w ww.e l s e v i e r . c o m / l o c a t e / b j p
Original
Article
Antibacterial
activity
of
(
−
)-cubebin
isolated
from
Piper
cubeba
and
its
semisynthetic
derivatives
against
microorganisms
that
cause
endodontic
infections
Karen
C.S.
Rezende
a,
Rodrigo
Lucarini
a,
Guilherme
V.
Símaro
a,
Patricia
M.
Pauletti
a,
Ana
H.
Januário
a,
Viviane
R.
Esperandim
a,
Carlos
H.G.
Martins
a,
Mislaine
A.
Silva
a,
Wilson
R.
Cunha
a,
Jairo
K.
Bastos
b,
Márcio
L.A.E.
Silva
b,∗aNúcleodeCiênciasExataseTecnológicas,UniversidadedeFranca,Franca,SP,Brazil
bDepartmentofPharmaceuticalSciences,FaculdadedeCiênciasFarmacêuticasdeRibeirãoPreto,UniversidadedeSãoPaulo,RibeirãoPreto,SP,Brazil
a
r
t
i
c
l
e
i
n
f
o
Articlehistory:
Received2October2015 Accepted15December2015 Availableonline22February2016
Keywords:
Lignans
Pipercubeba
(−)-cubebin
Semisyntheticderivatives Antibacterialactivity
a
b
s
t
r
a
c
t
Recentpublicationshavehighlightedthenumerousbiologicalactivitiesattributedtothelignan(−
)-cubebin(1),PipercubebaL.f.,Piperaceae,andongoingstudieshavefocusedonitsstructuraloptimization, inordertoobtainderivativeswithgreaterpharmacologicalpotential.Theaimofthisstudywasthe obtain-mentof(1),itssemisyntheticderivativesandevaluationofantibacterialactivity.Theextractoftheseeds ofP.cubebawaschromatographed,subjectedtorecrystallizationandwasanalyzedbyHPLCand spectro-metrictechniques.Itwasusedforthesynthesisof:(−)-O-methylcubebin(2),(−)-O-benzylcubebin(3), (−)-O-acetylcubebin(4),(−)-O-(N,N-dimethylamino-ethyl)-cubebin(5),(−)-hinokinin(6)and(−)-6.6′
-dinitrohinokinin(7).Theevaluationoftheantibacterialactivityhasbeendonebybrothmicrodilution techniquefordeterminationoftheminimuminhibitoryconcentrationandtheminimumbactericidal concentrationagainstPorphyromonasgingivalis,Prevotellanigrescens,Actinomycesnaeslundii,Bacteroides fragilisandFusobacteriumnucleatum.Itwaspossibletomakeananalysisregardingtherelationship betweenstructureandantimicrobialactivityofderivativesagainstmicroorganismsthatcause endodon-ticinfections.Themostpromisingwereminimuminhibitoryconcentration=50g/mlagainstP.gingivalis
by(2)and(3),andminimuminhibitoryconcentration=100g/mlagainstB.fragilisby(6).Cytotoxicity
assaysdemonstratedthat(1)anditsderivativesdonotdisplaytoxicity.
©2016SociedadeBrasileiradeFarmacognosia.PublishedbyElsevierEditoraLtda.Allrightsreserved.
Introduction
Inthelastdecade,therehasbeenintensificationinthesearch ofanti-bactericidalcompoundsfromnaturalsources,mainlyfrom plants,whichcontinuetobeamajorsourceofbiologicallyactive metabolitesthatmayprovideleadstructuresforthedevelopment ofnewdrugs(AyresandLoike,1990).
Thebiological propertiesoflignans areclosely relatedtothe stereochemistryofthevariousstereogeniccenterspresentintheir chemical structure, which makes these compounds interesting synthetictargets(Silva etal., 2009).In this context, many bio-logical activities have been reported in the literature (Saleem etal.,2005),suchasantitumor(AyresandLoike,1990;Lietal., 2006),antiviral(Rimandoetal.,1994),anti-PAF(Shenetal.,1985;
∗ Correspondingauthor.
E-mail:mlasilva@unifran.br(M.L.A.E.Silva).
BraquetandGodfroid,1986),antidepressant(Deyamaetal.,2001), anti-inflammatory(Souzaetal.,2004;Kassuyaetal.,2006)and car-diovascular(Rimandoetal.,1994),andantiviral(Charlton,1998; Piccinellietal.,2005),trypanocidal(DeSouzaetal.,2005),analgesic (DaSilvaetal.,2005).
Theliteraturereportstheaccumulationoflignoidsandother metabolitesthat possess significant pharmacologicalactivity in speciesofthegenusPiper,Piperaceaefamily,andespeciallyinP.
cubebaL.f.(Parmaretal.,1997;Arrudaetal.,2005).
Anexampleofthesesubstancesis(−)-cubebin(1),a dibenzyl-butyrolactoliclignanfoundinhighconcentrationsinseedsofPiper
cubeba(Elfahmi, 2006).Duetoitswell-knownbiological
activi-ties,1hasbecometheobjectofstudyofmanyresearchgroups overthelastdecade.Theseworkshaveculminatedinthe discov-eryofnewandimportantbiologicalactionsfor1(DeSouzaetal., 2005;Da Silva etal., 2005;Silva et al.,2007,2009; Usiaetal., 2005;Hirataetal.,2007a,b,2008;Saraivaetal.,2007;Yametal., 2008).
http://dx.doi.org/10.1016/j.bjp.2015.12.006
3 2
1 7
8
6
5 4
H
H O
O
OH
O
1 O
O
9'
1'
2'
3'
4' 5' 6'
8' 7'
Theestablishmentofainflammatoryprocessleadsthemajor forms of endodontic disease, gingivitis and periodontitis. The intensityoftheinflammatory responsevarieswiththestageof evolutionarydevelopmentofinfection,asithasinthebeginnings ofapathologicalprocessaminorreactionthattendstoincrease inproportiontothespreadofmicrobialinvasion,andmaylead totalnecrosisofthepulptissueprovidinganopportunityforthe developmentofsevereapicalperiodontitis(LuisiandFachin,1990). Theoralmicro-organismsarefoundinsalivawithinacomplex matrixcomposedofmicrobialextracellularproductsandsalivary compoundsonthesurfacesofgrowingenamel,knownasbiofilms (Marsh,2005).Thepathogenesisofpulpalinjuryisduetobacterial invasioninthistissue(BergenholtzandCrawford,1989).
Theharmfuleffectofmicroorganismsinthepulpandperiapical tissuesanditsinstallationhasbeenthesubjectofongoing stud-iestoshowtheirpresence,tocorrelatetheassociationbetween thesemicro-organismsandoralinfections(Kakehashietal.,1965; Wittgowand Sabiston,1975;Tani-Ishiiet al.,1994;Fine,2000; Socranskyand Haffajee,2002;De Lilloetal.,2004)anddevelop methodsthatleadtoitsdestructionwiththedevelopmentofnew antimicrobials.
Ideally, in addition to having antimicrobial activity, the endodontic formulation should not harm the host’s tissues (Baumgartner,1997).Inthisregard,theuseofanorabase formu-lationcontaining0.1%fluocinoloneacetonideandtheactivelignin derivativewouldenhancetheeffectivenessagainstoralinfections. However,thereis yetnodrugor substancecapableofbringing togetherthesetworequirements,whichjustifiestheresearchand developmentofnewantimicrobialagents.Additionally,he litera-turereportsthepossibilityofisolationoflignansfromP.cubeba
andotherspeciessuchasChamaecyparisobtusa(Marcotullioetal.,
2014),Linum(Schmidtetal.,2010).However,althoughthese
com-poundscanbeisolatedfromplants,theyareobtainedinlowyields inextractions,alsoresultingindifficultpurificationprocesswith severalsteps.Therefore,partialsynthesisfrom(−)-cubebinisthe mostviableway,withreactionsthatallowyieldsabove98%(Da Silvaetal.,2005)andsimplerpurificationsteps.
Takingaccountthevariousbiologicalpropertiesattributedto lignans, the previous studies obtained by our research group describingsatisfactoryresultsconcerningthesecompounds,this workaimstoexpandknowledgeoftherelationshipbetweenthe chemicalstructuresoflignansandtheirbiologicalactivitiesand thusthesearchforbioactivemolecules.
Materialsandmethods
Solventsandreagents
Anhydroussodiumsulfate (MerckCo.),dry dichloromethane (AcrosCo.),magnesiumsulfate(MerckCo.),pyridinium chlorochro-mate (Acros Co.), hexane (Mallinckrodt Co.), ethyl acetate (MallinckrodtCo.),ethanol (MerckCo.),aceticanhydride(Merck Co.),drytetrahydrofuran(THF)(MerckCo.),sodiumbicarbonate (MerckCo.),sodiumchloride(MerckCo.),drypyridine(AcrosCo.), toluene(MallinckrodtCo.),chloroform(MerckCo.),hexane(Merck
0 10 20 30 40 min
0 100 200
mAU
254nm,4nm (1.00)
21.783
23.426
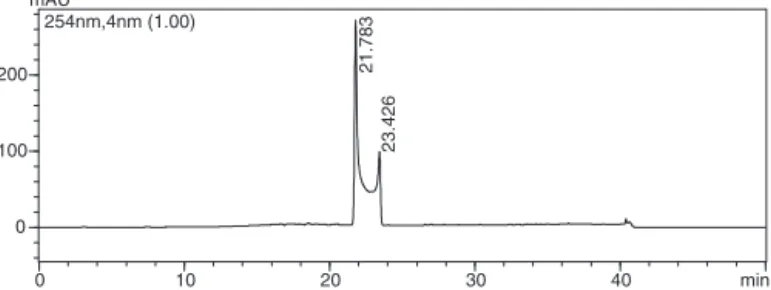
Fig.1. Chromatogramof theepimersof 1obtainedusing a.Shim-pack ODS, 250×4.20mm,5mColumn,volumeofinjection:20l,flow:1ml/min,detection at254nmandlineargradient:MeOH-H2O(50%)/MeOH(100%),in48min.
Co.), sodium hydride (NaH) (Acros Co.), methyl iodide (Merck Co.), dimethylethylaminechloride (MerckCo.), metallic sodium bars(MerckCo.),fumingnitricacid(MerckCo.)andethylacetate (MallinckrodtCo.).
Plantmaterialcollectionandisolationof1
TheseedsofPipercubebaL.f.,Piperaceae,usedinthis study wereimportedfromFloralSeedCompany,Dehradun,India(Proc. FAPESP05/01550-8).TheprocessofseedsofP.cubebaextraction wascarriedoutaccordingtoliterature(Souzaetal.,2004).Itwas used1kgofseeds,whichyieldedthecrudeethanolicextract.The partitionsoriginatedahydroalcoholicextract,285g.A chromato-graphiccolumnchromatographywasundertakenandthefractions werecollectedandcombinedaccordingtotheirchromatographic profiles.
HPLCanalysisoffraction10,obtainedfromthefiltrationcolumn chromatography,showedthepresenceof1.Thefractionwas sub-jectedtothecrystallizationprocess,solubilizingitwithacetone, andaddinghexaneuntilturbidityofthemedium.After crystalliza-tion,thesupernatantwasremoved,thusfurnishingcrystalsof1.
The recrystallization process was repeated five consecutive times, which made it possibleto obtain 13.9g 1. This method allowed excellent purification, which was confirmed by analy-sis using a binary system chromatograph Shimadzu CBM-20A, -ProminenceLC-6ADequippedwithamanualinjectorwith20ml loop,a“degasser”DGU-20A5coupledtoaUV–visdetectormodel SPD-20Aanddataacquisitionbyamicrocomputerandan analyti-calcolumnofShimadzu,Shim-packODS,250mm×4.20mm,5m, equippedwithpre-column.Thesolventsystemconsistedofa mix-tureofmethanolandwaterinalineargradient50–100%in48min. ThechromatographicconditionsdefinedforcompoundHPLC anal-ysiswere:254nm,injectionvolumeof20landflow rateof 1ml/min.
Thesecrystalswereanalyzedusingnuclearmagneticresonance techniquesofhydrogen(1H)andcarbon(13C)andHPLC(Fig.1), confirmingitsidentificationas(−)-cubebin.
Obtainmentofsemi-syntheticderivatives
Scheme1representsasummaryofthereactionsforobtainment ofthederivativesof1.
Obtainingthederivate2(a)
7
6
1
3
2
4
5
Scheme1.Obtainingsemisyntheticderivativesof1:2,where:CH3I,NaH,dryTHF,30min,TAatmN2;3,whereb:benzylbromide,NaH,THF,6h,TA;4,wherec:acetic
anhydride,drypyridine,toluene,dichloromethane;5,whered:dimethylethylaminechloride,sodiumethoxide,refluxfor8h,6wheree:PCC,dryDCM,atmN2,-60C,24h;
7,wheref:HNO36equiv,CHCl3,10◦C,4h.
v/v).ThereactionwasneutralizedwithdiluteHClandsubjected topartitionwithethylacetate(3×30ml).Theorganicfractionwas washedsuccessivelywith5%aqueousNaHCO3(3×20ml)and10% saline(3×20ml),driedwithMgSO4andfiltered.
Obtainingthederivate3(b)
Toobtainthebenzylderivative(Souzaetal.,2004),asolutionof 1(300mg)and10mlofdryTHFwasaddedtoamixtureofNaHand dryTHF(5ml),anditwasallowedunderrefluxfor30min.Benzyl bromide(0.12ml)wasaddedandleftunderthesameconditions for2h.Tocompletethereaction,waterwasslowlyaddedtothe flaskandbecameextractionswithether.
Obtainingthederivative4(c)
Toobtainthederivative4(Souzaetal.,2004),50mgof1and 3mlofaceticanhydridewereaddedto0.8mldrypyridine.After 5hofreactionatroomtemperature,themixturewasplacedinavial containingtolueneandevaporatedunderthereducedpressureto removepyridine.Subsequently,dichloromethanewasaddedand evaporatedunderreducedpressuretoremovetracesoftoluene.
Obtainingthederivate5(d)
To obtain 5 (Souza et al., 2004), 300mg of 1 was added a 10mlof sodiumethoxideand leftit underrefluxfor2h. Later,
itwasadded120mgof dimethylethylaminechloride.The reac-tion wasmaintainedunder thesame conditions for 6h. Water (5ml)wasadded and partitions weremade withethyl acetate (3×10ml).Theorganicphasewaswashedwith10%NaClaqueous solution(3×10ml),driedwithMgSO4andfiltered.Thesolventwas evaporatedandtheresiduewaspurifiedbycolumn chromatog-raphyonsilicagel60(0.063–0.200mm,MerckCo.)eluted with dichloromethane.
Obtainingthederivative6(e)
Inathree-burnerreactionflaskprotectedfromlightand main-tainedat0◦C,wasaddedto10mlofdichloromethaneand50mgof
1.Later,underinertatmosphere,wasadded2equivalentsof1to 1equivalentofPCC(pyridiniumchlorochromate),andthereaction wasmaintainedunderstirringfor12h(Souzaetal.,2004;DeSouza etal.,2005).
Obtainingthederivate7(f)
Toobtainanitratedderivative(DaSilvaetal.,2005;DeSouza et al.,2005), 200mg of 6wassubjected toreactionwith nitric acid(6EqM)for2hundermagneticstirringand0◦C.Later,itwas
Determinationofminimuminhibitoryconcentration(MIC)
Toobtaintheinoculum,thestrainswerestoredat−20◦Cand resuspendedinliquidmediumbrothSchaedlersupplementedwith hemin(5mg/ml) andmenadione(1mg/ml). Thenalltest bacte-ria weresubculturedin Schaedler agarplus defibrinated sheep blood(5%),hemin(5mg/ml)andmenadione(1mg/ml)were subse-quentlyincubatedintemperatureof36◦Cinananaerobicchamber
for72hinanatmospherecontaining10.5%H2,10%CO2and80–85% N2 toconfirmthe purityof thestrains. Afterconfirmation,the strainswereusedinthetestsfordeterminationofMIC.
Thedensityoftheinoculumwaspreparedtoscaleof0.5Mc FarlandsecondBier(1981).Allbacterialsuspensionswereadjusted inthesametransmittance.Compoundsweredissolvedindimethyl sulfoxide(DMSO)usinganultrasonicbathwhichwereusedforthe brothmicrodilutiontechnique.
The following “American Type Culture Collection” (ATCC) microorganismswere used in this study: Porphyromonas
gingi-valis(33277),Prevotellanigrescens(33563),Actinomycesnaeslundii
(19039),Bacteroidesfragilis(25285)andFusobacteriumnucleatum
(25586).
Methodofbrothmicrodilution
Thetechniquewasperformedaccordingtomethodology rec-ommendedbytheClinicalandLaboratoryStandardsInstitute(CLSI, 2007),withadaptations.
Inasterileplateof96holesweredepositedatotalof200lof themixtureofSchaedlerbroth,microbialsuspensionandtest sub-stanceinordertoobtainconcentrationsfrom20to400g/ml.It wasmadetheculturecontrol,thecontrolofsterilityofthebroth andcompounds.Thechlorhexidinedigluconatewasusedas posi-tivecontrolandDMSOasnegativecontrol.
Theplatewasincubatedat36◦Cinananaerobicchamberfor
72h.Laterwereaddedtoeachholeof30lresazurin0.02% pre-paredinaqueoussolution.Theplatewasreincubatedfor30min. Therewas,then,changingthecolorblue(nobacterialgrowth)and pink(bacterialgrowth).Thisprocedurewasperformedintriplicate.
Determinationofminimumbactericidalconcentration(MBC)
TodeterminetheMBCweusedthequantitativetechniquebased onthemethodofLifengetal.(2004).TheseriesusedforMIC deter-minationwereperformedinduplicateandonewasusedtoevaluate theMBCofthesubstancesobtained.10mlofthesolutionwhich showedinhibitionof bacterialgrowthintheMICmethodwere platedinSchaedleragar,andsubsequentlyincubatedfor24hat 37◦C.Themediawereanalyzedtoobservethepresenceorabsence
ofgrowthoforalbacteria.
Cytotoxicityassay
TheLLCMK2fibroblastcellsweregrowninRPMI(RoswellPark MemorialInstitute)1640mediumsupplementedwith100U/mlof penicillin,100g/mlofstreptomycin,and5%ofinactivatedfetal calf serum, and maintainedat 37◦C in 5% CO2. A cell
suspen-sionwasseededataconcentrationof1×106cells/mlina96-well microplatecontaining RPMI1640medium. Thereafter, thecells weretreatedwithPC-EOatdifferentconcentrations(200,100,50, 25,12.5,6.25,and3.125g/ml).Theplateswereincubatedat37◦C
for 24h, andthe biologicalactivitywasevaluated byusingthe MTTcolorimetricmethod[MTT; 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazoliumbromide]thatiscleavedbythemitochondrial enzymeresultingformazancrystalsdetectinglivingcellsina24h period in a microplate reader at 540nm. RPMI 1640 medium plusDMSO and RPMI1640medium wereused aspositive and
negative controls, respectively. All the experiments were per-formedintriplicate.Thepercentageofcytotoxicitywasdetermined bytheformula:%cytotoxicity=1−[(Y−N)/(N−P)]×100,where Y=absorbance of wells containing cells and PC-EO at different concentrations;N=negativecontrol;P=positivecontrol( Muellas-Serranoetal.,2000).
Resultsanddiscussion
Isolationof1
Regarding the method for extraction by percolation with ethanol,itcouldbeconcludedthatitisaneffectiveprocedure,in whichalargeamountofsubstancesofdifferentdegreesofpolarity intheextractcouldbeextracted.Thepartitionsmadewithorganic solventsfortheseparationoflesspolaroils,forfurther fraction-ationbychromatographictechniquesallowedabetterseparation ofsubstances.
In the analysisby HPLC, compound 1 presented two peaks, showingtheexistenceofand␣epimers indifferentamounts (Fig.1).Theepimersarediastereoisomersgeneratedbyhydroxyl linkedtothelactolring.Itisaconvertiblemixtureofbothepimers, inwhichitwaspossibletoassigntheirsignalsduetoalarger per-centageofeachconformation.Thetestedsampleisastablemixture ofbothisomers.
Thecrystalswereanalyzedusingtechniquesof1Hand13CNMR. [␣]D25◦=−8.12(c0.46,CHCl3).Thepurityof(−)-cubebinwas esti-matedtobehigherthan95%bybothHPLCand13CNMRanalyses, aswellasitsmeltingpoint,131–132◦C,1HNMR[ı,multiplicity
(JinHz),400MHz,CDCl3]:ArH(6.8–6.4;m;6H);H7(2.8–2.4;m; 4H);H7′(2.8–2.4;m;4H);H8(2.3–1.9;m;1H);H8′ (2.8–2.4;m;
1H);H9(5.2;s;1H);H9′[4.05(dd;6Hz;1H);3.72(dd;8Hz;1H)];
O CH2 O(5.9;s;4H).13CNMR[ı,multiplicity(JinHz),100MHz, CDCl3]:C1(132.3);C2(108.3);C3(147.5);C4(145.9);C5(109.5); C6(121.9);C7(39.5);C8(53.1);C9(103.5);C1′(133.9);C2′(109.3);
C3′(147.5);C4′(145.9);C5′(109);C6′(121.6);C7′(38.7);C8′(45.1);
C9′(72.7); O CH2 O (101.1).
Obtainingthesemi-syntheticderivatives
Thepuritiesoftheobtained(−)-cubebinderivativecompounds wereestimatedtobehigherthan95%bybothHPLCand13CNMR analyses.
Obtainingthederivative2
The compound was successfully obtained using methods
describedbyVenkateswarluetal.(1999),withayieldof approxi-mately95%(Fig.1S).
Thereactionoccursviaabimolecularnucleophilicsubstitution reaction,whichthereistheformationofthealkoxideion, nega-tivelycharged,inthetreatmentof1withastronglyreactivemetal. Thisintermediatereactionisusuallyusedasacatalyst,sothatthe centerislocatedinthenucleophiliccarbonatompartially nega-tivecharge.Ingeneral,it isareactioninwhich thenucleophile approaches thesubstrate fromthesideopposite thehalideion, directlyattackingthecarbonandatthesametime,shiftingthe leavinggrouploosingitthemolecule.Thenucleophilehasapairof electronsthatwillbeusedtomakethenewbondtocarbon.The reactionisaprocessinasinglestep,withoutintermediaries,that formswhenthecarbon-nucleophile–givingrisetotheexpected compound,2,breaksthecarbon-halogen.
isalsoanucleophile,becauseitalsohasapairofelectronstomake asubstitutionreaction.
Thecompoundobtainedwassubjectedtospectroscopicanalysis of1HNMR,13Canddeterminationof[␣]
D25◦[−120.4◦ (c0.0057,
CHCl3)].1H[multiplicity(JinHz)]:ARH(6.5m),H7andH15␦NMR at400MHzinCDCl3 [(5.8m)],H2inagreaterproportionofthe epimer(J=1.3Hz)(4.6d),H2epimerwaslower(J=4.6Hz)(4.55d). Theremainingsignalsareindicativeofepimers:3.9(m,H5aoftwo epimers),3.5(m,H5boftwoepimers),3.2and3.1(twosingletsof protonsof16twoepimers),andbetween2.7and1.9(m,H3,H8, H6andH4ofthetwoepimers).
Obtainingthederivative3
Thebenzylderivative(73.78%)wasobtainedfromthereaction between1andbenzylbromide.Bythinlayerchromatographywas possibletoobservethedisplacementoftheproductwithRfthan 1,indicatingthepossiblepresenceofpolaritylowerthanthatof1 (Fig.2S).
ThereactionoccursviatheWilliamsonsynthesis,which con-sistsofanalcoholatesbyreactinganalcoholwithareactivemetal, andsubsequenttreatmentofthealkoxidewithanarylhalide.It producesa nucleophilic substitutionreactionwithformation of ether.
Itisalsoabimolecularnucleophilicsubstitution,inwhichthe nucleophileapproachesthesubstratefromtheoppositesidetothe halideion.Theaffinityforoppositechargesallowsthedirectattack onthecarbonandthesimultaneousdisplacementoftheleaving group.
Thereactionisaprocessinasinglestep,without intermedi-aries,thatformswhenthecarbon-nucleophile–givingrisetothe expectedcompound,3,breaksthecarbon-halogen.
Thiswassubmittedtospectroscopicanalysisof1HNMR,13C anddeterminationof[␣]D25◦=−2.09(c0.008,CHCl3).1HNMR[ı, multiplicity(JinHz),400MHz,CDCl3]:ArH(6.8–6.4;m;6H);H7 [2.60–2.10(m;4H)];H7′[2.60–2.10(m;4H)];H8[2.15–1.80;(m;
1H)];H8′[2.60–2.70(m;1H)];H9[4.80(s;1H)];H9′[3.95(dd;12
and6Hz;1H)and3.70(dd;12and8Hz;1H)]; O CH2 O [5.90 (s;4H)];CH2[4.70(d;1H)and4.85(d;1H)]andAr[6.40–6.8(s;5H) and7.0(s)].13CNMR[ı,multiplicity(JinHz),100MHz,CDCl
3]:C1 (133.9);C2(108.2);C3(142.2);C4(146.3);C5(109.3);C6(121.8); C7(38.9);C8(52.5);C9(103.7);C1′(134.5);C2′(108.3);C3′(148.2);
C4′(146.3);C5′(109.3);C6′(121.7);C7′(39.4);C8′(46.2);C9′(72.3);
O CH2 O (100.8);Ar-CH2(69.0);Ar(109.7–108.2).
Obtainingthederivative4
Theproductappearedasayellowishoil,whichcanbeexplained bythepresenceoftwoforms(␣and)oflignanmodified,andthat theproductwasmixedwith1.Themassderivedfrompointedend ofthereactionyield(85%)(Fig.3S).
Theacetylationreactionwasnotdevelopedinaconventional mannerasitisforthesynthesisofacetylsalicylicacidandother estherificationreactions, because theyuse acidcatalysis which leadstodisruptionoftheringorsufferdehydrationcetalic non-characterizingthemoleculeoftheoriginal1.
Thus,theacetylationof1wasperformedusingpyridineasa catalystforitsabilitytomediateredoxreactions,actingas “elec-trontransport”,whichleadstotheformationofthenucleophile. Thenucleophilicsubstitutionoccurs,then,withtheattackofthe nucleophiletothecarbonpartiallypositivechargeofacetic anhy-dride.Atthesametime,thiscarbonatomattackstheoxygenatom whichhaspartialnegativecharge.Thesereactionsoccurinonestep, withoutformationofintermediates,givingriseto4andacetateion. ThiswassubjectedtoNMRspectroscopicanalysisof1H,13C,and determinationof[␣]D25◦.
[␣]D25◦=−123.33(c0.0057,CHCl3);1HNMR[␦,multiplicity(Jin Hz),400MHz,CDCl3]:Ar-H(6.65–6.40;m,6H);C-8(2.30–1.95;m, 1H);C-9′(4.04(dd;1H)and4.00(dd;1H)); O CH2 O (5.90;s,
4H); CH3(1.99,s,3H).13CNMR[ı,multiplicity(JinHz),100MHz, CDCl3]:C-1(132.21);C-2(107.63);C-3(147.42);C-4(145.72);C-5 (108.43);C-6(121.51);C-7(38.71);C-8(51.10);C-9(102.89);C-1′
(133.29);C-2′(109.01);C-3′(147.42);C-4′(145.92);C-5′(108.43);
C-6′(120.88);C-7′(37.40);C-8′(44.23);C-9′(73.32); O CH2 O
(100.49); O CO (169.91); CH3(20.89).
Obtainingthederivative5
Thesynthesiswascarriedoutwithoutcausingdamagetothe lactolicringof1(Fig.4S).Itwasobtainedin93%yield.
The technique of Williamson was also utilized, as it is an importantprocessforthepreparationofethers,symmetricaland asymmetricones. Thesodium alkoxidewasprepared by direct actionofethoxide,whichcanmakenucleophilicsubstitutions eas-ily,onalcohol.Replacedalkylhalidereactedwiththepreviously formed.Itconsistsofthenucleophilicsubstitutionofhalideionby thealkoxideion,withthesimultaneousattackoncarbonpartially loaded,andofthechloride.
ThiswassubjectedtoNMRspectroscopicanalysisof1H,13C,and determinationof[␣]D25◦.
[␣]D25◦=−4.38◦ (c 0.067, CHCl3); 1H-RMN ␦ (CDCl3): Ar-H [6.70–6.40(m;6H)];H7[2.80–2.30(m;4H)];H7′ [2.80–2.30(m;
4H)];H8[2.10–1.90(m;1H)];H8′[2.80–2.40(m;1H)];H9[4.75
(s;1H)];H9′[4.05(m;1H)and3.80(m;1H)]; O CH2 O [5.90
(s;4H)];O CH2 [3.66–3.52(m;2H)];CH2 N [1.12(t;7.07Hz; 1H) and 1.09 (t;7.07Hz;1H)]; N (CH3)2 [1.09 (s; 6H)]. 13 C-RMN␦(CDCl3):C1(134.5);C2(108.3);C3(147.8);C4(146.2);C5 (109.6);C6(122.0);C7(39.5);C8(52.5);C9(104.5);C1′ (133.8);
C2′(108.9);C3′ (147.2);C4′ (145.9);C5′ (109.3);C6′ (121.8);C7′
(39.0); C8′ (46.2); C9′ (72.3); O CH2 O (101.1); O CH2
(63.2); CH2 N (43.7); N (CH3)2(46.2).
Obtainingthederivative6
Theproductwaspurifiedbyproviding98%yield,anditspurity wasdeterminedbyHPLC(Fig.5S).Inanacidicenvironment, per-hapsthe1sufferlactolichydroxyldehydrationandelimination. Theoutputofthe OHgroupleadstoformationofadoublebond inthefuranring.Thiseffectofacidinordertoremovethehydroxyl lactolicof1ismoredifficulttooccur.Inanotherpossible behav-ioralmechanismof1inanacidmedium,protonationofthefuran oxygenandsubsequentopeningoflactolicringwiththeformation ofanaldehydeandanalcohol.
Thus,theoxidation requiresa detailed analysis.Possiblythe reaction with PCC led to the formation of an intermediate inorganicesterofanalcohol.Theresiduecontainsametalthatcan beeasilyreduced,andintheprocessthealcoholisoxidized.
Inthe1HNMRspectrumshowedamarkeddifferenceinthe structureof6withrespecttothestructureof1,which consists mainlyin the absenceof hydrogenH-9 and maybe notedthe absenceof signalin5.20ppm(s,1H),indicatinganoxidationat thisposition.
[␣]D25◦=−30 (c 0.99, CHCl3); 1H-RMN ␦ (CDCl3): Ar-H [6.80–6.40 (m; 6H)]; H7 [2.65–2.40 (m; 4H)]; H7′ [2.65–2.40
(m;4H)];C8[2.10–2.95(m;1H)];H8′ [2.85(m;1H)];H9′ [3.85
(dd; 7.4Hz; 1H) and 4.12 (dd; 7.0Hz; 1H)] and O CH2 O [5.90 (s; 4H)]. 13C-RMN ı (CDCl3): C1 (131.6); C2 (108.4); C3 (147.9);C4(146.3);C5(108.3);C6(121.6);C7(34.8);C8(46.5); C9(178.4);C1′ (131.3);C2′ (108.8);C3′ (147.9);C4′ (146.5);C5′
(108.3);C6′(122.2);C7′(38.4);C8′(41.3);C9′(71.2); O CH2 O
Obtainingthederivative7
Themechanismofnitrationmaybethroughamixtureofnitric andsulfuricacids.Inreactiontoobtain7(Fig.6S),whichhad97.6% of yield,it wasused fuming nitricacidwhich already contains nitroiliondissolved,whichistheelectrophilicparticlethatattacks thebenzenering.Solutionsofthesesaltsarestableincertain sol-ventssuchasnitromethaneoraceticacid,performthenitrationof aromatics,gentlyandwithhighyieldatroomtemperature.
Needingelectrons,thisionfindthemparticularlyavailablein thecloudofthebenzenering,soitwillbefixedtooneofthe carbonatomsoftheringbyacovalentbond,formingacarbocation, oftendesignatedasbenzenoidion(MorrisonandBoyd,1996).
Thismeansthatthepositivechargeisnotlocalizedinonecarbon atom,butdistributedthroughoutthemolecule,andisparticularly strongonthecarbonatomsinorthoandparapositioninrelationto thatwhichbindstheNO2group,thedispersionthepositivecharge throughoutthemolecule,theeffectofresonance,theionbecomes morestablethanitwouldhavehadapositivechargelocated. Prob-ablythisisthesamestabilizingeffectthatallowstheformationof carbocation,takingintoaccountthestabilityoftheoriginalbenzene molecule.
Thereactionissimilartotheadditiontoalkenes:anelectrophile species,drawnelectrons,isattachedtothebenzenemolecule form-ingacarbocation.Theinclusionofabasicgroupinbenzenóico ionwithformationofanadditionproductwoulddestroythe char-acterofthearomaticring.Instead,thebasicion,HSO4−,extractsa hydrogenionandformbenzenoidasubstituteproductthatstays intheringstabilizedbyresonance.Thelossofhydrogenisoneof thetypicalreactionsofcarbocations(MorrisonandBoyd,1996).
Theelectrophilic substitution,suchas electrophilicaddition, takesplaceinsuccessivesteps,involvingtheformationofa car-bocationintermediate. Bothreactions,however,differfromone another,thefateofthecarbocation.Althoughthemechanismof nitrationispossiblybetterthanelucidatedthemechanismsofother aromaticsubstitutionreactions,itseemsalmostcertainthatallof themareprocessedinthesamemanner(MorrisonandBoyd,1996). Aromaticsubstitutionreactionsarecharacterizedbythe addi-tionofNO2grouptothearomaticringleadingtothedesired prod-uct.Thearomaticcompoundsasmuchascanbenitratedaliphatic, formingimportantproductsinorganicsynthesis.Thenitrationof aromaticringdeactivatesit,inareactionwhoseproductsareeasily separatedandanalyzed,whichidentifiestheproportionsofortho, metaandparaproductsformed(Kouletal.,1983).
Thiswassubmittedtospectroscopicanalysisof1HNMRand 13Cand[␣]
D25◦.[␣]D25◦=−29.07(c0.008,CHCl3).1HNMR[ı, mul-tiplicity(JinHz),400MHz,CDCl3]:7.5(s,1H),7.48(s,1H),6.8(s, 1H);6.6(s,1H);6.1(m,2H);4.35(dd,1H,J=7.1and9.1Hz);4.0 (dd,1H,J=7.3and9.1Hz);3.26(d,2H,J=7.1Hz);3.2(dd,1H,J=6.3 and13.6Hz);3.0(dd,1H,J=7.8and13.6Hz);2.8(m,2H).13CNMR [ı,multiplicity(JinHz),100MHz,CDCl3]:178.00;152.3;152.2; 147.6;143.1;142.9;130.9;130.7;112.5;111.2;106.6;106.2;103.6; 103.5;71.4;45.7;41.7;37.2;34.2;EMAR(M+H)+Calculationfor C20H17N2O10:445.0884;found:445.0890.Analyticcalculationfor C20H16N2O10:C,54.0937;H,3.6315;N,6.3081;O,35.9667;found: C,53.9173;H,3.5462;N,6.2121;O,36.3244.
DeterminationofMIC
Silvaetal.(2007)studiedtheantimicrobialactivityof deriva-tives of 1 extracted fromPiper cubeba, compared toother oral micro-organisms.Theantimicrobialactivitywastestedonseven oftheoral cavity pathogens:Enterococcusfaecalis,Streptococcus
salivarius,Streptococcussanguinis,Streptococcusmitis,Streptococcus
mutans, Streptococcus sobrinus and Candida albicans. The
com-poundswereeffectiveonmostofthepathogensstudied.
Inthepresentwork,othermicroorganismsthatcause endodon-tic infection were tested, to expand knowledge regarding the chemicalstructureandbiologicalactivity.Assayswereperformed intriplicate,evaluatingthecompoundsseparately.TheMICvalues ofcompoundsareshowninTable1.
According to Rivers and Recio (2005), among the results obtainedinthiswork,compounds2and3showedsignificant activ-ity(50g/ml)comparedtothemicroorganismP.gingivalis(ATCC 33277)forcompounds1and7,whichalsoshowedsignificant activ-ity(200g/ml).However,5and6showedloweractivities,and4 wasnotactive(MIC>400g/ml).
Compound 6 displayed significant activity against B. fragilis
(ATCC 25285)(100g/ml).On theother hand, noactivity was observedfortheotherevaluatedcompounds(>400mg/ml). Com-pounds1,2,3and7showedlowactivity(400g/ml)againstP.
nigrescens(ATCC33563),andcompounds4,5and6werenotactive
MICvalues(>400g/ml).
Thecompounds werenotactiveagainst themicroorganisms
Actomycesnaeslundii(ATCC19039)andF.nucleatum(ATCC25586)
showingMICvalues>400g/ml.
The MICvalues displayedby compounds 2 and 3 were sig-nificant(50g/ml)comparedtothevaluesformicroorganismP.
gingivalis(ATCC33277)andforcompound6againstthe
microor-ganismB.fragilis(ATCC25285)(100g/ml).Theresultsobtained forantimicrobialactivityinthisstudyareinaccordancewiththe activitiesobtainedfortheanti-inflammatoryandanalgesic, accord-ing toreportsin theliterature (Da Silva etal., 2005), in which theanti-inflammatoryandanalgesiceffectsof dibenzylbutyrolac-tonelignanswereinvestigatedusingdifferentanimalmodels.It wasobserved that (−)-cubebin and (−)-hinokinininhibited the edemaformationintheratpawedemaassayandthatallresponses weredosedependent.Also,atthedoseof30mg/kg,compounds (−)-cubebin,(−)-hinokinin,(−)-6,6′-dinitrohinokinininhibitedthe
edemaformationby53%,63%and54%,respectively,atthethird houroftheexperiment.Intheaceticacid-inducedwrithingtest inmice,(−)-hinokininproducedinhibitionlevelof97%,while(− )-6,6′-dinitrohinokinindisplayedlowereffect(75%),whichwasstill
higherthan(−)-cubebin.
Compounds2and 3differfrom1bythepresenceofmethyl orbenzylgroupsreplacingthelactolichydrogen.Compound6is alignan-lactonethatdiffersfrom1bythepresenceofacarbonyl groupatC-9.
Theliterature reportsthat compoundshavinga lactonering with two methylenodioxyaril groups showed significant anti-inflammatoryandanalgesicactivities,andtheintroductionofpolar groupsonthearomaticringsisalsoadvantageousforthese activ-ities(DaSilvaetal.,2005).However,withrespecttotrypanocidal activity,theintroductionofnitrogroupsinthearomaticringsis harmfulforthisactivity(DaSilvaetal.,2005).
Althoughchlorhexidine have displayeda considerablylower valueoftheMICagainstalltestedmicro-organismsinrelationto thecompoundsobtainedinthiswork,theresultsseemtobe sat-isfactorycomparedwithotherpreviouslypublishedworksinthe literature.Inaddition,thecontinualsearchfor newcompounds against oral pathogens may be justified by the side effects of chlorhexidine,suchasdiscolorationoftheteethandtaste perver-sion.Itshouldbenotedthatthisstudyprovidesadditionaldatathat isrelevanttoshowthepotentialactivityagainstoralpathogens presentedbypurecompoundsobtainedfromP.cubeba,alongwith theirsemisyntheticderivatives.
DeterminationofMBC
Table1
MICvaluesofevaluatedcompounds.
Compounds Minimuminhibitoryconcentration(g/ml)
P.gingivalis A.naeslundii B.fragilis P.nigrescens F.nucleatum
1 200 >400 >400 400 >400
2 50 >400 >400 400 >400
3 50 >400 >400 400 >400
4 >400 >400 >400 >400 >400
5 400 >400 >400 >400 >400
6 400 >400 100 >400 >400
7 200 >400 >400 400 >400
Control(Chlorexidine) 0.922 1.844 7.375 0.922 1.844
Concentrationsofthetestedsubstances:20g/mland400g/ml.Concentrationsofpositivecontrol:0.0115g/mland8g/ml.
Table2
Minimumbactericidalconcentrationvaluesoftheevaluatedcompounds.
Compounds Minimumbactericidalconcentration(g/ml)
P.gingivalis A.naeslundii B.fragilis P.nigrescens F.nucleatum
1 400 >400 >400 >400 >400
2 200 >400 >400 >400 >400
3 300 >400 >400 >400 >400
4 >400 >400 >400 >400 >400
5 >400 >400 >400 >400 >400
6 400 >400 >400 >400 >400
7 >400 >400 >400 >400 >400
Control(Chlorexidine) 0.922 1.844 7.375 0.922 1.844
Concentrationsofthetestedsubstances:20g/mland400g/ml.Concentrationsofpositivecontrol:0.0115g/mland8g/ml.
humansgrowsfasterthanthedevelopmentofnewdrugs. There-fore,thesearchforalternativetherapiesisimperative.
An antibiotic may have bactericidal (cause cell death with reducedbacterialgrowthgreaterthanorequalto80%), bacterio-static(inhibitsbacterialgrowthwithreductionusuallylessthan 80%sinceitdoesnotoccurcelldeath),ornotpresentanyactivity, whenthebacteriumisresistanttoit,accordingtoCLSI(2007).
Thetestsconcerningtheminimumbactericidalconcentrationof thecompoundsobtainedareshowninTable2.Amongit,notethat therewassomeactivityof2and3againstP.gingivaliswithMBCof 200g/mland300g/ml,respectively.1and6presentedtheMBC of400g/ml.Asfortheothercompounds,theirMBCvalueswas notdetermined(>400g/ml).
However,comparedtoothermicroorganisms(P.nigrescens,A.
naeslundii,F.nucleatumandB.fragilis)itwasnotobservedactivity,
sinceallthecompoundsshowedMBCvalues>400g/ml.
Determinationofcytotoxicity
Theviabilityofthecultureswasdeterminedbyestablishinga relationbetweentheabsorbancevalues obtainedin thetreated
100
80
60
40
Viability
, %
20
(1) (2) (3) (4) (5) (6) (7)
6.25
µM
12.5
µM
25µ M
50µ M
100
µM
200
µM
400
µM
0
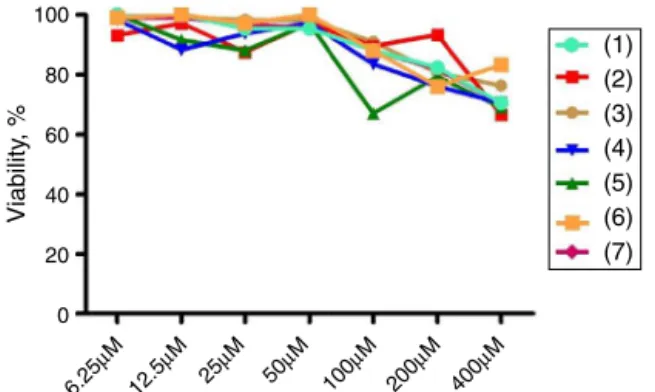
Fig.2.EffectsofthecompoundsontheviabilityofLLCMK2fibroblastcells. Cyto-toxicitywasdeterminedusingMTTassayafter24htreatmentwiththeindicated concentrations.Valuesareexpressedbymean±S.D.
anduntreated(control)groups,asshowninFig.2.Theseresults revealedthattheinvitroactivityofthetestedsubstancesmaynot berelateddocytotoxiceffects,sincetheydonotpresentsignificant cytotoxicity.
Althoughinvitroassayshavenotbeenconsideredsuitableto cover all the aspects of the activities of drugs, especially with respecttopharmacologicalandimmunologicalinteractions,they providefirstevidenceoftheeffectsandinsightintothemodeof action,thus,theymayleadtothedevelopmentofnewtherapeutic approacheslikedrugtargeting(Holtfreteretal.,2011).
Conclusions
Fromthisstudyitwaspossibletoobtainrelevantdata concern-ingtherelationshipbetweenstructureandantimicrobialactivity forlignansderivedfrom(−)-cubebin(1)againstmicroorganisms thatcauserootcanalinfections,ie,whichsubstituentgroupsare importantformaintenanceorincreaseofantimicrobialactivityfor thesecompounds.Investigationson1arealwaysgearedtoward findingoutnewbiologicalpropertiesandsynthesizingmorepotent derivativesfromapharmacologicalviewpoint.Itshouldbe high-lightedthatthederivativeswereobtainedwithhighyields,which allowedthecompletionofallstepsofpurification,identification andbiologicalevaluation.
Accordingtothecurrentstudy,thepresencesofcarbonylgroup atcarbon9,aswellastheintroductionofpolargroupsarebeneficial forantimicrobialactivity.Theanalysisoftheresultsalsosuggests thatthelignan1anditsderivatives,especially2,3and6, consti-tuteanimportantsourceofnewbioactivenaturalproducts,which encouragesfurtherstudies.
Authors’contributions
AHJ contributed tocritical reading of themanuscript. VRE and CHGMcontributedtobiologicalassays.MAS contributedto iso-lationandidentificationofcompounds.WRCcontributedtothe identificationofderivatives.JKBcontributedtocriticalreadingof themanuscript.MLASdesignedthestudyandsupervisedthe labo-ratory.Alltheauthorshavereadthefinalmanuscriptandapproved thesubmission.
Conflictsofinterest
Theauthorsdeclarenoconflictsofinterest.
Acknowledgements
ThisworkwassupportedbyFAPESP(forGrantsProcessNumber 2008/07089-9and 2009/05049-2),CNPq(forfellowshipsGrants ProcessNumber303349/2013-1)andCAPES.
AppendixA. Supplementarydata
Supplementarydataassociatedwiththisarticlecanbefound,in theonlineversion,atdoi:10.1016/j.bjp.2015.12.006.
References
Arruda,D.C.,D’alexandri,F.L.,Katzin,A.M.,Uliana,S.R.,2005.Antileishmanialactivity ofterpenenerolidol.Antimicrob.AgentsChemother.49,1679–1687.
Ayres,D.C.,Loike,J.D.,1990.Lignans:Chemical,BiologicalAndClinicalProperties. ChemistryandPharmacologyofNaturalProducts.CambridgeUniversityPress, Cambridge.
Baumgartner,J.,1997.Microbiologiaendodôntica.In:Walton,R.E.,Torabinejad,M. (Eds.),PrincípiosEPráticaEmEndodontia.SantosLivrariaEditora,SãoPaulo,pp. 277–291.
Bergenholtz,G.,Crawford,J.,1989.Endodonticmicrobiology.In:Walton,R.E., Tora-binejad,M.(Eds.),PrinciplesandPracticeofEndodontics.,1sted.W.B.Saunders Co.,Philadelphia,P.A.,USA,pp.267–282.
Bier,O.,1981.BacteriologiaEImunologia:EmSuasAplicac¸õesàMedicinaEA Higiene.Ed.Melhoramentos,SãoPaulo.
Braquet,P.,Godfroid,J.J.,1986.PAF-acetherspecificbindingsites:2.Designof spe-cificantagonists.TrendsPharmacol.Sci.7,397–403.
Charlton,J.L.,1998.Antiviralactivityoflignans.J.Nat.Prod.61,1447–1451.
CLSI,2007.MethodsforAntimicrobialSusceptibilityTestingofAnaerobicBacteria; ApprovedStandard–SeventhEdition.CLSIdocumentM11-A7[ISBN 1-56238-626-3]. Clinical and LaboratoryStandards Institute, Wayne,Pennsylvania, USA.
DaSilva,R.,deSouza,G.H.B.,daSilva,A.A.,deSouza,V.A.,Pereira,A.C.,Royo,V.A., Silva,M.L.A.,Donate,P.M.,Araujo,A.L.S.M.,Carvalho,J.C.T.,Bastos,J.K.,2005.
Synthesisandbiologicalactivityevaluationoflignanlactonesderivedfrom(− )-cubebin.Bioorg.Med.Chem.Lett.15,1033–1037.
DeLillo,A.,Booth,V.,Kyriacou,L.,Weightman,A.J.,Wade,W.G.,2004. Culture-independent identification of periodontitis-associated Porphyromonas and Tannerellapopulationsbytargetedmolecularanalysis.J.Clin.Microbiol.42, 5523–5527.
DeSouza,V.A.,daSilva,R.,Pereira,A.C.,Royo,V.A.,Saraiva,J.,Montanheiro,M.,de Souza,G.H.B.,daSilvaFilho,A.A.S.,Grando,M.D.,Donate,P.M.,Bastos,J.K., Albu-querque,S.,Silva,M.L.A.,2005.Trypanocidalactivityof(−)-cubebinderivatives againstfreeamastigoteformsofTrypanosomacruzi.Bioorg.Med.Chem.Lett.15, 303–307.
Deyama, T., Nishibe, S., Nakazawa, Y., 2001. Constituents and pharmacolog-ical effects of Eucommia and Siberian ginseng. Acta Pharm. Toxicol. 22, 1057–1070.
Elfahmi,Y.,2006.LignanprofileofPipercubeba,anIndonesianmedicinalplant. In:PhytochemicalandBiosyntheticStudiesofLignans.UniversiyofGroningen, Netherlands(Chapter4).
Fine,D.H.,2000.Chemicalagentstopreventandregulateplaquedevelopment. Periodontology8,87–107.
Hirata,N.,Naruto,S.,Ohguchi,K.,Akao,Y.,Nozawa,Y.,Iinuma,M.,Matsuda,H., 2007a.Mechanismofthemelanogenesisstimulationactivityof(−)-cubebinin murineB16melanomacells.Bioorg.Med.Chem.15,4897–4902.
Hirata,N.,Tokunaga,M.,Naruto,S.,Iinuma,M.,Matsuda,H.,2007b.Testosterone 5a-reductaseinhibitoryactiveconstituentsofPipernigrumLeaf.Biol.Pharm.Bull. 30,2402–2405.
Hirata,N.,Naruto,S.,Inaba,K.,Itoh,K.,Tokunaga,M.,Iinuma,M.,Matsuda,H.,2008.
HistaminereleaseinhibitoryactivityofPipernigrumleaf.Biol.Pharm.Bull.31, 1973–1976.
Holtfreter,M.C.,Loebermann,M.,Klammt,S.,Sombetzki,M.,Bodammer,P.,Riebold, D.,Kinzelbach,R.,Reisinger,E.C.,2011.Schistosomamansoni:schistosomicidal effectofmefloquineandprimaquineinvitro.Exp.Parasitol.127,270–276.
Kakehashi,S.,Stanley,H.R.,Fitzgerald,R.J.,1965.Theeffectsofsurgicalexposuresof dentalpulpsingerm-freeandconventionallaboratoryrats.OralSurg.OralMed. OralPathol.20,340–349.
Kassuya,C.A.,Silvestre,A.,Menezes-De-Lima,O.J.R.,Marotta,D.M.,Rehder,V.L., Calixto,.J.B.,2006.Antiinflammatoryandantiallodynicactionsofthelignan niranthinisolatedfromPhyllanthusamarus.Eur.J.Pharmacol.546,182–188.
Koul,S.K.,Taneja,S.C.,Dhar,K.L.,Atal,C.K.,1983.LignansofPiperclusii. Phytochem-istry22,99–1000.
Li,N.,Wu,J.,Hasegawa,T.,Sakai,J.,Wang,L.,Kakuta,S.,Furuya,Y.,Tomida,A.,Tsuruo, T.,Ando,M.,2006.Bioactivedibenzoylbutyrolactoneanddibenzoylbutanediol lignanesfromPeperomiaduclouxi.J.Nat.Prod.69,234–239.
Lifeng,Q.I.,Zirong,X.,Xia,J.,Caihong,H.,Xiangfei,Z.,2004.Preparationand antibac-terialactivityofchitosannanoparticles.Carbohyd.Res.339,2693–2700.
Luisi,S.B.,Fachin,E.V.F.,1990.Revisãoeenfoqueclínicosobreabacteriologiadas infecc¸õesendodônticasagudas.Rev.Fac.Odontol.UFGRS40,41–45.
Marcotullio,M.C.,Pelosi,A.,Curini,M.,2014.Hinokinin,anemergingbioactive lig-nan.Molecules19,14862–14878.
Marsh,P.D.,2005.Dentalplaque:biologicalsignificanceofabiofilmandcommunity lifestyle.J.Clin.Periodontol.32,7–15.
Morrison,R.,Boyd,R.,1996.QuímicaOrgânica:Hidrogenac¸ão,13aedic¸ão.Fundac¸ão CaloustreGulbenkian,Lisboa,375,380,382e384.
Muellas-Serrano,Nogal,S.,Martinez-Dias,J.J.,Escario,R.A.,Martinez-Fernandez,J.Á., Gomes-Barrio.,A.R.,2000.AinvitroscreeningofAmericanplantextractson
TrypanosomacruziandTrichomonasvaginalis.J.Ethnopharmacol.71,101–107.
Parmar,V.S.,Jain,S.C.,Bisht,K.S.,Jain,R.,Taneja,P.,Jha,A.,Tyagi,O.D.,Prasad, A.K.,Wengel,J.,Olsen,C.E.,Boll,P.M.,1997.PhytochemistryofthegenusPiper. Phytochemistry46,597–673.
Piccinelli,A.L.,Mahmood,N.,Mora,G.,Poveda,L.,Simone,F.,Rastrelli,L.,2005. Anti-HIVactivityofdibenzylbutyrolactone-typelignansfromPhenaxspeciesendemic inCostaRica.J.Pharm.Pharmacol.57,1109–1115.
Rimando, A.M.,Pezzuto, J.M.,Farnsworth, N.R.,Santisuk, T.R.V.,Kawanishi,K., 1994.Newlignansfromanogeissus-acuminatawithhiv-1reverse-transcriptase inhibitoryactivity.J.Nat.Prod.57,896–904.
Rivers, J.C., Recio, M.C., 2005. Medicinal plants and antimicrobial activity. J. Ethnopharmacol.100,80–84.
Saleem,M.,Kim,H.J.,Ali,M.S.,Lee,Y.S.,2005.Anupdateonbioactiveplantlignans. Nat.Prod.Rep.22,696–716.
Saraiva,J.,Veja,C.,Rolon,M.,Silva,R.,Silva,M.L.A.,Donate,P.M.,Bastos,J.K., Gomez-Barrio,A.,Albuquerque,S.,2007.Invitroandinvivoactivityoflignanlactones derivativesagainstTrypanosomacruzi.Parasitol.Res.100,791–795.
Schmidt,T.J.,Hemmati,S.,Klaes,M.,Konuklugil,B.,Mohagheghzadeh,A.,Ionkova,I., Fuss,E.,WilhelmAlfermann,A.,2010.LignansinfloweringaerialpartsofLinum
species–chemodiversityinthelightofsystematicsandphylogeny. Phytochem-istry71,1714–1728.
Shen,T.Y.,Hwang,S.B.,Chang,M.N.,Doebber,T.W.,Lam,M.H.,Wu,M.S.,Wang,X., Han,G.Q.,Li,R.Z.,1985.CharacterizationofaPAFreceptorantagonistisolated fromhaifenteng(Piperfutokadsura):SpecificinhibitorofinvitroandinvivoPAF inducedeffects.Proc.Natl.Acad.Sci.U.S.A.82,672–676.
Silva,M.L.A.,Albuquerque,S.,Souza,B.H.B.,Bastos,J.K.,Silva,R.,2009.Processto obtaindibenzylbutyrolactoniclignans,processtoobtainsyntheticderivatives fromlignansbearinganti-Chagaschemoprophylacticandtherapeutical activ-ities.Fundac¸ãodeAmparoàPesquisadoEstadodeSãoPaulo.US7,521,569 B2.
Silva,M.L.A.,Silva,R.,Rodrigues,V.,PereiraJúnior,O.S.,SilvaFilho,A.A.,Donate,P.M., Albuquerque,S.,Bastos,J.K.2007.Processtoobtainsyntheticandsemi-synthetic lignanderivatives,theirantiparasiticactivitiesandcorresponding pharmaceu-ticalformulations,includingthetherapeuticmethodusingsaidlignanforthe treatmentofparasitosis.Fundac¸ãodeAmparoàPesquisadoEstadoeSãoPaulo. WO2007/009201.
Socransky,S.S.,Haffajee,A.D.,2002.Dentalbiofilms:difficulttherapeutictargets. Periodontology28,12–55.
Souza,G.H.B.,Silva,A.A.,Souza,V.A.,Pereira,A.C.,Royo,V.A.,Silva,M.L.A.,Silva,R., Donate,P.M.,Carvalho,J.C.T.,Bastos,J.K.,2004.Analgesicandanti-inflammatory activitiesevaluationof(−)-O-acetyl,(−)-O-methyl,(−)-O-dimethylethylamine cubebinandtheirpreparationfrom(−)-cubebin.IIFarmaco59,55–61.
Tani-Ishii,N.,Wang,C.Y.,Tanner,A.,Stashenko,P.,1994.Changesinrootcanal micro-biotaduringthedevelopmentofratperiapicallesions.OralMicrobiol.Immunol. 9,129–135.
Usia,T.,Watabe,T.,Kadota,S.,Tezuka,Y.,2005.PotentCYP3A4inhibitory con-stituentsofPipercubeba.J.Nat.Prod.68,64–68.
Venkateswarlu,R.,Kamakshi,C.,Moinuddin,S.G.A.,Subhash,P.V.,1999. Transfor-mationsoflignans,PartV.ReactionsofDDQwithagmelinolhydrogenolysis productanditsderivatives.TetrahedronLett.55,13087–13108.
WittgowJr.,W.C.,SabistonJr.,C.B.,1975.Microorganismsfrompulpalchambersof intactteethwithnecroticpulps.J.Endod.1,168–171.