w w w . r b h h . o r g
Revista
Brasileira
de
Hematologia
e
Hemoterapia
Brazilian
Journal
of
Hematology
and
Hemotherapy
Original
article
Molecular
response
to
imatinib
mesylate
of
Brazilian
patients
with
chronic
myeloid
leukemia
Ana
Lucia
Vieira-Mion
∗,
Noemi
Farah
Pereira,
Vaneuza
Araujo
Moreira
Funke,
Ricardo
Pasquini
UniversidadeFederaldoParaná(UFPR),Curitiba,PR,Brazil
a
r
t
i
c
l
e
i
n
f
o
Articlehistory:
Received31August2016 Accepted5April2017 Availableonline18May2017
Keywords:
Imatinib
Molecularresponse Chronicmyeloidleukemia Q-PCR
Tyrosinekinaseinhibitor
a
b
s
t
r
a
c
t
Background:Imatinib mesylate has revolutionized the treatment of chronic myeloid
leukemialeadingtosignificantreductionsofBCR-ABL1transcriptlevelsinperipheralblood.
Objective:Toevaluatetheresponsetoimatinibmesylatetreatment(400mg/day)inBrazilian
patientsinthechronicphaseofchronicmyeloidleukemiamonitoredbyquantitativereal timepolymerasechainreaction.
Methods:BetweenOctober2002andOctober2010,3169peripheralbloodsampleswere
col-lectedfrom1403patientsfrom3to5months,6to11months,12to17months,18to23 monthsand≥24monthsafterbeginningimatinibtreatment.Eighty-twopatientshad
sam-plesavailableandanalyzedforalltimeintervals.BCR-ABL1quantificationwasperformedby quantitativerealtimepolymerasechainreactionusingtheABL1geneasthecontrol.Results
oftheBCR-ABL1ratioasapercentagewerereportedbytheinternationalscale(IS)usingthe
laboratoryconversionfactor(0.51).
Results:In the first interval, 80.8% of patients achieved the optimal response (
BCR-ABL1IS≤10%).Inthesecondperiod,69.1%achievedoptimalresponse(BCR-ABL1IS≤1%)and,
between12and17months,47.3%achievedmajormolecularresponse(BCR-ABL1IS≤0.1%).
Conclusions:Theresultsofthisretrospectivestudyshowthattheresponsetoimatinib
treat-ment(400mg/day)ofBrazilianpatientsinthechronicphaseofchronicmyeloidleukemiais withintheexpectedprofilewhencomparedtopatientsreportedininternationalprospective randomizedstudies.
©2017Associac¸ ˜aoBrasileiradeHematologia,HemoterapiaeTerapiaCelular.Published byElsevierEditoraLtda.ThisisanopenaccessarticleundertheCCBY-NC-NDlicense
(http://creativecommons.org/licenses/by-nc-nd/4.0/).
∗ Correspondingauthor at:Hospitalde Clínicas,UniversidadeFederaldoParaná(HC/UFPR),ImmunogeneticsLaboratory,RuaPadre
Camargo,280,80060-240Curitiba,PR,Brazil.
E-mailaddress:ana.mion@hc.ufpr.br(A.L.Vieira-Mion).
http://dx.doi.org/10.1016/j.bjhh.2017.04.007
Introduction
Theuse ofimatinib mesylate (IM), a first generation tyro-sine kinase inhibitor, has revolutionized the treatment of chronicmyeloidleukemia(CML)leadingtoasignificant
reduc-tionofthe breakpointclusterregion-Abelsonmurineleukemia1
(BCR-ABL1)transcriptlevelsinperipheralblood.1,2The
Inter-nationalrandomizedstudyofinterferonvs.STI571(IRIS)study showed that IM isable toreduce the amount of leukemic cellsquicklyandinlargeproportionsinmostpatientswith thisdisease.1–6Theintensityandspeedofresponseto
treat-mentareprognosticandtreatmentplanningparameters.The hematological,cytogeneticandmolecularresponses,the lat-terbasedonthenumberofBCR-ABL1transcriptsinperipheral blood,areusedtoquantifythelevelofreductionofleukemic cellsduringtherapy.5,6ByanalyzingtheIRISstudydata,the
EuropeanLeukemiaNetwork(ELN)establishedresponsegoals tobeachievedindifferentintervalsofdrugexposure, particu-larlyinthechronicphaseofCML.5Achievingmajormolecular
response(MMR),definedasBCR-ABL1transcripts≤0.1%,isthe
goaloftreatmentwithIMduetotheassociationbetweenthis level ofresponse and the higher likelihood ofdisease-free progression.7,8
The lack of data on the molecular response to treat-ment with IM in Brazilian patients and the availability of alargenumberofsamplestested byquantitativerealtime polymerase chainreaction (Q-PCR) in the Immunogenetics LaboratoryofHospitaldeClinicas,Universidade Federaldo Paraná(HC-UFPR)motivatedustoevaluatetheresultsinthis populationtoindirectlydemonstratetheresponseof Brazil-ianpatientstothisdrugindifferenttimeintervalsafterthe beginningoftherapy.
Methods
TheImmunogeneticsLaboratoryatHC-UFPRreceivedsamples from26Braziliancenters(23publicinstitutions)fromOctober 2002toOctober2010.Atotalof3169sampleswerecollected from1403patientsfrom3to5months,6to11months,12to17 months,18to23monthsand≥24monthsaftertheinitiationof
IMtreatment.Thesesampleswereselectedbecausetheymet theinclusioncriteriaforthisstudy:Brazilianpatientsaged≥18
yearsinthechronicphaseofCMLundertreatmentusingIM (400mg/day)andbeingmonitoredbyQ-PCR.Amongthe1403 patients,only82hadsamplesavailableforalltimeintervals. ThisstudywasapprovedbytheEthicsCommitteeofHC-UFPR.
RNAstabilizationandextraction
Sixteento20mLofperipheralblood collected in ethylene-diaminetetraacetic acid (EDTA) was treated with red blood cell lysis buffer (0.144M ofNH4Cl and 0.01M ofNH4HCO3)
within24hofcollection.9Forsamplesprocesseduntil
Septem-ber2006,RNAfrom 1×107 leukocytes was stabilizedusing
GTCsolution (4M guanidine thiocyanate, 5mM EDTA, 0.5% n-laurilsarcosil,25mMsodiumcitrate,pH7.0) with7.1% -mercaptoethanol and extracted with RNeasy MiniTM Kits
(Qiagen,UK)accordingtothemanufacturer’sinstructions.10,11
AfterSeptember2006,RNAfrom1×107leukocyteswas
sta-bilized using Trizol® (Invitrogen, USA) and isolated with isopropanol,chloroformandethanolaccordingtothe manu-facturer’sinstructions.ComplementaryDNAwassynthesized fromtotalRNAusingtheenzyme,Moloneymurineleukemia virus(M-MLV)reversetranscriptase(Invitrogen,USA)and ran-domizedhexameraspreviouslydescribed.11
IdentificationofthetypeofBCR-ABL1transcript
ThetypeofBCR-ABL1transcriptwasidentifiedbymultiplex
polymerasechainreaction(PCR)asoptimizedbyCrossetal.,10
excepttheicebathwasreplacedforPlatinumTaqDNA
poly-merase(Invitrogen,USA).Thesampleswithnegativeresults
bymultiplexPCRwereamplifiedbynestedPCRaspreviously described.11
QuantificationofBCR-ABL1transcripts
Thetranscriptsofall sampleswere quantifiedinduplicate byQ-PCR(ABIPRISM7500,LifeTechnologies,USA)usingthe hydrolysisTaqManTM probesystem.12Thecopynumbersof
BCR-ABL1andthecontrolgeneABL1werecalculatedby
com-paring the results with a standard curve based on serial dilutionsofthelinearizedplasmidwiththeBCR-ABL1insert (pNC210/G)engineeredbyCrossetal.13
Resultsarereportedasaratio(%)oftheBCR-ABL1toABL1
copy numbers (BCR-ABL1/ABL1×100). Theratio was
multi-plied by the conversion factor (CF) to reportvalues in the internationalscale(IS).Theconversionfactorofthe Immuno-geneticsLaboratoryatHC-UFPRis0.51,whichwasdetermined bycomparingtheresultsofBCR-ABL1transcriptquantification in30samplesanalyzedinboththeHC-UFPRlaboratoryand thereferencelaboratoryattheInstituteofMedicaland Veteri-naryScience,Adelaide,Australia.TheCFvaluewasconfirmed using asecond setof30 samplesfromthe same reference laboratory.Sampleswereconsideredacceptableforanalysis whenthenumberofABL1copieswas≥10.000.NestedPCRwas
performedonallsamplesthathadnotranscriptsdetectedby Q-PCRinordertoconfirmtheresults.
Statisticalanalysis
Descriptivestatisticalanalysiswasperformedforthegeneral parameters ofthesample: number ofsamplesper patient, typeoftranscript,ageatdiagnosisandgender.
Results
Patients’demographics(n=1403)aredetailedinTable1.The ageatdiagnosis,gender,stageofdisease,treatmentandIM dosewereobtainedfromtherequestformsentwiththe sam-plestotheImmunogeneticsLaboratoryatHC-UFPR.
Thenumber of samplesper patientranged from 1to5 (median=2).Samplesweregrouped intofivetimeintervals closetothosesetbytheELNtoassessresponsetoIM
treat-ment(Table2);theresponsesofpatientsineachintervalare
Table1–DemographicsofBrazilianpatientsinthe chronicphaseofchronicmyeloidleukemiaunder treatmentwithimatinibmesylate.
Characteristic n=1403
Ageatdiagnosis(years)a
Median 46.6
Range 19–85
Gender
Male 771
Female 632
Numberofsamplesperpatient
Median 2
Range 1–5
Transcript–n(%)b
e14a2(b3a2) 674(50.2)
e13a2(b2a2) 557(41.5)
e14a2ee13a2 112(8.3)
a Dataobtainedfromtheanalysisof1382patients.Thebirthdates
of21patientswerenotinformedontherequisitionformsentwith
bloodsamplestotheHospitaldeClinicas,UniversidadeFederal
doParaná(HC-UFPR)laboratory.
b Dataobtainedfromtheanalysisof1343patients.Thetranscript
wasnotidentifiedIn60outof1403patientsbecausetheywerein
deepmolecularresponsewhentheirfirstsamplewassenttothe
labformonitoringyettheywereincludedinthestudybecause
theyhadpreviousclinicaldiagnosisconsistentwithCMLand
weretaking400mg/dayimatinibmesylate.
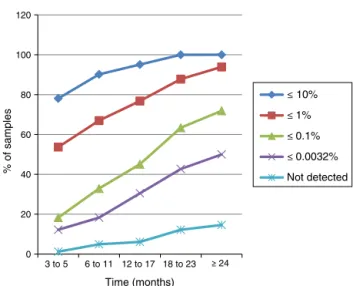
Amongthiscohort,only82patientshadsamplesavailable andanalyzedforeverytimeintervalestablishedinthisstudy; theirresponseprofileisshowninFigure1.Inthefirsttime interval,78.1%(64/82)ofpatientsachievedlevelsofBCR-ABL1IS
transcripts≤10%andallofthemreachedthislevelofresponse
inthelasttwotimeintervals.Betweensixand11months,67% ofpatients(55/82)achievedBCR-ABL1IStranscripts≤1%,and
from12to17months45.1%(37/82)hadtranscripts≤0.1%.
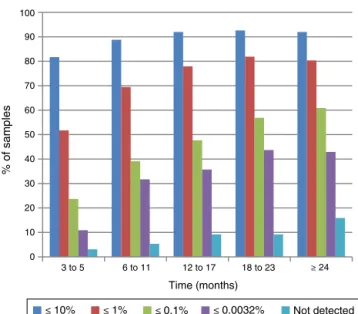
TheresponsetoIMtreatment inthe largercohort(1321 patients/2759samples)who didnothavesamplesavailable foralltimeintervalsisshowninFigure2.Inthefirst inter-val,81.7%(210/257)hadBCR-ABL1IS transcripts≤10%which
increasedto 92% (864/940) in the last interval. In the sec-ondtime interval,69.5% (342/492)ofthe patients achieved
0 20 40 60 80 100 120
12 to 17 6 to 11
3 to 5 ≥ 24
Time (months)
% of samples
≤ 10%
≤ 1%
≤ 0.1%
≤ 0.0032%
Not detected
18 to 23
Figure1–Responseof82patientsinthechronicphaseof
chronicmyeloidleukemiatoimatinibmesylatetreatment
whosesampleswereanalyzedinalltimeintervals.
BCR-ABL1IS transcripts≤1%,andbetween12and17months
47.7%(260/546)achievedresultsconsistentwithMMR(≤0.1%).
Discussion
ThisstudyshowedtheresponsetotreatmentusingIMina cohortofBrazilianpatientsinthechronicphaseofCML.One ofthedifficultieswastoestablishtimepointstoperformthe analysis,duetotheheterogeneityofsamplecollectiondates thatwerenotsystematicallyplanned.Thetimeintervalsin thepresentstudyweredeterminedtakingintoconsideration thetimesclosesttothoserecommendedbytheELNtomonitor responsetoIMtreatment.
Themolecularresponseprofileofpatientswithsamples inalltimeintervals(n=82)showedinthefirstinterval(three tofivemonths)that78.1%(64/82)had1logreductioninthe numberofBCR-ABL1IStranscripts;thisisconsideredan
opti-mal response toIM by the ELN.5 Hanfstein et al., in their
prospectiverandomizedstudy,foundthatafterthreemonths
Table2–Numberofsamplesdistributedaccordingtothetime(months)frominitiationofimatinibmesylatetreatment.
Time(months) 3–5 6–11 12–17 18–23 ≥24
Samplesna(%) 339(10.7) 574(18.1) 628(19.8) 606(19.1) 1022(32.2)
a Onlythesampleclosesttothebeginningofeachintervalwasselectedperpatient.
Table3–Responseof1403Brazilianpatientsinthechronicphaseofchronicmyeloidleukemiatoimatinibmesylate treatment.
BCR-ABLISTranscripts–n(%) Time(months)
3–5 6–11 12–17 18–23 ≥24
≥10% 65(19.2) 63(11) 48(7.7) 39(6.4) 76(7.4)
≤10to>1% 97(28.6) 114(19.9) 92(14.6) 66(10.9) 115(11.2) ≤1to>0.1% 101(29.8) 177(30.8) 191(30.4) 151(24.9) 200(19.6) ≤0.1 76(22.4) 220(38.3) 297(47.3) 350(57.8) 631(61.8)
0 10 20 30 40 50 60 70 80 90 100
18 to 23 12 to 17
6 to 11
3 to 5 ≥ 24
Time (months)
≤ 10% ≤ 1% ≤ 0.1% ≤ 0.0032% Not detected
% of samples
Figure2–Responseof1321patientsinthechronicphase
ofchronicmyeloidleukemiatoimatinibmesylate
treatmentwhosesampleswerenotavailableforanalysisin
alltimeintervals.
ofIMtreatment, 72%ofpatientshadthis levelofresponse and observed that early reduction in the number of BCR-ABL1IStranscriptsisastrongpredictorofresponsethroughout
treatment.14Inthisseries,53.7%(44/82)ofpatientshad
opti-malresponsesandachievedtranscriptlevels≤1%,and24.4%
wereintherangebetween1%and10%whileHanfsteinetal. detectedtheselevelsofresponsein31%and41%ofpatients, respectively.14 MMR was achieved in 18.3% (15/82) of this
patientsamplecomparedto15%foundintheIRISstudyat threemonthsoftreatment.4Atotalof12.2%(10/82)patients
withMMRachievedadeepmolecularresponse(DMR) with reductionsofatleast4.5logs,4,5andofthese,1.2%(1/82)had
noBCR-ABL1transcriptsdetectedeitherbyQ-PCRorbynested
PCR.
Atsixto11monthsfromthebeginningofIMtreatment, 90.2%(74/82)ofpatientsachieved1logreductionsintheBCR
-ABL1IS transcripts.Accordingtothe IRISstudy,this levelof
responseatsixmonthsisassociatedwithbetterevent-free survivalandprogression-freesurvival.3,4In67%(55/82)ofthe
patientsquantificationoftranscriptswas≤1%,consideredan
optimalresponsebytheELN.5Thisresultwassimilartothat
foundbyHanfsteinetal.andbythesevenyearsIRISstudy update, wherethis level ofresponse was observedin 63% and67.8%ofpatients,respectively.4,14 Inthis timeinterval,
23.2%(19/82)hadBCR-ABL1IStranscriptsbetween1%and10%, therebyincludingtheminthewarningsignsgroup,and9.8% (8/82)remainedwithtranscripts>10%denotingtreatment fail-ureaccordingtotheELN.5MMRoccurredin32.9%(27/82)of
patients,similartothe33.3%observedintheIRISstudyatsix monthsoftreatment4;ofthese18.3%(15/82)achievedDMR4,5
and4.9%(4/82)hadnoBCR-ABL1transcriptsdetectedeitherby Q-PCRorbynestedPCR.
MonitoringofBCR-ABL1IS transcriptsbetween12and 17
monthsshowedthat95.1%(78/82)ofpatientsachieved reduc-tionsofatleast1logand76.8%(63/82)ofatleast2logs,and
weresimilartotheIRISstudythatfound91.8%and79.9%of patients withtheselevelsofresponses, respectively.4 MMR
wasobservedin45.1%(37/82)ofpatientswithamedianof 14.2months;theseareconsideredoptimalrespondersbythe ELN.5Thisresultisclosetothe50.3%foundintheIRISstudy
update.4DeLavalladeetal.,SilveiraandMachadoetal.found
this level ofresponsein39.0%,37.1% and 40% ofpatients, respectively.15–17 Intheprospectiveand sequentialstudyof
deLavalladeetal.,patientsachievedMMRbetweentwoand 73months(median:15.7months)afterstartingtreatment.15
MMRwasachievedwithamediantimeof18months(6–78 months)accordingtoSilveira,buthisstudyincludedtoofew patientswithmolecularassessments(n=39).16Machadoetal.
foundthisresponselevelbetween0.4and31monthsof treat-ment (median: 8.5 months).17 In this time interval, 31.7%
(26/82)wereincludedinthewarningsignsgroup(BCR-ABL1IS
>0.1 and ≤1%), and 23.2% (19/82) in the treatment failure
groupasthenumberoftranscripts>1%accordingtotheELN criteria.5
Intheintervalbetween18and23months,allpatientshad atleast1logand87.8%(72/82)hadatleast2logreductions inBCR-ABL1IStranscripts.TheIRISstudy showedthese
lev-elsofresponsein93.7%and83.8%,respectively.4MMRwas
achievedby63.4%(52/82)ofpatients.Hughesetal.found64.8% ofpatientsinMMRat18monthsoftherapy.4
Theevaluationoftheperiod≥24monthsafterinitiationof
IMtreatmentshowedthatMMRwasachievedby72%(59/82)of patientsandDMR4,5by50%(41/82).Itwasobservedthat28%
(23/82)ofpatientswhodidnotachieveMMRat24monthswere stillunderIMtreatment.Onepossiblecauseisthedifficulty forpatientsatdistantcenterstoaccessspecialized laborato-riesthatperformquantificationofBCR-ABL1transcripts.Many were first monitoredforBCR-ABL1 transcriptlevels only18 monthsafterthebeginningofIMuse.Otherfactorsthatmay explain the unsatisfactory response are the misuse of the drugduetothesideeffects,thelackofadherenceto treat-mentorevensecondarydrugresistance.Anotherreasonto beconsideredinBrazilisthedifficultytoaccessother treat-ment strategies,suchassecond-generationtyrosinekinase inhibitorsandhematopoieticstemcelltransplantation.
VariationsinresponsestoIMtreatmentbetweenthisand other studies may reflect differences in the methods uti-lized.WhiletheIRISstudywasprospective,randomizedand included patients recently diagnosed who received IM as first-line treatment,this studyisretrospectiveand withno information availableabout previous therapiesor the time betweendiagnosisandbeginningoftreatment.Benditetal. showedthatthenumberofBrazilianpatientsachievingMMR wassignificantlyhigherwhenIMwasthefirst-linetreatment comparedtothosewhoreceivedIFN-␣associatedwithARA-C priortoIM.18Scernietal.foundthattheprobabilityof
achiev-ingMMRwassignificantlyhigher(60%)inBrazilianpatients whoreceivedIMwithinoneyearofdiagnosisthaninthose who started treatment morethan oneyear afterdiagnosis (40%).19
from 18 to 23 months and in the period ≥24 months. In
this group, all patients achieveda reductionof1 log from 18monthson. Yet,inthesecond group,thisdidnotoccur possibly because many patients had the first molecular testingperformedonlyafter18monthsofIMuse.Thisdata corroboratestheimportanceofsequentialmonitoring,every threeor six months,so that therapeuticinterventionscan takeplaceearlywhenthepatientdoesnothaveanadequate responsetothedrug.
Datafromthisretrospectivestudygiveanoverviewofthe molecularresponseinBrazilianpatientswithchronicphase CMLbeingtreatedwithIM(400mg/day),althoughthispatient sampledoesnothomogeneouslyrepresentthepopulationof Brazilianpatientswiththisdisease.Thiscanbebecausenot allpatientsunderIMtreatmenthavetheopportunitytobe monitoredforBCR-ABL1transcriptsastheylivelongdistances fromspecializedmolecularbiologylaboratoriesorbecauseof financiallimitationsinthecenterswheretheyaretreated.
The observation that sequential monitoring ofpatients isessentialforproperclinicalmanagementemphasizesthe importanceofincluding this kind of laboratorytest inthe listoftestsprovidedbytheBrazilianNationalHealthSystem. Furthermore,quantificationofBCR-ABL1transcriptsat differ-enttimepointsinpatientsunderIMtreatmentshouldbea requirementbyregulatoryagenciesofbothpublicandprivate healthcaresystems.ThesemeasureswillensurethatallCML patientsreceive propermonitoringoftheirresponsetoIM, andthepossibilityofreceivingothertherapeuticstrategiesin atimelymannerwheneverappropriate.
In conclusion, the results of this retrospective study showedthattheresponseofBrazilianpatientsinthechronic phaseofCML, treatedwithIM (400mg/day)isclosetothat expectedfrompatientsreportedinprospectiveand random-izedinternationalstudies.
Conflict
of
interest
Theauthorsdeclarenoconflictsofinterest.
Acknowledgements
ToDr.NicholasCrossandDr.JaspalKaedaourspecialthanks for generously providing the plasmids with the inserts of
theBCR-ABL1rearrangements,andtechnicaltrainingtoour
personnelmaking the implementation of the methods for BCR-ABL1transcriptquantificationfeasibleinthe Immuno-geneticsLaboratoryatHC-UFPRin2000.
r
e
f
e
r
e
n
c
e
s
1. O’BrienSG,GuilhotF,LarsonRA,GathmannI,BaccaraniM,
CervantesF,etal.Imatinibcomparedwithinterferonand
low-dosecytarabinefornewlydiagnosedchronic-phase
chronicmyeloidleukemia.NEnglJMed.
2007;348(11):994–1004.
2. HehlmannR,HochhausA,BaccaraniM,onbehalfofthe
EuropianLeukemiaNet.Chronicmyeloidleukaemia.Lancet.
2007;370(9584):342–50.
3.O’BrienSG,GuilhotF,GoldmanJM,HochhausA,HughesTP,
HadichJP,etal.Internationalrandomizedstudyofinterferon
versusSTI571(IRIS)7-yearfollow-up:sustainedsurvival,low
rateoftransformationandincreasedrateofmajormolecular
response(MMR)inpatients(pts)withnewlydiagnosed
ChronicMyeloidLeukemiainchronicphase(CML-CP)treated
withImatinib(IM)(abstract).Blood.2008;112(11):76.Abstract
186.
4.HughesTP,HochhausA,BranfordS,MüllerMC,KaedaJS,
ForoniL,etal.Long-termprognosticsignificanceofearly
molecularresponsetoimatinibinnewlydiagnosedchronic
myeloidleukemia:ananalysisfromtheInternational
RandomizedStudyofInterferonandSTI571(IRIS).Blood.
2010;116(19):3758–65.
5.BaccaraniM,DeiningerMW,RostiG,HochhausA,SoveriniS,
ApperleyJF,etal.EuropeanLeukemiaNetrecommendations
forthemanagementofchronicmyeloidleukemia:2013.
Blood.2013;122(6):872–84.
6.SaglioM,BaccaranIG,GoldmanJ,HochhausA,SimonssonB,
AppelbaumF,etal.Evolvingconceptsinthemanagementof
chronicmyeloidleukemia:recommendationsfromanexpert
panelonbehalfoftheEuropeanLeukemiaNet.Blood.
2006;108(6):1809–20.
7.HughesTP,KaedaJ,BranfordS,RudzkiZ,HochhausA,
HensleyM,etal.Frequencyofmajormolecularresponsesto
imatiniborinterferonalfapluscytarabineinnewlydiagnosed
chronicmyeloidleukemia.NEnglJMed.2003;349(15):1423–32.
8.HughesT,DeiningerM,HochhausA,BranfordS,RadichJ,
KaedaJ,etal.MonitoringCMLpatientsrespondingto
treatmentwithtyrosinekinaseinhibitors:reviewand
recommendationsforharmonizingcurrentmethodologyfor
detectingBCR-ABLtranscriptsandkinasedomainmutations
andforexpressingresults.Blood.2006;108(1):28–37.
9.BrandfordS,HughesTP,RudzkiZ.Monitoringchronic
myeloidleukaemiatherapybyreal-timequantitativePCRin
bloodisareliablealternativetobonemarrowcytogenetics.Br
JHaematol.1999;107(3):587–99.
10.CrossNC,HughesTP,LinF,O’SheaP,BunjeyJ,MarksDI,etal.
Minimalresidualdiseaseafterallogeneicbonemarrow
transplantationforchronicmyeloidleukemiainfirstchronic
phase:correlationswithacutegraft-versushostdiseaseand
relapse.BrJHaematol.1993;84(1):67–74.
11.CrossNC,FengL,ChaseA,BungeyJ,HughesTP,GoldmanJM.
Competitivepolymerasechainreactiontoestimatethe
numberofBCR-ABLtranscriptsinchronicmyeloidleukemia
patientsafterbonemarrowtransplantation.Blood.
1993;82(6):1929–36.
12.MarinD,KaedaJ,SzydloR,SaundersS,FlemingA,HowardJ,
etal.Monitoringpatientsincompletecytogeneticremission
aftertreatmentofCMLinchronicphasewithimatinib:
patternsofresidualleukaemiaandprognosticfactorsfor
cytogeneticrelapse.Leukemia.2005;19(4):507–12.
13.CrossNC,MeloJV,FengL,GoldmannJM.Anoptimized
multiplexpolymerasechainreaction(PCR)fordetection
BCR-ABLfusionmRNAsinhaematologicaldisorders.
Leukemia.1994;8(1):186–9.
14.HanfsteinB,MüllerMC,HehlmannR,ErbenP,LausekerM,
FabariusA,etal.Earlymolecularandcytogeneticresponseis
predictiveforlong-termprogression-freeandoverallsurvival
inchronicmyeloidleukemia(CML).Leukemia.
2012;26(9):2096–102.
15.deLavalladeH,ApperleyJF,KhorashadJS,MilojkovicD,Reid
AG,BuaM,etal.Imatinibfornewlydiagnosedpatientswith
chronicmyeloidleukemia:incidenceofsustainedresponses
inanintention-to-treatanalysis.JClinOncol.
2008;26(20):3358–63.
16.SilveiraCA[DistritoFederal[thesis]]Respostaaotratamento
mieloidecrônicadoHospitaldeBasedo.Brasília:
UniversidadedeBrasília;2011.p.98.
17.MachadoMP,TomazJP,Lorand-MetzeI,SouzaCA,Vigorito
AC,DelamainMT,etal.MonitoringofBCR-ABLlevelsin
chronicmyeloidleukemiapatientstreatedwithimatinibin
thechronicphase–theimportanceofamajormolecular
response.RevBrasHematolHemoter.2011;33(3):211–5.
18.BenditI,SanabaniSS,ConchonM,SerpaM,NovaesMM,
NardinelliL,etal.Evaluationoflong-termoutcomes,
cytogeneticandmolecularresponseswithimatinibmesylate
inearlyandlatechronic-phasechronicmyeloidleukemia:a
reportfromasingleinstitute.ActaHaematol.
2012;128(4):223–32.
19.ScerniAC,AlvaresLA,BeltrãoAC,BentesIR,AzevedoTC,
BentesAQ,etal.Influenceoflatetreatmentonhowchronic
myeloidleukemiarespondstoimatinib.Clinics.