O R I G I N A L A R T I C L E
Lowered quality of life in mood disorders is associated with
increased neuro
‐
oxidative stress and basal thyroid
‐
stimulating
hormone levels and use of anticonvulsant mood stabilizers
Caroline Sampaio Nunes MD, Doctor
1|
Michael Maes MD, PhD, Prof
2,3,4,5|
Chutima Roomruangwong MD, Associate Prof
3|
Juliana Brum Moraes MD, Lecturer
2|
Kamila Landucci Bonifacio MSc, Doctor
1|
Heber Odebrecht Vargas MD, PhD, Prof
1,2,6|
Decio Sabbatini Barbosa PhD, Prof
2|
George Anderson PhD, Director
7|
Luiz Gustavo Piccoli de Melo MD, Psychiatrist
1,2|
Stoyanov Drozdstoj MD, PhD, Prof
4|
Estefania Moreira PhD, Prof
2|
André F. Carvalho MD, PhD, Associate Prof
8,9|
Sandra Odebrecht Vargas Nunes MD, PhD, Prof
1,21Department of Psychiatry, Health Sciences
Center, Health Sciences Center, State University of Londrina, Londrina, Paraná, Brazil
2Health Sciences Graduation Program, Health
Sciences Center, State University of Londrina, Londrina, Paraná, Brazil
3Department of Psychiatry, Faculty of
Medicine, Chulalongkorn University, Bangkok, Thailand
4Department of Psychiatry, Medical
University of Plovdiv, Plovdiv, Bulgaria
5IMPACT Strategic Research Centre, Deakin
University, Geelong, VIC, Australia
6Center for Approach and Treatment for
Smokers, University Hospital, State University of Londrina, Londrina, Paraná, Brazil
7Clinical Research Center Scotland & London,
London, UK
8Department of Psychiatry, University of
Toronto, Toronto, ON, Canada
9Centre for Addiction & Mental Health,
Toronto, ON, Canada
Correspondence
Michael Maes, MD, PhD, Department Psychiatry, Faculty of Medicine,
Chulalongkorn University, Bangkok, Thailand. Email: dr.michaelmaes@hotmail.com
Funding information
Ministry for Science and Technology of Brazil (CNPq), Grant/Award Numbers: 465928/ 2014‐5 and 470344/2013‐0; Health Sciences Postgraduate Program at Londrina State University, Paraná, Brazil (UEL)
Abstract
Rationale, aims:
Major affective disorders including bipolar disorder (BD) and major
depressive disorder (MDD) are associated with impaired health
‐
related quality of life
(HRQoL). Oxidative stress and subtle thyroid abnormalities may play a
pathophysio-logical role in both disorders. Thus, the current study was performed to examine
whether neuro
‐
oxidative biomarkers and thyroid
‐
stimulating hormone (TSH) levels
could predict HRQoL in BD and MDD.
Methods:
This cross
‐
sectional study enrolled 68 BD and 37 MDD patients and 66
healthy controls. The World Health Organization (WHO) QoL
‐
BREF scale was used
to assess 4 QoL subdomains. Peripheral blood malondialdehyde (MDA), advanced
oxidation protein products, paraoxonaxe/CMPAase activity, a composite index of
nitro
‐
oxidative stress, and basal TSH were measured.
Results:
In the total WHOQoL score, 17.3% of the variance was explained by
increased advanced oxidation protein products and TSH levels and lowered CMPAase
activity and male gender. Physical HRQoL (14.4%) was associated with increased MDA
and TSH levels and lowered CMPAase activity. Social relations HRQoL (17.4%) was
predicted by higher nitro
‐
oxidative index and TSH values, while mental and
environ-ment HRQoL were independently predicted by CMPAase activity. Finally, 73.0% of
the variance in total HRQoL was explained by severity of depressive symptoms, use
of anticonvulsants, lower income, early lifetime emotional neglect, MDA levels, the
presence of mood disorders, and suicidal ideation.
Conclusions:
These data show that lowered HRQoL in major affective disorders
could at least in part result from the effects of lipid peroxidation, protein oxidation,
lowered antioxidant enzyme activities, and higher levels of TSH.
DOI: 10.1111/jep.12918
K E Y W O R D S
bipolar disorder, child abuse, depressive disorder, quality of life, suicide, tobacco use disorder
1
|I N T RO D U C T I O N
The World Health Organization (WHO) reported that bipolar disorder (BD) and major depressive disorder (MDD) are among the 10 leading sources of disability among young adults (aged 15 to 44 years) world-wide in either gender.1,2 Approximately 30% of bipolar‐1 (BP1) patients show severe impairment in their work role, while 15% of BP2 patients have some inter‐episode dysfunction.3 Patients with mood disorders show difficulties in work productivity and functioning, which may contribute to an impairment of health‐related quality of life (HRQoL).4-12Evidence suggests that HRQoL may be lower among BD patients7,13and may be associated with the use of certain psychotro-pic medications14 at least in part due to treatment
‐emergent side effects.15-17Furthermore, a growing body of evidence indicates that exposure to early lifetime trauma (ELT) may be related to worse clinical outcomes among patients with mood disorders.18-20
There is evidence that BD and MDD are associated with an aberrant activation of neuro‐oxidative pathways, including increased levels of malondialdehyde (MDA), indicating membrane damage medi-ated by free radicals–inducing peroxidation of polyunsaturated fatty acids.21-25Activated neuro
‐oxidative pathways promote neurotoxicity, excitotoxicity, apoptosis, attenuated neurogenesis, and autoimmu-nity.21,22 Moreover, major depression is associated with increased levels of advanced oxidation protein products (AOPP) indicating peroxynitrite and hypochlorous acid–induced protein oxidation.26,27 In patients with mood disorders, lowered activity levels of paraoxo-nase/CMPAase (PON1), an antioxidant enzyme, are inversely associ-ated with HRQoL.28,29
Furthermore, hypothalamic‐pituitary‐thyroid activity shows modest changes in MDD (lowered serum basal thyroid‐stimulating hormone [TSH] levels) and BD (increase in basal TSH),30-32while sub-clinical hyperthyroidism and hypothyroidism may impact HRQoL.33,34 Treatments for subclinical hypothyroidism with thyroxine may improve HRQoL.35 Nevertheless, there are no data as to whether increased nitro‐oxidative stress, increased MDA and AOPP levels, and alterations in basal TSH levels could impact HRQoL among patients with mood disorders.
Therefore, the current study aims to explore the role of bio-markers of nitro‐oxidative stress and TSH serum levels as putative pre-dictors of HRQoL among patients with BD and MDD and to determine whether those associations are independent of clinical characteristics of mood disorders and confounder sociodemographic variables.
2
|M E T H O D S
2.1
|Study population
This study included outpatients with BD (n = 68) and MDD (n = 37) who were recruited at the Psychiatric Outpatient Clinics at the State
University of Londrina (UEL), Brazil. We enrolled 66 healthy controls recruited from staff at UEL, Londrina, Brazil. Participants were men and women aged 18 to 65 years. All participants provided written informed consent to take part in the study, and the research procedures of the current study was approved by the Ethics Research Committee (number CAAE 34935814.2.0000.5231).
Exclusion criteria for patients were (1) other axis‐1 DSM‐IV‐TR diagnoses besides BD/MDD/tobacco use disorder (TUD) and anxiety disorders, (2) a current (hypo)manic episode, and (3) mood disorders due to a general medical condition or substance/drug use.36Controls were excluded if any major axis‐1 psychiatric disorder was evident. Further exclusion criteria for both patients and controls were the presence of (1) neurodegenerative disorders; (2) medical diseases including heart failure, neoplasm, autoimmune disorders, and infectious disorders; and (3) pregnancy. All patients and controls were assessed by trained research psychiatrists. Diagnoses of BD/MDD/current smoking TUD were made using the diagnostic criteria of the DSM‐IV‐TR by means of the Structured Clinical Inter-view for the DSM‐IV, translated and validated for the Brazilian population.37
2.2
|Measures
We measured HRQoL using the World Health Organization Quality of Life instrument‐abbreviated version (WHOQoL‐BREF).38 This instrument comprises 26 items and measures 4 HRQoL subdomains: (1) physical health: activities of daily living, dependence on medicinal substances and medical aids, energy and fatigue, mobility, pain and dis-comfort, sleep and rest, and work capacity; (2) psychological health: bodily image and appearance, negative/positive feelings, self‐esteem, spirituality/religion/personal beliefs, thinking, learning, and memory and concentration; (3) social relationships: personal relationships, social support, and sexual activity; and (4) environment: financial resources, freedom, physical safety and security, health and social care (accessibility and quality), home environment, opportunities for acquir-ing new information and skills, participation in, and opportunities for, recreation/leisure activities, and physical environment (pollution, noise, traffic, climate, and transport). We used the validated Brazilian Portuguese version of the WHOQoL‐BREF.39The raw scores on the 4 domains were used according to the WHOQoL‐BREF manual.38 Total HRQoL scores were estimated by summing up the raw scores of the 4 domains.
including number of lifetime depressive and manic episodes and use of psychopharmacological drugs.
Severity of depression was measured using the 17‐item version of the Hamilton Depression Rating Scale (HAM‐D).40 Exposure to ELT was assessed using the Childhood Trauma Questionnaire41 com-prising 5 domains, namely, sexual abuse, physical abuse, emotional abuse, emotional neglect, and physical neglect. The Columbia– Suicide Severity Rating Scale42 was used to quantify the severity of current suicidal ideation as well as prior suicide attempts. We used the ASSIST (Alcohol, Smoking and Substance Involvement Screening Test).43,44Body mass index (BMI) was computed as weight (in kg) divided by square of height (in m2). We made the diagnosis of metabolic syndrome using the International Diabetes Federation criteria (see Supporting Information). Blood was sampled at 8.00 am after an overnight fast for the assay of biomarkers. The assays have been described previously45-52 and are shown in Supporting Information.
2.3
|Statistical analyses
Differences in socio‐demographic and clinical characteristics between groups were checked using analyses of variance or analyses of contin-gency tables (χ2 tests). The effects of 3 study groups, divided accord-ing to the q25 and q75 WHOQoL‐BREF total score values, were examined, giving low (<74.30, n = 57), medium (between 74.30 and 92.66, n = 57), and high (>92.66, n = 57) WHOQoL‐BREF total score values. The cut‐off values were chosen in order to obtain an equal number of participants in each group (n = 57). The univariate statisti-cal results of analyses of variance and analyses of contingency analy-ses contrasting these 3 WHOQol‐BREF subgroups (see Table 1) were used—together with Pearson or point‐biserial–based intercorrelation matrixes between the variables—to select the explanatory variables that were used as determinants of independent association with the dependent variable (ie, WHOQoL‐BREF and domains) in the ultimate multivariate generalized linear model (GLM) and logistic regression analyses. Therefore, we did not analyse multiple post hoc compari-sons among the 3 treatment means but usedP‐corrections to check the multiple statistical analyses. The main aim of the study is to define the most significant biomarker predictors of the 4 HRQoL domains (while controlling for clinical mood disorder characteristics), and therefore, the primary outcome measurements are the 4 HRQoL domain scores. Multivariate GLM analyses were used to examine the effects of significant explanatory variables on the 4 WHOQoL‐ BREF domain scores. Associations between 1 dependent variable (total WHOQoL‐BREF score) and multiple explanatory variables were assessed using linear regression analysis and univariate GLM analyses (to compute the partial eta squared). Binary logistic regression analy-ses were used to delineate the most significant predictors of the group with the low WHOQoL‐BREF total score as a dependent vari-able and the remaining subjects as reference group. Malondialdehyde and AOPP were assessed in logarithmic transformation. Results of regression analyses were checked for multicollinearity. We used the IBM SPSS Windows version 22 to analyse all data. Statistical signifi-cance was set at .05, 2‐tailed.
3
|R E S U L T S
3.1
|Descriptive statistics
Table 1 shows the socio‐demographic, clinical, and biomarker data of our study population classified according to the q25 and q75 values of the total WHOQoL‐BREF score into low (<74.30), moderate (74.30 to 92.66), or high (>92.66) HRQoL score individuals. There were no significant differences among these 3 study groups in alcohol use, BMI, lithium use, antihypertensive drugs use, TUD, and MDA. The low HRQoL score group had a higher number of females and lower education and income; increased prevalence of depressive and manic episodes, current suicidal ideation and lifetime suicidal attempts; scored higher on the HAM‐D, ASSIST–hypnotics and childhood trauma; and showed an increased prevalence of mood disorders. In the low HRQoL score group, there were more participants using antidepressants, antipsychotics, lithium, and anticonvulsant mood stabilizers. There was a significant association between the HRQoL scores and AOPP, zLOOH + SOD + NOx + MDA + AOPP, and basal TSH values.
3.2
|Effects of biomarkers on HRQoL
Table 2 shows the results of multivariate regression analyses with the HRQoL domain subscores and total score as dependent vari-ables and all biomarkers as explanatory varivari-ables (with age, sex, BMI, and TUD entered as additional explanatory variables). In phys-ical health score, 14.4% of the variance was explained by the regression on CMPAase (positively) and MDA and basal TSH (both inversely). Psychological health and environment scores were posi-tively associated with CMPAase activity. In social relationships, 17.4% of the variance was explained by the nitro‐oxidative index and basal TSH (both inversely) and sex. In the total HRQoL score, 17.3% of the variance was explained by CMPAase activity, AOPP, basal TSH, and sex.
increased the risk of belonging to the low median‐split WHOQoL group (Χ2= 87.79,df= 5,P< .001, Nagelkerke = 0.694) with 88.5% of all participants being classified correctly (sensitivity: 74.4% and specificity: 94.6%).
3.3
|Effects of biomarkers, mood characteristics,
and other relevant predictors on HRQoL
To examine whether the effects of the biomarkers on HRQoL were still significant after considering the effects of mood disorders and
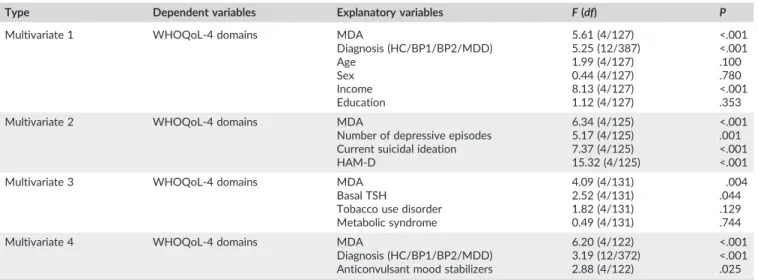
metabolic characteristics, we have performed multivariate GLM analy-ses with the 4 HRQoL domains as dependent variables and biomarkers together with clinical and metabolic characteristics as explanatory variables. In Table 4, regression 1 shows that there was a significant association among the 4 WHOQoL domain scores and MDA (partial Eta‐squared = 15.0%), mood disorders diagnosis (partial Eta‐ squared = 14.0%), and income (partial Eta‐squared = 20.0%), while age, gender, and education were not significant. Malondialdehyde was significantly and inversely associated with domains 1 and 3. The nitro‐oxidative stress index (F= 3.97,df= 4/127,P= .005), but not TABLE 1 Socio‐demographic data of the study population classified according to the World Health Organization quality of life (WHOQoL)‐BREF score in subjects divided into those with low (<74.3), moderate (74.3 to 92.66), or high (>92.66) scores
Total WHOQoL‐BREF score
P_correct
Low (n = 57) Moderate (n = 57) High (n = 57)
WHOQoL—Total score 64.1 (8.7) 83.6 (4.8) 101.5 (6.9) <.01
WHOQoL—Physical health 19.3 (4.3) 25.2 (3.5) 31.0 (2.8) <.01
WHOQoL—Psychological health 14.7 (3.1) 20.6 (2.7) 25.4 (2.1) <.01
WHOQoL—Social relationships 7.2 (2.1) 10.1 (1.9) 12.5 (1.6) <.01
WHOQoL—Environment 22.8 (4.4) 27.7 (3.3) 32.5 (3.5) <.01
Age, y 42.3 (10.3) 45.5 (10.9) 40.1 (11.2) <.05
Sex (F/M) 53/4 39/8 37/20 <.01
Education, y 9.4 (4.3) 11.2 (5.0) 13.0 (5.7) <.01
Income* 2.6 (1.4) 3.4 (1.3) 4.0 (1.3) <.01
Number of depressive episodes 6.5 (5.4) 2.2 (3.1) 1.0 (2.1) <.01
Number of manic episodes 4.4 (5.8) 3.0 (6.3) 0.6 (2.0) <.01
HAM‐D score 12.5 (6.3) 6.9 (5.5) 2.2 (2.7) <.01
Current suicidal ideation (N/Y) 31/21 50/7 55/2 <.01
Number suicidal attempts 1.4 (3.12) 0.2 (0.6) 0.1 (0.4) <.01
Assist‐alcohol score 5.1 (8.7) 4.0 (5.7) 2.7 (3.4) NS
Assist‐hypnotics score 3.3 (6.2) 1.3 (3.5) 0.3 (1.3) <.01
Early lifetime trauma score 59.1 (17.9) 41.6 (16.1) 34.3 (10.6) <.01
HC/BP1/BP2/MDD 3/28/13/13 21/12/7/17 42/5/3/7 <.01
HC/mood disorders (N/Y) 3/54 21/36 42/15 <.01
Antidepressants (N/Y) 32/23 39/16 42/9 <.05
Antipsychotics (N/Y) 38/17 45/10 46/5 <.05
Lithium (N/Y) 43/12 44/11 47/3 NS
Anticonvulsants (N/Y) 31/24 49/6 47/3 <.01
Antihypertensive drugs (N/Y) 44/13 44/13 45/11 NS
Statins (N/Y) 52/5 45/12 54/2 <.05
TUD (N/Y) 21/36 24/33 32/25 NS
Metabolic syndrome (N/Y) 44/14 28/27 33/24 <.05
BMI, kg/m2 27.3 (5.7) 26.3 (4.3) 25.9 (4.4) NS
MDA,μmol/mg proteins 64.88 (22.3) 69.3 (23.0) 59.5 (19.4) NS
AOPP,μM 80.3 (33.2) 94.4 (64.0) 67.5 (37.7) <.05
zSOD + LOOH + NOx + MDA + AOPP +0.09 (2.21) +0.86 (2.97) −1.02 (2.20) <.01
zCMPase ‐0.44 (0.81) +0.16 (1.06) +0.25 (0.98) <.01
Basal TSH,μIU/mL 2.89 (2.07) 2.37 (4.07) 1.79 (0.93) <.01
Data are shown as mean (±SD) and were analysed by ANOVA (continuous variables) or chi‐square test (nominal variables).P_correct,Pvalues corrected for false discovery rate; NS, non‐significant; WHOQ0L‐BREF, WHO quality of life‐BREF; HAM‐D, Hamilton Depression Rating Scale‐17 items; ASSIST, Alcohol, Smoking and Substance Involvement Screening Test; BMI, body mass index; HC, healthy controls; MDD, major depressive disorder; BP1, bipolar disorder type 1; BP2, bipolar disorder type II; TUD, tobacco use disorder; MDA, malondialdehyde; AOPP, advanced oxidation protein products; z CMPAase, adjusted values of serum paraoxonase activities as determined using CMPA and expressed in z score; basal TSH, baseline plasma concentrations of thyroid‐ stimulat-ing hormone; SOD + LOOH + NOx + MDA + AOPP, sum of z transformation of superoxide dismutase + peroxides + nitric oxide metabolites + MDA + AOPP.
TABLE 4 Results of different multivariate GLM analyses with the World Health Organization quality of life (WHOQoL)‐BREF domain scores as
dependent variables
Type Dependent variables Explanatory variables F(df) P
Multivariate 1 WHOQoL‐4 domains MDA
Diagnosis (HC/BP1/BP2/MDD) Age
Sex Income Education
5.61 (4/127) 5.25 (12/387) 1.99 (4/127) 0.44 (4/127) 8.13 (4/127) 1.12 (4/127)
<.001 <.001 .100 .780 <.001 .353
Multivariate 2 WHOQoL‐4 domains MDA
Number of depressive episodes Current suicidal ideation HAM‐D
6.34 (4/125) 5.17 (4/125) 7.37 (4/125) 15.32 (4/125)
<.001 .001 <.001 <.001
Multivariate 3 WHOQoL‐4 domains MDA
Basal TSH
Tobacco use disorder Metabolic syndrome
4.09 (4/131) 2.52 (4/131) 1.82 (4/131) 0.49 (4/131)
.004 .044 .129 .744
Multivariate 4 WHOQoL‐4 domains MDA
Diagnosis (HC/BP1/BP2/MDD) Anticonvulsant mood stabilizers
6.20 (4/122) 3.19 (12/372) 2.88 (4/122)
<.001 <.001 .025
HC/BP1/BP2/MDD, healthy controls, patients with bipolar disorder types 1 and 2, and major depression; MDA, malondialdehyde; TSH, thyroid‐stimulating hormone; HAM‐D, Hamilton Depression Rating Scale.
TABLE 2 Results of multivariate regression analyses with the World Health Organization quality of life (WHOQoL)‐BREF domain subscores as dependent variables and biomarkers as explanatory variables
Dependent variables Explanatory variables t P R(Model) F(df) P
Domain 1 Physical health CMPAase Basal TSH MDA
+2.86
−2.22 −2.22
.005 .028 .028
14.4% 7.30 (3/133) <.001
Domain 2 Psychological health CMPAase Sex
+2.86 +2.50
.005 .014
9.8% 7.15 (2/132) .001
Domain 3 Social relationships zLOOH + SOD + NOx + MDA + AOPP Basal TSH
Sex
−4.45 −2.93
+2.73
<.001 .004 .007
17.4% 9.37 (3/133) <.001
Domain 4: Environment CMPAase +2.68 .008 5.0% 7.17 (1/135) .008
Total WHOQoL‐BREF score CMPAase Sex AOPP Basal TSH
+3.34 +2.86
−2.17 −2.00
.001 .005 .031 .048
17.3% 6.86 (4/131) <.001
CMPAase, levels of serum paraoxonase activities as determined using CMPA (after adjusting for paraoxonase 1 genotypes); Basal TSH, baseline plasma con-centrations of thyroid‐stimulating hormone; MDA, malondialdehyde; zSOD + LOOH + NOx + MDA + AOPP, sum of z transformation of superoxide dismut-ase + peroxides + nitric oxide metabolites + MDA + AOPP; AOPP, advanced oxidation protein products.
TABLE 3 Results of binary logistic regression analyses with WHOQoL‐BREF groups as dependent variables
WHOQoL‐BREF dichotomies Significant explanatory variables Wald P Odds ratio 95% CI
1. Low + moderate versus high* Basal TSH 4.03 .045 2.06 1.02‐4.16
zLOOH + SOD + NOx + AOPP + MDA 4.74 .029 1.23 1.02‐1.47
AOPP 7.04 .008 3.19 1.35‐7.53
Age 4.42 .036 1.04 1.01‐1.08
2. Low versus moderate + high* Basal TSH 5.24 .022 2.30 2.30‐4.69
Res CMPAase 10.12 .001 0.45 0.28‐0.74
Sex 8.28 .004 0.11 0.02‐0.49
3. Median split* Basal TSH 4.26 .039 2.71 1.05‐6.98
CMPAase 6.52 .011 0.40 0.19‐0.81
Emotional abuse 17.55 <.001 4.05 2.11‐7.79
HAM‐D 10.28 .001 1.22 1.08‐1.38
Sex 5.12 .024 0.13 0.02‐0.77
Low, moderate, and high: see Table 1. 95% CI: 95% confidence interval with lower and upper interval. Alldf= 1. Basal TSH, baseline plasma concentrations of thyroid‐stimulating hormone; zSOD + LOOH + NOx + MDA + AOPP, sum of z transformation of superoxide dismutase + peroxides + nitric oxide metabo-lites + malondialdehyde + advanced oxidation protein products; CMPAase, levels of serum paraoxonase activities as determined using CMPA (after adjusting for paraoxonase 1 genotypes).
*Biomarkers, age, sex, body mass index, and tobacco use disorder were entered as explanatory variables.
AOPP, basal TSH, and CMPAase, had a significant effect but less important than MDA. The effects of basal TSH on HRQoL as detected in Table 2 were no longer significant after considering diagnosis. The explanation is that basal TSH is significantly higher in patients with BD (mean +/−SD = 2.70 +/−1.92 IU/mL) versus the other subjects
(2.14 +/−3.11 IU/mL;F= 8.73,df= 1/153,P= .004), while there are no significant differences (P= .493) between MDD (1.88 +/−1.27 IU/
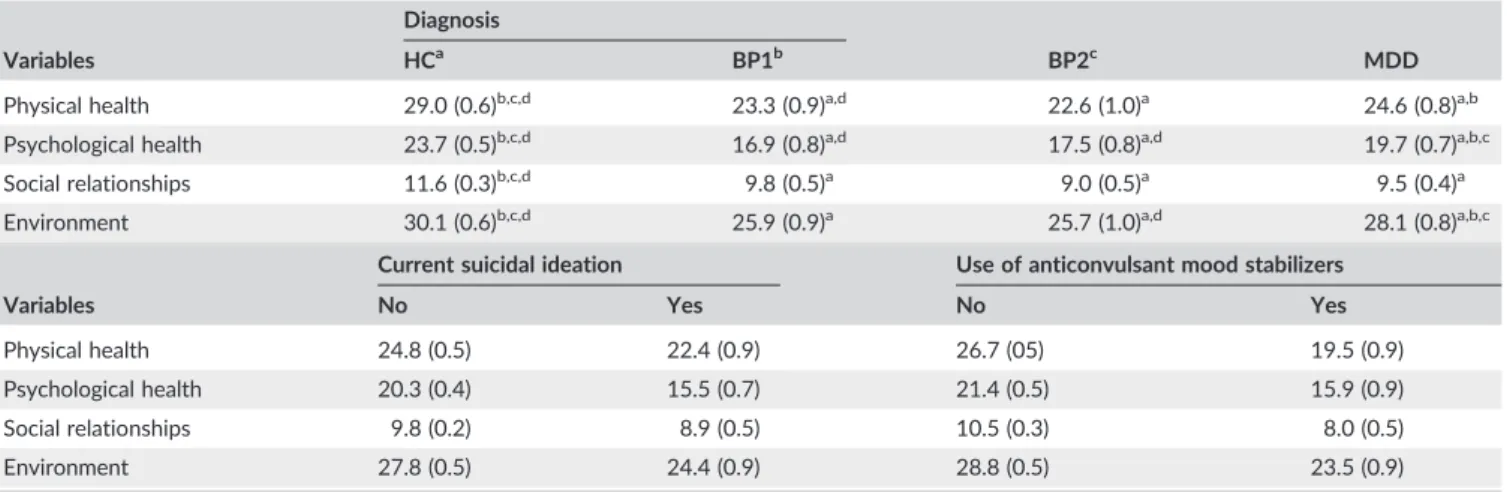
mL) and controls (2.28 +/−3.77 IU/mL). Table 5 shows the model‐ pre-dicted estimated marginal mean values (SE) of the 4 domains in healthy controls and BP1, BP2, and MDD patients. All domain scores were significantly lower in patients with mood disorders than in the control group. In addition, patients with BP1 and/or BP2 showed lower scores on physical health, psychological health, and environment as compared with MDD patients.
Consequently, we have examined the effects of biomarkers together with number of depressive and manic episodes, HAM‐D, and suicidal ideation on the 4 domains. Generalized linear model analysis 2 shows that the effects of MDA were still significantly associated with the 4 HRQoL domains after considering number of depressive episodes, suicidal ideation, and HAM‐D. None of the other biomarkers was significant. There were significant negative effects of number of depressive episodes and HAM‐D on all 4 HRQoL domains. Interestingly, the significant effects of basal TSH on HRQoL (as detected in Table 2) were no longer significant after considering the effects of number of depressive episodes, which could be explained by the significant correlation between number of depressive episodes and basal TSH levels (r = .268, P < .001; and manic episodes: r= .312,P< .001). Table 5 shows the model‐ predicted marginal means of subject with and without suicidal idea-tion. Suicidal ideation was accompanied by lowered levels of domain 1 (P= .025), domain 2 (P< .001), and domain 4 (P= .001), but not domain 3 (P= .130).
Regression 3 shows that MDA and basal TSH had significant neg-ative effects on the 4 HRQoL domains after adjusting for TUD and metabolic syndrome, which were both not significant. Finally, we have also examined whether the effects of the biomarkers still hold after adjusting for the drug state of the patients. Towards this end, we have
entered the biomarkers together with use of antidepressants, mood stabilizer, lithium and antipsychotics in the multivariate GLM analysis. Regression 4 shows that MDA together with mood stabilizers and diagnosis explained part of the variance in the 4 domains. There were no significant effects of the other 3 drug state variables. Table 5 shows that subjects who were treated with mood stabilizers had significantly lower levels on all 4 domains as compared with those without mood stabilizers (allP< .001). There were no significant differences in the biomarkers between those treated with antidepressants (P= .490), antipsychotics (P= .710), mood stabilizers (P = .649), and lithium (P= .3450).
3.4
|Best predictions of HRQoL domain scores and
total score
Table 6 shows the results of the same multivariate regression analyses as those listed in Table 2 but now with addition of other clinical explan-atory variables as described above. In domain 1, 63.4% of the variance was explained by HAM‐D, use of mood stabilizers, MDA, basal TSH, and age. Regression 2 shows that 66.7% of the variance in domain 2 was explained by HAM‐D, emotional abuse, income, suicidal ideation, use of anticonvulsants, and the presence of mood disorders (versus controls). Domain 3 is best predicted by HAM‐D, income, emotional neglect, MDA, and use of mood stabilizers. Domain 4 is best predicted by HAM‐D, mood stabilizers, income, and emotional neglect. Total HRQoL score is predicted (73.0%) by HAM‐D, mood stabilizers, income, emotional neglect, MDA levels, presence of mood disorders, and suicidal ideation.
4
|D I S C U S S I O N
The first major finding of this study is that MDA, AOPP, the nitro‐ oxidative stress index, and lowered CMPAase activity are associated with the different HRQoL domains, especially with physical and social relationships. We are not aware of any studies showing a relationship between MDA and HRQoL in mood disorders, while a prior study
TABLE 5 Model‐predicted estimated means (standard error) of the World Health Organization quality of life (WHOQoL‐BREF) 4 domain scores
after multivariate general linear model analysis (see Table 4)
Diagnosis
Variables HCa BP1b BP2c MDD
Physical health 29.0 (0.6)b,c,d 23.3 (0.9)a,d 22.6 (1.0)a 24.6 (0.8)a,b
Psychological health 23.7 (0.5)b,c,d 16.9 (0.8)a,d 17.5 (0.8)a,d 19.7 (0.7)a,b,c
Social relationships 11.6 (0.3)b,c,d 9.8 (0.5)a 9.0 (0.5)a 9.5 (0.4)a
Environment 30.1 (0.6)b,c,d 25.9 (0.9)a 25.7 (1.0)a,d 28.1 (0.8)a,b,c
Current suicidal ideation Use of anticonvulsant mood stabilizers
Variables No Yes No Yes
Physical health 24.8 (0.5) 22.4 (0.9) 26.7 (05) 19.5 (0.9)
Psychological health 20.3 (0.4) 15.5 (0.7) 21.4 (0.5) 15.9 (0.9)
Social relationships 9.8 (0.2) 8.9 (0.5) 10.5 (0.3) 8.0 (0.5)
Environment 27.8 (0.5) 24.4 (0.9) 28.8 (0.5) 23.5 (0.9)
HC/BP1/BP2/MDD, healthy controls, patients with bipolar disorder types 1 and 2, and major depression. a,b,c,dPair
found AOPP to be inversely associated with HRQoL in patients with depression due to periodontitis.26 In women with postmeno-pausal osteoporosis, HRQoL subdomains, including pain and mental and social functions, correlate with NO levels and antioxidant enzyme activities, but not MDA.45 Increased MDA levels are detected in MDD, BD, patients with prior suicidal attempts, severe depression, TUD, and metabolic syndrome,21,25,46,47while increased levels of AOPP are detected in suicide, major depression, and pre-natal depression.26,27,48Increased MDA and AOPP levels are oxida-tive damage biomarkers,21,22 which may cause (neuro)degenerative processes including arteriosclerosis and neurodegeneration,21,22 which both may be linked to lowered HRQoL scores. Increased MDA was the single best predictor of lowered HRQoL levels, sug-gesting that lipid peroxidation is a more significant mechanism explaining lowered HRQoL than protein oxidation. Interestingly, supplementation with antioxidants is effective at improving HRQoL measurements.49 By inference, future randomized controlled trials should examine the effects of treatments with antioxidants targeting lipid and protein oxidation to improve HRQoL in patients with mood disorders.
While increased nitro‐oxidative stress appears to exert its neg-ative effects from normal to moderately low HRQoL values, CMPAase activity and TSH levels may further decrease HRQoL to the very low HRQoL range. Previously, it was observed that thyroid disorders may negatively impact QoL50,53 and that treatment with thyroid hormones may improve QoL.35,51 One explanation is that thyroid hormones mediate symptoms that play a role in HRQoL,
including fatigue, feeling cold, and weight gain.51Interestingly, here, we report that basal TSH was significantly higher in patients with BD and increased with number of episodes, especially manic epi-sodes. Nevertheless, only 8 subjects showed basal TSH values higher than 5.0 IU/mL (1 control, 5 BP, and 2 MDD patients). Thus, our study sample is probably not characterized by subclinical hypo-thyroidism, and by inference, the results suggest that moderate increases in TSH are sufficient to deteriorate HRQoL. By inference, future randomized controlled trials should examine the effects of thyroid hormone treatments in patients to improve HRQoL in patients with BD.
A second major finding is that use of valproic acid, carbamaze-pine, or lamotrigine, but not antidepressants, lithium, or antipsychotic medications, had a significant effect on HRQoL. The use of these mood‐stabilizers has been approved for the acute treatment of mania and depression and prevention of relapses as well.52,54Mood stabi-lizers not only alleviate manic and depressive symptoms but may also improve HRQoL.55 Nevertheless, many aspects of HRQoL may be affected by use of psychotropic medications,56including detrimental side effects.15For example, the anticonvulsant mood stabilizers used in our study may cause sedation; loss of appetite; weight gain or weight loss; gastrointestinal effects including vomiting, nausea, dys-pepsia, constipation, and diarrhoea; diplopia; rash; and CNS effects, including sedation, ataxia, dizziness, confusion and clumsiness, alope-cia, and headache.57 Moreover, the older anticonvulsants including valproic acid and carbamazepine may cause overproduction of reac-tive oxygen species,58while long
‐term use of these drugs may cause TABLE 6 Results of automatic stepwise multivariate regression analyses with the World Health Organization quality of life (WHOQoL)‐BREF domain subscores as dependent variables and biomarkers and clinical data as explanatory variables
Dependent variables Explanatory variables t P R(Model) F(df) P
Domain 1 Physical health HAM‐D
Anticonvulsant mood stabilizers MDA TSH Age −8.88 −6.25 −4.25 −2.56 −2.07 <.001 <.001 <.001 .012 .041 63.4% 39.48 (5/114) <.001
Domain 2 Psychological health HAM‐D Emotional abuse Income
Current suicidal ideation Anticonvulsant mood stabilizers MOOD disorders −4.13 −2.73 +3.34 −3.66 −2.61 −2.43 <.001 .007 .001 <.001 .010 .017 66.7% 37.46 (6/112) <.001
Domain 3 Social relationships HAM‐D Income
Emotional neglect MDA
Anticonvulsant mood stabilizers
−5.48 +2.99 −3.84 −3.32 −2.93 <.001 .003 <.001 .001 .004 56.1% 29.14 (5/114) <.001
Domain 4 Environment HAM‐D
Anticonvulsant mood stabilizers Income Emotional neglect −4.18 −3.77 +3.56 −2.39 <.001 <.001 <.001 .019 46.0% 25.12 (4/115) <.001
WHOQoL‐BREF total score HAM‐D
Anticonvulsant mood stabilizers (−)
Income
Emotional neglect MDA
MOOD disorders Current suicidal ideation
−5.91 −5.28 +3.90 −2.81 −2.42 −2.14 −2.06 <.001 <.001 <.001 .006 .017 .034 .041
73.0% 43.19 (7/112) <.001
oxidative damage to neuronal cells.53,59,60In our study, we could not detect that these antiepileptic drugs impact O&NS biomarkers. Interestingly, newer antiepileptic drugs, including topiramate, may lower oxidative burden including reactive oxygen production.58 It follows that long‐term treatment with the older anticonvulsant mood stabilizers is probably not the best choice to treat individuals with BD.
The third major finding is that a large part of the variance in overall HRQoL, namely, 73.0%, is associated with MDA, use of anticonvulsant mood stabilizers, HAM‐D, lower income, ELT, suicidal ideation, and the presence of a mood disorder. In the multivariate analyses, the HRQoL domain scores were not significantly related with the clinical diagnoses of MDD or BD, number of depressive episodes, TUD, BMI, metabolic syndrome, age, or gender. Severity of depression is a far more important predictor of HRQoL domains than clinical diagnoses. These results extend those of previous studies showing that in nonremitted depressed patients, depressive symptoms were associated with impair-ments in HRQoL61and that residual symptoms appear to be associated with poor outcomes, including social functioning.3Therefore, recovery should not only be defined by symptomatic or syndromal remission but also by clinical remission together with functional recovery and a return to an acceptable HRQoL.62-64
Participants with ELT, including childhood abuse and neglect, experienced a lower HRQoL. These findings extend those of previ-ous studies showing that HRQoL in patients with mental disorders is associated with child abuse and neglect.65We found that partici-pants with lower income had lower HRQoL domain scores. These findings extend previous knowledge that, even during remission in BD and MDD, severe functional impairments may be present in work role functions and thus a lower income.3 Another finding is that suicidal behaviours are associated with a lower score on the psychological HRQoL domain. Previously, it was reported that HRQoL impairment is associated with suicidal ideation and suicide attempts.66
Interestingly, TUD was not significantly related to HRQoL. Previ-ous studies reported that smokers have a worse HRQoL as compared with never smokers.67-70The more negative findings reported here may be explained by our selection criteria, which excluded individuals with tobacco‐related chronic diseases, including chronic obstructive pulmonary disease and cardiovascular disorder, which are associated with loss of productivity and a worse HRQoL. Moreover, we were unable to find a significant positive association between Metabolic syndrome (MetS) and HRQoL domains. Some, but not all, previous studies reported a lower HRQoL in adults with MetS.71,72 Neverthe-less, we were unable to detect a significant association between BMI and HRQoL. Also, these negative findings may be explained by our criteria excluding individuals with CVD and diabetes.
The results of this study should be interpreted considering its strengths and limitations. Firstly, our study excluded patients with BD or MDD with comorbid medical conditions, whereas in the clinical practice, these comorbidities are common and probably worsen HRQoL.21Secondly, we used the raw scores on the WHOQoL
‐BREF domains rather than the transformed scores, which convert the lowest and highest scores to 0 and 100, respectively.38 Nevertheless, the transformed and raw data are highly correlated (r= .997).
5
|C O N C L U S I O NS
Our results suggest that HRQoL in mood disorder patients is highly predicted by lipid peroxidation, protein oxidation, lowered CMPAase activity, and increasing basal TSH values. These effects of MDA occur independently from the negative impact of severity of depression, childhood trauma, lowered income, and use of mood stabilizers. These findings open new perspectives to improve HRQoL in patients with mood disorders by combinatorial treatments using antioxidants (targeting lipid peroxidation and protein oxidation), thyroid hormones when BD is present, while avoiding the older anticonvulsant mood stabilizers.
A C K N O W L E D G E M EN T S
This study was supported by Health Sciences Postgraduate Program at Londrina State University, Paraná, Brazil (UEL), and Ministry for Science and Technology of Brazil (CNPq). CNPq number 470344/ 2013‐0 and CNPq number 465928/2014‐5. M.M. is supported by a CNPq–PVE fellowship and a Health Sciences Graduate Program fel-lowship, State University of Londrina. C.R. is funded by Chulalongkorn University, Government Budget.
C O N F L I C T O F I N T E R E S T
The authors declare no conflict of interest.
ET H I C A L A P P R O V A L
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent was obtained from all individual participants included in the study.
O R C I D
Michael Maes http://orcid.org/0000-0002-2012-871X
RE FE RE NC ES
1. Murray CJ, Vos T, Lozano R, et al. Disability‐adjusted life years (DALYs) for 291 diseases and injuries in 21 regions, 1990‐2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380(9859):2197‐2223.
2. Lopez AD, Mathers CD, Ezzati M, Jamison DT, Murray CJL. Global and regional burden of disease and risk factors, 2001: systematic analysis of population health data.Lancet. 2006;367(9524):1747‐1757.
3. American Psychiatric Association. Bipolar disorder. In: 5th ed, ed. Diag-nostic and Statistical Manual of Mental Disorders. Vol.2013:571‐577.
4. Young AS, Klap R, Sherbourne CD, Wells KB. The quality of care for depressive and anxiety disorders in the United States.Arch Gen Psychi-atry. 2001;58(1):55‐61.
5. Dean BB, Gerner D, Gerner RH. A systematic review evaluating health‐related quality of life, work impairment, and healthcare costs and utilization in bipolar disorder. Curr Med Res Opin. 2004; 20(2):139‐154.
7. Michalak EE, Yatham LN, Lam RW. Quality of life in bipolar disorder: a review of the literature.Health Qual Life Outcomes. 2005;3(1):72.
8. Michalak EE, Yatham LN, Kolesar S, Lam RW. Bipolar disorder and quality of life: a patient‐centered perspective. Qual Life Res. 2006; 15(1):25‐37.
9. Goossens PJ, Hartong EG, Knoppert‐van der Klein EA, et al. Self‐ reported psychopathological symptoms and quality of life in outpatients with bipolar disorder. Perspect Psychiatr Care. 2008; 44(4):275‐284.
10. Gutierrez‐Rojas L, Gurpegui M, Ayuso‐Mateos JL, et al. Quality of life in bipolar disorder patients: a comparison with a general population sam-ple.Bipolar Disord. 2008;10(5):625‐634.
11. Katon WJ, Lin EH, Von Korff M, et al. Collaborative care for patients with depression and chronic illnesses. N Engl J Med. 2010; 363(27):2611‐2620.
12. Ekman M, Granstrom O, Omerov S, et al. The societal cost of depres-sion: evidence from 10,000 Swedish patients in psychiatric care. J Affect Disord. 2013;150(3):790‐797.
13. Martin‐Subero M, Berk L, Dodd S, et al. Quality of life in bipolar and schizoaffective disorder—a naturalistic approach. Compr Psychiatry. 2014;55(7):1540‐1545.
14. Ryynanen OP, Kivela SL, Honkanen R, et al. Medications and chronic diseases as risk factors for falling injuries in the elderly.Scand J Soc Med. 1993;21(4):264‐271.
15. Balon R. SSRI‐associated sexual dysfunction.Am J Psychiatry. 2006; 163(9):1504‐1509.
16. Carvalho AF, Sharma MS, Brunoni AR, Vieta E, Fava GA. The safety, tol-erability and risks associated with the use of newer generation antidepressant drugs: a critical review of the literature.Psychother Psychosom. 2016;85(5):270‐288.
17. Solmi M, Murru A, Pacchiarotti I, et al. Safety, tolerability, and risks associated with first‐and second‐generation antipsychotics: a state‐ of‐the‐art clinical review.Ther Clin Risk Manag. 2017;13:757‐777.
18. Agnew‐Blais J, Danese A. Childhood maltreatment and unfavourable clinical outcomes in bipolar disorder: a systematic review and meta‐ analysis.Lancet Psychiatry. 2016;3(4):342‐349.
19. Nelson J, Klumparendt A, Doebler P, Ehring T. Childhood maltreatment and characteristics of adult depression: meta‐analysis.Br J Psychiatry. 2017;210(2):96‐104.
20. Jimenez E, Sole B, Arias B, et al. Impact of childhood trauma on cogni-tive profile in bipolar disorder.Bipolar Disord[Epub ahead of print]. 2017;19(5):363‐374.
21. Maes M, Galecki P, Chang YS, Berk M. A review on the oxidative and nitrosative stress (O&NS) pathways in major depression and their pos-sible contribution to the (neuro)degenerative processes in that illness.
Prog Neuropsychopharmacol Biol Psychiatry. 2011;35(3):676‐692.
22. Berk M, Kapczinski F, Andreazza AC, et al. Pathways underlying neuroprogression in bipolar disorder: focus on inflammation, oxidative stress and neurotrophic factors. Neurosci Biobehav Rev. 2011; 35(3):804‐817.
23. Gawel S, Wardas M, Niedworok E, et al. Malondialdehyde (MDA) as a lipid peroxidation marker.Wiad Lek. 2004;57(9‐10):453‐455.
24. Brown NC, Andreazza AC, Young LT. An updated meta‐analysis of oxidative stress markers in bipolar disorder. Psychiatry Res. 2014; 218(1‐2):61‐68.
25. Liu T, Zhong S, Liao X, et al. A meta‐analysis of oxidative stress markers in depression.PLoS One. 2015;10(10):e0138904.
26. Gomes C, Martinho FC, Barbosa DS, et al. Increased root canal endo-toxin levels are associated with chronic apical periodontitis, increased oxidative and nitrosative stress, major depression, severity of depres-sion, and a lowered quality of life.Mol Neurobiol 2018;55(4):2814– 2827.
27. Roomruangwong C, Barbosa DS, Matsumoto AK, et al. Activated neuro‐oxidative and neuro‐nitrosative pathways at the end of term are associated with inflammation and physio‐somatic and depression
symptoms, while predicting outcome characteristics in mother and baby.J Affect Disord. 2017;223:49‐58.
28. Moreira EG, Correia DG, Bonifácio KL, et al. Lowered PON1 activities are strongly associated with depression and bipolar disorder, recur-rence of (hypo)mania and depression, increased disability and lowered quality of life.World J Biol Psychiatry. 2017 [Epub ahead of print];1‐13.
29. Mackness MI, Abbott C, Arrol S, Durrington PN. The role of high‐ density lipoprotein and lipid‐soluble antioxidant vitamins in inhibiting low‐density lipoprotein oxidation.Biochem J. 1993;294(3):829‐834.
30. Maes M, Meltzer HY, Cosyns P, et al. An evaluation of basal hypothalamic‐pituitary‐thyroid axis function in depression: results of a large‐scaled and controlled study.Psychoneuroendocrinology. 1993; 18(8):607‐620.
31. Chakrabarti S. Thyroid functions and bipolar affective disorder. J Thyroid Res. 2011;2011:306367.
32. Wysokinski A, Kloszewska I. Level of thyroid‐stimulating hormone (TSH) in patients with acute schizophrenia, unipolar depression or bipo-lar disorder.Neurochem Res. 2014;39(7):1245‐1253.
33. Tognini S, Pasqualetti G, Calsolaro V, et al. Cardiovascular risk and qual-ity of life in elderly people with mild thyroid hormone deficiency.Front Endocrinol (Lausanne). 2014;5:153.
34. Bunevicius A, Laws ER, Deltuva V, Tamasauskas A. Association of thy-roid hormone concentrations with quality of life of primary brain tumor patients: a pilot study.J Neurooncol. 2017;131(2):385‐391.
35. Pandrc MS, Ristic A, Kostovski V, et al. The effect of early substitution of subclinical hypothyroidism on biochemical blood parameters and the quality of life.J med Biochem. 2017;36(2):127‐136.
36. Machado MO, Oriolo G, Bortolato B, et al. Biological mechanisms of depression following treatment with interferon for chronic hepatitis C: a critical systematic review.J Affect Disord. 2017;209:235‐245.
37. Del‐Ben CM, Vilela JAA, Crippa JAS, et al. Confiabilidade da“Entrevista Clinica Estruturada para o DSM‐IV–Versao Clinica”traduzida para o portugues.Rev Bras Psiquiatr. 2001;23(3):156‐159.
38. WHO. Study protocol for the World Health Organization project to develop a quality of life assessment instrument (WHOQOL).Qual Life Res. 1993;2(2):153‐159.
39. Fleck MP, Louzada S, Xavier M, et al. Application of the Portuguese version of the abbreviated instrument of quality life WHOQOL‐bref.
Rev Saude Publica. 2000;34(2):178‐183.
40. Moreno RA, Moreno DH. Escalas de depressao de Montgomery & Asberg (MADRS) e de Hamilton (HAM‐D)/Hamilton and Montgomery & Asberg depression rating scales. Rev Psiquiatr Clin. 1998; 25(5):262‐272.
41. Grassi‐Oliveira R, Stein LM, Pezzi JC. Translation and content valida-tion of the childhood trauma quesvalida-tionnaire into Portuguese language.
Rev Saude Publica. 2006;40(2):249‐255.
42. Posner K, Brown GK, Stanley B, et al. The Columbia‐Suicide Severity Rating Scale: initial validity and internal consistency findings from three multisite studies with adolescents and adults.Am J Psychiatry. 2011;168(12):1266‐1277.
43. WHO ASSIST Working Group. The alcohol, smoking and substance involvement screening test (ASSIST): development, reliability and feasi-bility.Addiction. 2002;97(9):1183‐1194.
44. Henrique IFS, De Micheli D, RBd L, et al. Validacao da versao Brasileira do teste de triagem do envolvimento com alcool, cigarro e outras substancias (ASSIST).Rev Assoc Med Bras. 2004;5(2):199‐206.
45. Ozgocmen S, Kaya H, Fadillioglu E, Aydogan R, Yilmaz Z. Role of antiox-idant systems, lipid peroxidation, and nitric oxide in postmenopausal osteoporosis.Mol Cell Biochem. 2007;295(1‐2):45‐52.
46. Nunes SO, Piccoli de Melo LG, Pizzo de Castro MR, et al. Atherogenic index of plasma and atherogenic coefficient are increased in major depression and bipolar disorder, especially when comorbid with tobacco use disorder.J Affect Disord. 2015;172:55‐62.
metabolic syndrome are not specific to mood disorders. Life Sci. 2014;102(1):65‐71.
48. Vargas HO, Nunes SO, Pizzo de Castro M, et al. Oxidative stress and lowered total antioxidant status are associated with a history of suicide attempts.J Affect Disord. 2013;150(3):923‐930.
49. Fuchs‐Tarlovsky V, Bejarano‐Rosales M, Gutierrez‐Salmean G, et al. Effect of antioxidant supplementation over oxidative stress and quality of life in cervical cancer.Nutr Hosp. 2011;26(4):819‐826.
50. Ott J, Promberger R, Kober F, et al. Hashimoto's thyroiditis affects symptom load and quality of life unrelated to hypothyroidism: a pro-spective case‐control study in women undergoing thyroidectomy for benign goiter.Thyroid. 2011;21(2):161‐167.
51. Winther KH, Cramon P, Watt T, et al. Disease‐specific as well as generic quality of life is widely impacted in autoimmune hypothyroid-ism and improves during the first six months of levothyroxine therapy.PLoS One. 2016;11(6):e0156925.
52. Schatzberg AF, Cole JO, Debattista C. Manual of clinical psychophar-macology. In:6th Ed: American Psychiatric Publishing; 2007.
53. Niketic V, Ristic S, Saicic ZS, et al. Activities of antioxidant enzymes and formation of the glutathione adduct of hemoglobin (Hb ASSG) in epi-leptic patients with long‐term antiepileptic therapy. Farmacoterapia. 1995;50(11):811‐813.
54. Yatham LN, Kennedy SH, Parikh SV, et al. Canadian Network for Mood and Anxiety Treatments (CANMAT) and International Society for Bipo-lar Disorders (ISBD) collaborative update of CANMAT guidelines for the management of patients with bipolar disorder: update 2013.Bipolar Disord. 2013;15(1):1‐44.
55. Vieta E. Mood stabilization in the treatment of bipolar disorder: focus on quetiapine.Hum Psychopharmacol. 2005;20(4):225‐236.
56. Ryynanen OP. Psychotropic medication and quality of life in the elderly.
Nord J Psychiatry. 1993;47(Suppl 28):67‐72.
57. Roy P, Lee H, Rao V. Antiepileptic medications in the treatment of neuropsychiatric symptoms associated with traumatic brain injury. In: McElroy SL, Keck PE, Post RM, eds.Antiepileptic Drugs to Treat Psychi-atric Disorders. CRC Press; 2008:103‐114.
58. Naziroglu M, Yurekli VA. Effects of antiepileptic drugs on antioxidant and oxidant molecular pathways: focus on trace elements.Cell Mol Neurobiol. 2013;33(5):589‐599.
59. Maertens P, Dyken P, Graf W, Pippenger C, Chronister R, Shah A. Free radicals, anticonvulsants, and the neuronal ceroid‐lipofuscinoses.Am J Med Genet. 1995;57(2):225‐228.
60. Schulpis KH, Lazaropoulou C, Regoutas S, et al. Valproic acid monotherapy induces DNA oxidative damage. Toxicology. 2006; 217(2‐3):228‐232.
61. Woo JM, Jeon HJ, Noh E, et al. Importance of remission and residual somatic symptoms in health‐related quality of life among outpatients with major depressive disorder: a cross‐sectional study.Health Qual Life Outcomes. 2014;12(1):188.
62. Harvey PD. Defining and achieving recovery from bipolar disorder.J Clin Psychiatry. 2006;67(Suppl 9):14‐18.
63. IsHak WW, Mirocha J, James D, et al. Quality of life in major depressive disorder before/after multiple steps of treatment and one‐year follow‐ up.Acta Psychiatr Scand. 2015;131(1):51‐60.
64. Avila CC, Cabello M, Cieza A, et al. Functioning and disability in bipolar disorders: a systematic review of literature using the ICF as a reference.
Bipolar Disord. 2010;12(5):473‐482.
65. Springer KW, Sheridan J, Kuo D, Carnes M. Long‐term physical and mental health consequences of childhood physical abuse: results from a large population‐based sample of men and women.Child Abuse Negl. 2007;31(5):517‐530.
66. Kim JH, Kwon JW. The impact of health‐related quality of life on suicidal ideation and suicide attempts among Korean older adults.J Gerontol Nurs. 2012;38(11):48‐59.
67. Castro MR, Matsuo T, Nunes SO. Clinical characteristics and quality of life of smokers at a referral center for smoking cessation. J Bras Pneumol. 2010;36(1):67‐74.
68. Mitra M, Chung MC, Wilber N, Klein Walker D. Smoking status and quality of life: a longitudinal study among adults with disabilities.Am J Prev Med. 2004;27(3):258‐260.
69. Schmitz N, Kruse J, Kugler J. Disabilities, quality of life, and mental disorders associated with smoking and nicotine dependence. Am J Psychiatry. 2003;160(9):1670‐1676.
70. Cougle JR, Hakes JK, Macatee RJ, Chavarria J, Zvolensky MJ. Quality of life and risk of psychiatric disorders among regular users of alcohol, nicotine, and cannabis: an analysis of the National Epidemiological Survey on Alcohol and Related Conditions (NESARC).J Psychiatr Res. 2015;66‐67:135‐141.
71. Ford ES, Li C. Metabolic syndrome and health‐related quality of life among U.S. adults.Ann Epidemiol. 2008;18(3):165‐171.
72. Lee YJ, Woo SY, Ahn JH, Cho S, Kim SR. Health‐related quality of life in adults with metabolic syndrome: the Korea national health and nutrition examination survey, 2007‐2008. Ann Nutr Metab. 2012; 61(4):275‐280.
S U P P O R T I N G I N F O R M A T I O N
Additional Supporting Information may be found online in the supporting information tab for this article.