Short communication
Differences between renal effects of venom from two
Bothrops
jararaca
populations from southeastern and southern Brazil
Roberta Jeane Bezerra Jorge
a,*, Ant
onio Rafael Coelho Jorge
^
b,
Ramon R
oseo Paula Pessoa Bezerra de Menezes
b, Clarissa Perdig
~
ao Mello
c,
Danya Bandeira Lima
c, Jo
~
ao Alison de Moraes Silveira
b, Natacha Teresa Queiroz Alves
b,
Aline Diogo Marinho
b, Rafael Matos Ximenes
d, Carlos Corr
^
ea-Netto
e,
Larissa Gonçalves Machado
e, Russolina Benedeta Zingali
e, Alice Maria Costa Martins
c,
Helena Serra Azul Monteiro
baCentro Acad^emico de Vitoria, Universidade Federal de Pernambuco, Vitoria de Santo Ant~ao, Pernambuco, Brazil bDepartamento de Fisiologia e Farmacologia, Universidade Federal do Ceara, Fortaleza, Ceara, Brazil
cDepartmento de Analises Clínicas e Toxicol ogicas, Universidade Federal do Ceara, Fortaleza, Ceara, Brazil dDepartmento de Antibioticos, Universidade Federal de Pernambuco, Recife, Pernambuco, Brazil eInstituto Nacional de Biologia Estrutural e Bioimagem, Instituto de Bioquímica Medica Leopoldo de Meis
eUniversidade Federal do Rio de Janeiro, Rio de Janeiro, Brazil
a r t i c l e
i n f o
Article history: Received 17 August 2016 Received in revised form 11 November 2016 Accepted 16 November 2016 Available online 17 November 2016
Keywords:
Bothrops jararacavenom Renal effects
MDCK
Isolated kidney perfusion
a b s t r a c t
Components from animal venoms may vary according to the snake's age, gender and region of origin. Recently, we performed a proteomic analysis ofBothrops jararacavenom from southern (BjSv) and southeastern (BjSEv) Brazil, showing differences in the venom composition, as well as its biological ac-tivity. To continue the study, we report in this short communication the different effects induced by the BjSEvandBjSvon isolated kidney and MDCK renal cells.BjSEvdecreased perfusion pressure (PP) and renal vascular resistance (RVR) and increased urinaryflow (UF) and glomerularfiltration rate (GFR), whileBjSvdid not alter PP and RVR and reduced UF and GFR. Both types of venom, more expressively BjSEv, reduced %TNaþ, %TKþand %Cl . In MDCK cells, the two types of venom showed cytotoxicity with IC50of 1.22mg/mL forBjSvand 1.18mg/mL forBjSEvand caused different profiles of cell death, withBjSv being more necrotic. In conclusion, we suggest thatBjSvis more nephrotoxic thanBjSEv.
©2016 Elsevier Ltd. All rights reserved.
1. Introduction
Snake venoms are a complex mixture of proteins, peptides and small inorganic molecules. Clinical manifestations of bothropic accidents consist in various local and systemic effects, mainly characterized by severe coagulopathy, necrosis, edema, sponta-neous bleeding and, in some cases, compartment syndrome (França et al., 2003; Santoro et al., 2008). Systemic complications occur in the absence of antivenom administration, leading to more severe cases associated with intense bleeding, shock and acute kidney injury (AKI), which are closely related to death (Ribeiro and Jorge, 1997; De Castro et al., 2004; Sgrignolli et al., 2011).
There are many cases of snake envenomation annually in Brazil,
most of them associated with theBothropsgenus (Queiroz et al.,
2008). Bothrops jararaca (Wied-Neuwied, 1824) is a medically-relevant, semi-arboreal pit viper, found from the state of Bahia to Rio Grande do Sul in Brazil and in parts of Paraguay and Argentina. This wide geographical distribution range is associated with the most populated areas in Brazil (Sazima, 1992).
Recently, a combined proteomic study was conducted using
venom pools from the outermost points ofB. jararacageographical
distribution in Brazil. These representative venom pools of the
southeastern and southern populations of B. jararaca revealed
distinct quantitative and some qualitative overall toxin composi-tions of proteomes and transcriptomes. The different composicomposi-tions
of the venoms ofB. jararacafrom the south and southeast have
demonstrated different biological actions, such as coagulant,
pro-teolytic and of phospholipase A2actions (Gonçalves-Machado et al.,
*Corresponding author.
E-mail address:robertajeanebj@gmail.com(R.J.B. Jorge).
Contents lists available atScienceDirect
Toxicon
j o u r n a l h o m e p a g e : w w w . e l s e v i e r . c o m / l o c a t e / t o x i c o n
2016). To continue this study, we report in this short communica-tion the differences in renal effects induced by both venoms on an isolated kidney perfusion model and cytotoxicity on MDCK renal epithelial cells.
2. Material and methods
B. jararacavenom samples from the southeastern snake
popu-lation were collected from a number (n¼22) of adult specimens
captured in the following states of Brazil: Rio de Janeiro, Espirito Santo and Minas Gerais. The specimens were maintained at the
serpentarium of Vital Brazil Institute (Niteroi, Rio de Janeiro, Brazil)
under IBAMA registration n. 26354-9, while the venom samples of
adult specimens of B. jararaca(n¼22) from the southern
pop-ulations were captured in localities of the state of Rio Grande do Sul (RS) and kept in captivity in the serpentarium of Center of Ophi-ology of Porto Alegre, Rio Grande do Sul, Brazil, under IBAMA registration n. 1/43/1999/000764-9.
We performed kidney perfusion tests to evaluate the renal effects with no interference of systemic factors. In this assay, adult male Wistar rats (260e320 g) were fasted for 24 h with free access to water. The rats were anesthetized with sodium pentobarbitone (50 mg/kg, i.p) followed by careful dissection of the right kidney. The right renal artery was cannulated via the mesenteric artery without
interrupting blood flow, as described by Bowman (1970). The
perfusate consisted of modified Krebs-Henseleit solution (MKHS)
consisting of (in mmol/L): 114.00 NaCl, 4.96 KCl, 1.24 KH2PO4, 0.5
MgSO4.7H2O, 2.10 CaCl2and 24.99 NaHCO3. Bovine serum albumin
(BSA 6 g%; fraction V), urea (0.075 g), inulin (0.075 g) and glucose
(0.15 g) were added to the solution, resulting in afinal perfusate
volume of 100 mL. The pH was adjusted to 7.4. In each experiment, 100 mL of MKHS were recirculated for 120 min. The perfusion pressure (PP) was measured at the tip of the stainless steel cannula in the renal artery. Urine and perfusate samples were collected at 10 min-intervals for analysis of sodium, potassium and chloride levels by ion-selective electrodes (RapidChem 744, Bayer Diagnostic, UK);
inulin, as described byWalser et al. (1955)and modified byFonteles
et al. (1983); and osmolality, which was measured in a vapor
pres-sure osmometer (Wescor 5100C, USA).BjSvorBjSEv(10
mg/mL) was
added to the system 30 min after the beginning of each perfusion. The perfusion pressure (PP), renal vascular resistance (RVR), urinary
flow (UF), glomerularfiltration rate (GFR), the percentage of sodium
(%TNaþ), potassium (%TKþ) and chloride (%TCl-) tubular transport
were determined (Martinez-Maldonado and Opava-Stitzer, 1978). The results were compared to the internal control group, at early
30 min in each experiment (n¼6). In this experiment, both right and
left kidneys werefixed with formaldehyde until subsequent
histo-logical analysis. The fragments were subjected to dehydration,
diaphanization and then cut into 3
mm thick slices. Then,
hematoxylin-eosin (HE) staining was performed and the slides analyzed using an optical microscope.
We also performed assays in cells to evaluate renal cytotoxicity. Epithelial MadineDarby Canine Kidney (MDCK) cells were cultured in RPMI 1640 medium (RPMI, Sigma-Aldrich; St. Louis, MO, USA) supplemented with 10% fetal bovine serum (FBS, Sigma-Aldrich, St.
Louis, MO, USA), 1% penicillin/streptomycin solution at 37C, 5%
CO2. MDCK cells were cultured and treated with different
concen-trations ofBjSvorBjSEv(3.12; 1.56; 0.78; 0.39
mg/mL), incubated at
37C for 24 h and mitochondrial function was measured by MTT
colorimetric assay. MTT reagent (Sigma-Aldrich, St. Louis, MO, USA;
5 mg/mL in PBS) was added and incubated for 4 h at 37C. Finally,
the medium was removed and the precipitated formazan crystals were dissolved in 10% Sodium dodecyl sulfate (Vetec, Rio de Janeiro, RJ, Brazil). After 17 h, absorbance was read at 570 nm in a
micro-plate reader (Biochrom®
Asys Expert Plus). Cell viability was
calculated in comparison with the control group. The IC50 was
determined by non-linear regression (Mosmann, 1983).
Finally, the cell death profile was evaluated with Annexin V-FITC
(AX) and 7AAD staining. Cells treated with IC50and 2IC50ofBjSv
(1.22 and 2.44
mg/mL) and
BjSEv(1.18 and 2.36mg/mL) incubated
during 12 h were stained with fluorescein isothiocyanate
(FITC)-conjugated to annexin V/7AAD according to the manufacturer's instructions (BD Pharmingen, CA, USA). The population of
AX-7AAD-viable cells was evaluated by flow cytometry. Data were
collected in a FACSCaliburflow cytometer and analyzed using Cell
Quest software (Becton-Dickinson, Mountain View, CA, USA).
Statistical analysis shows values as mean± S.E.M of triplicate
values for each cell experiment and hexaplicate values for perfused
renal experiments. The significance of the difference between the
experimental and control groups was assessed by one-way analysis
of variance (ANOVA). Significance was defined as p<0.05.
3. Results and discussion
Bothrops jararacavenom consists of a mixture of molecules, such as metalloproteinases, disintegrin, vasoactive peptides (VAP; including BPPs, bradykinin-potentiating peptides), phospholipase
A2(PLA2), serine proteinases (Serprot) and L-amino-acid oxidases
(LAAO). The relative occurrence (in percentage of total venom
proteins) of toxins from venom pools of B. jararaca populations
from the Southeastern and Southern Atlantic forest regions disclose
many differences.BjSEvhas 35% of metalloproteinases, 7% of
dis-integrin, 16% of VAP, 4% of PLA2, 14% of Serprot; whileBjSvhas 10%
of metalloproteinases, 0.2% of disintegrin, 22% of VAP, 20.2% of PLA2,
28% of Serprot, and (Gonçalves-Machado et al., 2016).
The intrinsic toxicity of toxins and their abundance in venom are relevant when evaluating their potential medical impact (Laustsen et al., 2015). Phospholipases and metalloproteinases, among several enzymes, are believed to contribute to renal toxicity (Sitprija and Sitprija, 2012). TheBjSEv has more metalloproteinases, while the
BjSv has a higher amount of venom PLA2(Gonçalves-Machado et al.,
2016).
The effects of Bothrops venoms on isolated kidney have been
previously demonstrated. The Bothrops leucurus (Morais et al.,
2013),Bothrops marajoensis(Evangelista et al., 2010) and Bothro-poides pauloensis(Marinho et al., 2015) cause reduction in PP, RVR, UF, GFR and tubular transport of electrolytes. Furthermore, Bothrops moojeni(Barbosa et al., 2002),Bothropoides erythromelas
(Martins et al., 2005) andBothrops jararacussuvenoms (Havt et al.,
2001) decrease PP, RVR, tubular transport of electrolytes and in-crease UF and GFR.
Our results showed different profiles of renal alterations on
isolated kidney.BjSEvdecreased PP (Fig. 1A) and RVR (Fig. 1B) at
60 min, increased UF (Fig. 1C) at 90 and 120 min and GFR (Fig. 1D) at
120 min. BjSv, in turn, did not alter PP, RVR (Fig. 1A and B) and
reduced UF and GFR (Fig. 1C and D) at 60, 90 and 120 min. Both venoms caused renal tubular dysfunction by altering the
percent-age of tubular transport of electrolytes. They reduced %TNaþ
(Fig. 1E), %TKþ
(Fig. 1F) and %TCl (Fig. 1G) at 60, 90 and 120 min,
with the reduction of %TNaþ
and %TCl induced byBjSEvbeing
more significant.
Bothrops erythromelas venom has shown alterations in the perfused kidney (Martins et al., 2005), similar to alterations
observed inBjSEv.Metalloproteinases are the major component in
B. erythromelasvenom (Jorge et al., 2015) andBjSEv,suggesting this
fraction may be responsible for part of the renal alterations.BjSEv
showed a diuretic effect, in turn,BjSvshowed a sharp decline of
glomerularfiltration rate together with tubular dysfunction.
Renal pathological changes in patients with AKI caused by
snakebites include tubular necrosis, cortical necrosis,
glomerulonephritis and vasculitis (Kanjanabuch and Sitprija, 2008; Sitprija and Sitprija, 2012). Histopathological analyses of kidneys
from rats exposed toB. jararacavenom also showed tubular necrosis
(Monteiro and Fonteles, 1999). In this work, the histological analysis
Fig. 1.Effects of theBothrops jararacavenom from southeastern (BjSEv) and southern Brazil (BjSv) (10mg/mL) on perfusion pressure (PP;A); renal vascular resistance (RVR;B); urinaryflow (UF;C); glomerularfiltration rate (GFR;D); percentage of sodium tubular transport (%TNaþ
;E); percentage of potassium tubular transport (%TKþ
of the kidneys perfused withBjSvdemonstrated higher toxicity. The
kidneys treated withBjSvshowed abundant proteinaceous material
in the urinary space and tubules, tubular dilation and vacuolar
hydropic degeneration.BjSEvalso showed deposition of
proteina-ceous material in renal tubules, tubular dilation and vacuolar hydropic degeneration, but the glomeruli showed no deposition in the urinary space (Fig. 2).
The loss of tubular epithelial cell function could lead to tubular
dysfunction (Dantas et al., 2015). Both BjSv and BjSEv showed
cytotoxicity on MDCK distal tubular epithelial renal cells. MDCK cells constitute a very-well established cell line and have been extensively employed in the investigation of several cell processes, including epithelial transport and cell response to toxic agents and venoms (Peixoto and Collares-Buzato, 2005; Kusma et al., 2008).
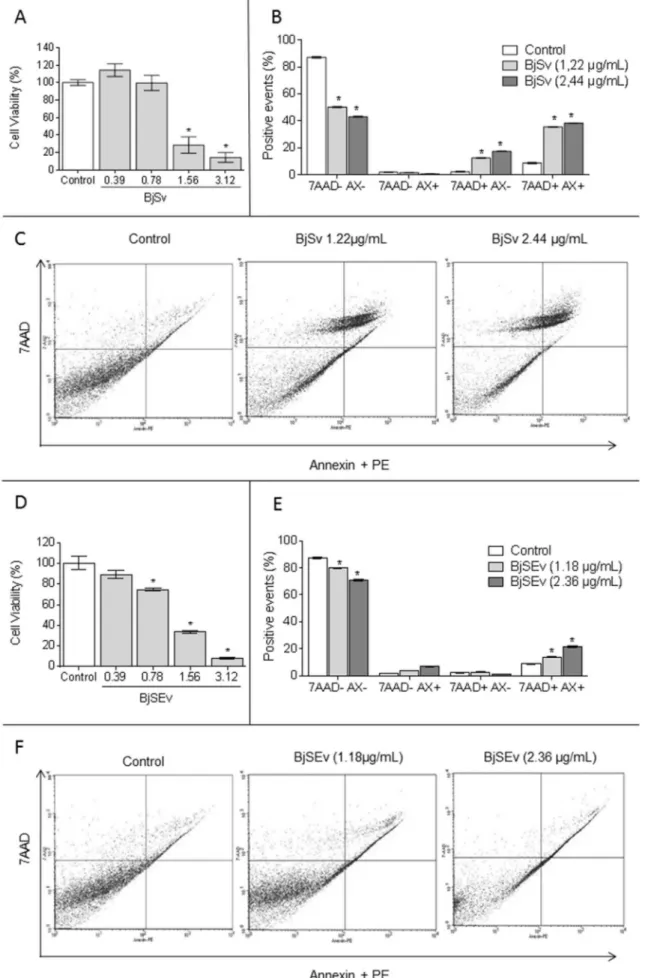
The concentration capable to kill 50% of MDCK cell of BjSvwas
1.22
mg/mL (Fig. 3A) and 1.18
mg/mL for
BjSEv(Fig. 3D). Despite thefact that IC50were very similar,BjSv(Fig. 3B and C) showed a higher
necrotic profile thanBjSEvinflow cytometry (3E and 3F).
Cellular injury can result from enzyme action, especially that of
PLA2and proteases on cell membrane. Cell injury caused by PLA2
consists of hemolysis, rhabdomyolysis, neuronal damage, renal tubular damage and endothelial necrosis, while metalloproteinases
can cause destruction of the cytoskeleton, leading to cell damage, cell adhesion disruption and apoptosis (Sitprija and Sitprija, 2012). Necrosis is characterized by rapid cytoplasmic swelling, culmi-nating in plasma membrane rupture and organelle breakdown
(Vanden Berghe et al., 2013).BjSvshowed an increase of 7AADþ/
AX- and 7AADþ/AX-, a predominantly necrotic profile, which can
be related to high concentrations of PLA2in its composition. PLA2is
toxic due to its effects on biological membranes through charge and van der Waals interaction, leading to membrane destabilization (Sitprija and Sitprija, 2012).
Apoptosis is a programmed cell death pathway, signaled by phosphatidylserine externalization. Apoptosis could become sec-ondary necrosis in the absence of phagocytosis (Vanden Berghe et al., 2013).BjSEvshowed only increase of 7AADþ/AXþ, a profile
of secondary necrosis, which can be related to the high concen-trations of metalloproteinases in its composition.
Bothrops venom such as,Bothrops pauloensis,Bothrops diporus
andBothrops pirajaivenoms have shown cytotoxic activity in tumor cells and the cytotoxic mechanism related to both necrosis and
apoptosis (Jorge et al., 2011).Bothrops leucurus(Morais et al., 2013),
B. erythromelas(De Sousa et al., 2016) andB. pauloensis(Marinho et al., 2015) venoms have shown an apoptotic profile, while
Fig. 2.Histological analysis of the kidneys perfused withBjSvandBjSEv. No changes were observed in glomeruli and tubules in controls: kidneys perfused with Krebs-Henseleit solution (A) and kidneys not perfused (B). Kidneys perfused withBjSEv(10mg/mL) displayed intratubular protein deposits (1) and vacuolar hydropic tubular degeneration (2) (C and D). Kidneys perfused withBjSv(10mg/mL) showed protein deposition in the urinary space (3), intratubular protein deposits (4) and vacuolar hydropic tubular degeneration (5) (E and F). 400 X. H/E.
Bothrops insularis(Mello et al., 2014) venom has shown a necrotic
profile predominantly on MDCK cells (Grazziotin et al., 2006).
Similar concentrations ofBjSEvandBjSvvenoms can result in
different profiles of cell death, in which the one induced byBjSvis
quicker and could be related to higher toxicity observed at 120 min of renal perfusion. It indicates the necessity of quickly establishing
the treatment of patients bitten byB jararacafrom south snakes.
The difference in nephrotoxicity found in these two bothrops venoms could be related to quantitative and qualitative differences
in their components.BjSvis rich in PLA2(Lys49-PLA2), which is an
exclusive component of this venom (Gonçalves-Machado et al., 2016). PLA2 plays an important role in cell injury through the
ability of mediating inflammatory response, catalyzing the
hydro-lysis of phosphoglycerides and leading to membrane damage (Sitprija and Sitprija, 2012). The presence of the strategic residues
known to constitute the Ca2þ-binding loop of PLA
2, such as Lys-49,
could be directly or indirectly involved in its catalytic activity
(Pereira et al., 1998). In the present study, BjSv showed more
toxicity in isolated kidney and quicker cell death pathway in MDCK
cells thanBjSEv. This fact could be related to high concentrations of
Lys49-PLA2inBjSv.
In conclusion, both types of venoms of the same species from different geographical regions showed differences regarding effects
on renal function parameters and cytotoxicity, being BjSv more
nephrotoxicity thanBjSEv. However, further studies with the
iso-lated toxins are needed to elucidate which toxin is involved in the pathophysiology of renal injury.
Ethical statement
This study was approved by the Ethics Committee on Animal Research (CEUA/UFPE) of Universidade Federal de Pernambuco (Authorization Number: 129/15).
Acknowledgements
We thank Moema Leit~ao de Araújo and Maria Lúcia Machado
Alves from Regional Center of Ophiology of Porto Alegre (NOPA)
and Leonora Brazil Mas from Vital Brazil Institute (IVB) for donating
the venoms. We thank Maria Silvia Helena Freire de França
(Uni-versidade Federal do Ceara) for their excellent technical assistance.
We thank National Council of Technological and Scientific
Devel-opment (CNPq) and Coordination for Enhancement of Higher
Ed-ucation Personnel (CAPES) for theirfinancial support.
Transparency document
Transparency document related to this article can be found
online athttp://dx.doi.org/10.1016/j.toxicon.2016.11.249.
References
Barbosa, P.S., Havt, A., Faco, P.E., Sousa, T.M., Bezerra, I.S., Fonteles, M.C., Toyama, M.H., Marangoni, S., Novello, J.C., Monteiro, H.S., 2002. Renal toxicity of
Bothrops moojenisnake venom and its main myotoxins. Toxicon 40, 1427e1435.
Bowman, R.H., 1970. Gluconeogenesis in the isolated perfused rat kidney. J.Biol. Chem. 245, 1604e1612.
Dantas, R.T., Jorge, A.R., Jorge, R.J., Menezes, R.R., Lima, D.B., Torres, A.F., Toyama, M.H., Monteiro, H.S., Martins, A.M.C., 2015. L-amino acid oxidase from
Bothrops marajoensiscauses nephrotoxicity in isolated perfused kidney and cytotoxicity in MDCK renal cells. Toxicon 104, 52e56.
De Castro, I., Burdmann, E.A., Seguro, A.C., Yu, L., 2004.Bothropsvenom induces direct renal tubular injury: role for lipid peroxidation and prevention by anti-venom. Toxicon 43, 833e839.
De Sousa, F.C., Jorge, A.R., De Menezes, R.R., Torres, A.F., Mello, C.P., Lima, D.B., Borges Nojosa, D.M., Havt, A., Alves, R.S., Martins, A.M., Monteiro, H.S., 2016.
Bothrops erythromelasvenom induces apoptosis on renal tubular epithelial cells. Toxicon 118, 82e85.
Evangelista, I.L., Martins, A.M., Nascimento, N.R., Havt, A., Evangelista, J.S., de
Nor~oes, T.B., Toyama, M.H., Diz-Filho, E.B., Toyama, D.O., Fonteles, M.C., Monteiro, H.S., 2010. Renal and cardiovascular effects of Bothrops marajoensis venom and phospholipase A2. Toxicon 55, 1061e1070.
França, F.O.S., Barbaro, K.C., Fan, H.W., Cardoso, J.L.C., Sano-Martins, I.S., Tomy, S.C., Lopes, M.H., Warrel, D.A., Theakston, R.G.D., 2003. The Butantan Institute an-tivenom study group. Envenoming byBothrops jararacain Brazil: association between venom antigenaemia and severity at admission to hospital. Trans. R. Soc. Trop. Med. Hyg. 97, 312e317.
Fonteles, M.C., Cohen, J.J., Black, A.J., Wertheim, S.J., 1983. Support of renal kidney function by long-chain fatty acids derived from renal tissue. Am. J. Phys. 244, 235e246.
Gonçalves-Machado, L., Pla, D., Sanz, L., Jorge, R.J., Leit~ao-De-Araújo, M., Alves, M.L., Alvares, D.J., De Miranda, J., Nowatzki, J., de Morais-Zani, K., Fernandes, W., Tanaka-Azevedo, A.M., Fernandez, J., Zingali, R.B., Gutierrez, J.M., Corr^ ea-Netto, C., Calvete, J.J., 2016. Combined venomics, venom gland transcriptomics, bioactivities, and antivenomics of two Bothrops jararaca populations from geographic isolated regions within the Brazilian Atlantic rainforest. J. Proteomics 135, 73e89.
Grazziotin, F.G., Monzel, M., Echeverrigaray, S., Bonatto, S.L., 2006. Phylogeography of the Bothrops jararaca complex (Serpentes: Viperidae): past fragmentation and island colonization in the Brazilian Atlantic forest. Mol. Ecol. 15, 3969e3982.
Havt, A., Fonteles, M.C., Monteiro, H.S., 2001. The renal effects of Bothrops jarar-acussu venom and the role of PLA(2) and PAF blockers. Toxicon 39, 1841e1846.
Jorge, R.J., Martins, A.M., Morais, I.C., Ximenes, R.M., Rodrigues, F.A., Soares, B.M., Evangelista, J.S., Toyama, M.H., Martins, A.M., Moraes Filho, M.O., Monteiro, H.S., 2011. In vitro studies on Bothrops venoms cytotoxic effect on tumor cells. J. Exp. Ther. Oncol. 9, 249e253.
Jorge, R.J., Monteiro, H.S., Gonçalves-Machado, L., Guarnieri, M.C., Ximenes, R.M., Borges-Nojosa, D.M., Luna, K.P., Zingali, R.B., Corr^ea-Netto, C., Gutierrez, J.M., Sanz, L., Calvete, J.J., Pla, D., 2015. Venomics and antivenomics of Bothrops erythromelas fromfive geographic populations within the Caatinga ecoregion of northeastern Brazil. J. Proteomics 114, 93e114.
Kanjanabuch, T., Sitprija, V., 2008. Snakebite nephrotoxicity in Asia. Semin. Nephrol. 28, 363e372.
Kusma, J., Chaim, O.M., Wille, A.C., Ferrer, V.P., Sade, Y.B., Donatti, L., Gremski, W., Mangili, O.C., Veiga, S.S., 2008. Nephrotoxicity caused by brown spider venom phospholipase-D (dermonecrotic toxin) depends on catalytic activity. Biochimie 90, 1722e1736.
Laustsen, A.H., Lohse, B., Lomonte, B., Engmark, M., Gutierrez, J.M., 2015. Selecting key toxins for focused development of elapid snake antivenoms and inhibitors guided by a Toxicity Score. Toxicon 104, 43e45.
Marinho, A.D., Morais, I.C., Lima, D.B., Jorge, A.R., Jorge, R.J., Menezes, R.R., Mello, C.P., Pereira, G.J., Silveira, J.A., Toyama, M.H., Orzaez, M., Martins, A.M., Monteiro, H.S., 2015.Bothropoides pauloensisvenom effects on isolated perfused kidney and cultured renal tubular epithelial cells. Toxicon 108, 126e133.
Martins, A.M., Sousa, F.C., Barbosa, P.S., Toyama, M.H., Toyama, D.O., Aprígio, C.C., Queiroz, M.G., Guarnieri, M.C., Havt, A., de Menezes, D.B., Fonteles, M.C., Monteiro, H.S., 2005. Action of anti-bothropic factor isolated from Didelphis marsupialis on renal effects of Bothrops erythromelas venom. Toxicon 46, 595e599.
Martinez-Maldonado, M., Opava-Stitzer, S., 1978. Free water clearance curves during saline, mannitol, glucose and urea diuresis in the rat. J. Physiol. 280, 487e497.
Mello, C.P., Morais, I.C., Menezes, R.R., Pereira, G.J., Torres, A.F., Lima, D.B., Pereira, T.P., Toyama, M.H., Monteiro, H.S., Smaili, S.S., Martins, A.M., 2014.
Bothropoides insularisvenom cytotoxicity in renal tubular epithelia cells. Tox-icon 88, 107e114.
Monteiro, H.S.A., Fonteles, M.C., 1999. The effect ofBothrops jararacavenom on rat kidney after short-term exposure: preliminary results. Pharmacol. Toxicol. 85, 198e200.
Morais, I.C., Torres, A.F., Pereira, G.J., Pereira, T.P., Pessoa Bezerra de Menezes, R.R., Mello, C.P., Coelho Jorge, A.R., Binda, A.H., Toyama, M.H., Monteiro, H.S., Smaili, S.S., Martins, A.M., 2013. Bothrops leucurus venom induces nephrotox-icity in the isolated perfused kidney and cultured renal tubular epithelia. Tox-icon 61, 38e46.
Mosmann, T., 1983. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J. Immunol. Methods 65, 55e63.
Peixoto, E.B., Collares-Buzato, C.B., 2005. Protamine-induced epithelial barrier disruption involves rearrangement of cytoskeleton and decreased tight junction-associated protein expression in cultured MDCK strains. Cell Struct. Funct. 29, 165e178.
Pereira, M.F., Novello, J.C., Cintra, A.C., Giglio, J.R., Landucci, E.T., Oliveira, B., Marangoni, S., 1998. The amino acid sequence of bothropstoxin-II, an Asp-49 myotoxin fromBothrops jararacussu(Jararacucu) venom with low phospholi-pase A2 activity. J. Protein Chem. 17, 381e386.
Queiroz, G.P., Pessoa, L.A., Portaro, F.C.V., Furtado, F.M.D.E., Tambourgi, D.V., 2008. Interspecific variation in venom composition and toxicity of Brazilian snakes from Bothrops genus. Toxicon 52, 842e851.
Ribeiro, L.A., Jorge, M.T., 1997. Bites by snakes in the genus Bothrops: a series of 3,139 cases. Rev. Soc. Bras. Med. Trop. 30, 475e480.
Santoro, M.L., Sano-Martins, I.S., Fan, H.W., Cardoso, J.L.C., Theakston, R.D.G., Warrell, D.A., Biasg, 2008. Haematological evaluation of patients bitten by the jararaca,Bothrops jararaca, in Brazil. Toxicon 51, 1440e1448.
southeastern Brazil. Biol. Pitvipers 199e216.
Sgrignolli, L.R., Mendes, G.E.F., Carlos, C.P., Burdmann, E.A., 2011. Acute kidney injury caused by bothrops snake venom. Nephron Clin. Pract. 119, c131ec136.
Sitprija, V., Sitprija, S., 2012. Renal effects and injury induced by animal toxins. Toxicon 60, 943e953.
Vanden Berghe, T., Grootjans, S., Goossens, V., Dondelinger, Y., Krysko, D.V., Takahashi, N., Vandenabeele, P., 2013. Determination of apoptotic and necrotic
cell death in vitro and in vivo. Methods 61, 117e129.
Walser, M., Davidson, D.G., Orloff, J., 1955. The renal clearance of alkali-stable inulin. J. Clin. Invest 34, 1520e1523.