Braz. J. of Develop.,Curitiba, v. 6, n. 7, p. 50417-50442 jul. 2020. ISSN 2525-8761
Characterization and Evaluation of Filmogenic, Polymeric, and Biofilm
Suspension Properties of Cassava Starch Base (Manihot esculenta Crantz)
Plasticized with Polyols
Caracterização e Avaliação das Propriedades Filmogénicas, Poliméricas e de
Suspensão de Biofilme da Base de Amido de Mandioca (Manihot esculenta
Crantz) Plastificado com Polióis
DOI:10.34117/ bjdv6n7-626
Recebimento dos originais: 23/06/2020 Aceitação para publicação: 23/07/2020
José de Arimateia Rodrigues do Rego
Doutor em Química pela Universidade Federal do Pará Instituição: Universidade Federal do Pará
Endereço: R. Augusto Corrêa, 01 - Guamá, Belém - PA, 66075-110, Brasil E-mail: jr2rego@gmail.com
Marcondes Lima da Costa
Doutorado em Mineralogia, geoquímica pelo Universitat Erlangen-Nurnberg (Friedrich-Alexander), Alemanha
Instituição: Universidade Federal do Pará
Endereço: R. Augusto Corrêa, 01 - Guamá, Belém - PA, 66075-110, Brasil E-mail: marcondeslc@gmail.com
Davi do Socorro Barros Brasil
Doutorado em Química pela Universidade Federal do Pará. Instituição: Universidade Federal do Pará
Endereço: R. Augusto Corrêa, 01 - Guamá, Belém - PA, 66075-110, Brasil E-mail: dsbbrasil18@gmail.com
Jorddy Neves Cruz
Graduando em Farmácia pela Universidade da Amazônia Instituição: Universidade Federal do Pará
Endereço: R. Augusto Corrêa, 01 - Guamá, Belém - PA, 66075-110, Brasil E-mail: jorddynevescruz@gmail.com
Cristiane Maria Leal Costa
Doutorado em Engenharia Química pela Universidade Estadual de Campinas, Instituição: Universidade Federal do Pará
Endereço: R. Augusto Corrêa, 01 - Guamá, Belém - PA, 66075-110, Brasil E-mail: cmlc@ufpa.br
Braz. J. of Develop.,Curitiba, v. 6, n. 7, p. 50417-50442 jul. 2020. ISSN 2525-8761 Elza Brandão Santana
Doutorado em Engenharia de Recursos Naturais da Amazônia Instituição: Universidade Federal do Pará
Endereço: R. Augusto Corrêa, 01 - Guamá, Belém - PA, 66075-110, Brasil E-mail: santanaeb@yahoo.com.br
Sarah Vasconcelos Furtado
Graduação em Engenharia Química pela Universidade Federal do Pará. Instituição: Universidade Federal do Pará
Endereço: R. Augusto Corrêa, 01 - Guamá, Belém - PA, 66075-110, Brasil E-mail: sarahvfurtado@gmail.com
Alessandra Santos Lopes
Doutorado em Tecnologia de Alimentos pela Universidade Estadual de Campinas. Instituição: Universidade Federal do Pará
Endereço: R. Augusto Corrêa, 01 - Guamá, Belém - PA, 66075-110, Brasil E-mail: alessalopes@ufpa.br
ABSTRACT
This study investigated the gelatinization process of polymeric suspensions of cassava starch (Manihot esculenta Crantz) plasticized with glycerol or ethylene glycol and used for biofilm production. Scanning electron microscopy confirmed that the starch, used as raw material for suspensions, consists of granule-forming clods and granular aggregates. Physical parameters such as viscosity, density, and temperature can be evaluated and used to accurately characterize and identify the gelatinization point of the polyol-plasticized starch. Upon reaching the gelatinization point, the suspensions went underwent retrogradation and had a kinetic viscosity of 19 to 23.508 mPa·s for the starch–glycerol suspension and 13.56 to 16.12 mPa·s for the starch–ethylene glycol suspension. However, the density of the suspensions slightly decreased during this process, ranging from 1.01 to 0.98 g/cm3. The starch–glycerol biofilm was more malleable and resistant, while the starch–ethylene glycol biofilm was inflexible and brittle. The use of different polyols facilitated the modification of the solubilization capacity of the biofilms. The starch–glycerol biofilm had a solubility value three times higher than that of the starch–ethylene glycol biofilm.
Keywords: Biofilms, starch, polyols, gelatinization, crystallinity. RESUMO
Este estudo investigou o processo de gelatinização de suspensões poliméricas de amido de mandioca (Manihot esculenta Crantz) plastificado com glicerol ou etileno glicol e utilizado na produção de biofilme. A microscopia eletrônica de varredura confirmou que o amido, usado como matéria-prima para suspensões, consiste em torrões formadores de grânulos e agregados granulares. Parâmetros físicos como viscosidade, densidade e temperatura podem ser avaliados e utilizados para caracterizar e identificar com precisão o ponto de gelatinização do amido poliol plastificado. Ao atingir o ponto de gelatinização, as suspensões foram submetidas a retrogradação e tiveram uma viscosidade cinética de 19 a 23,508 mPa · s para a suspensão de amido-glicerol e 13,56 a 16,12 mPa · s para a suspensão de amido-etileno-glicol. No entanto, a densidade das suspensões diminuiu ligeiramente durante esse processo, variando de 1,01 a 0,98 g / cm3. O biofilme de amido-glicerol era mais maleável e resistente, enquanto o biofilme de amido-etileno-glicol era inflexível e quebradiço. O uso de diferentes polióis facilitou a modificação da capacidade de solubilização dos
Braz. J. of Develop.,Curitiba, v. 6, n. 7, p. 50417-50442 jul. 2020. ISSN 2525-8761
biofilmes. O biofilme de amido-glicerol apresentou um valor de solubilidade três vezes maior que o do biofilme de amido-etileno-glicol.
Palavras-chave: Biofilmes, amido, polióis, gelatinização, cristalinidade.
1 INTRODUCTION
Cassava (Manihot esculenta Crantz) is an important part of food culture around the world and is produced by over 100 countries (Gillman & Erenler, 2009). Cassava is easy to cultivate and requires few inputs (with yields of approximately 10 tons per hectare), so it also is a good raw material for production of starch products, bioethanol, animal feed, sweeteners, and biodegradable products (Delcour et al., 2010; Fonseca-Florido et al., 2019). The physicochemical properties of starch extracted from cassava, its ease of production, and its low cost have promoted research on the use of cassava as a raw material for biofilm production (Kechichian, Ditchfield, Veiga-Santos, & Tadini, 2010; Shah, Naqash, Gani, & Masoodi, 2016). The use of such biofilms is aligned with the growing need to replace synthetic polymers, which pollute the environment, with biopolymers that degrade more quickly in the environment (Souza, Goto, Mainardi, Coelho, & Tadini, 2013; “The future of plastic,” 2018).
Biofilms are thin films made from biological materials that provide a barrier to external elements. Biofilms can protect a packaged product from physical and biological damage as well as extend its service life (Famá, Flores, Gerschenson, & Goyanes, 2006). The main biopolymers used to prepare biofilms are proteins and polysaccharides, especially starch because of its low cost and ability to be produced at a large-scale (Cazón, Velazquez, Ramírez, & Vázquez, 2017). Starch-based materials can also reduce the environmental impact of synthetic plastics (Bonilla, Atarés, Vargas, & Chiralt, 2013).
In vegetables, starch is stored as granules with a low degree of molecular disorganization, making the structure semi-crystalline, with crystallinity degrees ranging from 20 to 45% (Smith, 2010). Heating an aqueous starch solution causes several processes, including gelatinization, solubilization, glass transition, crystallization, crystal structure change, volume expansion, molecular degradation, and water mobility (Baker, Miles, & Helbert, 2001). The solubilization of starch molecules, especially amylose, promotes swelling of the granules. Solubilization occurs when water and other solvents convert the gel into an amorphous granular form, which is then dispersed into solution (Franco, Wong, Yoo, & Jane, 2002; Martelli, Moore, & Laurindo, 2006).
According to Gatenholma and coworkers (1997) (Rindlava, Hulleman, & Gatenholma, 1997), the degree of crystallization of a polymer depends on the ability of its chains to form crystals, and
Braz. J. of Develop.,Curitiba, v. 6, n. 7, p. 50417-50442 jul. 2020. ISSN 2525-8761
also on the mobility of the chain during recrystallization. At low drying rates, the polymer chains have more time to become arranged in a stable conformation, favoring the formation of crystals. The interactions established between the amylose and amylopectin molecules composing starch are important for biofilm formation. If these interactions are strong, the biofilms formed are rigid and become brittle (Maria Martelli, Moore, Silva Paes, Gandolfo, & Laurindo, 2006; Rocha Plácido Moore, Maria Martelli, Gandolfo, José do Amaral Sobral, & Borges Laurindo, 2006; Zhang et al., 2019).
To solve this problem, in this paper we describe the production and characterization of filmogenic suspensions and cassava starch based biofilms (Manihot esculenta Crantz) plasticized with glycerol and ethylene glycol polyols. These polyols interact with the amylose and amylopectin monomers, causing the interaction between the monomers to be less intense, and altering the physical characteristics of the biofilms such as plasticity and rigidity.
2 MATERIALS AND METHODS
2.1 CASSAVA STARCH AND FILM SUSPENSION 2.1.1 Material
Cassava starch samples (Manihot esculenta Crantz) were obtained from the municipality of Castanhal in the state of Pará, Brazil. The plasticizers used in the production of filmogenic suspensions and biofilms were glycerol and ethylene glycol (Sigma-Aldrich, Brazil).
2.1.2 Chemical treatment and storage of cassava starch
The starch was washed with absolute ethyl alcohol to remove lipids, then vacuum filtered and dried in a forced circulation oven (FABBE) at 45 °C to constant mass. The starch was cooled in desiccators at room temperature and stored in bottles until use.
2.1.3 Chemical composition of starch samples
The chemical composition of each sample was measured in triplicate. The protein, moisture, and ash content were analyzed by standard AOAC methods (Horwitz & Association of Official Analytical Chemists., 1970). The Bligh and Dyer method was used for lipid determination (Bligh & Dyer, 1959). The carbohydrate content was calculated by subtraction of protein, moisture, ash, and lipids from the total mass.
Braz. J. of Develop.,Curitiba, v. 6, n. 7, p. 50417-50442 jul. 2020. ISSN 2525-8761 2.1.4 Scanning electron microscopy (SEM)
The samples were placed on aluminum stubs and coated with gold to make them electrically conductive (Quorum Technologies, SC7620). The starch micrographs were obtained using the Tescan VEGA3 Scanning Electron Microscope under an acceleration voltage of 15 kV.
2.1.5 Production of filmogenic suspensions
Two different filmogenic suspensions were produced using the glycerol and ethylene glycol plasticizers. The suspensions were prepared using 100 g of distilled water, 4 g of cassava starch and 1.2 g of plasticizer (glycerol or ethylene glycol), and were stirred until completely homogenized. The suspension was then placed in a water bath at 70 °C with constant stirring until gelatinized.
2.1.6 Viscosity and density of filmogenic suspensions
Filmogenic solutions of glycerol and ethylene glycol were analyzed according to ASTM D445-06 for viscosity (ASTM International, 2006) and ASTM D3505-96 for density (ASTM International, 2000). The analyses were performed with a SVM 3001 Stabinger Viscometer, according to the ASTM D7042-04 method (ASTM International, 2004). The rheological parameters were measured in M9-TEMP/RANGE SCAN mode. The data were obtained at 5 °C intervals, scanning a temperature range of 25 °C to 80 °C, with a fixed solution volume of 2.5 mL.
2.1.7 Crystallinity index analysis of starch by X-ray diffraction (XRD)
The starch samples were placed in a circular sample holder and analyzed on a PW7310 Philips-PANalytical diffractometer equipped with a copper anode (λCuKα1 = 1.54060 Å) and monochromator (45 kV voltage and 40 mA current).
The crystallinity index of the biofilm was determined by the method of Rindlava and coworkers (1999) (Rindlava et al., 1997), where the crystallinity index (ΙCr) is determined according to Equation 1:
ΙCr = 1 − (Ιam/Ι002) × 100 (1)
where Ιam is the intensity of amorphous diffraction and Ι002 is the maximum intensity of diffraction.
2.1.8 Thermogravimetric analysis of cassava starch
Thermogravimetric analysis was performed on TGA-50 Shimadzu thermal analyzer. The starch samples were weighed in a platinum crucible heated from an equilibrium temperature of 27 °C to 600 °C at a rate of 10 °C/min, under flow of 50 mL nitrogen/min.
Braz. J. of Develop.,Curitiba, v. 6, n. 7, p. 50417-50442 jul. 2020. ISSN 2525-8761 2.1.9 Fourier Transform Infrared (FTIR) Spectroscopy
FTIR spectra were measured using a Vertex 70 Bruker spectrophotometer in the region of 400 to 4000 cm-1.
2.2 BIOFILMS
2.2.1 Biofilm production
Starch–glycerol and starch–ethylene glycol plasticized biofilms were produced by placing 40 mL of the filmogenic solutions in silicone molds (25 cm diameter), then drying in a forced circulation oven (FABBE) at 30 °C for 24 h.
2.2.2 thickness
Film thickness was measured with a digital micrometer (0.001 mm resolution; Insize, model IP54). Five random spots were measured on each film, each at least 60 mm from the edge (Zavareze, Halal, Marques e Silva, Dias, & Prentice-Hernández, 2014).
2.2.3 Water activity (aw)
Water activity analysis was performed with the AquaLab Vapor Sorption Analyzer (Decagon Devices).
2.2.4 Water solubility
The solubility of biofilms is measured by the percentage of matter that dissolves in distilled water within 24 h. We followed the method described by Bertuzzi and coworkers (2007) (Bertuzzi, Castro Vidaurre, Armada, & Gottifredi, 2007), using biofilm samples (2 cm x 2 cm) previously stored in a silica gel desiccator for seven days. The samples were weighed (± 0.0001 g) and immersed in 50 mL distilled water for 24 h. The biofilm material that did not dissolve was placed in a forced circulation oven at 105 °C to determine the dry mass. The solubility of biofilms in water was expressed as a percentage of solubilized mass, according to Equation 2.
𝑀𝑆 = (𝑀0− 𝑀𝑓)
𝑀0 × 100
(2)
Braz. J. of Develop.,Curitiba, v. 6, n. 7, p. 50417-50442 jul. 2020. ISSN 2525-8761 2.2.5 Thermogravimetric analysis of biofilms
Thermogravimetric analysis was performed on TGA-50 Shimadzu thermal analyzer. The biofilms samples were weighed in a platinum crucible heated from an equilibrium temperature of 27 °C to 600 °C at a rate of 10 °C/min, under flow of 50 mL nitrogen/min.
2.2.6 Fourier Transform Infrared (FTIR) Spectroscopy
FTIR spectra were measured using a Vertex 70 Bruker spectrophotometer in the region of 400 to 4000 cm-1.
2.2.7 XRD analysis of biofilms
The biofilms were placed in a circular sample holder and analyzed on a PW7310 Philips-PANalytical diffractometer equipped with copper anode (λCuKα1 = 1.54060 Å), and monochromator (45 kV voltage and 40 mA current).
The crystallinity index of the biofilm was determined as described by Rindlava and coworkers (1997) (Rindlava et al., 1997), where the crystallinity index (ΙCr) is determined according to Equation 3:
ΙCr = 1 − (Ιam/Ι002) × 100 (3)
Where Ιam is the intensity of amorphous diffraction and Ι002 is the maximum intensity f diffraction.
3 RESULTS AND DISCUSSION
3.1 CASSAVA STARCH AND FILMOGENIC SUSPENSIONS 3.1.1 Chemical composition of starch
The protein content in the starch was 0.085% (≤ 0.01), the mean moisture value was 6.88% (± 0.02), the ash content was 0.25% (± 0.01), the lipid content was 0.6% (± 0.02), and carbohydrate content was calculated to be 92.11% (± 0.05). Our samples had above average lipid content when compared to cassava root composition from the literature (average is 0.2%).
3.1.2 Starch morphological analysis by SEM
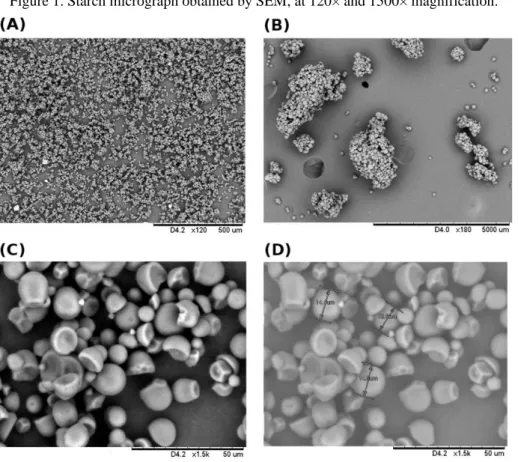
To analyze the morphology of the starch aggregates, the material was subjected to SEM analysis after chemical treatment (Figure 1).
Braz. J. of Develop.,Curitiba, v. 6, n. 7, p. 50417-50442 jul. 2020. ISSN 2525-8761
Figure 1. Starch micrograph obtained by SEM, at 120× and 1500× magnification.
Scanning electron microscopy (SEM) of the cassava starch (120× and 1500×) revealed that the starch consists of apparently homogeneous granules (Figure 1A), including clods (Figure 1B) and granular aggregates. The granules are predominantly round oval shapes, excavated at the top, with concave to convex shapes (Figure 1C). They appear homogeneous when compared to each other, and are an average of 14 µm in diameter (Figure 1D). This morphology is similar to that found by Franco and coworkers (2002) (Franco et al., 2002) and Luchese and coworkers (2017) (Luchese, Spada, & Tessaro, 2017), who found granules with a mean diameter of 12.2 µm. The diameter of our granules are also similar to Sriroth and coworkers (1999) (Sriroth et al., 1999) (8 to 22 µm).
3.1.3 Preparation of aqueous polyol starch
The cassava starch suspensions were prepared by dissolving the starch in water and adding glycerol and ethylene glycol plasticizers. Prior to the heating, these mixtures had an off-white appearance because the starch is not completely soluble in water (Figure 2).
Braz. J. of Develop.,Curitiba, v. 6, n. 7, p. 50417-50442 jul. 2020. ISSN 2525-8761
Figure 2. Starch and water solution with polyol addition.
After heating at 70 °C for 20 minutes, the mixture became translucent and viscous (Figure 3), indicating gelatinization of starch granules. The filmogenic suspensions produced with the plasticizers, glycerol and ethylene glycol, were similar in appearance.
Figure 3. Suspensions of starch–glycerol (right) and starch–ethylene glycol (left).
3.1.4 Viscosity variation of the filmogenic suspension with temperature
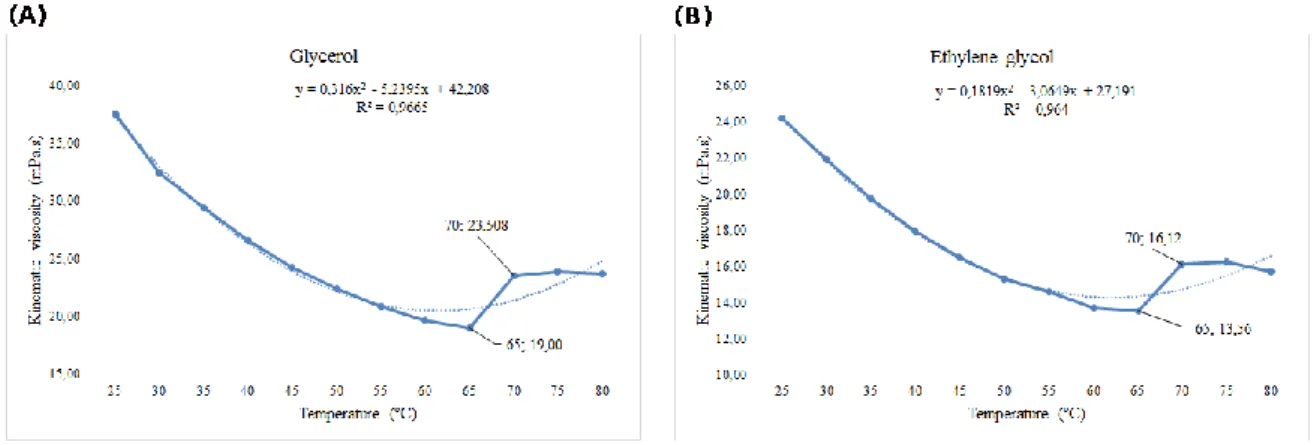
The viscosity of the starch–glycerol and starch–ethylene glycol plasticized suspensions varied over the temperature range of 65 to 70 °C (Table 1 and Figure 4). The gelatinization point occurred at 65 °C, within the characteristic temperature range reported for gelatinization of cassava starch (58 °C to 70 °C) (Ai & Jane, 2015).
Braz. J. of Develop.,Curitiba, v. 6, n. 7, p. 50417-50442 jul. 2020. ISSN 2525-8761
Table 1. Viscosity and density of aqueous suspensions of starch containing glycerol or ethylene glycol.
Figure 4. Viscosity of aqueous suspensions of starch containing glycerol or ethylene glycol with changes in temperature.
Although the suspensions both underwent gelatinization at 65 °C, the kinematic viscosities were different. The starch–glycerol suspension (19.00 mPa·s) was one-third higher than that of starch–ethylene glycol (13.56 mPa·s), and was clearly influenced by the polyol used. This indicates that the number of hydroxyls in the plasticizer (glycerol has 3 hydroxyls; ethylene glycol has 2 hydroxyls) interferes with the characteristics of the starch–polyol product, a fact corroborated by Miladinov and Hanna (Miladinov, 2001).
T (ºC)
Aqueous suspension of starch - glycerol
Aqueous suspension of
starch - ethylene glycol Viscosity (mPa.s) Especific mass (g.cm-3) Viscosity (mPa.s) Especific mass (g.cm-3) 25 37.49 1.01 24.21 1.01 30 32.48 1.01 21.88 1.01 35 29.39 1.01 19.7 1.01 40 26.6 1.01 17.95 1.01 45 24.29 1.01 16.49 1,0 50 22.37 1.01 15.32 1,0 55 20.83 1 14.56 1,0 60 19.67 1 13.71 1,0 65 19 1 13.56 0.99 70 23.51 0.99 16.12 0.99 75 23.89 0.99 16.23 0.99 80 23.68 0.99 15.73 0.98
Braz. J. of Develop.,Curitiba, v. 6, n. 7, p. 50417-50442 jul. 2020. ISSN 2525-8761
The kinematic viscosity of the starch–glycerol and starch–ethylene glycol suspension decreases with increasing temperature from 15 °C to 65 °C. However, between 65 °C and 70 °C the kinematic viscosity increases due to the swelling capacity of starch granules, with a kinematic viscosity of 4.51 mPa·s for starch–glycerol and 2.56 mPa·s for starch–ethylene glycol.
The starch–glycerol suspension had a higher viscosity at all analyzed points, with constant linearity and high correlations. The good correlation values (R2=0.96 for both the starch–glycerol and starch–ethylene glycol suspensions) means that viscosity can be used to differentiate polyol-plasticized starch suspensions.
The viscosity variation at 65 °C corresponds to the gelatinization of the suspensions. During this process, the breakdown of starch granules exposes the amylose and amylopectin monomers to the aqueous solvent. These monomers establish hydrogen bonds with water molecules and are responsible for starch retrogradation, which increases the viscosity at 65 °C to 70 °C from 19 to 23.508 mPa·s in the starch–glycerol suspension and from 13.56 to 16.12 mPa·s in the starch– ethylene glycol suspension. Although plasticizers influence the viscosity of the suspensions, the polyols used in this study did not change the starch gelatinization temperature in the range of 65 °C to 70 ºC.
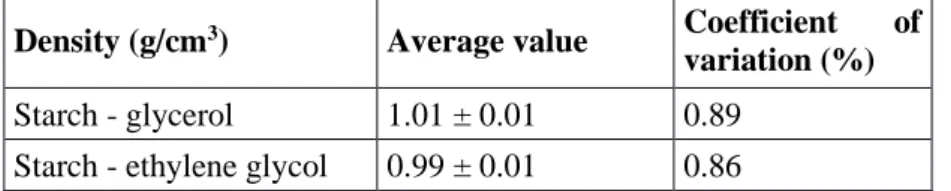
3.1.5 Variation of suspension density vs. temperature
The density of the starch–glycerol suspension was higher than that of the starch–ethylene glycol suspension (Table 2). The density of both the starch–glycerol suspension and starch–ethylene glycol suspension both decrease with increasing temperature throughout the analysis range (25 °C to 80 °C; Figure 5).
Table 2. Density of aqueous suspension starch–glycerol and starch–ethylene glycol.
Density (g/cm3) Average value Coefficient of
variation (%)
Starch - glycerol 1.01 ± 0.01 0.89
Braz. J. of Develop.,Curitiba, v. 6, n. 7, p. 50417-50442 jul. 2020. ISSN 2525-8761
Figure 5. Linear relationship of the densities of (A) starch–glycerol suspensions and (B) ethylene glycol starch as a function of temperature.
The suspension density was directly influenced by temperature, and was higher in the starch– glycerol suspension than in the starch–ethylene glycol suspension at all analyzed points, with constant linearity and high correlations (R2= 0.98 for both suspensions). This means that density measurements can be used to evaluate the starch suspensions plasticized with glycerol and ethylene glycol.
The density variation versus temperature was plotted over all temperature ranges (Figure 6), and displays an inflection point at the starch gelatinization temperature (65 to 70 °C). An inflection point at the same temperature was observed in the plot of viscosity as a function of temperature (Figure 4). The inflection point of Figure 6 was not observed by plotting the absolute values of density as a function of temperature as shown in Figure 5.
Figure 6. Density variation as a function of temperature for (A) starch–glycerol and (B) starch–ethylene glycol suspensions.
Braz. J. of Develop.,Curitiba, v. 6, n. 7, p. 50417-50442 jul. 2020. ISSN 2525-8761 3.2 BIOFILMS
3.2.1 Preparing biofilms
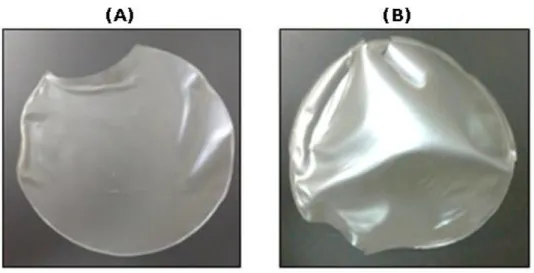
The starch–glycerol and starch–ethylene glycol suspensions were poured into round silicone forms and dried in an oven at 30 °C for 24 h. The consistency and appearance of these biofilms was similar to commercial plastics made from petroleum (Figure 7). The starch–glycerol biofilm (Figure 7A) showed greater malleability whereas the starch–ethylene glycol biofilm was more inflexible and brittle (Figure 7B).
Figure 7. Biofilms produced from filmogenic suspensions: (A) starch–glycerol biofilm and (B) starch–ethylene glycol biofilm.
3.2.2 SEM analysis of biofilms
The biofilms were examined with SEM (Figure 8), and the SEM micrographs show the continuity (Figure 8A) and roughness (Figure 8B; 200×) of the starch–glycerol biofilm.
Figure 8C and 8D show SEM images of starch–ethylene glycol biofilm with 20× to 200× magnification, respectively. This biofilm exhibits brittle points and glassy regions, which explain the rigidity and brittle behavior of this biofilm.
Braz. J. of Develop.,Curitiba, v. 6, n. 7, p. 50417-50442 jul. 2020. ISSN 2525-8761
Figure 8. Micrographs of plasticized starch biofilms (A and B) with glycerol and (C and D) ethylene glycol.
3.2.3 Biofilm thickness
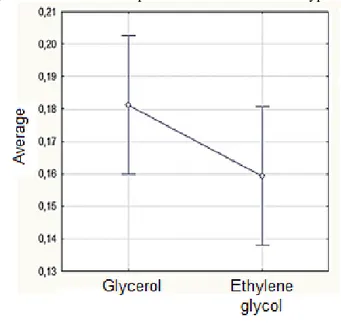
Table 3 presents the average thickness of the biofilms, with small variations between the three samples. The biofilms are thicker than others described in the literature, which may be related to the drying method or the material used to produce the biofilms. Chen and coworkers (2014) (Chen et al., 2015) produced starch–glycerol biofilms on Teflon forms with thicknesses around 25 to 30 µm. The thickness of the different biofilms in this study were not statistically significantly different, according to the Tukey test results with a reliability of 95% (Figure 9).
Table 3. Thickness and standard deviation measured in triplicate for each of the starch–glycerol and starch–ethylene glycol biofilms.
Biofilms Thickness (mm) Average (mm)
Starch – glycerol
1 0.1822 ± 0.0096
0.1813 ± 0.0103
2 0.1836 ± 0.0092
3 0.1780 ± 0.0131
Starch - ethylene glycol
1 0.1800 ± 0.0093
0.1593 ± 0.0211
2 0.1630 ± 0.0100
Braz. J. of Develop.,Curitiba, v. 6, n. 7, p. 50417-50442 jul. 2020. ISSN 2525-8761
Figure 9. Effective decomposition of thickness mean hypotheses.
It is difficult to control the thickness of the biofilms with the casting method since factors such as solution temperature, drying conditions, amount of suspension used, and the material of the casting form can influence the final biofilm thickness.
3.2.4 Water activity (aw)
Table 4 presents the values of aw obtained for the dried cassava starch and for the biofilms produced.
Table 4. Water activity of cassava starch, starch–glycerol biofilms, and starch–ethylene glycol biofilms.
Samples Aw
Starch 0.2303
Starch - glycerol biofilm 0.5411
Starch - ethylene glycol biofilm 0.5830
The water activity (aw) of a material is determined by the relationship between the partial water pressure and the vapor pressure of pure water at the same temperature. The water activity is related to the availability of free water in the material, which contributes to physical, chemical, and biological reactions causing food spoilage (Hamad, 2012).
Van den Berg and Bruin (van den Berg & Bruin, 1981) correlated water activity values with susceptibility to lipid oxidation, non-enzymatic browning, enzymatic activity, and growth of microorganisms such as fungi, yeast, and bacteria. A food with water activity above 0.7 is subject to attack by microorganisms, causing it to deteriorate.
Braz. J. of Develop.,Curitiba, v. 6, n. 7, p. 50417-50442 jul. 2020. ISSN 2525-8761
Cassava starch has a water activity of 0.23, and biofilms plasticized with glycerol and ethylene glycol have water activities of 0.54 and 0.58, respectively. Therefore, these biofilms have low water activity (aw < 0.7) and are not likely to be microbially degraded. These biofilms can be used to produce biodegradable packaging that prevents the growth of fungi, yeast, and bacteria.
3.2.5 Water solubility
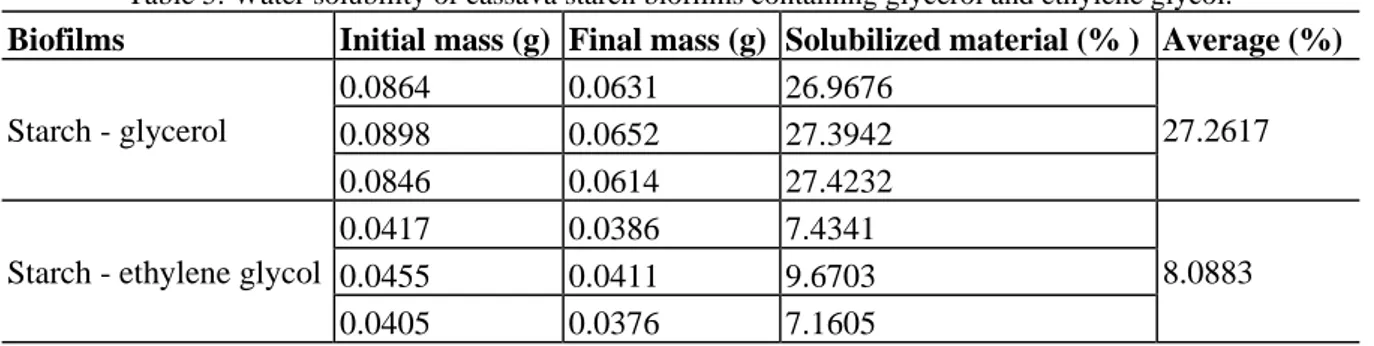
Water solubility is an important property of biofilms made from biodegradable raw material, because the use of biofilm can be affected by how soluble it is. For example, water-soluble biofilms can be used for nutrient encapsulation and food additives (Jiménez, Fabra, Talens, & Chiralt, 2012). To evaluate the solubilization capacity of our starch–glycerol and starch–ethylene glycol plasticized biofilms, we used the methodology described by Bertuzzi and coworkers (2007) (Bertuzzi et al., 2007). The solubilization capacity of the biofilms was tested in triplicate; the average values are presented in Table 5.
Table 5. Water solubility of cassava starch biofilms containing glycerol and ethylene glycol.
Biofilms Initial mass (g) Final mass (g) Solubilized material (% ) Average (%)
Starch - glycerol
0.0864 0.0631 26.9676
27.2617
0.0898 0.0652 27.3942
0.0846 0.0614 27.4232
Starch - ethylene glycol
0.0417 0.0386 7.4341
8.0883
0.0455 0.0411 9.6703
0.0405 0.0376 7.1605
The addition of different polyols to starch modified the biofilm solubility. The starch–glycerol biofilm had an average solubility value three times higher than that of the starch–ethylene glycol biofilm.
The solubilization capacity of the biofilm is closely related to the molecular properties of the polyols used as plasticizers. Glycerol has three hydroxyls in its molecular structure, while ethylene glycol has only two. The different number of hydroxyl groups on these molecules influences the intermolecular hydrogen connections between the biofilm polyol and water. Since glycerol has more hydroxyl groups, it makes more connections with water molecules and has a higher solubility. Glycerol can also increase the biofilm intrachain space, allowing more water molecules to access to the biofilm internal structure, and the material is more hydrophilic (Laohakunjit & Noomhorm, 2004). However, the solubility of the biofilm is much less when ethylene glycol is used as a plasticizer, and this film has more crystalline characteristics.
Braz. J. of Develop.,Curitiba, v. 6, n. 7, p. 50417-50442 jul. 2020. ISSN 2525-8761 3.2.6 Thermogravimetry
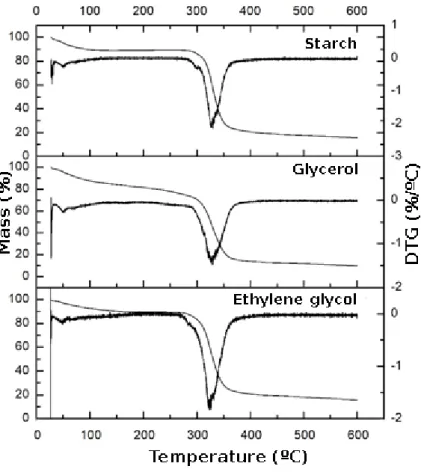
The mass loss with increased temperature was evaluated to determine the temperature the biofilms can withstand. The thermograms obtained for cassava starch and the plasticized biofilms have a similar thermal profile, with mass loss occurring in two stages (Figure 10).
Figure 10. Thermograms of (A) cassava starch and biofilms produced with (B) glycerol and (C) ethylene glycol.
The first stage (A) of mass loss is caused by dehydration of the sample. The second stage (B) is caused by thermal decomposition of organic matter.
The water loss in Stage A occurs slowly, starting at approximately 30 °C for the cassava starch and the biofilms. More mass was lost in Stage A by the starch–glycerol biofilm, indicating that this material initially had more moisture.
The final degradation temperatures in Stages A and B were higher for the biofilms than for the starch sample, suggesting that the organization of the biofilm polymer matrix was strengthened by the hydrogen bonds between the plasticizers, water, and starch.
The decomposition in Stage B started around 306 °C for all biofilms, indicating that the materials are highly resistant to thermal decomposition.
Braz. J. of Develop.,Curitiba, v. 6, n. 7, p. 50417-50442 jul. 2020. ISSN 2525-8761
The residual masses at 600 °C of starch, the starch–glycerol biofilm, and the starch–ethylene glycol biofilm were 15.72%, 9.77%, and 15.70%, respectively.
Table 6. Beginning, peak, and ending temperatures of the mass loss stages of the starch and biofilm samples.
Internship Sample Initial temperature
(°C) Final temperature (°C) Weight loss (%) A Starch 32.84 72.23 10.76 Starch - glycerol 35.52 90.32 17.851 Starch - ethylene glycol 34.33 98.98 10.215 B Starch 313.8 346.88 68.39 Starch - glycerol 305.98 352.92 69.01 Starch - ethylene glycol 307 347.62 70.28 3.2.7 FTIR Spectroscopy
FTIR analysis was used to investigate the structural changes in starch after biofilm formation. The spectral peaks of the biofilms are similar to that of starch, suggesting that the gelatinization process and biofilm formation do not involve modification of the chemical structure of cassava starch (Figure 11).
Braz. J. of Develop.,Curitiba, v. 6, n. 7, p. 50417-50442 jul. 2020. ISSN 2525-8761
The spectra show slight differences in peak intensity and width (Figure 11). These differences are related to the different polyols used as plasticizers in the biofilm formation.
The absorption band at approximately 3290 cm-1, which is related to the OH group stretching vibrations of starch, water, and polyols, is the most intense band in the starch–glycerol biofilm. The absorption bands at 2889 and 2928 cm-1, which are related to stretching of the CH group, are also more intense in the starch–glycerol biofilm because there are more CH groups present in the glycerol molecule.
The region from 900 to 1200 cm-1 (Figure 12) is sensitive to the conformation of polysaccharides in aqueous solution, and the absorption bands here are due to the stretching of the COC and C-OH groups present in the glycosidic ring. In general, the peaks are more intense in this region for the starch–glycerol biofilm, suggesting that this biofilm has more amorphous regions in its polymer matrix.
Figure 12. FTIR spectrum of starch (black), ethylene glycol (blue) and glycerol (red) in the region of 900 to 1200 cm-1.
3.3 XRD OF STARCH AND BIOFILMS
Figure 13 shows the X-ray diffractograms of starch, and the starch–glycerol, and starch– ethylene glycol biofilms. The diffractograms showed residual crystallinity because these biofilms are not completely amorphous. The starch diffractogram presents diffraction peaks around 2θ = 15, 18, and 23º with intensity of 7500, 8000, and 6500, respectively. The starch–glycerol plasticized biofilm has diffraction peaks around 2θ = 18, 20, and 22º with an intensity of 6000, 5750, and 5000,
Braz. J. of Develop.,Curitiba, v. 6, n. 7, p. 50417-50442 jul. 2020. ISSN 2525-8761
respectively, while the starch–ethylene glycol plasticized biofilm presents two crystallization peaks at 2θ = 20 and 25º with an intensity of 5500 and 5750, respectively.
Figure 13. X-ray diffractograms for in natura cassava starch, starch–glycerol biofilms, and starch–ethylene-glycol biofilms.
According to the starch diffraction peaks, starch can be classified as a type B standard, mainly by the presence of intense 2 peaks, represented by a singlet at 15º and a doublet at 17º (Rindlava et al., 1997).
Starch had a crystallinity index of 36.33%, while the starch–glycerol and starch–ethylene glycol biofilms had crystallinity indices of 20.83% and 87.50%, respectively. The different crystallinity indices indicate that the starch–ethylene glycol biofilm has a more defined crystal structure. The starch–glycerol biofilm had peaks close to those of in natura starch with lower crystallinity, while the starch–ethylene glycol biofilm had a single peak, with high crystallinity compared to starch and the starch–glycerol biofilm.
The diffractograms of the biofilms showed less amorphous characteristics than the in natura starch, with greater crystal structure in the starch–ethylene glycol biofilm. The crystalline structure of biofilms occurs due to the recrystallization of amylopectin and crystallization of amylose (amylose does not present crystallinity in its native state).
4 CONCLUSION
The physical characteristics, such as rigidity and plasticity, of the aqueous starch suspensions can be identified by measuring changes in viscosity and density with temperature. Starch–glycerol and starch–ethylene glycol suspensions formed biofilms with distinct physical characteristics, with
Braz. J. of Develop.,Curitiba, v. 6, n. 7, p. 50417-50442 jul. 2020. ISSN 2525-8761
differences that were related to the plasticizers used. Biofilms produced from the starch–glycerol suspension were more plastic, malleable, resistant, uniform, and roughened, whereas starch– ethylene glycol biofilms were more inflexible, brittle, and glassy. Starch–glycerol biofilms are more plastic and malleable because glycerol interacts more effectively with the amylose and amylopectin in starch. Glycerol has more hydroxyl groups than ethylene glycol, so it forms more hydrogen bonds with the starch molecules.
The SEM micrograph of cassava starch revealed round, oval, and polyhedral granules, and the analysis of the biofilm microstructure revealed a homogeneous surface, which indicates that there is good interaction between starch and polyols. Furthermore, the addition of different plasticizers to starch modifies the internal structure of the polymeric matrix, indicated by the surface cracks in the starch–ethylene glycol biofilm. The starch and biofilms had similar bands in the FTIR spectra, indicating that gelatinization and formation of biofilms does not alter the chemical structure of cassava starch. The XRD patterns of biofilms show residual crystallinity, indicating that the materials are not completely amorphous. The starch–glycerol biofilm has XRD peaks with similar intensity to the in natura starch.
The thermograms for starch and biofilms reveal a similar thermal profile. The final degradation temperatures were high for all biofilms, indicating that the structural organization of the polymeric matrix is strengthened by the hydrogen bonds between plasticizers, water, and starch.
CONFLICT OF INTEREST
The authors declare that there are no conflicts of interest regarding the publication of this paper.
ACKNOWLEDGMENTS
The authors appreciate the support of the National Council for Scientific and Technological Development (CNPq) and Coordination of Superior Level Staff Improvement (CAPES).
Braz. J. of Develop.,Curitiba, v. 6, n. 7, p. 50417-50442 jul. 2020. ISSN 2525-8761 REFERENCES
Ai, Y., & Jane, J. L. (2015, March 1). Gelatinization and rheological properties of starch. Starch/Staerke, Vol. 67, pp. 213–224. https://doi.org/10.1002/star.201400201
ASTM International. (2000). ASTM D3505-96, Standard Test Method for Density or Relative Density of Pure Liquid Chemicals. https://doi.org/10.1520/D3505-96
ASTM International. (2004). ASTM D7042-04, Standard Test Method for Dynamic Viscosity and Density of Liquids by Stabinger Viscometer (and the Calculation of Kinematic Viscosity). https://doi.org/10.1520/D7042-04
ASTM International. (2006). ASTM D445-06, Standard Test Method for Kinematic Viscosity of
Transparent and Opaque Liquids (and Calculation of Dynamic Viscosity).
https://doi.org/10.1520/D0445-06
Baker, A. A., Miles, M. J., & Helbert, W. (2001). Internal structure of the starch granule revealed by AFM. Carbohydrate Research, 330(2), 249–256. https://doi.org/10.1016/S0008-6215(00)00275-5
Bertuzzi, M. A., Castro Vidaurre, E. F., Armada, M., & Gottifredi, J. C. (2007). Water vapor permeability of edible starch based films. Journal of Food Engineering, 80(3), 972–978. https://doi.org/10.1016/j.jfoodeng.2006.07.016
Bligh, E. G., & Dyer, W. J. (1959). A rapid method of total lipid extraction and purification. Canadian Journal of Biochemistry and Physiology, 37(8), 911–917. https://doi.org/10.1139/o59-099
Bonilla, J., Atarés, L., Vargas, M., & Chiralt, A. (2013). Properties of wheat starch film-forming dispersions and films as affected by chitosan addition. Journal of Food Engineering, 114(3), 303– 312. https://doi.org/10.1016/j.jfoodeng.2012.08.005
Cazón, P., Velazquez, G., Ramírez, J. A., & Vázquez, M. (2017). Polysaccharide-based films and coatings for food packaging: A review. Food Hydrocolloids, 68, 136–148. https://doi.org/10.1016/j.foodhyd.2016.09.009
Chen, Q., Yu, H., Wang, L., Ul Abdin, Z., Chen, Y., Wang, J., … Chen, X. (2015). Recent progress in chemical modification of starch and its applications. RSC Advances, 5(83), 67459–67474. https://doi.org/10.1039/c5ra10849g
Delcour, J. A., Bruneel, C., Derde, L. J., Gomand, S. V., Pareyt, B., Putseys, J. A., … Lamberts, L. (2010). Fate of Starch in Food Processing: From Raw Materials to Final Food Products. Annual
Review of Food Science and Technology, 1(1), 87–111.
https://doi.org/10.1146/annurev.food.102308.124211
Famá, L., Flores, S. K., Gerschenson, L., & Goyanes, S. (2006). Physical characterization of cassava starch biofilms with special reference to dynamic mechanical properties at low temperatures. Carbohydrate Polymers, 66(1), 8–15. https://doi.org/10.1016/j.carbpol.2006.02.016
Braz. J. of Develop.,Curitiba, v. 6, n. 7, p. 50417-50442 jul. 2020. ISSN 2525-8761
Fonseca-Florido, H. A., Méndez-Montealvo, G., Velázquez de la Cruz, G., Rodríguez-García, M. E., Bello-Pérez, L. A., Hernández-Hernández, E., & Gómez-Aldapa, C. A. (2019). Physicochemical characteristics of stored gels from starch blends. LWT, 114, 108408. https://doi.org/10.1016/j.lwt.2019.108408
Franco, C. M. L., Wong, K. S., Yoo, S. H., & Jane, J. L. (2002). Structural and functional characteristics of selected soft wheat starches. Cereal Chemistry, 79(2), 243–248. https://doi.org/10.1094/CCHEM.2002.79.2.243
Gillman, M., & Erenler, H. (2009). The genetic diversity and cultural importance of cassava and its contribution to tropical forest sustainability. Journal of Integrative Environmental Sciences, 6(3), 189–200. https://doi.org/10.1080/19438150903090509
Hamad, S. H. (2012). Factors Affecting the Growth of Microorganisms in Food. In Progress in Food Preservation (pp. 405–427). https://doi.org/10.1002/9781119962045.ch20
Horwitz, W., & Association of Official Analytical Chemists. (1970). Official methods of analysis of the Association of Official Analytical Chemists. The Association.
Jiménez, A., Fabra, M. J., Talens, P., & Chiralt, A. (2012, August). Edible and Biodegradable Starch Films: A Review. Food and Bioprocess Technology, Vol. 5, pp. 2058–2076. https://doi.org/10.1007/s11947-012-0835-4
Kechichian, V., Ditchfield, C., Veiga-Santos, P., & Tadini, C. C. (2010). Natural antimicrobial ingredients incorporated in biodegradable films based on cassava starch. LWT - Food Science and Technology, 43(7), 1088–1094. https://doi.org/10.1016/j.lwt.2010.02.014
Laohakunjit, N., & Noomhorm, A. (2004). Effect of plasticizers on mechanical and barrier
properties of rice starch film. Starch/Staerke, 56(8), 348–356.
https://doi.org/10.1002/star.200300249
Luchese, C. L., Spada, J. C., & Tessaro, I. C. (2017). Starch content affects physicochemical properties of corn and cassava starch-based films. Industrial Crops and Products, 109, 619–626. https://doi.org/10.1016/j.indcrop.2017.09.020
Maria Martelli, S., Moore, G., Silva Paes, S., Gandolfo, C., & Laurindo, J. B. (2006). Influence of plasticizers on the water sorption isotherms and water vapor permeability of chicken feather keratin
films. LWT - Food Science and Technology, 39(3), 292–301.
https://doi.org/10.1016/j.lwt.2004.12.014
Martelli, S. M., Moore, G. R. P., & Laurindo, J. B. (2006). Mechanical properties, water vapor permeability and water affinity of feather keratin films plasticized with sorbitol. Journal of Polymers and the Environment, 14(3), 215–222. https://doi.org/10.1007/s10924-006-0017-4
Miladinov, V. D. (2001). Temperatures and ethanol effects on the properties of extruded modified starch. Industrial Crops and Products, 13(1), 21–28. https://doi.org/10.1016/S0926-6690(00)00048-0
Braz. J. of Develop.,Curitiba, v. 6, n. 7, p. 50417-50442 jul. 2020. ISSN 2525-8761
crystallinity. Carbohydrate Polymers, 34(1–2), 25–30. https://doi.org/10.1016/S0144-8617(97)00093-3
Rocha Plácido Moore, G., Maria Martelli, S., Gandolfo, C., José do Amaral Sobral, P., & Borges Laurindo, J. (2006). Influence of the glycerol concentration on some physical properties of feather keratin films. Food Hydrocolloids, 20(7), 975–982. https://doi.org/10.1016/j.foodhyd.2005.11.001 Shah, U., Naqash, F., Gani, A., & Masoodi, F. A. (2016). Art and Science behind Modified Starch Edible Films and Coatings: A Review. Comprehensive Reviews in Food Science and Food Safety, 15(3), 568–580. https://doi.org/10.1111/1541-4337.12197
Smith, A. M. (2010). Starch and Starch Granules. In Encyclopedia of Life Sciences. https://doi.org/10.1002/9780470015902.a0001294.pub2
Souza, A. C., Goto, G. E. O., Mainardi, J. A., Coelho, A. C. V., & Tadini, C. C. (2013). Cassava starch composite films incorporated with cinnamon essential oil: Antimicrobial activity, microstructure, mechanical and barrier properties. LWT - Food Science and Technology, 54(2), 346– 352. https://doi.org/10.1016/j.lwt.2013.06.017
Sriroth, K., Santisopasri, V., Petchalanuwat, C., Kurotjanawong, K., Piyachomkwan, K., & Oates, C. G. (1999). Cassava starch granule structure-function properties: Influence of time and conditions at harvest on four cultivars of cassava starch. Carbohydrate Polymers, 38(2), 161–170. https://doi.org/10.1016/S0144-8617(98)00117-9
The future of plastic. (2018, June). Nature Communications, Vol. 9, p. 2157. https://doi.org/10.1038/s41467-018-04565-2
van den Berg, C., & Bruin, S. (1981). Water activity and its estimation in food systems: theoretical aspects. In Water Activity: Influences on Food Quality (pp. 1–61). https://doi.org/10.1016/b978-0-12-591350-8.50007-3
Zavareze, E. da R., Halal, S. L. M. El, Marques e Silva, R., Dias, A. R. G., & Prentice-Hernández, C. (2014). Mechanical, barrier and morphological properties of biodegradable films based on muscle and waste proteins from the whitemouth croaker (Micropogonias Furnieri). Journal of Food Processing and Preservation, 38(4), 1973–1981. https://doi.org/10.1111/jfpp.12173
Zhang, Y., Zhang, Y., Li, B., Xu, F., Zhu, K., Tan, L., … Li, S. (2019). Retrogradation behavior of amylopectin extracted different jackfruit cultivars seeds in presence on the same amylose. LWT, 114, 108366. https://doi.org/10.1016/j.lwt.2019.108366
Braz. J. of Develop.,Curitiba, v. 6, n. 7, p. 50417-50442 jul. 2020. ISSN 2525-8761 FIGURE CAPTIONS
Figure 1. Starch micrograph obtained by SEM, at 120× and 1500× magnification. Figure 2. Starch and water solution with polyol addition.
Figure 3. Suspensions of starch–glycerol (right) and starch–ethylene glycol (left).
Figure 4. Viscosity of aqueous suspensions of starch containing glycerol or ethylene glycol with changes in temperature.
Figure 5. Linear relationship of the densities of (A) starch–glycerol suspensions and (B) ethylene glycol starch as a function of temperature.
Figure 6. Density variation as a function of temperature for (A) starch–glycerol and (B) starch– ethylene glycol suspensions.
Figure 7. Biofilms produced from filmogenic suspensions: (A) starch–glycerol biofilm and (B) starch–ethylene glycol biofilm.
Figure 8. Micrographs of plasticized starch biofilms (A and B) with glycerol and (C and D) ethylene glycol.
Figure 9. Effective decomposition of thickness mean hypotheses.
Figure 10. Thermograms of (A) cassava starch and biofilms produced with (B) glycerol and (C) ethylene glycol.
Figure 11. Infrared spectra of cassava starch and starch–glycerol and starch–ethylene glycol plasticized biofilms.
Figure 12. FTIR spectrum of starch (black), ethylene glycol (blue) and glycerol (red) in the region of 900 to 1200 cm-1.
Figure 13. X-ray diffractograms for in natura cassava starch, starch–glycerol biofilms, and starch– ethylene-glycol biofilms.
Braz. J. of Develop.,Curitiba, v. 6, n. 7, p. 50417-50442 jul. 2020. ISSN 2525-8761 TABLE CAPTIONS
Table 1. Viscosity and density of aqueous suspensions of starch containing glycerol or ethylene glycol.
Table 2. Density of aqueous suspension starch–glycerol and starch–ethylene glycol.
Table 3. Thickness and standard deviation measured in triplicate for each of the starch–glycerol and starch–ethylene glycol biofilms.
Table 4. Water activity of cassava starch, starch–glycerol biofilms, and starch–ethylene glycol biofilms.
Table 5. Water solubility of cassava starch biofilms containing glycerol and ethylene glycol. Table 6. Beginning, peak, and ending temperatures of the mass loss stages of the starch and biofilm samples.