Detection of N. meningitidis
Group B
Antigens by MB-Dot-ELISA
in Patients
with Meningitis1
MARIA DAS GRA~AS ADELINO
ALKMIN,
ILKA MARIA LANDGRAF,~ & SUMIE HOSHINO
SHIMIZU~Infection with Neisseria meningitidis group B has been dt$f?cult to detect, partly because this bacterial group’s polysaccharide is a weak immunogen. This article describes work carried out to test a new procedure (MB-Dot-ELlSA) employing a high-titered horse antiserum for detec- tion of N. meningitidis group B antigens. The study assayed cerebrospinalfluid samplesfrom 585 subjects, 574 with suspected meningitis cases and 11 with neurologic disorders. The re- sults of the assay indicated a sensitivity of 0.991 and a specificity of 0.826. These results were superior to those obtained with latex agglutination and in substantial agreement with the results of counterimmunoelectrophoresis and bacteriologic methods. Overall, the MB-Dot-ELISA was found to be sensitive, inexpensive, and suitable for public health laboratory investigations.
E
pidemic outbreaks of cerebrospinal meningitis caused by AJeisseriu menin- gitidis group B have occurred in the city of STio Paulo, Brazil, since 1988. Recent data (2) suggest that the incidence of the disease is decreasing somewhat, only 9.56 cases per 1000 000 residents being reported in 1993 as compared to 14.25 in 1990.Detection of N. meningitidis group B an- tigens in patient cerebrospinal fluid (CSF) involves some difficulties. These arise from lack of a good group-specific antiserum, which in turn is due to the low immunoge- nicity of the group’s bacterial polysaccha- ride (2, 3). Overall, data available on this subject are scarce.
A dot-immunobinding assay (DIA) us- ing a monoclonal antibody to detect group B meningococcal polysaccharide antigens in CSF and urine from infected patients has
been described (2). While apparently more sensitive than counterimmunoelectro- phoresis (CIE), latex agglutination (LA), or enzyme-linked immunosorbent assay (ELISA), its positivity did not exceed 67%. Regarding polyclonal antisera to N. menin- gitidis group B, no references were found in the literature.
In attempting to improve the tools avail- able for detecting this meningitis agent, we previously produced a high-titered poly- clonal antiserum to N. meningitidis group B in horses (4) that is being used routinely in diagnosis by CIE. The work reported here standardized and evaluated another test, the meningococcus B dot-ELISA (MB-Dot- ELISA), and compared the results yielded by it with those of microscopy, culture methods, and other immunologic tech- niques such as CIE and LA.
Imunologia, Institute Adolf0 Lutz, Av. Dr. Amaldo, r This article will also be publishedin Spanish in the 355,01246-902, S%o Paula, SF’, Brazil.
Revisfa Panamericana de Salud Ptiblica/Pan American *Immunology Section, Adolf0 Lutz Institute, Sao Journal ofPublic Health, Vol. 1,1997. Reprint requests Paulo, SE’, Brazil.
and other correspondence should be addressed 3 Bacteriology Section, Adolf0 Lutz Institute, SPo to Maria das Gracas Adelino Alkmin, Se+o de Paulo, Sl?, Brazil.
MATERIALS
AND METHODS
Cerebrospinal
Fluid (CSF)
A total of 585 CSF samples were ana- lyzed. Of these, 574 had been sent to the Adolf0 Lutz Institute for immunobac- teriologic diagnosis after being collected from patients hospitalized at the Instittlto de Infectologia Emilio Ribas with suspected meningitis cases. The 11 other CSF samples were collected at the polyclinic of the SBo Paul0 University Medical School from pa- tients with neurologic disorders.
Within the group of 574 samples, 113 were from patients infected with N. menin- gitidis group B. Another 287 yielded posi- tive results when examined by microscopy (Gram staining), culture, or other bacterio- logic methods (5, 6) for other etiologic agents; while the remaining 174 yielded negative results. (The 11 samples from the Sao Paula University Hospital all yielded negative results.) All of the CSF samples were processed within 24 to 48 hours by bacteriologic methods and CIE and were then stored at temperatures below -20 “C for about a year for use in other immuno- logic tests.
Conventional
Immunologic
Techniques
The CIE was carried out as described previously (7). The LA test employed a commercial reagent kit (Biolab Diagnostic0 SA, biohlerieux 69260 Charbonnieres les Bains, France) kindly donated by Dr. Anto- nio Walter Ferreira of the Institute of Tropi- cal Medicine of Sao Paulo.
MB-Dot-ELISA
The MB-Dot-ELISA was standardized as follows: Areas of 0.5 cm X 0.5 cm were out- lined on 1.0 cm X 11.0 cm nitrocellulose
strips using a waterproof pen. A 1.0 PL CSF sample was then dotted onto the center of
each square. Positive controls consisted of N. meningitidis group B polysaccharide an- tigen (8) and a CSF sample from a patient infected with this agent. Negative controls consisted of CSF samples from patients with unrelated infections. After drying the strips at room temperature, the membrane was blocked with 3% gelatin in phosphate- buffered saline (PBS) at pH 7.4 for 30 min- utes at room temperature and was washed three times in distilled water. Each strip was then incubated with 2.5 mL of horse anti- serum to N. meningi~idis group B (5) diluted 1:600 in PBS containing 1% gelatin (PBS-G), for 2 hours at room temperature, using a rocking platform shaker. After that the strips were washed twice in PBS contain- ing 0.05% Tween 20 and were incubated for 2 hours with 2.5 mL antihorse IgG peroxi- dase conjugate (Sigma Chemical Company, U.S.A.) diluted 1:500 in PBS-G. Following two more cycles of washing with distilled water and PBS, the strips were incubated with a chromogenic solution (3-3’ diarnino- benzidine-tetrahydrochloride and H,O,) for 5 minutes. The strips were then washed three times in distilled water. A positive reaction was recorded when a colored dot was seen against the white filter-paper background, while a negative result was recorded if no stained dot was observed.
Antiserum absorption with antigens of heterologous bacteria was developed as de- scribed by Alkmin et al. (4). To evaluate interassay reproducibility, 50 CSF samples were tested by MB-Dot-ELISA on different days. To evaluate intra-assay reproducibil- ity, each of 10 negative and 10 positive CSF samples was processed five times.
Statistical Analysis
to an association of the three using the fol- lowing criteria: All the CSF samples positive The diagnostic features of the MB-Dot-ELISA technique were evaluated in terms
by culture alone or in combination with one or both of the other two tests were consid- of sensitivity, specificity, positive and nega- ered true positives. Also, all the samples posi- tive predictive values (including 95% con-
fidence intervals), and kappa (K) index
tive by both microscopy and CIE were con- sidered true positives. However, all samples
agreement (9-22). testing positively only by microscopy or only
by CIE were considered negative. When these
RESULTS
criteria were applied, the sensitivity and specificity of the MB-Dot-ELISA improved to The results obtained with the MB-Dot- 0.991 and 0.826, respectively.ELISA are shown in Table 1. As may be seen, The degree of agreement between the 112 of the 113 proven group B meningococ- MB-Dot-ELISA and other diagnostic tech- cus cases yielded positive MB-Dot-ELISA niques, either individually or in combina- results (indicating a sensitivity of 0.991). tion, was determined in terms of the kappa When 39 samples, including 30 from these index (K), as shown in Table 3. All of the 113 cases and 9 from cases caused by other comparisons yielded significant K values, infectious meningitis agents, were tested by since the resulting Z values ranged from LA and MB-Dot-ELISA, they yielded over- 4.155 to 7.101, considerably exceeding the all positivity rates of 66.7% by LA and 71.8% critical Z value (1.96) for the 0.05 confidence by MB-Dot-ELISA. In terms of true posi- level. In each case the agreement between tivity the two tests yielded rates of 89.3% the MB-Dot-ELISA results and the bacterio- and lOO%, respectively. logic (microscopy or culture) or CIE results
Out of 98 CSF samples that were posi- was considered substantial.
tive by microscopy for Gram-negative The interassay and intra-assay reproduc- diplococci but were negative by culture and ibility testing by MB-Dot-ELISA yielded CIE, 83 (84.7%) yielded positive results results in complete agreement with the ini- when tested by MB-Dot-ELISA (see Table 1). tial tests. Finally, regarding the stability of There were also 33 cases (17.8%) of cross- the nitrocellulose membranes, it was found reactivity with unrelated bacteria. In the that after dotting with CSF samples the ni- group of 185 negative CSF samples, 38 trocellulose strips could be stored for 30 (20.5%) yielded positive results by MB-Dot- days at 37 “C or for 6 months at room tem-
ELISA. perature without loss of reactivity.
These data provided a basis for evaluat-
ing the diagnostic performance of the MB- DISCUSSION AND Dot-ELISA and other assays. As Table 2 CONCLTJSIONS shows, the diagnostic performance of the
MB-Dot-ELISAm relation to microscopy, cul- In the diagnosis of group B meningococ- turn,, and CIE was similar to these procedures, cal infections, the immunologic assays de- since the values of 95% confidence intervals signed for the rapid detection of bacterial determined for each technique overlapped, antigens in patient biologic fluids play a indicating that the differences between them relevant role in conjunction with bacterio- were not statistically significant. logic techniques, contributing to the insti- The Table 2 data compare the MB-Dot- tution of appropriate chemotherapy and ELISA to the other three tests individually. other epidemic control measures.
Since these tests tend to have low sensitiv- In this study, MB-Dot-ELISA using ity, the MB-Dot-ELISA was then compared polyclonal antiserum was standardized
Table 1. Positive and negative MB-Dot-ELISA results obtained with 400 cerebrospinal fluid samples from patients infected with Neisseria meningitidis group B or other disease agents and 185 samples from patients with no detected disease agents.
Infectious agents revealed by microscopy, culture, and/or counterimmuno- electrophoresis
Results of MB-Dot-ELISA with cerebrospinal fluid (CSF) Total No. No.
No. positive negative
Positive tests:
Neisseria meningitidis
group B
Gram-negative diplococci Other microorganisms:
Neisseria meningitidis
group A
Neisseria meningitidis
group C
Neisseria meningitidis
group WI 35
Neisseria meningitidis
group Y
Haemophilus influenzae type b
Streptococcus pneumoniae Streptococcus sp.
Staphylococcus aureus Escherichia co/i Klebsiella sp.
Proteus sp.
Listeria monocytogenes
Gram-positive diplococci Gram-positive cocci Gram-negative rods Gram-positive rods Yeast
Positive subtotal
Negative tests: Negative subtotal Total
113 112 1
98 83 15
4
11 3 8
11 5 6
2
80 a 72
16 3 13
4 1 3
13 2 11
1 0 1
1 0 1
2 1 1
2 0 2
21 3 18
3 1 2
12 2 10
2 1 1
4 0 4
400 228 172
185 585
1
2
38 266
3
0
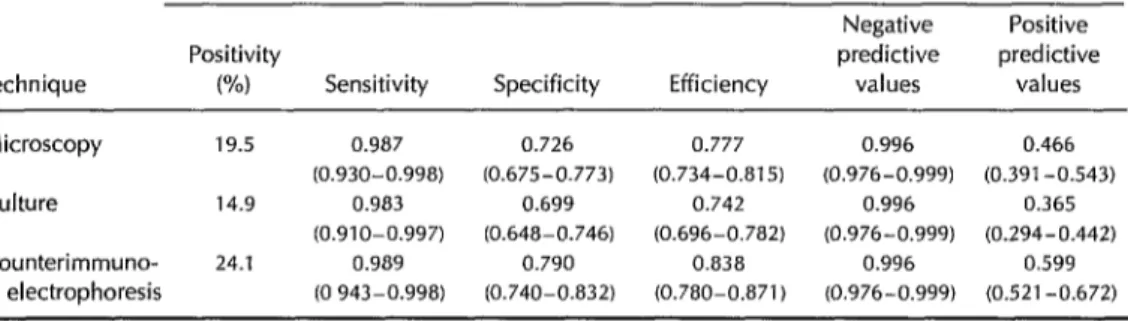
Table 2. Diagnostic performance of MB-Dot-ELISA relative to microscopy, culture, and
counterimmunoelectrophoresis techniques (13) in the study of 585 cerebrospinal fluid samples. Sensitivity = true positives detected/(true positives + false negatives); specificity = true negatives detected/(true negatives + false positives); efficiency = (true positives detected + true negatives detected)/total number tested; predictive value of positives = true positives detected/(true positives + false positives); and predictive value of negatives = true negatives detected/(true negatives + false negatives). In each case the 95% confidence interval is shown in parentheses.
MB-Dot-ELISA (95% confidence intervals in parentheses)
Negative Positive
Positivity predictive predictive
Technique W) Sensitivity Specificity Efficiency values values Microscopy 19.5 0.987 0.726 0.777 0.996 0.466
(0.930-0.998) (0.675-0.773) (0.734-0.815) (0.976-0.999) (0.391-0.543)
Culture 14.9 0.983 0.699 0.742 0.996 0.365 (0.910-0.997) (0.648-0.746) (0.696-0.782) (0.976-0.999) (0.294-0.442)
Counterimmuno- 24.1 0.989 0.790 0.838 0.996 0.599
electrophoresis (0 943-0.998) (0.740-0.832) (0.780-0.871) (0.976-0.999) (0.521-0.672)
and evaluated for the detection of N. meningitidis group B antigen in CSF samples from patients with meningitis.
Coll et al. (2), using monoclonal antibod- ies, reported a 67% positivity by DIA when studying bacteriologically confirmed group B meningococcus meningitis cases. In this report no positive reactions were detected in the 13 CSF samples from meningitis cases with other etiologic agents, indicating a specificity of 100%.
In contrast, the tests reported here using the MB-Dot-ELISA and polyclonal antibod- ies detected nearly all (99.1%) of the group B infections. Some cross-reactivity was ob- served, but the specificity was improved by additional absorptions of horse antiserum with individual types of bacteria or a mix- ture of different cross-reactive bacteria, leading to a specificity of 0.826. It is worth noting that 83 (84.7%) of the CSF samples yielding results positive only by micros-
Table 3. Degree of kappa (K) index agreement between the MB-Dot-ELISA and other bacteriologic and immunologic techniques for the diagnosis of Neisseria meningitidis group B infection.
MB-Dot-ELISA
Other techniques
Kappa* index
(K)
Strength
of K zo+ Culture 0.636 Substantial 4.155 Microscopy
Counterimmuno- electrophoresis
0.669 Substantial 5.494 0.741 Substantial 7.101
*All the K values are statistically significant.
‘Observed 2 (critical 2 = 1.96 for 0.05 confidence level). See references 70- 12.
copy for Gram-negative diplococci tested positive for N. meningitidis group B by MB- Dot-ELISA.
Besides being highly sensitive, the MB- Dot-ELISA was found to have some advan- tages over CIE: namely, the amounts of an- tigen and antiserum needed were greatly reduced; there was no need for special
equipment; and it was easy to send the dot-
ted strips by regular mail to distant labora- tories for processing. Overall, the MB-Dot- ELISA employed was found to be sensitive,
inexpensive, and suitable for public health laboratory investigations.
REFERENCES
1.
2.
3.
4.
5.
Panachio MRI, Kallas EG, Barbosa HA, et al. Group B meningococcal disease in SBo Paula, Brazil: an epidemiological and mi- crobiological study In: Evans JS, Yost SE, Maiden MCJ, Feavers IM, eds. Neisseria 94: proceedings of the Ninth Internafional Patho- genic Neisseria Conference. Winchester, En- gland: Guidhall; 1994:395.
Coil I?, Borche L, Ausina V, Mirelis B, Prats G. Dot-immunobinding assay with a mono- clonal antibody for detection of Group B meningococcal antigen. Eur J Clin Microbial 1986;5:44-46.
Ghanassia JE Slim A, Bergogne-Berezin E, Modai J. Failure of diagnosing group B men- ingococcal meningitis by immunoelectro- phoresis. Stand ] Infect Dis 1977;9:313-314. Alkmin MGA, Ho&no-Shimizu S, Landgraf IM, et al. Production and immunochemical characterization of Neisseria meningifidis group B antiserum for the diagnosis of pu- rulent meningitis. Braz J Med Biol Res 1994;27:16-34.
6.
7.
8.
9.
10.
11.
12.
13.
clinical microbiology. 5th ed. Washington, DC: American Society for Microbiology; 1991. Bras& Divisao National de Laboratorios de Sadde Pdblica. Normas fe’cnicas para o diagnostico das meningites bacferianas. Brasilia: Centro de Documentacao do Ministerio da Sadde; 1986:49p. (Serie A, normas e manuais tecnicos, 32).
Palhares M, Gelli DS, Abneida MCR, et al. Pesquisa de polissacarideos de Neisseria meningifidis do grupo C no liquid0 cefa- lorraquidiano por imunoeletroforese cruz- ada em acetato de celulose. Rev lnsf Adolf0 Lufz 1984;33:85-89.
Gotschlich EC, Liu TY,Amstein MS. Human immunity to the meningococcus: III, prepa- ration and immunochemical properties of the group A, group B, and group C menin- gococcal polysaccharides. J Exp Med 1969; 129:1349-1365.
Galen RJ, Gambino JR. Beyond normality: the predictive value and eficiency of medical diag- nosis. New York: John Wiley; 1975:235. Eleiss JL. Statistical methods for rates and pro- portions. New York: Wiley; 1981:217-225. Maclure M, Willet WC. Misinterpretation and misuse of the kappa statistic. Am J Epidemiol1987;126:161-169.
Feinstein AR. Clinical epidemiology. In: The architecture of clinical research. Philadelphia: Saunders; 1985:185-186.
Rothman KJ, Boice J. Epidemiologic analysis with programmable calculator. Boston: Epide- miology Resources; 1982.
Manuscript submitted for publication on 6 February 1996. Accepted for publication (following revision) in the Boletin de la Oficina Sanitaria Panamericana on 3 November 1995. Accepted for publication in the
Bullefin of fhe Pan American Health Organization on 14 Balows A, Hausler WJ Jr, Herrmann KL,