w ww.e l s e v i e r . c o m / l o c a t e / b j p
Original
Article
␣
-Glucosidase
and
pancreatic
lipase
inhibitory
activities
and
glucose
uptake
stimulatory
effect
of
phenolic
compounds
from
Dendrobium
formosum
Prachyaporn
Inthongkaew
a,
Nutputsorn
Chatsumpun
b,
Chonlakan
Supasuteekul
a,
Tharita
Kitisripanya
c,
Waraporn
Putalun
c,
Kittisak
Likhitwitayawuid
a,
Boonchoo
Sritularak
a,∗aDepartmentofPharmacognosyandPharmaceuticalBotany,FacultyofPharmaceuticalSciences,ChulalongkornUniversity,Bangkok,Thailand
bDepartmentofPharmacognosy,FacultyofPharmacy,MahidolUniversity,Bangkok,Thailand
cFacultyofPharmaceuticalSciences,KhonKaenUniversity,KhonKaen,Thailand
a
r
t
i
c
l
e
i
n
f
o
Articlehistory:
Received11March2017 Accepted26May2017 Availableonline11July2017
Keywords:
Dendrobiumformosum
Glucoseuptake
␣-Glucosidase Pancreaticlipase
a
b
s
t
r
a
c
t
AmethanolextractfromthewholeplantofDendrobiumformosumRoxb.exLindl.,Orchidaceae,showed inhibitorypotentialagainst␣-glucosidaseandpancreaticlipaseenzymes.Chromatographicseparationof theextractresultedintheisolationoftwelvephenoliccompounds.Thestructuresofthesecompounds weredeterminedthroughanalysisofNMRandHR-ESI-MSdata.Alloftheisolateswereevaluatedfor their␣-glucosidaseandpancreaticlipaseinhibitoryactivities,aswellasglucoseuptakestimulatory effect.Amongtheisolates,5-methoxy-7-hydroxy-9,10-dihydro-1,4-phenanthrenequinone(12)showed thehighest␣-glucosidaseandpancreaticlipaseinhibitoryeffectswithanIC50valuesof126.88±0.66M and69.45±10.14M,respectively.AnenzymekineticsstudywasconductedbytheLineweaver-Burk plotmethod.Thekineticsstudiesrevealedthatcompound12wasanon-competitiveinhibitorof␣ -glucosidaseandpancreaticlipaseenzymes.Moreover,lusianthridinat1and10g/mlandmoscatilin at100g/mlshowedglucoseuptakestimulatoryeffectwithouttoxicityonL6myotubes.Thisstudy isthefirstreportonthephytochemicalconstituentsandanti-diabeticandanti-obesityactivitiesofD. formosum.
©2017SociedadeBrasileiradeFarmacognosia.PublishedbyElsevierEditoraLtda.Thisisanopen accessarticleundertheCCBY-NC-NDlicense(http://creativecommons.org/licenses/by-nc-nd/4.0/).
Introduction
Diabetesmellitus(DM)isametabolicdisordercharacterizedby chronichyperglycemiathatcancausefurtherserioushealth prob-lemssuchasneurologicalandcardiovascularcomplications(Peng etal.,2016).Therearethreemaintypesofdiabeteswhicharetype I,typeII,andgestationaldiabetes.TypeIIdiabetes,whichisdueto insulinresistance,affectsthemajority(90–95%)ofdiabeticpatients (AmericanDiabetesAssociation,2006;RosakandMertes,2012).␣ -Glucosidaseisacarbohydrate-hydrolyzingenzymesecretedfrom theintestinalchorionicepithelium.Inhibitionof thisenzyme is oneofthetherapeuticapproachesfortypeIIdiabetessinceitcan causeretardationofcarbohydratedigestion,whichleadstothe pre-ventionofexcessglucoseabsorption(Youetal.,2012;Pengetal., 2016).Insulinplaysakeyroleinreductionoftheglucoselevelby stimulatingtheglucosetransportfrombloodintoskeletalmuscle
∗ Correspondingauthor.
E-mail:boonchoo.sr@chula.ac.th(B.Sritularak).
cells.Itiswellestablishedthatinsulin-stimulatedglucoseuptake isimpairedintypeIIdiabeticpatients(Yapetal.,2007;Choietal., 2013).Therefore,searchingforcompoundsthatcanenhance glu-coseuptakeisanimportantapproachtofacilitatethedevelopment ofnewmethodsforinsulinresistancetreatment(Choietal.,2013). Inaddition,typeIIdiabetescanbecausedbythedysfunction ofinsulin-producingpancreaticcells,whichcouldbeinstigated bytheexcessiveaccumulationoflipidsinthepancreas(Tushuizen etal.,2007;Youetal.,2012).Pancreaticlipaseisthekeyenzymein lipiddigestion,responsibleforabsorptionofdietaryfatsthrough thebreakdownoftriacylglycerolsintofreefattyacidsand monoa-cylglycerolsintheintestinallumen(Yangetal.,2014).Recently, inhibitorsofpancreaticlipasehaveattractedmuchresearch inter-est due to their anti-obesity activity by delaying the lipolytic process.Thisactionwouldleadtothedecreaseinlipidabsorption andthusprotectthepancreas,whichwillrestoreregularinsulin productionfromthecells(Tushuizenetal.,2007;Youetal.,2012; Yangetal.,2014).
DendrobiumisalargegenusintheOrchidaceaefamilywhich includeabout1100species,and150specieshavebeenidentified
http://dx.doi.org/10.1016/j.bjp.2017.05.005
in Thailand (Lam et al., 2015; Limpanit et al., 2016). Several
Dendrobiumspeciesarewell-knownfortheirtraditionalmedicinal properties. The stems of several species of Dendrobium have beenusedinfolkChinesemedicinecalled“Shi-Hu”assourcesof tonic,antipyretic, astringent, and anti-inflammatory substances (Hu et al., 2008; Lam et al., 2015). Previous studies revealed that Dendrobium plants contain diverse groups of secondary metabolites and possess various biological activities, including cytotoxic,antioxidant,anticancer,antimalarial,antifibrotic, hypo-glycemic, and neuroprotective activities (Lam et al., 2015). A numberofphenoliccompoundsfromDendrobiumtortile,suchas 3,4-dihydroxy-5,4′-dimethoxybibenzyl,(2S)-eriodictyoland
den-drofalconerolA,showedstrong␣-glucosidaseinhibitoryactivity inourrecentinvestigation(Limpanitetal.,2016).
Dendrobium formosumRoxb.ex Lindl.isa rare orchidnative to Himalayas and Indochina. It has one of the largest flowers amongthedendrobes(Dohlingetal.,2008).Anearlierreportof
D. formosum described potential antitumor activityof its etha-nolicextract(PrasadandKoch,2014).However,priortothisstudy, there have been no reports of the phytochemical constituents andanti-diabeticandanti-obesityactivitiesofthisplant.Aspart ofourongoingresearchonbioactiveconstituentsfrom Dendro-bium species (Sukphan et al., 2014; Klongkumnuankarn et al., 2015), a methanol extract from the whole plant of D. formo-sumataconcentrationof50g/ml wasevaluatedandfoundto exhibit95%inhibitionagainstboth␣-glucosidaseandpancreatic lipaseenzymes.Inthiscommunication,wewishtoreportthefirst studyonthechemicalconstituentsofD.formosumandtheir␣ -glucosidaseandpancreaticlipaseinhibitoryactivities,aswellas theirglucoseuptakestimulatorypotential.
Materialandmethods
General
MassspectrawererecordedonaBrukermicroTOFmass spec-trometer(ESI-MS).NMRspectrawererecordedonaBrukerAvance DPX-300FT-NMRspectrometerMicrotiterplatereadingwas per-formed on a Perkin-Elmer VictorTM 1420 multilabel counter.
Vacuum-liquidcolumnchromatography(VLC)andcolumn chro-matography(CC)wereperformedonsilicagel60(Merck,Kieselgel 60,70–320m),silicagel60(Merck,Kieselgel60,230–400m) andSephadexLH-20(25–100m,GEHealthcare).
Chemicals
Alphaminimalessentialmedium(␣-MEM),fetalbovineserum (FBS)andpenicillin-streptomycin(10000IU/ml)werepurchased fromThermoFisher Scientific (GrandIsland, NY,USA). Glucose Oxidase (GO) assay kit, sodium dodecyl sulfate (SDS), 3-(4,5-dimethyl thiazol-2-yl)-5-diphenyl tetrazolium bromide (MTT),
␣-glucosidasefromSaccharomycescerevisiae,lipasefromporcine pancreas,4-methylumbelliferyloleate(4MUO),p-nitrophenyl-␣ -D-glucopyranoside(pNPG), acarboseand orlistatwere obtained fromSigmaAldrich(StLouis,MO,USA).Insulin(100IU/ml)was obtainedfromBiocon(Bangalore,India).Allotherchemicalsused wereofanalyticalgrade.
Celllinesandculturemedium
L6(Ratskeletalmuscle,ATCC®CRL-1458)cellculturewas pur-chasedfromtheAmericanTypeCultureCollection(Manassas,VA, USA).StockcellsofL6wereculturedin␣-MEMsupplementedwith 10%FBS,1%penicillin-streptomycin,thegrowthmedium,at37◦C
under5%CO2.
Plantmaterial
ThewholeplantofDendrobiumformosumRoxb.exLindl., Orchi-daceae, waspurchased from Jatujakmarket, Bangkok, Thailand in September2015. It wascollectedfromMaeSot district,Tak province,Thailand.PlantidentificationwasperformedbyProf. Tha-treePhadungcharoen(FacultyofPharmacy,RangsitUniversity).A voucherspecimen(BS-DF-092558)isdepositedattheDepartment ofPharmacognosyandPharmaceuticalBotany,Facultyof Pharma-ceuticalSciences,ChulalongkornUniversity.
Extractionandisolation
ThedriedandpowderedwholeplantofD.formosum(2kg)was extractedwithMeOH(3×10l)atroomtemperaturetogivea vis-cousmassofdriedextract(115g)afterremovalofthesolvent.This materialwassuspendedinwaterandthenpartitionedwithEtOAc andn-butanoltogiveanEtOAcextract(57g),abutanolextract (25g)andanaqueousextract(30g),respectively,after evapora-tionofthesolvent.TheEtOAcextractwassubjectedtovacuum liquidchromatography(silicagel,EtOAc-hexane,gradient)togive eightfractions(A-H).FractionF(6.8g)wasfractionatedona sil-ica gel column (EtOAc-hexane, gradient)to give nine fractions (FI-FIX).FractionFII(271mg)wasseparatedbycolumn chromatog-raphy(CC)oversilicagel,elutedwithaCH2Cl2-hexanegradientto
givefourfractions(FII1-FII4).Confusarin(1)(3mg)wasobtained fromfractionFII2.Hircinol(2)(15mg)wasobtainedfromfraction FII4(21mg)afterpurificationonSephadexLH-20(MeOH). Purifi-cation offractionFIII(85mg)onSephadex LH-20(MeOH)gave erianthridin(3)(45mg).FractionFV(648mg)wasseparatedbyCC (silicagel,CH2Cl2-hexane,gradient)togivesixfractions(FV1-FV6).
Gigantol(4)(94mg)wasobtainedfromfractionFV3.FractionFV2 (17mg)wasfurtherpurifiedonSephadexLH-20(MeOH)toafford nudol(5)(10mg).FractionFV5(193mg)wassubjectedtoCC(silica gel,EtOAc-hexane,gradient)andthenpurifiedonSephadex LH-20(MeOH)toyieldlusianthridin(6)(8mg).FractionFVI(361mg) was fractionatedby CC (silica gel, CH2Cl2-hexane, gradient) to
givesixfractions(FVI1-FVI6).Coelonin(7)(75mg)wasobtained from fraction FVI2. Dihydroconiferyl dihydro-p-coumarate (8) (25mg)andbatatasinIII(9)(18mg)wereyieldedfromfractions FVI4(57mg)andFVI5(29mg),respectively,afterpurificationon Sephadex LH-20(MeOH).FractionFVIII(272mg)wasseparated by CC(silica gel, EtOAc-CH2Cl2,gradient) and then purified on
Sephadex LH-20 (MeOH)toafford 2,5,7-trihydroxy-4-methoxy-9,10-dihydrophenanthrene (10) (22mg). Fraction G (10g) was fractionatedbyCCoversilicagel,elutedwithanEtOAc-hexane gra-dienttogivesevenfractions(GI-GVII).FractionGIII(508mg)was separatedonSephadexLH-20(MeOH)togiveeightfractions (GIII1-GIII8). Fraction GIII2 (51mg) wasfurther purified by CC (silica gel,CH2Cl2-hexane, gradient)toyieldmoscatilin(11)(5mg).
5-Methoxy-7-hydroxy-9,10-dihydro-1,4-phenanthrenequinone(12) (11mg)wasobtainedfromfractionGIII4(52mg)afterpurification byCC(silicagel,MeOH-CH2Cl2,gradient).
Confusarin(1):yellowamorphoussolid;C17H16O5;HR-ESI-MS
m/z299.0919[M−H]−(calc.forC
17H15O5 requires299.0919).Its
structurewasidentifiedbycomparisonofNMRdatawithpublished values(MajumderandKar,1987).
Hircinol(2):yellowamorphoussolid;C15H14O3;HR-ESI-MSm/z
265.0847 [M+Na]+ (calc.forC
15H14O3Narequires265.0840).Its
structurewasidentifiedbycomparisonofNMRdatawithpublished values(Fischetal.,1973).
Erianthridin(3):yellowamorphoussolid;C16H16O4;HR-ESI-MS
m/z295.0949[M+Na]+(calc.forC
16H16O4Narequires295.0946).Its
Gigantol(4):brownamorphoussolid;C16H18O4;HR-ESI-MSm/z
297.1111[M+Na]+ (calc.for C
16H18O4Narequires 297.1103).Its
structurewasidentifiedbycomparisonofNMRdatawithpublished values(Chenetal.,2008).
Nudol(5):yellowamorphoussolid;C16H14O4;HR-ESI-MSm/z
293.0793[M+Na]+ (calc.for C
16H14O4Narequires 293.0790).Its
structurewasidentifiedbycomparisonofNMRdatawithpublished values(Bhandarietal.,1985).
Lusianthridin (6): brown amorphous solid; C15H14O3;
HR-ESI-MS m/z 265.0847 [M+Na]+ (calc. for C
15H14O3Na requires
265.0841).ItsstructurewasidentifiedbycomparisonofNMRdata withpublishedvalues(Guoetal.,2007).
Coelonin(7):brownamorphous solid;C15H14O3;HR-ESI-MS
m/z265.0845[M+Na]+(calc.forC
15H14O3Narequires265.0841).Its
structurewasidentifiedbycomparisonofNMRdatawithpublished values(Majumderetal.,1982).
Dihydroconiferyldihydro-p-coumarate(8):yellowamorphous solid; C19H22O5; HR-ESI-MS m/z 353.1368 [M+Na]+ (calc. for
C19H22O5Narequires 353.1365).Its structure was identified by
comparisonofNMRdatawithpublishedvalues(Zhangetal.,2006). BatatasinIII(9):brownamorphoussolid;C15H16O3;HR-ESI-MS
m/z267.0995[M+Na]+(calc.for267.0997,C
15H16O3Na).Its
struc-turewasidentified bycomparison ofNMR datawithpublished values(SachdevandKulshreshtha,1986).
2,5,7-Trihydroxy-4-methoxy-9,10-dihydrophenanthrene (10): brown amorphous solid; C15H14O4; HR-ESI-MS m/z 281.0791
[M+Na]+(calc.forC
15H14O4Narequires281.0790).Itsstructurewas
identifiedbycomparisonofNMRdatawithpublishedvalues(Hu etal.,2008).
Moscatilin(11):brownamorphoussolid;C17H20O5;HR-ESI-MS
m/z327.1219[M+Na]+(calc.forC
17H20O5Narequires327.1208).Its
structurewasidentifiedbycomparisonofNMRdatawithpublished values(MajumderandSen,1987).
5-Methoxy-7-hydroxy-9,10-dihydro-1,4-phenanthrenequinone (12): red amorphous solid; C15H12O4;
HR-ESI-MSm/z279.0642[M+Na]+(calc.forC
15H12O4Narequires
279.0633).ItsstructurewasidentifiedbycomparisonofNMRdata withpublishedvalues(Sritularaketal.,2011).
Assayfor˛-glucosidaseinhibitoryactivity
Theassaywasperformedasdescribedpreviouslywithaslight modification(Sunetal.,2014).Theenzymeactivitywasassessedby monitoringthereleaseofp-nitrophenolfromthep-nitrophenyl-␣ -D-glucopyranoside(pNPG)substrate.Eachtestsamplewasinitially evaluatedataconcentrationof50g/ml,andthentwo-foldserial dilutionwasperformedforIC50determination.Inbrief,10lof
testsample(1.56–50g/ml)and40lof0.1U/ml␣-glucosidase weremixedandallowedtoreactat37◦Cfor10minina96-well
microtiterplate.Then,50lof2mMpNPGwasaddedandthe reac-tionmixturewasfurtherincubatedfor20min.Finally,100lof1M Na2CO3solutionwasaddedtoterminatethereaction.The
absorp-tionat405nmwasthenmeasuredusingamicroplatereader.The percentageof␣-glucosidaseinhibitoryactivitywascalculatedas follows:
% ␣-glucosidaseinhibitoryactivity=
Ac−As Ac ×100whereAcistheabsorbanceofthecontrolandAsistheabsorbance
ofthesample.Acarbose(15.6–1000g/ml)wasusedasa posi-tivecontrolandtreatedunderthesameconditionsasthesamples. Enzymeinhibitionreactionsforallsampleswerecarriedoutin trip-licate(n=3),andeachexperimentconsistedofthreerepetitions.
Theenzyme kineticsstudywasperformed usingthe double reciprocalLineweaver–Burkplot.Theexperimentwasconducted by varying the pNPG substrate concentration (0.125, 0.25, 1.0,
2.0mM)intheabsenceandpresenceofdifferenttestsample con-centrations(80and160M).
Assayforpancreaticlipaseinhibitoryactivity
Evaluation of pancreatic lipase inhibitory activity was done bymeasuringthereleaseof4-methylumbelliferone(4MU)from thesubstrate4-methylumbelliferyloleate(4MUO)(Sergentetal., 2012).Eachtestsamplewasinitiallyevaluatedataconcentrationof 50g/ml,andthentwo-foldserialdilutionwasperformedforIC50
determination.Briefly,25loftestsample(1.56–50g/ml),50l of0.25mM4MUO,and25lof0.125mg/mlpancreaticlipasewere mixedandincubatedatroomtemperaturefor30minina96-well microtiterplate.Then,100lof0.1Msodiumcitratewasadded tostopthereaction.Fluorescencefromtherelease of4MUwas measuredusingamicroplatereaderwithexcitationandemission wavelengthsof355and460nm,respectively.Thepercentageof pancreaticlipaseinhibitoryactivitywascalculatedasfollows:
% pancreaticlipaseinhibitoryactivity=
Ac−As Ac ×100whereAcistheabsorbanceofthecontrolandAsistheabsorbance
ofthesample.Orlistat(0.0008–50g/ml)wasusedasapositive controland treated under thesame conditions asthe samples. Enzymeinhibitionreactionsforallsampleswerecarried outin triplicate(n=3),andeach experimentconsistedofthree repeti-tions.Theenzymekineticsstudywasconductedusingthedouble reciprocalLineweaver–Burkanalysis.Theexperimentwas exam-inedbyvaryingthe4MUOsubstrateconcentration(0.0625,0.125, 0.25,0.5,1mM)intheabsenceandpresenceofdifferenttestsample concentrations(40and80M).
Glucose-uptakeassay
Theglucose-uptakeassaywasperformedfollowingthe meth-ods (Zhou et al., 2007; Jantaramanant et al., 2014) with some modification. Briefly, rat L6 myoblasts were maintained in ␣ -MEM supplementedwith 10%fetal bovine serum(FBS)and 1% penicillin-streptomycinat37◦Cunder5%CO
2.Fortreatmentwith
testcompounds,thecellswereplatedin24-wellplatesata den-sityof2×104 cells/well. Oncethecellreached90%confluence,
themediawasswitchedto␣-MEMwith2%FBSand1% penicillin-streptomycin(thedifferentiatemedium).Thecellswereallowedto differentiateintomyotubesfor5–7dayswithmediachangedevery otherday.Then,themyotubeswereincubatedat37◦Cunder5%
CO2for24hwiththevariousconcentrations(1,10and100g/ml)
oftestcompoundand500nMofinsulin.Thedifferentiatemedium plus0.1%DMSOwasusedasthediluentandnegativecontrol.Then, themediumwascollectedandanalyzedfortheglucoselevelusing aglucoseoxidaseassaykit.Theglucose-uptakewaspresentedas theratioofinsulinrelativewhichcalculatedasfollowed:
Theratioofinsulinrelative
=% glucoseuptakeofthetestcompounds
% glucoseuptakeofinsulin
Cytotoxicity
Continuously, after 24h of the cell treatment for the glu-cosedetermination,cytotoxicitytestwasperformedfollowingthe methodof Rissetal.withsomemodification(Rissetal.,2004). The medium was adjusted to 200l per well. The cells were treatedwith20loftheMTTsolution(5mg/ml)andincubatedat 37◦Cunder5%CO
2for2h.Todissolvetheformazancrystal,each
glacialaceticacid,16%w/vSDSindistilledwater)andshakenfor 20min.Then,thesupernatantswerecollectedandmeasuredfor absorbanceat595nmusingmodel550microplatereader(Biorad). Thecytotoxicitywasshownasthe%cellviability.
Resultsanddiscussion
Asmentionedearlier,aMeOHextractpreparedfromthewhole plantof D.formosum,at a concentrationof50g/ml, exhibited potentinhibitionof95%againstboth␣-glucosidaseandpancreatic lipaseenzymes,respectively.Theextractwasthenseparatedby sol-ventpartitiontogiveanEtOAc,abutanolandanaqueousextracts. Theseextractswereevaluatedfor their␣-glucosidaseand pan-creaticlipaseinhibitoryactivities.OnlytheEtOAcextractshowed stronginhibitoryeffectsataconcentrationof50g/ml,with83% againstpancreaticlipaseand96%against␣-glucosidaseenzymes whilethebutanolandanaqueousextractwereinactive(lessthan 50%inhibition).Therefore,theEtOAcextractwasselectedfor fur-therchemicalinvestigation.Throughchromatographicseparation, twelvephenoliccompoundswereidentified.
Currently,onlyafew␣-glucosidaseinhibitors,suchasacarbose andvoglibose,havebeenapprovedforthetreatmentofdiabetes mellitus,andtheirstructuresaremainlycomposedofsugar moi-eties(Yinetal.,2014).Theproductionprocessesoftheseinhibitors are,however,rather complexandinvolve multistepprocedures (Yinetal.,2014).Withregardtopancreaticlipaseenzyme,orlistat isapowerfulinhibitor,clinicallyusedforthetreatmentofobesity (BirariandBhutani,2007;Jangetal.,2008).In recentyears,the numbersofreportsonnaturalcompoundswith␣-glucosidaseand lipaseinhibitoryactivitieshavecontinuouslyincreased(Kimetal., 2000;Jangetal.,2008;Yinetal.,2014).Manyresearcheshavebeen focusedonthesearchforalternative␣-glucosidaseinhibitorswith non-sugarcorestructure,particularlythepolyphenolsduetotheir abundantavailabilityinthenatureandtheirpromisingbiological activities(Yinetal.,2014).
Table1
IC50valuesofcompounds1–12isolatedfromD.formosumfor␣-glucosidaseand
pancreaticlipaseinhibitoryactivities.
Compounds ␣-Glucosidase(M) Pancreaticlipase(M)
Confusarin(1) 189.78±1.11 154.61±8.58
Hircinol(2) NA NA
Erianthridin(3) NA NA
Gigantol(4) NA NA
Nudol(5) NA NA
Lusianthridin(6) NA NA
Coelonin(7) NA NA
Dihydroconiferyl dihydro-p-coumarate(8)
NA NA
BatatasinIII(9) NA NA
2,5,7-Trihydroxy-4-methoxy-9,10-dihydrophenanthrene (10)
NA NA
Moscatilin(11) NA NA
5-Methoxy-7-hydroxy-9,10-
dihydro-1,4-phenanthrenequinone (12)
126.88±0.66 69.45±10.14
Acarbose 745.9±88.4 –
Orlistat – 0.013±0.004
NAmeansnoinhibitoryactivity.
In this study, compounds 1–12 were evaluated for their
␣-glucosidaseandpancreaticlipaseinhibitoryactivities.Each com-poundwasinitially testedat aconcentrationof 50g/ml.Only
compounds1 and12 showed>50%inhibitionand werefurther
analyzedtodeterminetheirIC50values(Table1).Duetoitshigh
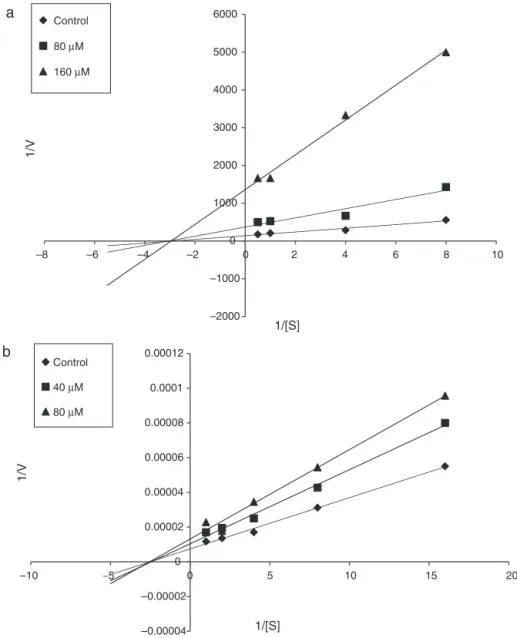
potencyandavailability,compound12wasinvestigatedfor inhibi-tionmechanismsagainst␣-glucosidaseandpancreaticlipaseusing theLineweaver–Burkplots,andkineticparameterswithrespectto thetwoenzymeswereobtained,aslistedinTable2.
Intheassayof␣-glucosidaseinhibitoryactivitywithpNPGas thesubstrate,themaximumvelocity(Vmax)valuewasdetermined
as 7.10×10−3 A
405/min, and the Michaelis–Menten constant
(Km)as0.3mM(Fig.1A).Thepresenceofcompound12at
differ-enceconcentrations(80Mand160M)reducedtheVmaxvalues
to2.70×10−3and7.35×10−4respectively,butdidnotaffectK mof
theenzyme.Theseresultssuggestedthat12isanon-competitive inhibitorofthisenzyme.
In experiments on pancreatic lipase inhibitory activity with 4MUOasthesubstrate,theVmaxvaluewasfoundtobe1.35×105 A355,460/min,andtheKmvaluewas0.4mM(Fig.1B).Thepresence
ofcompound12ataconcentrationof40Mand80Mdecreased theVmaxvaluesto9.60×104and7.60×104,respectively,buthad
noeffectonKm.Theseobservationsindicatedthat12isalsoa
non-competitiveinhibitorofpancreaticlipase.
The non-competitive mode of ␣-glucosidase and pancreatic lipaseinhibitionsobtainedfromtheLineweaver–Burkplots sug-geststhatcompound12doesnotcompetewithpNPGand4MUO substratesforbindingtotheactivesiteofenzymes,butitwould ratherbindtoothersitesoftheenzymestoretardthecarbohydrate andlipiddigestion(Kazeemetal.,2013;Martinez-Gonzalezetal., 2017).TheKmvalueswereunaffectedbecausetheinhibitor(12)did
notcauseanychangesattheactivesite.TheVmaxofthereactions
decreasedbecausethisnon-competitiveinhibitor(12)reducedthe quantityof activeenzymes(Balbaaand ElAshry,2012;Kazeem etal.,2013).
Table2
Kineticparametersinof␣-glucosidaseandpancreaticlipaseinthepresenceof5-methoxy-7-hydroxy-9,10-dihydro-1,4-phenanthrenequinone(12).
Inhibitors ␣-Glucosidase Pancreaticlipase
Dose(M) Vmax(A405/min) Km(mM) Dose(M) Vmax(A355,460/min) Km(mM)
None – 7.10×10−3 0.3 – 1.35×105 0.4
Compound12 80 2.70×10−3 0.3 40 9.60×104 0.4
160 7.35×10−4 0.3 80 7.60×104 0.4
concentrationsofthesubstrateascompared tothecompetitive inhibitorssuchasacarboseandorlistat,whichmayrequirelarge amountsofinhibitorstocompletewiththesubstrate(Ghadyale etal.,2012).
ThetreatmentforpatientswithtypeIIdiabetesrequires sev-eral types of medications to control the blood glucose level (Nathanetal.,2009).Thisimpliesthatnewglycemiccontrolagents withnovelmechanismsareindeedneeded.RecentstudiesinL6 skeletalmusclecellsshowedthatthestilbenoidsresveratroland piceatannoldisplayedantidiabeticactivitybypromotingglucose uptake (Breen et al., 2008;Minakawa et al.,2012).This ledus toinvestigatethestilbenederivatives1–12 inthehopeof find-ingnewglucose-uptakestimulators.Ourpreliminaryevaluationof
thesecompoundsrevealedthathircinol(2),lusianthridin(6)and moscatilin(11)showedhigher glucoseuptake stimulationthan insulin (insulinrelative value >1.0)(Fig.2A).However, hircinol (2)displayedtheactivityattheconcentrationlevelthatshowed toxicityonL6myotubeswhereaslusianthridin(6)andmoscatilin (11)didnot(Fig.2B).Whenweconsideredtheglucose-uptake stim-ulationpotenciesofthetestcompoundsatnon-toxicconcentration levels(≥100%cellviability),wefoundthatcompound6at1g/ml exhibitedrecognizableglucose-uptakestimulatoryactivity,with insulinrelativevalue≥0.8.
Maintainingthebalanceinbloodglucoselevelisanimportant processinhumanphysiology,andthisisregulatedbyhormones suchasinsulinandglucagon(Hanhinevaetal.,2010).Failureof
a
b
–2000 –1000 0 1000 2000 3000 4000 5000 6000
10 8
6 4 2 0 –2 –4 –6 –8
1/
V
1/[S]
Control
80 µM
160 µM
–0.00004 –0.00002 0 0.00002 0.00004 0.00006 0.00008 0.0001 0.00012
–10 –5 0 5 10 15 20
1/
V
1/[S]
Control
40 µM
80 µM
a
b
0 0.2 0.4 0.6 0.8 1 1.2 1.4
100 10 1 100 10 1 100 10 1 100 10 1 100 10 1
Compound 11 Compound 6
Compound 5 Compound 4
Compound 2
Insulin relative
Concentration (µg/ml)
0 20 40 60 80 100 120 140
100 10 1 100 10 1 100 10 1 100 10 1 100 10 1
Compound 11 Compound 6
Compound 5 Compound 4
Compound 2
% Cell viability
Concentration (µg/ml)
Fig.2. Stimulationofglucoseuptake(A)andcytotoxicity(B)ofL6myotubesbyhircinol(2),gigantol(4),nudol(5),lusianthridin(6)andmoscatilin(11)atadifferent concentrationintherangeof1–100g/ml.
thiscontrolcancauseanincidenceofmetabolicsyndrome(MetS), aclusterofmetabolicabnormalitiesthatincludeinsulinresistance, hyperglycaemia or type II diabetes, obesity, hypertension, and dyslipidemia(Hanhinevaet al.,2010;Lietal.,2013;Mohamed, 2014). MetS is known to increase the risk of atherosclerotic cardiovasculardisease,eventuallyleadingtomorbidityand mor-tality. Numerous dietary components and over 800 plants are foundtopreventorreduce MetS byassisting thecarbohydrate metabolism and glucose homeostasis through the inhibition of carbohydrate digestion, stimulation of insulin secretion from thepancreatic -cells, suppression of glucose release fromthe liver storage, activation of insulin receptors and improvement glucoseuptakeintheperipheraltissues(Hanhinevaetal.,2010; Mohamed,2014).Inthisstudy,theinhibitionofcarbohydrateand lipidhydrolyzingenzymesbycompound12mayreducetherate oftheircleavage,componentreleaseandabsorptioninthesmall intestine,andconsequentlysuppresspostprandialhyperglycemia andhyperlipidemia.Moreover,compounds6and11atnon-toxic concentrations may find a role in helping to control glucose metabolismbystimulatingglucoseuptakeinskeletalmuscle,the largestsiteofglucosedisposal,leadingtothepreventionofMetS
and type II diabetes.However, furtherinvestigations in animal modelsarestillneededtoconfirmthesesuggestions.
Conclusions
So far, there have been a few attempts to investigate the constituents of Dendrobium for ␣-glucosidase and pancreatic lipase inhibitory activities,andglucose-lowering effects. In this study,thechromatographicseparationofthemethanolicextract from the whole plant of D. formosum led tothe isolation and identificationoftwelvecompounds,includingconfusarin(1), hir-cinol(2),erianthridin(3),gigantol(4),nudol(5),lusianthridin(6), coelonin(7),dihydroconiferyldihydro-p-coumarate(8),batatasin III (9), 2,5,7-trihydroxy-4-methoxy-9,10-dihydrophenanthrene (10),moscatilin(11),and 5-methoxy-7-hydroxy-9,10-dihydro-1,4-phenanthrenequinone(12).Amongthesecompounds,compounds
Withregardtoglucose-uptakestimulationeffects, lusianthridin (6) and moscatilin (11) at non-toxic concentration (10 and 100g/ml,respectively)hadhigheractivitythaninsulin.In addi-tion, lusianthridin (6) at 1g/ml showed recognizable glucose uptakestimulationeffectwithouttoxicityonL6myotubes.Tothe bestofourknowledge,thisisthefirstreportonthephytochemical investigationandinvitrostudiesonglucoseuptakestimulation, and ␣-glucosidase and pancreatic lipase inhibitory activities of thisplant.Theresultsfromourinvestigationhaveformedabasis forthefuturedevelopmentofanti-diabeticandanti-obesitydrugs.
Authors’contributions
PIcontributedinisolationandpurificationofthecompounds andrunningthelaboratorywork.NCandCScontributionincluded theanalysisofthedataand draftedthepaper.TKandWP con-tributedincell-basedassayofglucoseuptakeandcytotoxicity.KL andBScontributedinsupervisionofthelaboratoryworkand crit-icalreadingofthemanuscript.Alltheauthorshavereadthefinal manuscriptandapprovedthesubmission.
Conflictsofinterest
Theauthorsdeclarenoconflictsofinterest.
Ethicaldisclosures
Protectionofhumanandanimalsubjects. Theauthorsdeclare thatnoexperimentswereperformedonhumansoranimalsfor thisstudy.
Confidentialityofdata. Theauthorsdeclarethatnopatientdata appearinthisarticle.
Righttoprivacyandinformedconsent. Theauthorsdeclarethat nopatientdataappearinthisarticle.
Acknowledgements
Thisworkwassupportedbya 2016researchgrantfromthe National Research Council of Thailand (NRCT) through Chula-longkornUniversity(GB-A600183303).WethankTheResearch Instrument Center of Faculty of Pharmaceutical Sciences, Chu-lalongkornUniversity,for providing researchfacilitiesand Prof. ThatreePhadungcharoen(RangsitUniversity,Bangkok,Thailand) forplantidentification.
References
AmericanDiabetesAssociation,2006.Diagnosisandclassificationofdiabetes mel-litus.DiabetesCare33,62–69.
Balbaa,M.,ElAshry,E.S.H.,2012.Enzymeinhibitorsastherapeutictools.Biochem. Physiol.1,1–8.
Bhandari,S.R.,Kapadi,A.H.,Majumder,P.L.,Joardar,M.,Shoolery,J.N.,1985.Nudol, aphenanthreneoftheorchidsEulophianuda,EriacarinataandEriastricta. Phy-tochemistry24,801–804.
Birari,R.B.,Bhutani,K.K.,2007.Pancreaticlipaseinhibitorsfromnaturalsources: unexploredpotential.DrugDiscov.Today12,879–889.
Breen,D.M.,Sanli,T.,Giacca,A.,Tsiani,E.,2008.Stimulationofmusclecell glu-coseuptakebyresveratrolthroughsirtuinsandAMPK.Biochem.Biophys.Res. Commun.374,117–122.
Chen,Y.,Xu,J., Yu, H.,Qing,C., Zhang,Y.,Wang, L.,Liu,Y., Wang,J., 2008.
Cytotoxic phenolics from Bulbophyllum odoratissimum. Food Chem. 107, 169–173.
Choi,S.S.,Cha,B.Y.,Choi,B.K.,Lee,Y.S.,Yonezawa,T.,Teruya,T.,Nagai,K.,Woo,J.T., 2013.Fargesin,acomponentofFlosMagnoliae,stimulatesglucoseuptakeinL6 myotubes.J.Nat.Med.67,320–326.
Dohling,S.,Kumaria,S.,Tandon,P.,2008.Optimizationofnutrientrequirementsfor asymbioticseedgerminationofDendrobiumlongicornuLindl.andD.formosum Roxb.Proc.IndianNat.Sci.Acad.74,167–171.
Fisch,M.H.,Flick,B.H.,Arditti,J.,1973.Structureandantifungalactivityofhircinol, loroglossolandorchinol.Phytochemistry12,437–441.
Ghadyale,V.,Takalikar,S.,Haldavnekar,V.,Arvindekar,A.,2012.Effectivecontrol ofpostprandialglucoselevelthroughinhibitionofintestinalalphaglucosidase byCymbopogonmartinii(Roxb.).Evid.BasedComplement.Alternat.Med.,6p., ArticleID841752.
Guo, X.Y., Wang, J., Wang, N.L., Kitanaka, S., Yao, X.S., 2007. 9,10-DihydrophenanthrenederivativesfromPholidotayunnanensisandscavenging activityonDPPHfreeradical.J.AsianNat.Prod.Res.9,165–174.
Hanhineva,K.,Törrönen,R.,Bondia-Pons,I.,Pekkinen,J.,Kolehmainen,M., Mykkä-nen,H.,Poutanen,K.,2010.Impactofdietarypolyphenolsoncarbohydrate metabolism.Int.J.Mol.Sci.11,1365–1402.
Hu,J.M.,Chen,J.J.,Yu,H.,Zhao,Y.X.,Zhou,J.,2008.Fivenewcompoundsfrom Dendrobiumlongicornu.PlantaMed.74,535–539.
Jang,D.S.,Lee,G.Y.,Kim,J.,Lee,Y.M.,Kim,J.M.,Kim,Y.S.,Kim,J.S.,2008.Anew pan-creaticlipaseinhibitorisolatedfromtherootsofActinidiaarguta.Arch.Pharm. Res.31,660–670.
Jantaramanant,P.,Sermwittayawong,D.,Noipha,K.,Hutadilok-Towatana,N., Witit-suwannakul,R.,2014.-Glucan-containingpolysaccharideextractfromthe greyoystermushroom[Pleurotussajor-caju(Fr.)Sing.]stimulatesglucoseuptake bytheL6myotubes.Int.FoodRes.J.21,779–784.
Kazeem,M.I.,Adamson,J.O.,Ogunwande,I.A.,2013.Modesofinhibitionof␣ -amylaseand␣-glucosidasebyaqueousextractofMorindalucidaBenthleaf. BiomedRes.Int.,http://dx.doi.org/10.1155/2013/527570.
Kim,J.S.,Kwon,C.S.,Son,K.H.,2000.Inhibitionofalpha-glucosidaseandamylaseby luteolin,aflavonoid.Biosci.Biotechnol.Biochem.64,2458–2461.
Klongkumnuankarn,P.,Busaranon,K.,Chanvorachote,P.,Sritularak,B., Jongbun-prasert,V.,Likhitwitayawuid,K.,2015.Cytotoxicandantimigratoryactivitiesof phenoliccompoundsfromDendrobiumbrymerianum.Evid.BasedComplement. Alternat.Med.,http://dx.doi.org/10.1155/2015/350410.
Lam, Y., Ng, T.B., Yao, R.M., Shi, J., Xu, K., Sze, S.C.W., Zhang, K.Y., 2015. Evaluationofchemicalconstituentsandimportantmechanismof pharmaco-logicalbiologyinDendrobiumplants.Evid.BasedComplement.Alternat.Med.,
http://dx.doi.org/10.1155/2015/841752.
Li,M.,Li,Y.,Liu,J.,2013.Metabolicsyndromewithhyperglycemiaandtheriskof ischemicstroke.YonseiMed.J.54,283–287.
Limpanit,R.,Chuanasa,T.,Likhitwitayawuid,K.,Jongbunprasert,V.,Sritularak,B., 2016.␣-GlucosidaseinhibitorsfromDendrobiumtortile.Rec. Nat.Prod.10, 609–616.
Majumder,P., Laha,S.,Datta, N.,1982.Coelonin,a 9,10-dihydrophenanthrene fromtheorchidsCoelogyneochraceaandCoelogyneelata.Phytochemistry21, 478–480.
Majumder,P.L.,Joardar,M.,1985.Erianthridin,anew9,10-dihydrophenanthrene derivativefromtheorchidsEria carinata&Eria sticta. IndianJ.Chem.24, 1192–1194.
Majumder,P.L.,Kar,A.,1987. Confusarinandconfusaridin,twophenanthrene derivativesoftheorchidEriaconfusa.Phytochemistry26,1127–1129.
Majumder,P.L.,Sen,R.C.,1987.Moscatilin,abibenzylderivativefromtheorchid Dendrobiummoscatum.Phytochemistry26,2121–2124.
Martinez-Gonzalez,A.I.,Díaz-Sánchez,A.G.,Rosa,L.A.,Vargas-Requena,C.L., Bustos-Jaimes,I.,Alvarez-Parrilla,E.,2017.Polyphenolic compoundsanddigestive enzymes:invitronon-covalentinteractions.Molecules22,1–27.
Minakawa,M.,Miura,Y.,Yagasaki,K.,2012.Piceatannol,aresveratrolderivative, promotesglucoseuptakethroughglucosetransporter4translocationtoplasma membraneinL6myocytesandsuppressesbloodglucoselevelsintype2diabetic modeldb/dbmice.Biochem.Biophys.Res.Commun.422,469–475.
Mohamed,S.,2014.Functionalfoodsagainstmetabolicsyndrome(obesity, dia-betes,hypertensionanddyslipidemia)andcardiovasulardisease.TrendsFood Sci.Technol.35,114–128.
Nathan,D.M.,Buse,J.B.,Davidson,M.B.,Ferrannini,E.,Holman,R.R.,Sherwin,R., Zinman,B.,2009.Medicalmanagementofhyperglycemiaintype2diabetes:a consensusalgorithmfortheinitiationandadjustmentoftherapy.DiabetesCare 32,193–203.
Peng,X.,Zhang,G.,Liao,Y.,Gong,D.,2016.Inhibitorykineticsandmechanismof kaempferolon␣-glucosidase.FoodChem.190,207–215.
Prasad,R.,Koch,B.,2014.AntitumoractivityofethanolicextractofDendrobium formosuminT-celllymphoma:aninvitroandinvivostudy.BiomedRes.Int. 2014,1–11.
Riss,T.L.,Moravec,R.A.,Niles,A.L.,Benink,H.A.,Worzella,T.J.,Minor,L.,2004.Cell ViabilityAssays,AssayGuidanceManual.EliLilly&CompanyandtheNational CenterforAdvancingTranslationalSciences.
Rosak,C.,Mertes,G.,2012.Criticalevaluationoftheroleofacarboseinthetreatment ofdiabetes:patientconsiderations.DiabetesMetab.Syndr.Obes.5,357–367.
Sachdev,K.,Kulshreshtha,D.K.,1986.PhenolicconstituentsofCoelogyneovalis. Phy-tochemistry25,499–502.
Sergent,T.,Vanderstraeten,J.,Winand,J.,Beguin,P.,Schneider,Y.J.,2012.Phenolic compoundsandplantextractsaspotentialnaturalanti-obesitysubstances.Food Chem.135,68–73.
Sritularak,B.,Anuwat,M.,Likhitwitayawuid,K.,2011.Anewphenanthrenequinone fromDendrobiumdraconis.J.AsianNat.Prod.Res.13,251–255.
Sukphan,P.,Sritularak,B.,Mekboonsonglarp,W.,Lipipun,V.,Likhitwitayawuid,K., 2014.ChemicalconstituentsofDendrobiumvenustumandtheirantimalarialand anti-herpeticproperties.Nat.Prod.Commun.9,825–827.
Tushuizen,M.E.,Bunck,M.C.,Pouwels,P.J.,Bontemps,S.,Waesberghe,J.K.V., Schind-helm,R.K.,Mari,A.,Heine,R.J.,Diamant,M.,2007.Pancreaticfatcontentand
-cellfunctioninmenwithandwithouttype2diabetes.DiabetesCare30, 2916–2921.
Yang,M.H.,Chin,Y.W.,Yoon,K.D.,Kim,J.,2014.Phenoliccompoundswithpancreatic lipaseinhibitoryactivityfromKoreanyam(Dioscoreaopposita).J.EnzymeInhib. Med.Chem.29,1–6.
Yap,A.,Nishiumi,S.,Yoshida,K.I.,Ashida,H.,2007.RatL6myotubesasaninvitro modelsystemtostudyGLUT4-dependentglucoseuptakestimulatedbyinositol derivatives.Cytotechnology55,103–108.
Yin,Z.,Zhang,W.,Feng,F.,Zhang,Y.,Kanga,W.,2014.␣-Glucosidaseinhibitors isolatedfrommedicinalplants.FoodSci.Hum.Wellness3,136–174.
You,Q.,Chen,F.,Wang,X.,Jiang,Y.,Lin,S.,2012.Anti-diabeticactivitiesofphenolic compoundsinmuscadineagainstalpha-glucosidaseandpancreaticlipase.LWT –FoodSci.Technol.46,164–168.
Zhang,X.,Gao,H.,Wang,N.,Yao,X.,2006.PhenoliccomponentsfromDendrobium nobile.Chin.Trad.Herb.Drugs37,652–655.