Study of the safety of methylphenidate: Focus on nephrotoxicity aspects
Luiza Herbene Macedo Soares Salviano
a, Maria Isabel Linhares
b, Klistenes Alves de Lima
d,
Alana Gomes de Souza
d, Dânya Bandeira Lima
c, Antônio Rafael Coelho Jorge
e, Marcus Felipe Bezerra da Costa
c,
Adriano José Maia Chaves Filho
e, Alice Maria Costa Martins
a,b,c,d, Helena Serra Azul Monteiro
b,e,
Teresa Maria de Jesus Ponte Carvalho
a,d, Marta Maria de França Fonteles
a,b,c,d,⁎
aPostgraduation Program in Development and Technological Innovation in Medicines (PPGDITM), Federal University of Ceará (UFC), Brazil bPostgraduation Program in Pharmacology/UFC, Brazil
cPostgraduation Program in Pharmaceutical Sciences/UFC, Brazil dPharmacy Course/UFC, Brazil
eDepartment of Physiology and Pharmacology/UFC, Brazil
a b s t r a c t
a r t i c l e
i n f o
Article history:
Received 14 February 2015
Received in revised form 13 August 2015 Accepted 21 September 2015 Available online 25 September 2015
Keywords:
Methylphenidate Nephrotoxicity Renal perfusion
Aims:Methylphenidate (MPD) is increasingly prescribed for the treatment of Attention Deficit Hyperactivity Dis-order and there are concerns about its appropriate use. Furthermore, little is known about the potential nephro-toxicity in patients using MPD. This study aimed to investigate the safety of MPD, with focus on the possible effects of this drug on renal function.
Main methods:We investigated the effects of MPD on renal perfusion system and renal tubular cells throughin vivoandin vitroexperimental models.
Keyfindings:In thein vivoexperiments, 24 h and 48 h after MPD administration, urea, creatinine, creatinine clear-ance, and the fractional excretion of sodium and potassium were not changed. In the isolated kidney perfusion, MPD significantly reduced urinaryflow, glomerularfiltration rate and the percentage of tubular sodium port. However, the perfusion pressure, renal vascular resistance and the percentage of tubular potassium trans-port were unchanged in this system. In the canine renal epithelial cell line MDCK culture, MPD was not cytotoxic and, in histopathological analysis, MPD did not promote alterations.
Significance:Ourfindings suggest a possible nephrotoxic effect of MPD, since it altered renal function by reducing the glomerular activity, urinaryflow and sodium transport. These effects need to be further investigated in order to minimize potential harms associated with the use of MPD.
© 2015 Elsevier Inc. All rights reserved.
1. Introduction
Methylphenidate (MPD) is the drug offirst choice for the treatment of Attention Deficit Hyperactivity Disorder (ADHD). The MPD is the most consumed psychostimulant in the world[16]. Its production rose from 2.8 tons in 1990 to nearly 38 tons in 2006; this is not only due to its link to ADHD, but mainly due to intense publicity about the drug [16]. In Brazil, in line with this global trend, consumption has also grown in each year. In 2007, nearly 1.15 million boxes of MPD were bought in this country[15].
There is widespread concern about the pharmaceutical industry's aggressive marketing of MPD, which only highlights the potential ben-efits of the drug, thus increasing possibility of inappropriate and unsafe use. These advertisements may also lead to abusive consumption of
MPD due to its putative effect of enhancement of cognitive function (one of the reasons young university students reported for using it), in addition to inappropriate use in misdiagnosed ADHD[29].
The diagnosis of ADHD is clinical and there are no objective mea-surements to confirm diagnosis[19,39]. This situation allows that ADHD may be overdiagnosed, leading to an increase in the prescription of MPD[19,29,39]. Methylphenidate is indicated for ADHD for children from six years of age, adolescents and adults; however, its‘unapproved’
use in children under six years has also been increasing[12].
MPD is completely and rapidly absorbed when administered orally, and it readily crosses the blood–brain barrier[8,40]. This drug is metab-olized primarily through deesterification in the liver, which generates the main metabolite ritalinic acid, which is excreted renally. The half-life of MPD is approximately 2 h after absorption, and the half-half-life of its metabolites is about 7 h[8]. The pharmacokinetic of MPD in children is essentially the same as for adults, and the bioavailability in humans is similar to that observed in rats and monkeys[40].
Although methylphenidate has a satisfactory safety profile[1,28], several adverse reactions have been reported including headaches,
–
⁎ Corresponding author at: Department of Pharmacy, Faculty of Dentistry, Nursing and Pharmacy, Federal University of Ceará, Street Capitão Francisco Pedro, 1210, CEP: 60430-375, Fortaleza, Ceará, Brazil.
E-mail address:martafonteles@yahoo.com.br(M.M. de França Fonteles).
http://dx.doi.org/10.1016/j.lfs.2015.09.014 0024-3205/© 2015 Elsevier Inc. All rights reserved.
Contents lists available atScienceDirect
Life Sciences
Despite this, little is known about the effect on renal function in pa-tients using MPD. Therefore, it is important to examine these aspects of its safety for its use by the general population[31].
The objective of this study was to investigate the safety of Ritalin® (methylphenidatehydrochloride) byin vitroandin vivoassays, with a particular focus on the possible effects of this drug on renal function.
2. Material and methods
2.1. Animals and drug used
The animals used in this study were adult maleWistarrats, weighing 250 to 300 g, from the Vivarium of the Department of Physiology and Pharmacology, Federal University of Ceará (UFC). The animals were kept in standard cages, at a controlled temperature (24 ± 2°C) with a 12-h dark/light cycle and food and water ad libitum. Twenty-four hours prior to the kidney perfusion experiments, the animals were put on a fast with free access to water. Studies were carried out in compli-ance with national and international laws and guidelines for the use of animals in biomedical research and under the consent and surveillance of Ethics Committee on Animal Research (Protocol number: 09/2012/ UFC). Moreover, the experiments were performed to minimize the number of rats used and their suffering, following the ethical doctrine of the three“R”s—reduction, refinement and replacement.
The methylphenidatehydrochloride(Ritalin®) used in the experi-ment was purchased from its manufacturer (Novartis Laboratory) and dissolved in distilled water.
2.2. In vitro assays
2.2.1. Isolated kidney perfusion
The rats were fasted for 24 h with free access to water. The animals were anesthetized with sodium pentobarbital (50 mg/kg, ip) and after careful dissection of the right kidney, the right renal artery was cannu-lated via the mesenteric artery without interrupting the flow, as
artery. The inulin of the perfusate and of the urine was measured using direct hydrolysis, as in Walser et al.[38]and Fonteles et al.[9]. The pho-tometric readings were carried out in a spectrophotometer and osmo-larity was measured using an osmometer (Vapor pressure osmometer —Model 5520 ESCOR).
The experiments were started after stabilization of the organ to the new conditions. MPD (10μg/mL) was added to the system at 30 min after the beginning of the renal perfusion. Every 5 min the perfusion pressure and perfusionflow were recorded for 120 min. Urine and per-fusionfluid samples were collected every 10 min for determination of renal parameters: Urinary Flow (UF), Glomerular Filtration Rate (GFR), Perfusion Pressure (PP), Renal Vascular Resistance (RVR) and percentage of transported tubular sodium (%TNa+) and potassium (%TK+), in line with Martinez-Maldonado et al.[22], and Fonteles et al.[9]. The results were compared to the internal control group, at 30 min early in each experiment. The treatment group was also com-pared to the control group, in which the kidneys were perfused with MKHS alone.
The renal perfusion system used in our experiments was developed by Bowman and Maack[2]and Ross[33]and modified by Fonteles et al. [9]by adapting the Silastic artificial lung, based on the Hamilton et al. [13]model.
2.2.2. Cell culture
MDCK (Madin–Darby Canine Kidney) renal epithelial tubular cells were used. TheMDCKcells were kindly donated by Chemistry Laborato-ry from BiochemistLaborato-ry Institute of São Paulo University—USP, São Paulo, Brazil. They were grown in cell cultureflasks with RPMI 1640 supple-mented with 10% fetal bovine serum (FBS) and 1% antibiotics (penicil-lin/streptomycin). Cells were maintained at 37°C in an atmosphere of 95% O2/5% CO2,and then exposed to trypsin–EDTA (0.25/0.02%v/v). Af-terwards, the cells were suspended in culture medium with FBS and quantified in a Neubauer chamber. MDCK cells were plated at a concen-tration of 1 × 105cells/mL in 96-well plates for 24 h, after which the ex-periments were initiated.
2.2.3. Cytotoxicity assay
Cell viability was assessed using the MTT ((4,5-dimetilazil-2-yl)-2,5 diphenyltetrazolium hydroxide) assay as described by Mosmann[26]. The MDCK cells were treated with MPD (80, 40, 20, 10, 5, 2.5 and 1.25μg/mL). After 24 h of treatment with MFD, the cells were incubated with tetrazolium salt (MTT, 2.5 mg/mL, Sigma) for 4 h. The formazan crystals resulting from the reduction of MTT were dissolved by adding sodium dodecyl sulfate, SDS (10%). The plates were maintained at 37°C and, after 17 h, the reading was carried out in a spectrophotometer at a wavelength of 570 nm.
2.3. In vivo assays
2.3.1. Renal biochemistry tests
Additional experiments were carried out to determine the biochem-ical renal parameters at 24 h and 48 h after administration of MPD. The animals were randomized into two groups (n = 6): MPD (10 mg/kg) and control group (aqueous solution). The drug was orally administered (gavage).This dose was chosen because of the large number of previous studies that used this dose or related doses to investigate the behavioral effects and the mechanisms involved in the central action of the MPD, which justify both its therapeutic use as abuse[4,5,11,18,21,42]. The an-imals were kept in metabolic cages in the last 24 h of the experiment, after which their urine was collected. Right after obtaining the urine vol-ume, the animals were anesthetized with sodium pentobarbital (50 mg/kg) and a laparotomy was carried out; the abdominal vena
cava was dissected in order to collect the blood. The extracted plasma was used to measure the creatinineclearance(CrCl) and the concentra-tions of urea (U), creatinine (Cr), sodium (Na+), and potassium (K+). These measurements were also carried out at 24 h. Creatinineclearance (CrCl) was calculated to determine the kidney function of animals and to estimate the glomerularfiltration rate (GFR). Urea and creatinine were measured using the colorimetric method, in which a semi-automatic device (LabQuest®) and diagnostic kits (LABTEST®, Brazil) were employed.
The dosage of sodium and potassium was carried using an ion-selective electrode system (RapidChem 744, Bayer Diagnostics). With sodium and potassium plasma and urinary concentrations, fractional excretion of Na+and K+(FENa+, FEK+) were calculated. These param-eters were used to evaluate the renal tubular functional capacity. All biochemical tests were carried out at the Clinical Research Unit, Federal University of Ceará.
2.3.2. Histological analysis
At the end of thein vivoexperiments, a longitudinal section of the kidney was biopsied and placed on 10% formalin for histological exam-ination. The fragments were dehydrated and diaphanized and then cut to a thickness of 5μm. Hematoxylin–eosin (HE) slides were analyzed using an optical microscope by an experienced histologist blinded to the treatment regimen. The control group consisted of kidney section perfused with saline. The slides were made at Biopse-Pathological Anat-omy Laboratory.
Fig. 2.Determination of perfusion pressure (a) and renal vascular resistance—RVR (b) of control and treated with methylphenidate (MPD) groups (N = 6). Results were expressed as mean and standard error of the mean (SEM). *pb0.05 compared with the control group (ANOVA and Bonferroni test).
Fig. 3.Percentage of tubular sodium transport—%TNa+(a) and percentage of tubular potassium transport—%TK+(b) of control and treated with methylphenidate (MPD) groups (N = 6). Results were expressed as mean and standard error of the mean (SEM). *pb0.05 compared with the control group (ANOVA and Bonferroni test).
2.4. Statistical analysis
All data are expressed as the mean ± S.E.M. Statistical tests used to determine significant differences between treatment and control groups are indicated in thefigure legends. For all experiments, the treat-ment and control groups were blinded to the investigator, and mice were randomized between groups (N as indicated in the figure legends).
3. Results
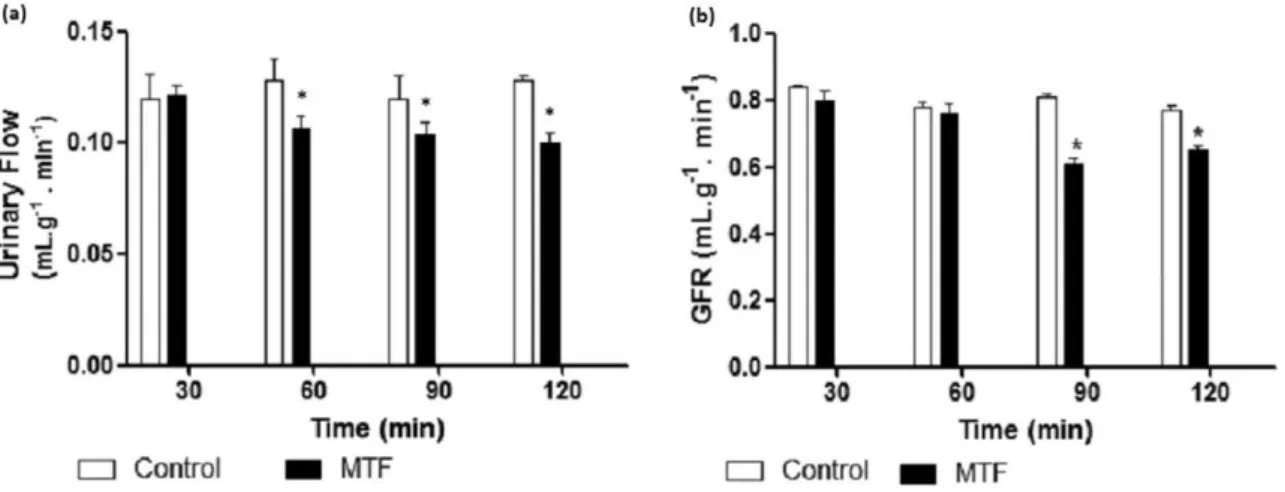
The parameters that were evaluated in isolated kidney perfusion with MPD (10μg; n = 6) were: urinaryflow rate of glomerularfi ltra-tion, perfusion pressure and renal vascular resistance, percentage of tu-bular transport of sodium (%TNa+) and the percentage of tubular transport of potassium (%TK+).
Continuous perfusion with MPD in the isolated kidney system signif-icantly reduced the urineflow (Fig. 1a) and the glomerularfiltration rate of isolated kidney in rats (Fig. 1b) compared with the control group (pN0.05, ANOVA). However, the reduction was not statistically significant for the perfusion pressure (Fig. 2a) or renal vascular resis-tance (Fig. 2b). The values of %TNa+was also signi
ficantly reduced (pN0.05, ANOVA) (Fig. 3a), but the %TK+remained unchanged (Fig. 3b).
In the cytotoxicity tests, the MPD did not reduce the viability of the epithelial cells of the renal distal tubules in any of the MDCK cell culture concentrations, showing no cytotoxic effect (Fig. 4). In the in vivo exper-iments with animals treated with MPD (10 mg/kg; n = 6), 24 and 48 h after treatment, there were no significant changes for the other param-eters of renal function (urea, creatinine, creatinine clearance, FENa+and
ported as adverse effects, there is little scientific evidence about the drug's effect on the physiological functions of the renal system[24].
Renal impairment is a major contributor to the high rate of morbid-ity and mortalmorbid-ity associated with the indiscriminate use of medicines [24]. Thus, it is important to investigate the effects of MPD on renal func-tion to ensure the safe use of the drug.
The kidneys receive approximately 25% of the entire cardiac output and are the primary organ of excretion[17]. Filtration and urine produc-tion depend on different factors. The most important is the self-regulation of bloodflow through the glomeruli. Thus, if the glomerular function is impaired, urinaryflow will be altered[36].
In this context, several conditions may affect glomerularfiltration. Changes in renal bloodflow affect thefiltration coefficient and the amount of urine produced, and the vascular tone of the arterioles of the glomeruli affectsfiltration pressure and also influences the volume of urine produced. Some drugs produce intense constriction of the arte-rioles, reducing the glomerular pressure, thefiltration of plasma and thus, the formation of urine[36].
Ourfindings demonstrated that MPD reduced the glomerularfi ltra-tion rate, urineflow and the percentage of tubular transport of sodium during perfusion experiments. The self-regulation of blood flow through the glomeruli and the tubular balance depend on the integrity of sodium reabsorption mechanisms. In this context, this effect on the kidney may be related to activation of renin–angiotensin–aldosterone system, since aldosterone, similar to the action of MPD, is able to reduce the elimination of sodium and cause renal vasoconstriction[36].
Furthermore, prostaglandins, as PGI2, PGE2 and PGD2, are also fun-damental to maintain a normal renal bloodflow and glomerularfi ltra-tion rate. These substances are able to cause vasodilaltra-tion and reduction in vascular resistance, improving the renal perfusion, with the redistribution of bloodflow from the cortex to the juxtamedullary region[30].
Prostaglandin synthesis can be altered in some renal diseases or con-ditions that affect renal function such as glomerular disease, renal fail-ure, hyperkalemia, situations of depletion of the effective plasma volume, heart failure, cirrhosis and hypovolemic shock. In some these circumstances, as extreme hypovolemic situations, the renin– angioten-sin–aldosterone system is also stimulated, resulting in vasoconstriction and an increase in the renal reabsorption of sodium and water. The vas-cular tone increases as result of the stimulation of the sympathetic sys-tem. In this condition, prostaglandins can provide a compensatory Fig. 4.Effect of different concentrations of methylphenidate (80; 40; 20; 10; 5; 2.5; 1.25
μg/mL) on cells MDCK by the method of MTT. Data were expressed as mean ± SEM and analyzed using ANOVA and Dunnett test (*pb0.05).
Table 1
Effect of MPD (10 mg/kg) on renal function of animals after 24 h and 48 h (N = 6).
Groups Urea (U) Creatinine (Cr) Creatinine Cl (CrCl) (%) Excretion Na+(FENa+) (%) Excretion K+(FEK+)
Mean ± SD Mean ± SD Mean ± SD Mean ± SD Mean ± SD
24 h
CT 39.25 ± 2.1 0.65 ± 0.05 0.76 ± 0.15 0.42 ± 0.4 1.72 ± 0.2
10 mg/kg 37.88 ± 4.6 0.60 ± 0.08 0.97 ± 0.12 0.40 ± 0.09 1.30 ± 0.25
48 h
CT 37.5 ± 1.1 0.7 ± 0.06 0.72 ± 0.05 0.44 ± 0.12 1.55 ± 0.24
10 mg/kg 35.8 ± 3.9 0.62 ± 0.1 0.92 ± 0.23 0.37 ± 0.06 1.41 ± 0.31
vasodilation, to ensure adequate perfusion and prevent impairment of the glomerularfiltration and renal function[37].
In our study, the MPD may have interfered with the vasodilator ef-fect of prostaglandins, since it reduced the urinaryflow and glomerular filtration rate. This effect may cause renal vasoconstriction and, after long-term treatment, cause renal injury. Moreover, such a reduction in the abovementioned parameters may also suggest changes in the effects of vasopressin, via the inhibition of renal prostaglandin synthesis or the reduction of the renal ability of urine dilution, leading to hyponatremia [34].
No studies were found that associated kidney disease with the use of MPD. However, electrolyte disturbances, renal failure and damage to tu-bular cells are often associated with the nephrotoxic effects of some medications, especially their inhibitory action on the synthesis of pros-taglandins[6,17,24]. Similar results to our study were found for the par-enteral analgesic ketorolac, which reduced the glomerularfiltration rate in some patients, had nephrotoxic potential and increased the risk of renal failure[32].
Despite this, the perfusion pressure, renal vascular resistance and the percentage of tubular transport of potassium did not change signif-icantly. Studies on the antidepressant sertraline reported no change in renal parameters after treatment of animals, similar to our results[6]. Furthermore, in our work, kidney perfusion with Krebs–Henseleit solu-tion alone did not affect the assessed renal parameters, which corrobo-rates previous studies[7,10].
On the other hand,in vivoexperiments, urea, creatinine, creati-nineclearance, FENa+and FEK+showed no signi
ficant changes compared to the other parameters of renal function at the two ob-servation periods (24 h and 48 h). Nevertheless, the results were presented, because in vivo studies on animal renal biochemistry and MPD were not found.
At the start of a drug-induced renal syndrome, the levels of serum urea, creatinine and potassium are high. However, in some cases, for ex-ample, of anti-inflammatory agents, the increase in serum creatinine may be observed only after thefirst three to seven days of use. This amount of time is required before a drug reaches its maximum levels and, therefore, a maximum inhibition of prostaglandin synthesis[3]. Ourfindings demonstrate that the MPD did not alter these serum pa-rameters in the amount of time covered in this study. However, moni-toring of renal function for more time may be required for changes to be detected.
When the cytotoxicity potential of MPD for these cells was assessed, it did not alter the cell viability in MDCK cultured cells. The MDCK cells are a useful model for studying cell growth regulation, metabolism, and transport mechanisms in distal renal epithelium[14], so the capacity of MPD did not cause damage to these cells suggest that its potential ad-verse effect on the kidney is not in distal renal structures.
Moreover, the histopathological analysis of kidneys, 24 h after MPD administration in animals, also showed that the MPD did not have harmful effect on this organ, mainly, on the renal tubules. Thisfinding,
together with the above results, reinforces that the possible nephrotoxic effect of MPD is probably not mediated by changes in the renal tubules. Finally, although this study provides an evidence indicating the pos-sible nephrotoxic effect of methylphenidate hydrochloride in adult ani-mals, future studies with chronic dosing schedule and longer follow-up periods of analysis are needed, since some changes in renal function and histology may appear only late and chronically to treatment with this drug. Then, to investigate the effect of MPD on renal parameters in in-fant and adolescent animals may be a promising direction for future re-search due to the considerable use of MPD in earlier ages of human development.
5. Conclusion
MPD did not show cytotoxicity or the potential to damage the kid-ney, mainly renal tubules, in histopathological analysis, and did not change the biochemical parameters of renal functionin vivostudies. De-spite this, the continuous perfusion with MPD reduced the urineflow and glomerularfiltration rate in the isolated rat kidney, and reduced the percentage of tubular transport of sodium.
Therefore, our results showed that although methylphenidate seems to be a safe option for the treatment of ADHD, it should be used with caution, since changes in renal function parameters were found. This suggests a possible nephrotoxic effect of MPD, but it should be investi-gated in greater depth in order to evaluate the intensity of these effects, and to minimize risks when this drug is taken.
Conflict of interest statement
The authors report no conflicts of interest.
Acknowledgments
The authors thank to Brazilian National Research Council (CNPq) and Foundation for Support of Scientific and Technological Develop-ment of Ceará (FUNCAP) by scholarships for postgraduate students. Also, the authors are grateful to David J. Woods (University of Otago, New Zealand) for his valuable comments and review of this work.
References
[1] E.P. Askenasy, K.H. Taber, P.B. Yang, N. Dafny, Methylphenidate (Ritalin): behavioral studies in the rat, Int. J. Neurosci. 117 (2007) 757–794.
[2] R.H. Bowman, T. Maack, Effect of albumin concentration and ADH on H2O and
elec-trolyte in perfused rat kidney, Am. J. Physiol. 226 (2) (1974) 426–430.
[3] L.F. Bricks, C.A.A. Da Silva, Toxicidade dos anti-inflamatórios não hormonais, Pediatria (São Paulo). 27 (3) (2005) 181–193.
[4] J. Cheng, Z. Xiong, L.J. Duffney, J. Wei, A. Liu, S. Liu, G.J. Chen, Z. Yan, Methylphenidate exerts dose-dependent effects on glutamate receptors and behaviors, Biol. Psychia-try 76 (12) (2014) 953–962.
[5] C.M. Claussen, N. Dafny, Acute administration of methylphenidate alters the pre-frontal cortex neuronal activity in a dose–response characteristic, J. Exp. Pharmacol. 11 (6) (2014) 1–9.
[12]T. Grund, K. Lehmann, N. Block, A. Rothenberger, G. Teuchert-Noodt, Influence of methylphenidate on brain development—an update of recent animal experiments, Behav. Brain Funct. 2 (2) (2006).
[13]R.L. Hamilton, et al., A simple and inexpensive membrane“lung”for small organ perfusion, J. Lipid Res. 15 (1974) 182–186.
[14] J. Irvine, L. Takahashi, K. Lockhart, J. Cheong, J.W. Tolan, H.E. Selick, J.R. Grove, MDCK (Madin–Darby Canine Kidney) cells: a tool for membrane permeability screening, J. Pharm. Sci. 88 (1) (1999) 28–33.
[15] Itaborahy, C., 2009. A Ritalina no Brasil: uma década de produção, divulgação e consumo. Rio de Janeiro, RJ: Universidade do Estado do Rio de Janeiro (UERJ), (Dissertação).
[16] C. Itaborahy, F. Ortega, O metilfenidato no Brasil: Uma década de publicações, Ciência&Saúde Coletiva. 18 (3) (2013) 803–816.
[17] R. John, A.M. Herzenberg, Renal toxicity of therapeutic drugs, J. Clin. Pathol. 62 (2009) 505–515.
[18]Z. Jones, N. Dafny, Acute and chronic dose–response effect of methylphenidate on ventral tegmental area neurons correlated with animal behavior, J. Neural Transm. 121 (3) (2014) 327–345.
[19] D. Keen, I. Hadjikoumi, ADHD in children and adolescents, BMJ Clin. Evid. 10 (2008) 312.
[20]B. Levine, Y.H. Caplan, G. Kauffman, Fatality resulting from methylphenidate over-dose, J. Anal. Toxicol. 10 (5) (1986) 209–210.
[21]M.I. Linhares, E.T. Venâncio, C.N.C. Lima, M.L. Feitosa, L.H.M.S. Salviano, A.G. Souza, K.A. Souza, F.C.F. Souza, D.J. Woods, L.M.V. Aguiar, M.M.F. Fonteles, Methylpheni-date: proconvulsant effect and action on acetyl cholinesterase activity in young and adult mice, Int. J. Pharm. Pharm. Sci. 6 (7) (2014) 547–551.
[22] M. Martinez-Maldonado, A. Stawroulaki-Tsapara, N. Tsapara, W.N. Suki, G. Eknoyan, Renal effects of lithium administration in rats: alteration in water and electrolyte metabolism and the response to vasopressin and cyclic-adenosine monophosphate during prolonged administration, J. Lab. Clin. Med. 80 (3) (1978) 445–461. [23] W. Massello, D.A. Carpenter, A fatality due to the intranasal abuse of
methylpheni-date (Ritalin), J. Forensic Sci. 44 (1) (1999) 220–221.
[31] Pastura, G., Mattos, P., 2004. Efeitos colaterais do metilfenidato.Rev. Psiq. Clin. 31 (2), 100–104.
[32] Rose, B.D., Post, T.W., 2010. NSAIDs: acute kidney injury (acute renal failure) and ne-phritic syndrome. UpToDate.www.uptodate.com(acessado em 20 de abril de 2014).
[33] B.D. Ross, The isolated perfused rat kidney, Clin. Sci. Mol. Med. 55 (1978) 13–21. [34] M.H. Rosner, J. Kirven, Exercise-associated hyponatremia, Clin. J. Am. Soc. Nephrol. 2
(2007) 151–161.
[35]O. Stecyk, T.A. Loludice, S. Demeter, J. Jacobs, Multiple organ failure resulting from intravenous abuse of methylphenidate hydrochloride, Ann. Emerg. Med. 14 (6) (1985) 597–599.
[36]J.R. Utley, Renal effects of cardiopulmonary bypass, in: G.P. Gravlee, R.F. Davis, J.R. Utley (Eds.), Cardiopulmonary Bypass Principles and Practice, Williams & Wilkins 1993, pp. 488–508.
[37] H.E. Vonkeman, M.A.F.J. Van de Laar, Nonsteroidal anti-inflammatory drugs: adverse effects and their prevention, Semin. Arthritis Rheum. 39 (2010) 294–312. [38]M. Walser, D.G. Davidson, J. Orloff, The renal clearance of alkali-stable inulin, The
Clin. Invest. 34 (10) (1955) 1520–1523.
[39] Wannmacher, L., 2006. DHDA: correto diagnóstico para real indicação de psicoestimulantes. In: OPAS. Uso racional de medicamentos. PAHO. Rational use of medicines. Temas Selecionados. Brasília. 3 (10), 1–6.www.saude.gov.br/portal/ arquivos/pdf/v3n10/dhda/psicoestimulantes/pdf(accessed April 12, 2014). [40]W. Wargin, K. Patrick, C. Kilts, C.T. Gualtieri, K. Ellington, R.A. Mueller, G. Kraemer,
G.R. Breese, Pharmacokinetics of methylphenidate in man, rat and monkey, J. Pharmacol. Exp. Ther. 226 (2) (1983) 382–386.
[41] J. Weiss, Autoregulation offlow in isolated rat kidney in the absence of red cells, Am. J. Psysiol. 195 (5) (1959) 1115–1118.