and visualized in Quanta
450 Scanning Electron Microscope (UFC, Brazil).
Antioxidant activity
Sequestration of the 2,2-diphenyl-1-picrylhydrazyl (DPPH)
The ability of CLF1 in sequestering 1,1-Diphenyl-2-picryl-hydrazyl (DPPH) radicals was determined according to the method described by (Duan et al. 2006). A methanolic solution containing 0.16 mM DPPH was added to CLF1 at concentrations ranging from 500 to 7.8μg/mL. The data obtained were compared to blank solution (a mixture of CLF1 and methanol) and control of the assay (0.16 mM DPPH solution). The samples were incubated in the absence of light at 25 °C for 30 min and the optical density was measured at 517 nm by a microplate reader (Biochrom Asys UVM 340). Quercetin was used as a positive control. The
percentage of DPPH radical sequestration was determined by the following equation:
Scavenging effectð Þ%
¼ 1 Abssample Abssample blank
Abscontrol
100%
Ferrous ion chelating assay (FIC)
The FIC assay was performed according to Wang et al. (2009). CLF1 was suspended in deionized water to obtain concentrations ranging from 500 to 7.8μg/mL. Then, 2 mM ferrous chloride (FeCl2) and 5 mM ferrozine were also
added to the suspension. Both, blank and control were performed with ferrozine solution plus CLF1 and ferrous chloride solution, respectively. Samples were incubated at 25 °C for 10 min and the optical density was measured at 562 nm using a microplate reader (Biochrom Asys UVM 340). EDTA (ethylenediaminetetraacetic acid) was used as positive control. The FIC activity was calculated by the following equation:
Ferrous ion chelating activityð Þ%
¼ Abscontrol Abssample Abssample blank
Abscontrol 100%
Iron reduction method
The Ferric-Reducing Antioxidant Power (FRAP) assay was performed according to methodology described by Ganesan et al. (2008). First, CLF1 was diluted in 0.2 mol/L sodium phosphate buffer (pH 6.6) with 1% potassium ferricyanide at concentrations ranging from 500 to 7.8μg/mL. Samples were incubated at 50 °C for 20 min. After cooling, 10% trichloroacetic acid was added. An aliquot was mixed with 1% iron hydrochloride in distilled water. After 10 min, the optical density was measured at 700 nm in the microplate reader (Biochrom Asys UVM 340). Butylhydroxyanisole (BHA) was used as positive control.
Method of inhibition ofβ-carotene oxidation
evaporated using a rotary evaporator, and then ultra-pure water was added to the solution. CLF1 was tested in con-centrations ranging from 500 to 7.8μg/mL. At the first
moment, the optical density of the solutions measured at 470 nm (Biochrom Asys UVM 340 microplate reader) and again after 3 h of incubation at 50 °C. BHA was used as a positive control for this assay. The antioxidant activity was calculated by the following equation:
Antioxidant activityð Þ ¼% Abs3h
Absinitial
100%
Statistical analysis
All tests were performed in triplicates and with significance
levelp<0.05. For the antimicrobial assays the difference between the means of the triplicates was verified by
one-way ANOVA test with Bonferroni post-test, executed by GraphPad Prism version 5.0 for Windows (San Diego, California, USA). For antioxidant assays, percentiles obtained in all concentrations tested were converted to absolute values, submitted to angular transformation and compared through Student’s t test for independent values.
Results
Effect of CLF1 on planktonic growth
The MIC and MBC obtained forS. mutans and S. para-sanguiniswere 3.9 and 15.6μg/mL, respectively, for both species (Table1).
Bacterial death time
The Fig.2shows the bacterial death kinetics ofS. mutans (A) andS. parasanguinis (B) in the presence of CLF1. In both concentrations, the treatment with CLF1 resulted in a progressive reduction in the number of CFUs from thefirst
2 h of contact to a complete reduction at 24 h. In addition, no significant differences were observed when comparing
both concentrations tested.
CLF1 activity on preformed biofilms
As showed in Fig.3a, none of the concentrations tested was able in reducing the biofilm biomass ofS. mutans. On the
other hand, CLF1 significantly reduced the biomass of
S. parasanguinis in all concentrations tested (Fig.3c). The treatment with the concentration of 500μg/mL reduced the biomass to approximately zero. Regarding the number of viable cells in biofilms, CLF1 reduced the number of CFUs
of S. mutans in all concentrations tested, except for 15.6μg/mL (Fig.3b). In addition, the concentrations of 500 and 250μg/mL reduced the cell viability by approximately 3.0 log forS. mutansbiofilms (Fig.3b). A better effect was
seen on S. parasanguinis (Fig. 3d), since almost all con-centrations tested reduced the number of viable cells. The treatment of S. parasanguinis with 500μg/mL led to a reduction in the number of viable cells of approximately 5.3 log.
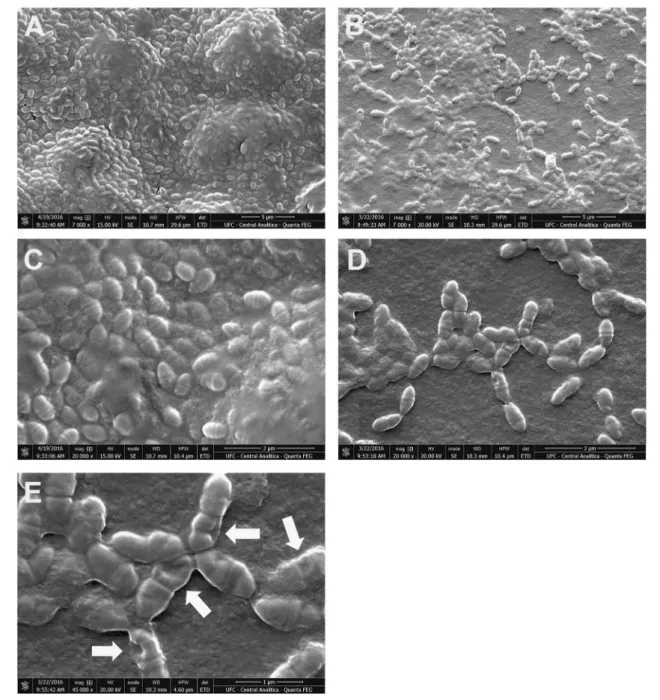
Analysis of preformed biofilms by scanning electron microscopy
As shown in Fig.4b, d, e), the number ofS. mutansbiofi
lm-entrapped cells was significantly reduced by the treatment
with CLF1. Similarly, the content of extracellular matrix (EPS) was also decreased. In fact, the images allow us to believe that CLF1 probably caused major changes in topology of biofilms as well as in morphology of biofi
lm-entrapped cells. The bacteria present in the untreated
bio-films show regularity in bacterial morphology (Fig. 4a, c).
On the other hand, biofilms treated with CLF1 at the
con-centration of 500μg/mL clearly show a disruption in the integrity of the bacterial membrane, with the apparent for-mation of pores on the surface (Fig. 4e).
Antioxidant activity
For the purpose of evaluating antioxidant activity of CLF1 the antioxidant assays DPPH, FIC, FRAP, and BCB were performed. The results are shown in Fig.5.
In the DPPH method, CLF1 at all concentrations sequestered ~40% free radicals, while the positive control (quercetin) showed a 100% DPPH sequestration.
In the FIC assay, CLF1 showed an iron ion chelating activity of ~20% in almost all tested concentrations, except for the concentration of 7.8μg/mL. The positive control EDTA presented a chelation activity of 100%.
In the assay that evaluated the ability of CLF1 to donate electrons to the medium converting Fe3+
to Fe2+
it can be seen that CLF1 demonstrated a low antioxidant activity when compared to the BHA control.
Regarding the β-carotene oxidation inhibition test, data obtained with CLF1 were very similar to the DPPH assay
Table 1 Minimal inhibitory concentration and minimum bactericidal
concentration of 3β,6β,16β-Trihydroxylup-20(29)-ene against S. mutansandS. parasanguinis
Strain MIC MBC
S. mutansATCC 700610 3.9 15.6 S. parasanguinisATCC 903 3.9 15.6
showing 40% inhibition for all concentrations tested. For the BHA control, the reduction varied between 70 to 90%.
Discussion
Species from the genus Streptococcus are responsible for the emergence of several oral pathologies, such as dental caries. This disease affects almost 100% of the world’s adult population, and 60 to 90% of school-age children (Aimutis 2012). Epidemiological studies have shown that many compounds obtained from medicinal plants present several
biological activities, among them antibacterial (Owen et al. 2000; Sala et al. 2002; Sasidharan et al.2011).
In this study, the MIC of CLF1 on S. mutans and S. parasanguinis was 3.9μg/mL. Previously, Evaristo et al. (2014) reported a MIC of 7.8μg/mL for both bacteria. The differences found in this study could be explained by the lower concentration of bacteria used in assays. Moreover, bacterial death time assay showed that CLF1 at concentra-tions of 62.5 and 31.25μg/mL reduced bacterial cell growth shortly afterfirst 2 h contact, leading to a fully reduction of
planktonic cells after 24 h. Corroborating with our results, Kim et al. (2011) reported the antimicrobial activity of a
Fig. 3 Biofilm massaandcand
viability of biofilm-entrapped cellsbanddofS. mutans ATCC 700610 andS. parasanguinisATCC 903 biofilms treated with CLF1.
Error barsindicate the standard deviation (SD). *p<0.05 compared to the control group
Fig. 2 Effect of CLF1 on death
triterpene ursolic acid againstS. mutans ATCC 25175 and S. sobrinusATCC 33478, with MIC of 2μg/mL and MBC of 4μg/mL, respectively. Concerning the death time assay, they reported that at 24 h, the number ofS. mutans andS. sobrinus was reduced to approximately zero by the treat-ment with triterpene at 8μg/mL.
Despite the promising results on planktonic cells, it is known that microorganisms preferentially live in commu-nities forming biofilms (Costerton and Wilson 2004;
Wol-cott et al. 2013). Furthermore, when incorporated in biofilms, bacterial cells are able to adapt to different
environments, to withstand external aggressions such as
predator attack and chemical treatments (Gupta et al.2016). The literature shows that triterpenes have been considered as promising molecules in the fight against microbial bio-films (Wojnicz et al. 2015).
Crucially, the age of biofilms influences its susceptibility,
in general, the increasing age of biofilm shows increased
resistance to antimicrobials (Sedlacek and Walker 2007; Wong et al. 2010; Stewart 2015). For this purpose, CLF1 was tested on preformed (mature) biofilms ofS. mutansand S. paransanguinis. In this study, biofilms ofS. mutansand S. paransanguinis grown for 48 h showed more resistance to CLF1 compared to data obtained by Evaristo et al.
Fig. 4Electro micrographs of Preformed biofilm ofS. mutansATCC 700610 treated with CLF1 at 500μg/mLb,d, andeand untreated biofilms
(2014), which tested the development of biofilms in the
presence of CLF1 during 24 h. According Stewart (2015), biofilm age and cell density are strongly correlated and
these factors contribute to biofilm tolerance. Jiang et al.
(2017)showed that mature biofilms from different strains of
S. mutans, included caries-active and caries-free adults strains, showing more acid-resistant when compared to young biofilms. Still according to the authors, the results
could be explained by the fact that mature biofilms are
denser, compact and complex compared with young
bio-films, supporting that mature biofilms are more difficult to
treat and more cariogenic than young ones.
S. parasanguinisshowed a higher susceptibility to CLF1, with a significant reduction of biomass and cell viability
(Fig.3c, d). According to Costerton et al. (1999), biofilms
of different species, but belonging to the same genus can present a degree of resistance differentiated to external factors, considering that the extracellular matrix of the biofilm can suffer variations in its composition and
struc-ture. Nevertheless, Kim et al. (2015) state that among the complex oral microbiota,S. mutansis not always the most abundant species. However, this bacterium is more tolerant to stress factors, in addition, it has a high capacity to form and modulate its cariogenic biofilms when exposed to
sucrose. TheS. mutansEPS matrix provides the creation of well-compacted biofilms and high adhesion to substrates
while acting as a physical barrier making it difficult to
penetrate antimicrobial agents into biofilms. In fact, the
results presented in this study show that biofilms formed by
S. mutans presented a significantly higher amount of
bio-mass when compared to S. parasanguinis, which may explain the smaller susceptibility ofS. mutans.
Regarding differences of susceptibility observed among species, the literature shows thatS. mutansalso has a higher resistance to antimicrobial agents due to its ability to act in the synthesis of genes resistant to antimicrobial agents (William et al. 2012). The genes (MbrABCD) comprise a complex system that play some roles in stimulus perception, in the transduction of chemical signals, in the regulation of other genes, in the transport of ATP ligase and in the dis-persion of substrates in response to stress factors (Ouyang et al. 2010). In addition, the CiaRH genes that act on the resistance of antimicrobial agents, mutacine production, competence development, stress tolerance, and acid envir-onments, and increased biofilm production (Mazda et al.
2012). Moreover, some proteins (Clp protease, DnaK, ser-ine protease and chaperones) present in S. mutans partici-pate concomitantly in resistance to stress factors and in the ability of cells to produce biofilms (Ahn et al.2006).
Studies with bioactive metabolites show that antibacterial activity may be related to damages on the plasma membrane (perforation and/or reduction in membrane fluidity),
inhi-bition of nucleic acid synthesis (inhiinhi-bition of topoisome-rase) and inhibition of energy metabolism (caused by
Fig. 5 Antioxidant activity of
inhibition of the reductase and NADH-cytochrome c) (Bernard et al. 1997; Tsuchiya and Iinuma 2000; Plaper et al.2003; Cushnie and Lamb2005). In fact the literature has shown that CLF1 and several other pentacyclic tri-perpenes, such as lupeol and betulinic acid, have inhibitory effects on the enzymes topoisomerases I and II (Moriarity et al.1998; Ma et al.2000; Wada et al.2001; Teles et al. 2015).
S. mutans biofilms treated with CLF1 and analyzed by
SEM clearly demonstrate the presence of pore-like struc-tures on the bacterial surface, suggesting a impairment of the bacterial membrane and subsequently loss of cellular
fluid, thus causing a possible disruption.
Several studies have suggested that triterpenes can be inserted into the bacterial membrane causing significant
functional and structural changes (Kurek et al.2010; Naz-zaro et al. 2013). Ursolic and oleanolic acids have been widely cited in the literature for their antibacterial and antibiofilm activities against Gram-positive bacteria,
includingS. mutans(Kozai et al.1999; Horiuchi et al.2007; Jimenez-Arellanes et al.2007; Fontanay et al. 2008; Park et al.2015).
Assays performed to determine the mechanism of action of ursolic acid and oleanolic acid have shown that tri-terpenes may also act by compromising the synthesis of peptidoglycans through the production of proteins involved in the cell wall, in the hydrolysis or modifications that allow
to decrease the cross-linking of the peptidoglycan opti-mizing its destabilization and consequently causing a compromise of the integrity of the cellular wall (Korsak et al.2005; Kurek et al.2010). In addition, due to their role in the development and aggravation of diseases, free radi-cals have been the target of the attention of scientists and practitioners involved with human health (Halliwell2006). The literature has been describing the antioxidant prop-erties of extracts, fractions and secondary metabolites of plant origin (Pannangpetch et al.2006; Gouveia et al.2011; Aderogba et al.2012).
For the antioxidant assays, CLF1 was able to reduce DPPH free radicals, as well as inhibitβ-carotene oxidation on a level of 40% for both assays, demonstrating moderate antioxidant ability when compared with controls quercetin and BHA.
The literature shows different pentacyclic triterpenes, such as erythrodiol, uvaol, oleanolic acid, maslinic acid, ursolic acid, lanthanum-A with antioxidant activity by DPPH assays (Grace-Lynn et al.2012; do Nascimento et al. 2014; do Nascimento-Neto et al. 2015).
In this study, we showed that CLF1 acting as an anti-oxidant agent can also contribute in the fight against
infections of the oral cavity, given that antioxidant agents minimize the biological effects of free radicals on tissues and cells, as well as assist the immune system by indirectly
fighting against microbial infections (Hughes 1999;
Ader-ogba et al.2012).
In conclusion, CLF1 obtained from the leaves of C. leprosum possess antibacterial activity against planktonic cells and is able in disrupting mature biofilms from S. mutans and S. parasanguinis. In addition, electron micro-graphs of S. mutans cells in mature biofilms treated with
CLF1 showed impairment in the integrity of the cell membrane. In addition, CLF1 showed antioxidant effect in different assays. Furthermore, future use of CLF1 as anti-oxidant agent and a potential antimicrobial compound against oral infections caused by biofilms from S. mutans and S. parasanguiniscan be suggested.
Acknowledgements The authors are grateful to the Fundação
Cearense de Apoio ao Desenvolvimento Científico e Tecnológico
(FUNCAP), Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), and Coordenação de Aperfeiçoamento de Pes-soal de Nível Superior (CAPES). E. H. Teixeira is a member of the Brazilian Academy of Sciences and senior investigators of CNPq.
Compliance with ethical standards
Conflict of interest The authors declare that they have no competing
interests.
References
Achtman M, Wagner M (2008) Microbial diversity and the genetic nature of microbial species. Nat Rev Microbiol 6(6):431–440 Aderogba MA, Kgatle DT, McGraw LJ, Eloff JN (2012) Isolation of
antioxidant constituents from Combretum apiculatum subsp. apiculatum. S Afr J Bot 79:125–131
Agarwal P, Nagesh L (2011) Comparative evaluation of efficacy of 0.2% chlorhexidine, listerine and tulsi extract mouth rinses on salivary streptococcus mutans count of high school children--RCT. Contemp Clin Trials 32(6):802–808
Ahn SJ, Wen ZT, Burne RA (2006) Multilevel control of competence development and stress tolerance in streptococcus mutans UA159. Infect Immun 74(3):1631–1642
Aimutis WR (2012) Lactose cariogenicity with an emphasis on childhood dental caries. Int Dairy Jo 22(2):152–158
Almaz ME, Sonmez IS, Okte Z, Oba AA (2017) Efficacy of a
sugar-free herbal lollipop for reducing salivary streptococcus mutans levels: a randomized controlled trial. Clin Oral Investig 21 (3):839–845
Asokan S, Rathan J, Muthu MS, Rathna PV, Emmadi P, Raghuraman, Chamundeswari (2008) Effect of oil pulling on streptococcus mutans count in plaque and saliva using dentocult SM Strip mutans test: a randomized, controlled, triple-blind study. J Indian Soc Pedod Prev Dent 26(1):12–17
Bernard FX, Sablé S, Cameron B, Provost J, Desnottes JF, Crouzet J, Blanche F (1997) Glycosylatedflavones as selective inhibitors of topoisomerase IV. Antimicrob Agents Chemother 41(5):992–998 Chew YL, Lim YY, Omar M, Khoo KS (2008) Antioxidant activity of three edible seaweeds from two areas in South East Asia. Lwt-Food Sci Technol 41(6):1067–1072
Costerton JW, Stewart PS, Greenberg EP (1999) Bacterial biofilms: a
common cause of persistent infections. Science 284 (5418):1318–1322
Costerton JW, Wilson M (2004) Introducing biofilms. Biofilms 1:1–4 Cushnie TP, Lamb AJ (2005) Antimicrobial activity offlavonoids. Int
J Antimicrob Agents 26(5):343–356
Davey ME, O’Toole GA (2000) Microbial biofilms: from ecology to molecular genetics. Microbiol Mol Biol Rev 64(4):847–867 do Nascimento PG, Lemos TL, Bizerra AM, Arriaga AM, Ferreira
DA, Santiago GM, Braz-Filho R, Costa JG (2014) Antibacterial and antioxidant activities of ursolic acid and derivatives. Mole-cules 19(1):1317–1327
do Nascimento-Neto LG, Evaristo FF, Alves MF, Albuquerque MR, dos Santos HS, Bandeira PN, Arruda FV, Teixeira EH (2015) Effect of the triterpene 3beta, 6beta, 16beta-trihydroxylup-20 (29)-ene isolated from the leaves of combretum leprosum mart. on cutaneous wounds in mice. J Ethnopharmacol 171:116–120 Duan X, Zhang W, Li X, Wang B (2006) Evaluation of antioxidant
property of extract and fractions obtained from a red alga, poly-siphonia urceolata. Food Chem 95(1):37–43
Evaristo FF, Albuquerque MR, dos Santos HS, Bandeira PN, Avila Fdo N, da Silva BR, Vasconcelos AA, Rabelo Ede M, Nascimento-Neto LG, Arruda FV, Vasconcelos MA, Carneiro VA, Cavada BS, Teixeira EH (2014) Antimicrobial effect of the triterpene 3beta,6beta,16beta-trihydroxylup-20(29)-ene on planktonic cells and biofilms from gram positive and gram negative bacteria. Biomed Res Int 2014:729358
Facundo VA, Andrade CHS, Silveira ER, Braz-Filho R, Hufford CD (1993) Triterpenes and flavonoids from combretum leprosum. Phytochemistry 32(2):411–415
Fontanay S, Grare M, Mayer J, Finance C, Duval RE (2008) Ursolic, oleanolic and betulinic acids: antibacterial spectra and selectivity indexes. J Ethnopharmacol 120(2):272–276
Forssten SD, Bjorklund M, Ouwehand AC (2010) Streptococcus mutans, caries and simulation models. Nutrients 2(3):290–298 Ganesan P, Kumar CS, Bhaskar N (2008) Antioxidant properties of
methanol extract and its solvent fractions obtained from selected Indian red seaweeds. Bioresour Technol 99(8):2717–2723 Gouveia MG, Xavier MA, Barreto AS, Gelain DP, Santos JP, Araujo
AA, Silva FA, Quintans JS, Agra MF, Cabral AG, Tavares JF, Silva MS, Quintans-Junior LJ (2011) Antioxidant, anti-nociceptive, and anti-inflammatory properties of the ethanolic
extract of combretum duarteanum in rodents. J Med Food 14 (11):1389–1396
Grace-Lynn C, Darah I, Chen Y, Latha LY, Jothy SL, Sasidharan S (2012) In vitro antioxidant activity potential of lantadene A, a pentacyclic triterpenoid of Lantana plants. Molecules 17 (9):11185–11198
Gupta P, Sarkar S, Das B, Bhattacharjee S, Tribedi P (2016) Biofilm, pathogenesis and prevention--a journey to break the wall: a review. Arch Microbiol 198(1):1–15
Halliwell B (2006) Reactive species and antioxidants. Redox biology is a fundamental theme of aerobic life. Plant Physiol 141 (2):312–322
Herrero ER, Slomka V, Bernaerts K, Boon N, Hernandez-Sanabria E, Passoni BB, Quirynen M, Teughels W (2016) Antimicrobial effects of commensal oral species are regulated by environmental factors. J Dent 47:23–33
Horiuchi K, Shiota S, Hatano T, Yoshida T, Kuroda T, Tsuchiya T (2007) Antimicrobial activity of oleanolic acid from Salvia offi
-cinalis and related compounds on vancomycin-resistant enter-ococci (VRE). Biol Pharm Bull 30(6):1147–1149
Hu JF, Garo E, Goering MG, Pasmore M, Yoo HD, Esser T, Sestrich J, Cremin PA, Hough GW, Perrone P, Lee YS, Le NT, O’ Neil-Johnson M, Costerton JW, Eldridge GR (2006) Bacterial biofilm inhibitors from diospyros dendo. J Nat Prod 69(1):118–120
Hughes DA (1999) Effects of dietary antioxidants on the immune function of middle-aged adults. Proc Nutr Soc 58(1):79–84 Jeon JG, Rosalen PL, Falsetta ML, Koo H (2011) Natural products in
caries research: current (limited) knowledge, challenges and future perspective. Caries Res 45(3):243–263
Jiang S, Chen S, Zhang C, Zhao X, Huang X, Cai Z (2017) Effect of the biofilm age and starvation on acid tolerance of biofilm formed by streptococcus mutans isolated from active and caries-free adults. Int J Mol Sci 18(4):E713
Jimenez-Arellanes A, Meckes M, Torres J, Luna-Herrera J (2007) Antimycobacterial triterpenoids from Lantana hispida (Verbena-ceae). J Ethnopharmacol 111(2):202–205
Katerere DR, Gray AI, Nash RJ, Waigh RD (2003) Antimicrobial activity of pentacyclic triterpenes isolated from African com-bretaceae. Phytochemistry 63(1):81–88
Kim D, Hwang G, Liu Y, Wang Y, Singh AP, Vorsa N, Koo H (2015) Cranberryflavonoids modulate cariogenic properties of
mixed-species biofilm through exopolysaccharides-matrix disruption.
PLoS One 10(12):1–13
Kim MJ, Kim CS, Park JY, Lim YK, Park SN, Ahn SJ, Jin DC, Kim TH, Kook J (2011) Antimicrobial effects of ursolic acid against mutans streptococci isolated from Koreans. Int J Oral Bio 36:7–11
Kolenbrander PE, Palmer Jr. RJ, Periasamy S, Jakubovics NS (2010) Oral multispecies biofilm development and the key role of cell-cell distance. Nat Rev Microbiol 8(7):471–480
Koo H, Falsetta ML, Klein MI (2013) The exopolysaccharide matrix: a virulence determinant of cariogenic biofilm. J Dent Res 92 (12):1065–1073
Korsak D, Popowska M, Markiewicz Z (2005) Analysis of the murein of a Listeria monocytogenes EGD mutant lacking functional penicillin binding protein 5 (PBP5). Pol J Microbiol 54 (4):339–342
Kozai K, Suzuki J, Okada M, Nagasaka N (1999) Effect of oleanolic-acid-cyclodextrin inclusion compounds on carries by in vitro experiment and rat-carries model. Microbios 97:179–188 Kt S, Kmk M, NB, Jimson S, RS (2013) Dental caries vaccine-a
possible option? J Clin Diagn Res 7(6):1250–1253
Kurek A, Grudniak AM, Szwed M, Klicka A, Samluk L, Wolska KI, Janiszowska W, Popowska M (2010) Oleanolic acid and ursolic acid affect peptidoglycan metabolism in Listeria monocytogenes. Antonie Van Leeuwenhoek 97(1):61–68
Lamont RJ, Demuth DR, Davis CA, Malamud D, Rosan B (1991) Salivary-agglutinin-mediated adherence of streptococcus mutans to early plaque bacteria. Infect Immun 59(10):3446–3450 Lira SRS, Almeida RN, Almeida FRC, Oliveira FS, Duarte JC
(2002) Preliminary studies on the analgesic properties of the ethanol extract of combretum leprosum. Pharmaceut Biol 40 (3):213–215
Longhi-Balbinot DT, Lanznaster D, Baggio CH, Silva MD, Cabrera CH, Facundo VA, Santos AR (2012) Anti-inflammatory effect of
triterpene 3beta, 6beta, 16beta-trihydroxylup-20(29)-ene obtained from combretum leprosum Mart & Eich in mice. J Ethno-pharmacol 142(1):59–64
Ma ZZ, Hano Y, Nomura T, Chen YJ (2000) Three new triterpenoids from Peganum nigellastrum. J Nat Prod 63(3):390–392 Maeda H, Akaike T (1998) Nitric oxide and oxygen radicals in
infection, inflammation, and cancer. Biochemistry 63(7):854–865 Marinho VC, Chong LY, Worthington HV, Walsh T (2016) Fluoride mouthrinses for preventing dental caries in children and adoles-cents. Cochrane Database Syst Rev 7:CD002284
antimicrobial peptides in biofilms. Mol Oral Microbiol 27
(2):124–135
McGaw LJ, Rabe T, Sparg SG, Jager AK, Eloff JN, van Staden J (2001) An investigation on the biological activity of combretum species. J Ethnopharmacol 75(1):45–50
Moriarity DM, Huang J, Yancey CA, Zhang P, Setzer WN, Lawton RO, Bates RB, Caldera S (1998) Lupeol is the cytotoxic principle in the leaf extract of dendropanax cf. querceti. Planta Med 64 (4):370–372
Nazzaro F, Fratianni F, De Martino L, Coppola R, De Feo V (2013) Effect of essential oils on pathogenic bacteria. Pharmaceuticals 6 (12):1451–1474
Nunes PH, Cavalcanti PM, Galvao SM, Martins MC (2009) Anti-ulcerogenic activity of combretum leprosum. Pharmazie 64 (1):58–62
Ouyang J, Tian XL, Versey J, Wishart A, Li YH (2010) The BceABRS four-component system regulates the bacitracin-induced cell envelope stress response in Streptococcus mutans. Antimicrob Agents Chemother 54(9):3895–3906
Owen RW, Giacosa A, Hull WE, Haubner R, Spiegelhalder B, Bartsch H (2000) The antioxidant/anticancer potential of phenolic com-pounds isolated from olive oil. Eur J Cancer 36(10):1235–1247 Pannangpetch P, Theansuwan W, Kongyingyoes B, Kukongviriyapan
V, Kukongviriyapan U (2006) The ethanolic extract of com-bretum decandrum Roxb. improved glucose tolerance and increased glucose uptake of hyperinsulinemic rats. Encorine Abst 11:300
Park SN, Ahn SJ, Kook JK (2015) Oleanolic acid and ursolic acid inhibit peptidoglycan biosynthesis in streptococcus mutans UA159. Braz J Microbiol 46(2):613–617
Pham-Huy LA, He H, Pham-Huy C (2008) Free radicals, antioxidants in disease and health. Int J Biomed Sci 4(2):89–96
Pietrovski EF, Rosa KA, Facundo VA, Rios K, Marques MC, Santos AR (2006) Antinociceptive properties of the ethanolic extract and of the triterpene 3beta,6beta,16beta-trihidroxilup-20(29)-ene obtained from the flowers of Combretum leprosum in mice.
Pharmacol Biochem Behav 83(1):90–99
Plaper A, Golob M, Hafner I, Oblak M, Solmajer T, Jerala R (2003) Characterization of quercetin binding site on DNA gyrase. Bio-chem Biophys Res Commun 306(2):530–536
Rabe P, Twetman S, Kinnby B, Svensater G, Davies JR (2015) Effect offluoride and chlorhexidine digluconate mouthrinses on plaque
biofilms. Open Dent J 9:106–111
Sala A, Recio M, Giner RM, Manez S, Tournier H, Schinella G, Rios JL (2002) Anti-inflammatory and antioxidant properties of heli-chrysum italicum. J Pharm Pharmacol 54(3):365–371
Sasidharan S, Chen Y, Saravanan D, Sundram KM, Yoga Latha L (2011) Extraction, isolation and characterization of bioactive compounds from plants’extracts. Afr J Tradit Complement Altern Med 8(1):1–10
Sedlacek MJ, Walker C (2007) Antibiotic resistance in an in vitro subgingival biofilm model. Oral Microbiol Immunol 22
(5):333–339
Smina TP, Mathew J, Janardhanan KK, Devasagayam TP (2011) Antioxidant activity and toxicity profile of total triterpenes
iso-lated from Ganoderma lucidum (Fr.) P. Karst occurring in South India. Environ Toxicol Pharmacol 32(3):438–446
Stepanovic S, Vukovic D, Dakic I, Savic B, Svabic-Vlahovic M (2000) A modified microtiter-plate test for quantification of
sta-phylococcal biofilm formation. J Microbiol Methods 40 (2):175–179
Stewart PS (2015). Antimicrobial tolerance in biofilms. Microbiol Spectr 3(3):1–13
Teles CB, Moreira-Dill LS, Silva Ade A, Facundo VA, de Azevedo Jr. WF, da Silva LH, Motta MC, Stabeli RG, Silva-Jardim I (2015) A lupane-triterpene isolated from Combretum leprosum Mart. fruit extracts that interferes with the intracellular development of Leishmania (L.) amazonensis in vitro. BMC Complement Altern Med 15:165
Tredwin CJ, Scully C, Bagan-Sebastian JV (2005) Drug-induced disorders of teeth. J Dent Res 84(7):596–602
Tsuchiya H, Iinuma M (2000) Reduction of membrane fluidity by
antibacterial sophoraflavanone G isolated from Sophora exigua.
Phytomedicine 7(2):161–165
Varoni E, Tarce M, Lodi G, Carrassi A (2012) Chlorhexidine (CHX) in dentistry: state of the art. Minerva Stomatol 61(9):399–419 Viau CM, Moura DJ, Facundo VA, Saffi J (2014) The natural
tri-terpene 3beta,6beta,16beta-trihydroxy-lup-20(29)-ene obtained from theflowers of Combretum leprosum induces apoptosis in MCF-7 breast cancer cells. BMC Complement Altern Med 14:280
Wada S, Iida A, Tanaka R (2001) Screening of triterpenoids isolated from Phyllanthus flexuosus for DNA topoisomerase inhibitory activity. J Nat Prod 64(12):1545–1547
Wang T, Jónsdóttir R, Ólafsdóttir G (2009) Total phenolic com-pounds, radical scavenging and metal chelation of extracts from Icelandic seaweeds. Food Chem 116(1):240–248
William B, Rwenyonyi CM, Swedberg G, Kironde F (2012) Cotri-moxazole prophylaxis specifically selects for cotrimoxazole
resistance in streptococcus mutans and streptococcus sobrinus with varied polymorphisms in the target genes folA and folP. Int J Microbiol 2012:916129
Wojnicz D, Tichaczek-Goska D, Kicia M (2015) Pentacyclic tri-terpenes combined with ciprofloxacin help to eradicate the bio-film formed in vitro by Escherichia coli. Indian J Med Res 141
(3):343–353
Wolcott R, Costerton JW, Raoult D, Cutler SJ (2013) The poly-microbial nature of biofilm infection. Clin Microbiol Infect 19 (2):107–112
Wong MC, Glenny AM, Tsang BW, Lo EC, Worthington HV and Marinho VC (2010). Topical fluoride as a cause of dental
fluorosis in children. Cochrane Database Syst Rev (1): CD007693 Wong HS, Townsend KM, Fenwick SG, Maker G, Trengove RD, O’Handley RM (2010) Comparative susceptibility of salmonella typhimurium biofilms of different ages to disinfectants.