w ww . e l s e v i e r . c o m / l o c a t e / b j p
Original
Article
Some
triterpenic
compounds
in
extracts
of
Cecropia
and
Bauhinia
species
for
different
sampling
years
Marcella
Emilia
Pietra
Schmidt
a,
Fernanda
Brum
Pires
b,
Lucas
Paines
Bressan
a,
Fábio
Vieira
da
Silva
Jr.
a,
Osmar
Lameira
c,
Marcelo
Barcellos
da
Rosa
a,b,∗aProgramadePós-graduac¸ãoemQuímica,UniversidadeFederaldeSantaMaria,Camobi,SantaMaria,RS,Brazil
bProgramadePós-graduac¸ãoemCiênciasFarmacêuticas,UniversidadeFederaldeSantaMaria,Camobi,SantaMaria,RS,Brazil cEmbrapaAmazôniaOriental,Belém,PA,Brazil
a
r
t
i
c
l
e
i
n
f
o
Articlehistory: Received9October2017 Accepted24November2017 Availableonline12December2017
Keywords: HPLC-UV Validation Triterpenes
Whiteandpinkpata-vaca Redandwhite-embaúba Amazonianmedicinalplants
a
b
s
t
r
a
c
t
Theaimofthispaperistoprovideanoverviewonthechemicalcompositionoftriterpenesinwidespread usedfolkmedicinespecies,throughthedevelopmentandvalidationofelevencompoundsusing HPLC-UVdetection.Thecompoundswereseparatedusingisocraticelution,onareversephasecolumn(Kinetex C18,250mm×4.6mm,5m)withmobilephaseconsistedofacetonitrile:tetrahydrofuran(90:10,v/v), flow-rateof0.5ml/minanddetectionin210nm.Diversevalidationparametersweresuccessfully eval-uated.ThesamplesofBauhiniavariegataL.,B.variegatavar.candidaVoigt,Fabaceae,Cecropiapalmata Willd.andC.obtusaTrécul,Urticaceae,collectedin2012,2013and2014fromAmazonweretreatedwith twodifferentsolvents(ethylacetateandchloroform)andanalyzedbytheproposedmethod. Stigmas-terol,lupeol,-sitosterol,-amirinand␣-amirinwerefoundinallthestudiedplants.Highlightingthe presenceofoleanolicacid,maslinicacidinC.obtusaandC.palmataextracts,erythrodiolonlyinC. pal-mata,stigmasteolinB.variegataand␣-amirininB.variegatavar.candida.Overall,ethylacetateshowed betterperformanceastheextractorsolventthanchloroform.Moreover,itcouldbeusedforthequality controlofmedicinalplantsandtoassesspotentialmarkercompounds.
©2017SociedadeBrasileiradeFarmacognosia.PublishedbyElsevierEditoraLtda.Thisisanopen accessarticleundertheCCBY-NC-NDlicense(http://creativecommons.org/licenses/by-nc-nd/4.0/).
Introduction
Triterpenes,presentsinvegetableoils,cereals,fruitsandbarkof treesarewidespreadinthehumandiet(Rhourri-Frihetal.,2009; Saleem,2009;SiddiqueandSaleem,2011;Szakieletal.,2012)and one of thelargestclasses of secondary metabolites, withmore than30,000differenttriterpenes reported(Muffleret al.,2011; Thimmappaetal.,2014).
Thislargenumberofcompoundsisrelatedtotheversatilityof theirstructure,consistedofacycles,bi-,tri-,tetra-andpentacycles (Dias et al., 2011; Muffler et al., 2011). Among those, penta-cyclictriterpenespresentedpromisingpharmacologicalproperties (Szakieletal.,2012;GhoshandSil,2013;Shanmugametal.,2013) suchas,anti-inflammatory(Saleemetal.,2008;Martelancetal., 2009), hepatoprotector (Kumari and Kakkar, 2012; Pollier and Goossens, 2012), anti-tumor (Saleem, 2009; Shanmugamet al., 2013), anti-viral(Sánchez-Ávilaet al.,2009; Kong etal., 2013),
∗ Correspondingauthor.
E-mail:marcelo.b.rosa@ufsm.br(M.B.Rosa).
anti-HIV(Chenget al.,2011; Wójciak-Kosioret al.,2013), anti-microbial (Pai et al., 2011), anti-fungal (Rocha et al., 2004), anti-diabetic (Manna et al., 2010), gastroprotective (Sánchez et al., 2006; Quílez et al., 2010), anti-hyperlipidemic (Claude et al., 2004), neuroprotector (Silva et al., 2011), antiarthritic (SiddiqueandSaleem,2011),antioxidant(Alloucheetal.,2010), cholesterol-reducingproperties(Chauhanetal.,2013), cardiopro-tective(Somovaet al.,2003)andtrypanocidal activity(Ferreira etal.,2010).
Medicinalplantshavebeenusedforcenturiesinfolkmedicine associatedwithhealthpromotion,preventionandcureofhuman diseases(Laszczyk,2009;Romeroet al.,2010).Withmorethan 50,000plantspecies,Brazilhasthegreatestlevelsofbiodiversity intheplanet(Giuliettietal.,2005)andAmazoniaisaregionwith oneoftherichestfloraintheworldwithalargepotentialdiscovery andresearchofnewdrugs(Giuliettietal.,2005;Coelho-Ferreira, 2009).Despitethediversityandthewidespreaduseof phytother-apicsinBrazilthescientificknowledgeaboutthisfloraproperties islimited(Figueredoetal.,2014).
BauhiniavariegataL.,popularknowninBrazilas“pata-de-vaca” or “unha-de-boi”, member of the Fabaceae family, it has been
https://doi.org/10.1016/j.bjp.2017.11.005
usedinpopularmedicineasaresultoftheirhypoglycemic(Silva and Filho, 2002;Parekhet al., 2006; Murillo etal., 2007; Silva etal.,2007),anti-cholesterol,anti-elephantiasis(Silvaetal.,2007), antibacterial(SilvaandFilho,2002;Parekhetal.,2006),anti-tumor (Rajkapooretal.,2003),antifungal(SilvaandFilho,2002;Rajkapoor etal.,2003),diuretic,tonic,depurativeactivities(Pizzolattietal., 2003), while alsobeing usefulagainst skindiseases and ulcers (Reddyetal.,2003)inbronchitis,leprosy(Rajkapooretal.,2003), andinthemanagementofinflammatorydiseases(SilvaandFilho, 2002;Raoetal.,2008).
AnothermedicinalplantsincludedinthisstudyisCecropia pal-mata Willd. and C. obtusaTrécul,popularly known in Brazilas “embaúba-vermelha”and“embaúba-branca”fromtheUrticaceae family,traditionalusedasanti-rheumatic(Silvaetal.,2007), anti-inflammatory(Rochaetal.,2007;Costaetal.,2011;Nicasio-Torres etal.,2012;Pelaezetal.,2013),anti-oxidantactivities( Nicasio-Torresetal.,2012),anti-tumor(Rochaetal.,2007),actincentral nervoussystem,includinganxiolyticandantidepressant-like activ-ities(Silvaetal.,2007;Costaetal.,2011),againstasthma,highblood pressure(Costaetal.,2011),aswellusedinthetreatmentoftype 2diabetes(Rochaetal.,2007;Nicasio-Torresetal.,2012;Pelaez etal.,2013).
This wide diversity of pharmacological properties reported inCecropia andBauhiniaisrelated tosecondarymetabolites, as flavonoids,phenolicacids,carotenoids,tocopherols,alkaloids, lig-nans,tannins,salicylates,glucosinolatesandtriterpenes(Szakiel etal.,2012).
Severalpapersdescribedthedetectionandseparationof triter-penes in medicinal plants. Some methods include preparative thin-layerchromatography(TLC)(Martelancetal.,2007; Marte-lancet al.,2009), gaschromatography (GC) withderivatization step(Zhangetal.,2012), capillaryelectrophoresis(CE)(Cheung and Zhang, 2008; Li et al., 2011), evaporative light-scattering detectors (Lesellier et al.,2012), high-performance liquid chro-matography(HPLC)(Martelancetal.,2009;Lietal.,2011;Lesellier etal.,2012;Zhangetal.,2012)withUVdetectorormass spectro-metricdetectorsusingatmosphericpressurechemicalionization (APCI)atmosphericpressurephotoionization(APPI)and electro-sprayionization(ESI)(Sánchez-Ávilaetal.,2009).Thesimultaneous determinationof triterpenes rendera difficulttaskconsidering, theirsimilarstructure,lackofchromophores,verylowUV absorp-tionandsimilarpolarity.Accordingtotheliterature,thereisonly onepublishedreportthatseparatesmorethansixtriterpenesin a singleHPLC run (Bedneret al., 2008;Martelanc et al., 2009; Sánchez-Ávilaetal.,2009;Lietal.,2011;SlavinandYu,2012;Li etal.,2013).
Inthisstudythedevelopmentofa methodforsimultaneous determinationofeleventriterpeneswithisocraticelutionandUV detectionwasproposed. Thedevelopedmethodwasappliedto theanalysisoffourdifferentmedicinalplantsfromtheAmazon region(Cecropiaobtusa,C.palmata,B.variegataandB.variegatavar.
candida)HPLCwithUVdetection.
Materialsandmethods
Chemicals
All the chemical standards used, ␣-amirin (98%), -amirin (98.5%),-sitosterol(85%),stigmasterol(95%),lupeol(90%),uvaol (95%),erythrodiol(97%),oleanolicacid(97%),betulinicacid(97%), arjunicacid(88%)and maslinicacid(95%)usedwereof analyti-calgradefromSigma–Aldrich(St.Louis,MO,USA).Thesolvents acetonitrile(ACN),methanol(MeOH)andtetrahydrofuran(THF) wereofHPLCgradefromTedia(Fairfield,OH,EUA).Chloroform (CHCl3)andethylacetate(EtOAc)wereofanalyticalgradefrom
Merck (Darmstadt, Germany). Stock solutions of ␣-amirin,  -amirin,uvaol,erythrodiol,oleanolicacid,arjunicacidandmaslinic acid1000mg/l, stigmasterol481mg/l, lupeol365mg/l, betulinic acid196mg/land-sitosterol873mg/lwerepreparedinmethanol. Theworkinganalyticalsolutionsforanalyticalcurvewereobtained bydilutingtheanalyticalsolutionsinacetonitrilewiththe follow-ingconcentrations0.7,6.5,12.2,18.0,23.6,29.3and35.0mg/l.All thesolutionswerestoredat−20◦Cuntilanalysis.
HPLC-UVanalysis
ChromatographicmeasurementswereperformedonaDionex®
modelP680(Sunnyvale,CA,USA)liquidchromatographequipped withaUV-visdetectormodelUVD170U,Rheodyne®
injectionvalve model 8125 (Cotati, CA, USA) with loop of 100l. The analy-seswerecarriedoutwithaKinetexreversed-phaseC18 column
(250mm×4.6mm,5mparticlesize;Phenomenex,Torrance,CA, USA) which was precededby a Security Guard C18.pre-column
(Phenomenex,Torrance,CA,USA).Themobilephaseconsistedof acetonitrile:tetrahydrofuran(90:10,v/v)andtheflow-ratewasset at0.5ml/min.Spectrophotometrydetectionofanalyteswas per-formedat210nmwavelengths.Evaluationandquantificationwere madeonaChromeleon6.7Workstation.Thesamesampleswere previouslystudiedalsobyperforminganultra-high-performance liquid chromatography – atmospheric pressure photoionization sourcemassspectrometry(UHPLC-APPI-MS/MS).Therefore,a com-parisonofthespeciesdetectedusingHPLC-UVwasperformedby UHPLC-APPI-MS/MS,describedindetailsbyGoboetal.(2016).
Plantmaterial
ThemedicinalplantspeciesBauhiniavariegataL.(depositn◦IAN
185932),B.variegatavar.candidaVoigt(depositn◦IAN185831), CecropiaobtusaTrécul(depositn◦IAN185555)andC.palmataWilld
(depositn◦ IAN 185556)wereobtainedfromtheherbal
collec-tionoftheBrazilianAgriculturalResearchCorporation,Embrapa AmazôniaOriental,Belém,PA,Brazil.Thegeographicallocationof thecollectionsiteis1◦27′21′′Slatitudeand48◦30′14′′Wlongitude.
TheAmazonregionhasahotandhumidcharacteristicclimate withsmalltemperaturegradients.Thereare twowellestablish seasonsinthatregion,adry-period(July–October)andrainy sea-son (December–May); the months of June and November are considered transition periods (Ananias et al., 2010). According
Gobbo-NetoandLopes(2007)thereisapositiveinfluenceof rain-fall ontheconcentration of secondary metabolites(cyanogenic glycosides,glucosinolates,terpenes,anthocyaninsand alkaloids) therefore,thesamplesstudiedwerecollectedduringtherainy sea-soninthreedifferentyears(2012,2013and2014).
Samplepreparationandextractionprocedure
Thefreshplantspecimenswerecleaned,driedat40◦Cfor12h,
groundintoafinepowderinalaboratorymillandusedasadry powderedmaterial.Allplantswerereceivedasafinepowdered driedleafmaterial.Driedsampleswerestoredindesiccatorsunder vacuumatroomtemperatureuntilsampletreatment.
Ultrasound-assisted extraction was performed in a reactor thermostaticwater bath(temperatureaccuracy of±1.0◦C).The
experimental setup consists of an ultrasonic bath USC 1800A (UniqueInc.,Brazil,BR)equippedwithatransducerwith longitu-dinalvibrations.Theultrasonicunithasanoperatingfrequencyof 40kHzandamaximum-ratedultrasoundpoweroutputof132W. Theultrasonictransducer(surfaceareaof282.2cm2)isfittedatthe
andsonicatedfor30minat37◦C.Extractionwascarriedoutthree
timeswithfreshportionsofsolventintheaboveconditions(Pai etal.,2011;Wójciak-Kosioretal.,2013).Theextractremainedwas driedwithN2anddissolvedin10mlofmobilephase.Allthe
sam-plesweredilutedtoa2%(m/v)andfilteredthroughChromafilXtra PEFT-20/25filtersfromMacherey-Nagel(Düren,Germany)before injection.Forconstructionofthecalibrationcurves,sevendifferent mixedsolutionswereinjectedinthreereplicates.
Validationprocedure
Theanalyticalmethodwasvalidatedforlinearity,limitof detec-tion(LOD),limitofquantification(LOQ),accuracy,precisionand robustnessfollowingtheREn◦899/2003(Anvisa,2003).The
cal-ibration curves were preparedusing sevenconcentrations (0.7, 6.5,12.2,18.0,23.6,29.3and35.0mg/l)ofthe11stockstandard solutionsintherange0.21–40mg/lwhichwereinjectedin trip-licate. The mean peak areas were taken for the construction thecalibrationcurve.Thedata wereanalyzed bylinear regres-sionleastsquaremodel.TheLODandLOQ,underthepresented chromatographicconditions weredeterminedvisually.The pre-cisionwasdetermined byintraand inter-dayvariation andthe recoverywereevaluatedbystandardadditionmethod,the vari-ationswereexpressedbyRSD %asthefollowing formula:RSD (%)=(detected amount−original amount)/amount spiked×100.
Allsolutionswerekeptat−20◦Cbeforeanalysis.
Resultsanddiscussion
HPLCanalysis
Basedinmethodsreportedintheliterature(Martelancetal., 2007;Martelancetal.,2009;Sánchez-Ávilaetal.,2009;Yangetal., 2009;Lietal.,2013),chromatographicconditionswereoptimized inordertoobtainthebestseparationoftheanalytesintheshortest time.Themainvariablesevaluatedinthechromatographic sepa-rationwereratioofthemobilephase,flow-rateofmobilephase, temperatureoftheoperationalroomanddetectionwavelength.
Table1showsthesevariables,therangesstudiedandtheir opti-mumvaluesaswellasthesolventsusedintheoptimizationofthe mobilephasecomposition.
Table1
Optimization of the chromatographic conditions for separation of triterpenic compounds.
Variable Tastedvalue Optimumvalue
Flowrate(ml/min) 0.5,0.8and1.0 0.5
Roomtemperature(◦C) 21.0and23.0 21.0
Compositionmobilephase
MeOH:H2O 90:10(pH3.0)
80:20(pH3.0)
ACN:H2O 90:10(pH3.0) ACN:THF(90:10)
THF:ACN:H2O 30:60:10
ACN:THF 90:10
Wavelength(nm) 200,205,210,220
and225
210
ACN,acetonitrile;MeOH,methanol;THF,tetrahydrofuran;H2O,water.
Table2
Molarabsorptivitycoefficient(ε)inl/molcmoftriterpenesindifferentwavelengths.
Compound ε200nm ε210nm ε220nm
Arjunicacid 2.4·104 1.6·104 6.4·103
Betulinicacid 5.0·103 5.7·103 2.2·103
Erythrodiol 7.1·104 2.8·104 1.2·104
Lupeol 1.2·105 6.1·104 3.1·104
Maslinicacid 2.2·105 1.5·105 7.1·104
Oleanolicacid 1.6·105 9.7·104 5.4·104
Stigmasterol 1.1·105 5.0·104 2.8·104
Uvaol 1.1·105 5.1·104 2.9·104
␣-Amirin 1.4·105 8.1·104 4.7·104
-Amirin 1.3·105 4.6·104 2.4·104
-Sitosterol 1.3·104 7.2·104 4.3·104
ThebestmobilephasewasACN/THF(90:10;v/v).Theoptimum flowrateandroomtemperaturewere0.5ml/minand21◦C, respec-tively.Thedetectionwavelengthwaschosenat210nmaccordingly toexperimentaldata(Table2)and theliterature(Holen,1985; Schneideretal.,2009;Romeroetal.,2010;SlavinandYu,2012; Xuet al.,2012; Zhanget al.,2012)due tobetterabsorptionat the selected wavelength. The representative chromatogram for standard solutions under the proposed conditions is shown in
Fig.1.Itisnoteworthytomentionthatthecompleteseparation oftheeleventriterpenescouldbeachievedin45min,comparedto otherseparationmethods,itcanbesaidthatthenew methodol-ogydevelopedseparatesmorecompoundsthantheothersreported
120 mAU
Arjunic acid
Maslinic acid
Betulinic acid
Oleanolic acid
Er
ythrodiol
Lupeol
a-amir
in
b-amir
in
Stigmasteol b - stigmasteol
Uv
aol
100
80
60
40
20
-20 0
0.0 5.0 10.0 15.0 20.0 25.0 30.0 35.0 40.0 45.0 min
Table3
AnalyticalparametersforthevalidatedmethodbyHPLC-UV.
Compound LOD(mg/kg) LOQ(mg/kg) Linearrange
(mg/kg)
Correlation
coefficient(r2) Intra-day(RSD%) precision Inter-day(RSD%) precision Accuracy(%)
␣-Amirin 0.37 0.75 0.74–40.0 0.996 3.60 3.21 95–110
-Amirin 1.04 2.12 0.74–40.0 0.996 2.73 2.15 100–105
Oleanolicacid 0.22 0.43 0.74–35.0 0.990 8.92 3.65 98–102
Betulinicacid 0.09 0.20 0.74–35.0 0.996 9.38 3.40 93–97
-Sitosterol 0.08 0.16 0.74–40.0 0.992 3.95 3.19 97–110
Stigmasterol 0.06 0.13 0.74–40.0 0.991 2.01 2.12 85–96
Lupeol 0.12 0.25 0.74–40.0 0.996 2.43 2.54 92–102
Uvaol 0.12 0.24 0.74–35.0 0.991 2.38 2.29 95–108
Arjunicacid 0.07 0.15 0.74–35.0 0.990 4.01 3.75 97–103
Maslinicacid 0.13 0.27 0.74–40.0 0.996 4.39 4.40 96–99
Erythrodiol 0.08 0.17 0.74–35.0 0.994 2.16 1.85 90–96
LOD,limitofdetection;LOQ,limitofquantification;RSD,relativestandarddeviation.
A
B
Bauhinia variegata
Bauhinia variegatavar.candida
Maslinic acid
Stigmasterol
Lupeol
Stigmasterol
Lupeol
Chlorof
or
m
Chlorof
or
m
Chlorof
or
m
Eth
yl acetate
Eth
yl acetate
Eth
yl acetate
2012 2013 2014
Chlorof
or
m
Chlorof
or
m
Chlorof
or
m
Eth
yl acetate
Eth
yl acetate
Eth
yl acetate
2012 2013 2014 10000
1000
100
10
1
1000
Concentr
ation (mg/kg)
Concentr
ation (mg/kg)
100
10
1
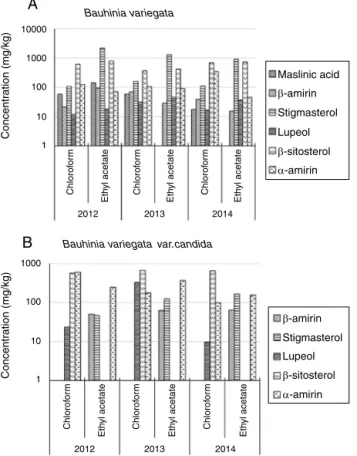
Fig.2.TriterpeniccompoundsfoundintheBauhiniaspecies viaHPLC-UV(A) Bauhiniavariegataand(B)B.variegatavar.candida.
inliteratureonisocraticmode(Liuetal.,2003;Ávilaetal.,2007; Razborˇseketal.,2007;CheungandZhang,2008).
Methodvalidation
Thecalibrationcurvesshowedgoodlinearityandthe correla-tioncoefficientswerefoundintherangeof0.990–0.996forallofthe testedcompounds.Therecoveriesoftheelevenanalyteswereinthe rangeof85–110%.Therelativestandarddeviation(RSD)between samplemeasurementswasusedasprecision,varyingfrom2.01 to9.37%forintradayprecisionandfrom1.84to4.04%tointerday forprecision.Robustnesswastested intermsofflowdifference (±0.1ml/min),%mobilephase(±5%THF),andwavelength(±1nm).
Thevariationdoesnotexceed20%.Overall,theseresults demon-stratethatthedevelopedmethodhasenoughaccuracy,precision, andsensitivityforthesimultaneouslyquantitativeanalysisofthe elevencompounds.Therecoveryresultsforevaluatingtheaccuracy ofthemethodweresatisfactoryaccordingtotheAnvisa(2003),
Maslinic acid Oleanolic acid
B
Cecropia obtusa
Cecropia palmata
Stigmasterol
Lupeol
Maslinic acid Oleanolic acid
Stigmasterol
Lupeol
Concentr
ation mg/kg
Concentr
ation mg/kg
10000 10000
A
1000
100
10
1 1 1000
100
10
Chlorof
or
m
Chlorof
or
m
Chlorof
or
m
Eth
yl acetate
Eth
yl acetate
Eth
yl acetate
2012 2013 2014
Chlorof
or
m
Chlorof
or
m
Chlorof
or
m
Eth
yl acetate
Eth
yl acetate
Eth
yl acetate
2012 2013 2014
Fig.3.TriterpeniccompoundsfoundintheCecropiaspecies viaHPLC-UV(A) Cecropiaobtusaand(B)Cecropiapalmata.
whichallowsarangeof80–120%foramazonplantsextracts.The obtainedvalidationparameterssuchascorrelationcoefficient, lin-earrange,recovery,LODandLOQaresummarizedinTable3.
ApplicationtoBauhiniasamples
Previous studies in phytochemical analysis reported the presence of glycosides, triterpene, flavonoid,lactones, steroids, alkaloids,coumarinsandsaponinsinBauhiniaspecies(Silvaand Filho, 2002; Pizzolatti et al., 2003; Rajkapoor et al., 2003; Rao etal.,2008).Sampleanalysis(Fig.2)indicatedthepresenceofsix (maslinicacid,stigmasterol,lupeol,-sitosterol,-amirinand␣ -amirin)outoftheelevencompoundsintheBauhiniaspecies.The presenceofthefourcompoundspreviouslylisted,usingthesame sample, was also confirmed previously by UHPLC-APPI-MS/MS analysis(Goboetal.,2016).
Bauhinia variegata shows greater amounts of triterpenes in
lupeol (+35%) and -sitosterol (+13%) in comparison with the chloroform.
ApplicationtoCecropiasamples
Regardingthestudiedcompounds,C.obtusa(Fig.3)exhibited a greater diversity when compared tothe otherspecies ofthe samegenus analyzedC. palmata.Among theeleventriterpenes separatedinthismethodoleanolicacid,maslinicacid,-amirin, stigsmasterol,lupeoland-sitosterolwerefoundinthesesamples.
-amirin,lupeoland-sitosterolwerealsoidentifiedby UHPLC-APPI-MS/MSanalysis(Goboetal.,2016).
Noteworthy is thepresence of-sitosterol (+55%),␣-amirin (+60%),-amirin(+60%)andoleanolicacid(+20%),increasing con-centrationsalong theyears, and theethyl acetatewas a better extractsolventthanchloroformforthesecompounds.This obser-vation can be a result of some environmental factors such as ultravioletradiation,rainfall,temperature(Gobbo-NetoandLopes, 2007).
Conclusions
Anewanalyticalmethodhasbeendevelopedforthe simulta-neousidentificationandquantificationoftriterpenescompounds usingHPLC-UVandisocraticelution,toanalyzethesecompounds
inCecropiaand Bauhiniaspecies.TheHPLC-UVmethodis
effec-tivetoseparateandquantifythemedicinalplantsextractswith good validationparameters, suchaslinearity, LOD,LOQ, recov-ery, accuracy, precision,robustness and repeatability.Although triterpenoidscontributesignificantlytothebioactivityand phar-macologyofBauhiniaandCecropia,nostudywasreportedsofar forthequantitativedetermination ofthesecompoundsinthese folkmedicineplants.Triterpenescompoundssuchasmaslinicacid, oleanolicacid,␣-amirin,-amirinand-sitosterolwerefoundas majorcompoundsinchloroformandethylacetateextracts. Fur-thermore, the presence of these triterpenic compounds in the extractsreinforcesthepharmacologicalaction,andthemedicaluse ofsuchplantsinfolkmedicine.Basedontheresults,thespecies withthehighestvarietyofcompoundsandconcentrationwereB. variegataandC.obtusa.Thepresentmethoddevelopedcanbeused inresearchofchemicalmarkersinmedicinalplantsaswellasin thequalitycontrolofherbalmedicineswidelyusedinBraziland infolkmedicine.TheidentifiedcompoundsinBauhinia(lupeol, -sitosterol,-amirinand␣-amirin),aswellinCecropia(-amirin,
lupeoland-sitosterol)extractswerealsoobservedandusingthe samesampleby HPLC-APCI-MS/MSanalysis,therefore,ratifying theimportanceofthiswork.
Authors’contributions
MEPS,FBPandLPBdevelopedandtestedtheproposedmethod. FVJr. gavea graphical andcalculationsupport.OL collectedthe plantsandperformedthevoucher.MBRwastheadvisorofMEPS, FBP,LPBandFVSJr.Alltheauthorshavereadthefinalmanuscript andapproveditssubmission.
Conflictsofinterest
Theauthorsdeclarenoconflictsofinterest.
Acknowledgements
This work was supported by grants from the Brazilian NacionalResearchCouncilCNPq,(processes487028/2012-0and
440132/2014-2).TheauthorsalsothankEmbrapaAmazônia Ori-ental(Belém/PA)forsupplyingthemedicinalplantsstudied.
References
Allouche,Y.,Beltrán,G.,Gaforio,J.J.,Uceda,M.,Mesa,M.D.,2010.Antioxidantand antiatherogenicactivitiesofpentacyclictriterpenicdiolsandacids.FoodChem. Toxicol.48,2885–2890.
Ananias,D.,Souza,E.,Souza,P.,Souza,A.,Vitorino,M.,Teixeira,G.,Ferreira,D.,2010. ClimatologiadaestruturaverticaldaatmosferaemnovembroparaBelém-PA. Rev.Bras.Meteorol.25,218–226.
Anvisa,2003.Resoluc¸ão–REn◦899,de29deMaiode2003.AgênciaNacional deVigilânciaSanitária,http://redsang.ial.sp.gov.br/site/docsleis/vm/vm1.pdf (accessed13.05.17).
Ávila,N.S.,Capote,F.P.,deCastro,M.L.,2007.Ultrasound-assistedextractionand sily-lationpriortogaschromatography–massspectrometryforthecharacterization ofthetriterpenicfractioninoliveleaves.J.Chromatogr.A1165,158–165. Bedner,M.,Schantz,M.M.,Sander,L.C.,Sharpless,K.E.,2008.Developmentofliquid
chromatographicmethodsforthedeterminationofphytosterolsinstandard referencematerialscontainingsawpalmetto.J.Chromatogr.A1192,74–80. Chauhan,R.,Ruby,K.M.,Dwivedi,J.,2013.SecondarymetabolitesfoundinBergenia
species:acompendiousreview.Int.J.Pharm.Pharm.Sci.5,9–16.
Cheng,X.R.,Jin,H.Z.,Qin,J.J.,Fu,J.J.,Zhang,W.D.,2011.Chemicalconstituentsof plantsfromthegenusGeum.Chem.Biodivers.8,203–222.
Cheung,H.Y.,Zhang,Q.F.,2008.Enhancedanalysisoftriterpenes,flavonoidsand phenoliccompoundsinPrunellavulgarisL.bycapillaryzoneelectrophoresis withtheadditionofrunningbuffermodifiers.J.Chromatogr.A1213,231–238. Claude,B.,Morin,P.,Lafosse,M.,Andre,P.,2004.Evaluationofapparentformation constantsofpentacyclictriterpeneacidscomplexeswithderivatized-and␥ -cyclodextrinsbyreversedphaseliquidchromatography.J.Chromatogr.A1049, 37–42.
Coelho-Ferreira,M.,2009.Medicinalknowledgeandplantutilizationinan Amazo-niancoastalcommunityofMarudáParáState(Brazil).J.Ethnopharmacol.126, 159–175.
Costa,G.M., Ortmann,C.F.,Schenkel,E.P.,Reginatto, F.H.,2011. AnHPLC-DAD methodtoquantificationofmainphenoliccompoundsfromleavesofCecropia species.J.Braz.Chem.Soc.22,1096–1102.
DalPrá,V.,Dolwitsch,C.B.,Lima,F.O.,deCarvalho,C.A.,Viana,C.,doNascimento,P.C., daRosa,M.B.,2015.Ultrasound-assistedextractionandbiologicalactivitiesof extractsofBrassicaoleraceavar.capitata.FoodTechnol.Biotechnol.53,102–109. Dias,M.O.,Hamerski,L.,Pinto,A.C.,2011.Semi-preparativeseparationof␣and -amyrinbyhighperformanceliquidchromatographic.Quim.Nova34,704–706. Ferreirada,D.S.,Esperandim,V.R.,Toldo,M.P.A.,Saraiva,J.,Cunha,W.R., Albu-querquede,S.,2010.Trypanocidalactivityandacutetoxicityassessmentof triterpeneacids.Parasitol.Res.106,985–989.
Figueredode,C.A.,Gurgel,I.G.D.,GurgelJunior,G.D.,2014.APolíticaNacionalde PlantasMedicinaiseFitoterápicos:construc¸ão,perspectivasedesafios.Physis 24,381–400.
Ghosh,J.,Sil,P.C.,2013.Arjunolicacid:anewmultifunctionaltherapeuticpromise ofalternativemedicine.Biochimie95,1098–1109.
Giulietti,A.M.,Queirozde,L.P.,Wanderley,M.D.G.L.,VanDenBerg,C.,2005. Biodi-versidadeeconservac¸ãodasplantasnoBrasil.Megadiversidade1,52–61. Gobbo-Neto,L.,Lopes,N.P.,2007.Plantasmedicinais:fatoresdeinfluênciano
con-teúdodemetabólitossecundários.Quim.Nova30,374–381.
Gobo,L.A.,Viana,C.,Lameira,O.A.,deCarvalho,L.M.,2016.Aliquid chromatography-atmosphericpressurephotoionizationtandemmassspectrometric (LC-APPI-MS/MS)methodfor thedeterminationoftriterpenoidsinmedicinal plant extracts.J.MassSpectrom.51,558–565.
Holen,B.,1985.Rapidseparationoffreesterolsbyreversed-phasehighperformance liquidchromatography.J.Am.OilChem.Soc.62,1344–1346.
Kong,L.,Li,S.,Liao,Q.,Zhang,Y.,Sun,R.,Zhu,X.,Zhang,Q.,Wang,J.,Wu,X.,Fang, X.,2013.Oleanolicacidandursolicacid:novelhepatitisCvirusantiviralsthat inhibitNS5Bactivity.AntiviralRes.98,44–53.
Kumari,A.,Kakkar,P.,2012.Lupeolprotectsagainstacetaminophen-induced oxida-tivestressandcelldeathinratprimaryhepatocytes.FoodChem.Toxicol.50, 1781–1789.
Laszczyk,M.N.,2009.Pentacyclictriterpenesofthelupane,oleananeandursane groupastoolsincancertherapy.PlantaMed.75,1549–1560.
Lesellier,E.,Destandau,E.,Grigoras,C.,Fougère,L.,Elfakir,C.,2012.Fastseparationof triterpenoidsbysupercriticalfluidchromatography/evaporativelightscattering detector.J.Chromatogr.A1268,157–165.
Li,G.L.,You,J.M.,Song,C.H.,Xia,L.,Zheng,J.,Suo,Y.R.,2011.Developmentofa newHPLCmethodwithprecolumnfluorescentderivatizationforrapid,selective andsensitivedetectionoftriterpenicacidsinfruits.J.Agric.FoodChem.59, 2972–2979.
Li,J.R.,Li,M.,Xia,B.,Ding,L.S.,Xu,H.X.,Zhou,Y.,2013.Efficientoptimizationof ultra-high-performancesupercriticalfluidchromatographicseparationofRosa sericeabyresponsesurfacemethodology.J.Sep.Sci.36,2114–2120.
Liu,H.,Shi,Y.,Wang,D.,Yang,G.,Yu,A.,Zhang,H.,2003.Determinationofoleanolic acidandursolicacidisomersinLigustrumlucidumAit.J.Pharm.Biomed.Anal. 32,479–485.
Martelanc,M., Vovk, I., Simonovska,B., 2007. Determination of three major triterpenoidsinepicuticularwaxofcabbage(BrassicaoleraceaL.)by high-performanceliquidchromatographywithUVandmassspectrometricdetection. J.Chromatogr.A1164,145–152.
Martelanc,M.,Vovk,I.,Simonovska,B.,2009.Separationandidentificationofsome commonisomericplanttriterpenoidsbythin-layerchromatographyand high-performanceliquidchromatography.J.Chromatogr.A1216,6662–6670. Muffler,K.,Leipold,D.,Scheller,M.C.,Haas,C.,Steingroewer,J.,Bley,T.,Neuhaus,
H.E.,Mirata,M.A.,Schrader,J.,Ulber,R.,2011.Biotransformationoftriterpenes. ProcessBiochem.46,1–15.
Murillo,E.,Lombo,O.,Tique,M.,Méndez,J.J.,2007. Potencialantioxidantede BauhiniakalbreyeriHarms(Fabaceae).Inf.Tecnol.18,65–74.
Nicasio-Torres,M.delP., Meckes-Fischer,M.,Aguilar-Santamaría,L., Gardu˜no-Ramírez,M.L.,Chávez-Ávila,V.M.,Cruz-Sosa,F.,2012.Productionofchlorogenic acidandisoorientinhypoglycemiccompoundsinCecropiaobtusifoliacalliandin cellsuspensioncultureswithnitratedeficiency.ActaPhysiol.Plant.34,307–316. Pai,S.R.,Nimbalkar,M.S.,Pawar,N.V.,Dixit,G.B.,2011.Optimizationofextraction techniquesandquantificationofbetulinicacid(BA)byRP-HPLCmethodfrom AncistrocladusheyneanusWall.ExGrah.Ind.CropsProd.34,1458–1464. Parekh,J.,Karathia,N.,Chanda,S.,2006.Evaluationofantibacterialactivityand
phytochemicalanalysisof Bauhinia variegataL. bark. Afr. J. Biomed.Res., http://dx.doi.org/10.4314/ajbr.v9i1.48773.
Pelaez,G.L.M.,Sierra,J.A.,Alzate,F.,Holzgrabe,U.,Ramirez-Pineda,J.R.,2013. Penta-cyclictriterpenesfromCecropiatelenitidawithimmunomodulatoryactivityon dendriticcells.Rev.Bras.Farmacogn.23,754–761.
Pizzolatti,M.G.,CunhaJr.,A.,Szpoganicz,B.,Sousa,E.d.,Braz-Filho,R.,Schripsema,J., 2003.FlavonoidsglycosidesfromleavesandflowersofBauhiniaforficata (Legu-minosae).Quim.Nova26,466–469.
Pollier,J.,Goossens,A.,2012.Oleanolicacid.Phytochemistry77,10–15.
Quílez,A.,Berenguer,B.,Gilardoni,G.,Souccar,C.,Mendonc¸a,S.,Oliveirade,L., Martín-Calero,M.,Vidari,G.,2010.Anti-secretory,anti-inflammatoryand anti-HelicobacterpyloriactivitiesofseveralfractionsisolatedfromPipercarpunya Ruiz&Pav.J.Ethnopharmacol.128,583–589.
Rajkapoor,B.,Jayakar,B.,Murugesh,N.,2003.AntitumouractivityofBauhinia var-iegataonDalton’sasciticlymphoma.J.Ethnopharmacol.89,107–109. Rao,Y.K.,Fang,S.H.,Tzeng,Y.M.,2008.Antiinflammatoryactivitiesofflavonoids
andatriterpenecaffeateisolatedfromBauhiniavariegata.Phytother.Res.22, 957–962.
Razborˇsek,M.I.,Vonˇcina,D.B.,Doleˇcek,V.,Vonˇcina,E.,2007.Determinationofmajor phenolicacids,phenolicditerpenesandtriterpenesinRosemary(Rosmarinus officinalisL.)bygaschromatographyandmassspectrometry.ActaChim.Slov. 54,60–67.
Reddy,M.V.,Reddy,M.K.,Gunasekar,D.,Caux,C.,Bodo,B.,2003.Aflavanoneanda dihydrodibenzoxepinfromBauhiniavariegata.Phytochemistry64,879–882. Rhourri-Frih,B.,Chaimbault,P.,Claude,B.,Lamy,C.,André,P.,Lafosse,M.,2009.
AnalysisofpentacyclictriterpenesbyLC–MS.AcomparativestudybetweenAPCI andAPPI.J.MassSpectrom.44,71–80.
Rocha,A.D.,Oliveirade,A.B.,SouzaFilhoda,J.D.,Lombardi,J.A.,Braga,F.C.,2004. AntifungalconstituentsofClytostomaramentaceumandMansoahirsuta. Phy-tother.Res.18,463–467.
Rocha,G.daG.,Simoes,M.,Lúcio,K.A.,Oliveira,R.R., Kaplan,M.A.C.,Gattass, C.R.,2007.NaturaltriterpenoidsfromCecropialyratilobaarecytotoxictoboth sensitiveandmultidrugresistantleukemiacelllines.Bioorg.Med.Chem.15, 7355–7360.
Romero,C.,García,A.,Medina,E.,Ruíz-Méndez,M.V.,Castrode,A.,Brenes,M.,2010. Triterpenicacidsintableolives.FoodChem.118,670–674.
Saleem,M.,2009.Lupeol,anovelanti-inflammatoryandanti-cancerdietary triter-pene.CancerLett.285,109–115.
Saleem,M.,Maddodi,N.,Zaid,M.A.,Khan,N.,binHafeez,B.,Asim,M.,Suh,Y.,Yun, J.-M.,Setaluri,V.,Mukhtar,H.,2008.Lupeolinhibitsgrowthofhighlyaggressive humanmetastaticmelanomacellsinvitroandinvivobyinducingapoptosis. Clin.CancerRes.14,2119–2127.
Sánchez, M., Theoduloz, C., Schmeda-Hirschmann, G., Razmilic, I., Yá˜nez, T., Rodríguez,J.A.,2006.Gastroprotectiveandulcer-healingactivityofoleanolic acidderivatives:invitro–invivorelationships.LifeSci.79,1349–1356. Sánchez-Ávila,N.,Priego-Capote,F.,Ruiz-Jiménez,J.,deCastro,M.L.,2009.Fast
andselectivedeterminationoftriterpeniccompoundsinoliveleavesbyliquid chromatography–tandemmassspectrometrywithmultiplereaction monitor-ingaftermicrowave-assistedextraction.Talanta78,40–48.
Schneider,P.,Hosseiny,S.,Szczotka,M.,Jordan,V.,Schlitter,K.,2009.Rapid sol-ubilitydeterminationofthetriterpenesoleanolicacidandursolicacidby UV-spectroscopyindifferentsolvents.Phytochem.Lett.2,85–87.
Shanmugam,M.K.,Dai,X.,Kumar,A.P.,Tan,B.K.,Sethi,G.,Bishayee,A.,2013.Ursolic acidincancerpreventionandtreatment:moleculartargets,pharmacokinetics andclinicalstudies.Biochem.Pharmacol.85,1579–1587.
Siddique,H.R.,Saleem,M.,2011.Beneficialhealtheffectsoflupeoltriterpene:a reviewofpreclinicalstudies.LifeSci.88,285–293.
Silva,E.,Souza,J.,Rogez,H.,Rees,J.F.,Larondelle,Y.,2007.Antioxidantactivities andpolyphenoliccontentsoffifteenselectedplantspeciesfromtheAmazonian region.FoodChem.101,1012–1018.
Silvada,K.A.S.,Paszcuk,A.F.,Passos,G.F.,Silva,E.S.,Bento,A.F.,Meotti,F.C.,Calixto, J.B.,2011.Activationofcannabinoidreceptorsbythepentacyclictriterpene␣ -amyrininhibitsinflammatoryandneuropathicpersistentpaininmice.Pain152, 1872–1887.
Silvada,K.L.,Filho,V.,2002.PlantasdogêneroBauhinia:composic¸ãoquímicae potencialfarmacológico.Quim.Nova25,449–454.
Slavin,M.,Yu,L.L.,2012.AsingleextractionandHPLCprocedureforsimultaneous analysisofphytosterols,tocopherolsandluteininsoybeans.FoodChem.135, 2789–2795.
Somova,L.,Nadar,A.,Rammanan,P.,Shode,F.,2003.Cardiovascular, antihyper-lipidemicandantioxidanteffectsofoleanolicandursolicacidsinexperimental hypertension.Phytomedicine10,115–121.
Szakiel,A.,P˛aczkowski,C.,Pensec,F.,Bertsch,C.,2012.Fruitcuticularwaxesasa sourceofbiologicallyactivetriterpenoids.Phytochem.Rev.11,263–284. Thimmappa,R.,Geisler,K.,Louveau,T.,O’Maille,P.,Osbourn,A.,2014.Triterpene
biosynthesisinplants.Annu.Rev.PlantBiol.65,225–257.
Wójciak-Kosior,M.,Sowa,I.,Kocjan,R.,Nowak,R.,2013.Effectofdifferentextraction techniquesonquantificationofoleanolicandursolicacidinLamiialbiflos.Ind. CropsProd.44,373–377.
Xu,X.H.,Su,Q.,Zang,Z.H.,2012.Simultaneousdeterminationofoleanolicacidand ursolicacidbyRP-HPLCintheleavesofEriobotryajaponicaLindl.J.Pharm.Anal. 2,238–240.
Yang, G., Fen,W., Xiao, W., Sun, H., 2009. Study on determination of pen-tacyclictriterpenoidsinChaenomel by HPLC-ELSD.J. Chromatogr. Sci.47, 718–722.