w w w . e l s e v ie r . c o m / l o c a t e / b j i d
The
Brazilian
Journal
of
INFECTIOUS
DISEASES
Original
article
Molecular
diagnosis
of
symptomatic
toxoplasmosis:
a
9-year
retrospective
and
prospective
study
in
a
referral
laboratory
in
São
Paulo,
Brazil
Lilian
Muniz
Camilo
a,
Vera
Lucia
Pereira-Chioccola
a,∗,
Ricardo
Gava
a,
Cristina
da
Silva
Meira-Strejevitch
a,
Jose
Ernesto
Vidal
b,c,d,
Cinara
Cássia
Brandão
de
Mattos
e,
Fábio
Batista
Frederico
f,
Luiz
Carlos
De
Mattos
e,
Lígia
Cosentino
Junqueira
Franco
Spegiorin
e,g,
FAMERP
Toxoplasma
Research
Group,
Fernando
Henrique
Antunes
Murata
h,
Marina
Neves
Ferreira
h,m,
Deusenia
Machado
Ulisses
Barbosa
h,
Fausto
da
Silva
Gonc¸alves
j,
Cristiane
Moraes
Dias
j,
Marcia
Wakai
Catelan
j,
Rubens
Camargo
Siqueira
h,
Mariana
Previato
h,k,
Amanda
Pires
Barbosa
i,l,
Danilo
Cavallini
iaCentrodeParasitologiaeMicologia,InstitutoAdolfoLutz,SãoPaulo,SP,Brazil bInstitutodeInfectologiaEmilioRibas,SãoPaulo,SP,Brazil
cFaculdadedeMedicina,HospitaldasClínicas,daUniversidadedeSãoPaulo,SãoPaulo,SP,Brazil
dLaboratóriodeInvestigac¸ãoMédica(LIM)49,InstitutodeMedicinaTropicaldaUniversidadedeSãoPaulo,SãoPaulo,SP,Brazil eFaculdadedeMedicinadeSãoJosédoRioPreto,SãoJosédoRioPreto,SP,Brazil
fAmbulatóriodeOftalmologia,Fundac¸ãoFaculdadeRegionaldeMedicina-HospitaldeBase,SãoJosédoRioPreto,SP,Brazil gHospitaldaCrianc¸aeMaternidade,SãoJosédoRioPreto,SP,Brazil
hFaculdadedeMedicinadeSãoJosédoRioPreto,SãoJosédoRioPreto,SP,Brazil
iAmbulatóriodeOftalmologia,Fundac¸ãoFaculdadeRegionaldeMedicina-HospitaldeBase,SãoJosédoRioPreto,SP,Brazil jHospitaldaCrianc¸aeMaternidade,SãoJosédoRioPreto,SP,Brazil
kDepartamentodeOftalmologia,FaculdadedeMedicinadeMarília,Marília,SP,Brazil lDepartamentodeOftalmologia,UniversidadedeSãoPaulo,RibeirãoPreto,SP,Brazil
mFaculdadedeMedicinaVeterináriaeZootecnia,UniversidadedeSãoPaulo,SãoPaulo,SP,Brazil
a
r
t
i
c
l
e
i
n
f
o
Articlehistory:
Received17May2017
Accepted23July2017
Availableonline29September2017
a
b
s
t
r
a
c
t
Symptomaticforms oftoxoplasmosisarea seriouspublichealth problemandoccurin
around10–20% of the infected people.Aiming to improve the molecular diagnosis of
symptomatictoxoplasmosisinBrazilianpatients,thisstudyevaluatedtheperformance
ofrealtimePCRtestingtwoprimersets(B1andREP-529)indetectingToxoplasmagondii
DNA.Themethodology wasassayed in807clinicalsamples withknownclinical
diag-nosis,ELISA, andconventional PCR results in a 9-year period. All samples werefrom
∗ Correspondingauthor.
E-mailaddress:pchioccola@gmail.com(V.L.Pereira-Chioccola).
http://dx.doi.org/10.1016/j.bjid.2017.07.003
1413-8670/©2017SociedadeBrasileiradeInfectologia.PublishedbyElsevierEditoraLtda.ThisisanopenaccessarticleundertheCC
Keywords: Toxoplasmagondii
Symptomatictoxoplasmosis
Diagnosis
Real-timepolymerasechain
reaction
patientswithclinicalsuspicionofseveralfeaturesoftoxoplasmosis.Accordingtothe
min-imumdetectionlimitcurve(inCT),REP-529hadgreatersensitivitytodetectT.gondiiDNA
thanB1.Bothprimersetswereretrospectivelyevaluatedusing515DNAfromdifferent
clin-icalsamples.The122patientswithouttoxoplasmosisprovidedhighspecificity(REP-529,
99.2%andB1,100%).Fromthe393sampleswithpositiveELISA,146hadclinicaldiagnosis
oftoxoplasmosisandpositiveconventionalPCR.REP-529andB1sensitivitieswere95.9%
and83.6%,respectively.ComparisonofREP-529andB1performanceswasfurtheranalyzed
prospectivelyin292samples.Thus,fromatotalof807DNAanalyzed,217(26.89%)had
posi-tivePCRwith,atleastoneprimersetandsymptomatictoxoplasmosisconfirmedbyclinical
diagnosis.REP-529waspositivein97.23%,whereasB1amplifiedonly78.80%.After
com-paringseveralsamplesinaBrazilianreferrallaboratory,thisstudyconcludedthatREP-529
primersethadbetterperformancethanB1one.Theseobservationswerebasedafterusing
caseswithdefinedclinicaldiagnosis,ELISA,andconventionalPCR.
©2017SociedadeBrasileiradeInfectologia.PublishedbyElsevierEditoraLtda.Thisis
anopenaccessarticleundertheCCBY-NC-NDlicense(http://creativecommons.org/
licenses/by-nc-nd/4.0/).
Introduction
The infection by Toxoplasma gondii in humans is generally
asymptomatic.Duringthechronicphase,theparasites
per-sistencystedinthebrainandmuscleinthemajorityofthe
cases.1–3However,symptomaticformsmayoccurinaround
10–20%oftheinfectedpeople.Thus,toxoplasmosiscouldbe
aseriouspublichealthproblem,asitmayleadtomoresevere
symptoms.4–7
Infectionsoccurringduringpregnancycanresultinsevere
neonatalimpairmentinthefetus.Intheocularform,lesions
resultfromcongenitalorafterbirth-acquiredinfectionsand
normallyarenecrotic.Theselesionsmaydestroythe
architec-tureoftheneuralretinaandsometimesinvolvethechoroid
(retinochoroiditis).8–13
Thereactivationoflatentinfectionasresultofan
immuno-deficiencysuchasseverelyimmunosuppressedHIV-infected
patients in absence of highly active antiretroviral therapy
andprophylaxisresultsmorefrequentlyincerebral
toxoplas-mosis.Inrarecases,disseminatedtoxoplasmosiscanoccur,
whichinvolvesatleasttwoorgans,infailureofaneffective
Th1immuneresponse.5,6,14–18
The laboratory diagnosis is based on serological and
moleculartests (PCR).Inocularform, thediagnosis is
typ-ically clinical but the laboratory tests normally provide
confirmation.13,19 Serological diagnosis regularly monitors
seronegativepregnantwomenandseroconversionofchildren
under congenital toxoplasmosis investigation.20 In case of
seroconversion,detection ofT. gondiiDNAbyPCRin
amni-oticfluid(AF) confirmscongenitaltoxoplasmosis.21–24 After
birth,congenitaldiagnosiscanbemadeincerebrospinalfluid
(CSF)orblood(BL)samplesfromnewborns.Incasesof
suspi-cionofcerebralordisseminatedtoxoplasmosis,thetreatment
isusuallyinitiatedbasedonclinical,radiological,and
sero-logicalfeatures.6,15PCRhasbeenshowntobeanimportant
diagnostictoolsinceitisusedinallformsofsymptomatic
toxoplasmosis.25–29Suchdiagnosisleadstorapidandspecific
treatment,thusavoiding,orreducingseriousdamagecaused
byT.gondii.
Real-timequantitativePCR(qPCR)hasbeenusedfor
molec-ulardiagnosisandstudiesdemonstratedqPCRapplicabilityin
toxoplasmosisdiagnosisusingdifferenttargets.24–26,28,30–34
Aiming toimprove molecular diagnosis ofsymptomatic
toxoplasmosis in Brazilian patients, this study evaluated
the performanceof qPCR in detecting T. gondii DNA using
two primer sets for routine diagnosis. These markers are
used in laboratories in European countries for routine
diagnosis.23,29,35
Material
and
methods
Patientsandclinicalsamples
This retrospective and prospectivestudy evaluated clinical
samplesfrom807patients.InstitutoAdolfoLutz(SãoPaulo,
Brazil) received these samples from January 2008 through
November 2016 for molecular diagnosis of toxoplasmosis.
Clinical samplesoriginated frompatients withknown final
clinical diagnosis were chosen. The clinical samples
ana-lyzedduringthisperiodwereasfollows:173(2008–2010),204
(2011–2013), 138 (2014–2015), and 292 (2016). These clinical
samplessent tothe laboratoryincluded CSF(302), BLwith
EDTA (439), AF (47), and formalin-fixedparaffin-embedded
(FFPE)tissuessectionedin4-m-thick(19)(AU).Forserological
tests,BLsampleswerecollectedintubeswithno
anticoag-ulants. Clinical samplesforPCRand serology weresent to
thelaboratorywithin48haftercollection, andimmediately
processed.Intheretrospectivepartofthisstudy,theroutine
diagnosisof515clinicalsamplescollecteduntil2015included
conventionalPCR(cPCR)andenzyme-linkedimmunosorbent
assay(ELISA).Intheprospectivestudy,the292clinicalsamples
senttothelaboratoryin2016wereanalyzedbyqPCRusingthe
primerssetsREP-529andB1.
The samples evaluated in this study were from
symp-tomatic patients with the following clinical diagnosis of
toxoplasmosis: cerebral(404HIV-patients),ocular(193with
ocular alterations), congenital infection by transplacental
women),anddisseminated(19brainautopsiesfromdeceased HIV-patients).
HIV-infected patients with suspicion of cerebral
toxo-plasmosis presented progressive neurological deficits and
contrast-enhancing mass lesion(s) on computed
tomogra-phy or magnetic resonance imaging. These cases were
includedin“definitionforcerebraltoxoplasmosis”previously
described.6,15Thosewhodiedfromseveredisseminated
toxo-plasmosishadpositiveserologyandimmunohistochemistry
fortoxoplasmosisofatleasttwoorgans.Bothgroupofpatients
were admitted and treated atthe Instituto de Infectologia
EmilioRibasbyDrJoseE.Vidal’sgroup.Patientswithsuspicion
ofoculartoxoplasmosishadocularalterations,suchas
tox-oplasmicretinochoroiditisscarsorretinalexudativelesions.
They were admitted and treated at Ambulatorio de
Oftal-mologia,byDrFábio B.Frederico’sgroup.Pregnant women
withsuspicionofacutetoxoplasmosisinfectionwere
admit-tedand treated atthe High RiskAntenatal Careand Fetal
Medicine Service. After enrollingin a high-risk pregnancy
clinic,pregnantwomenwereroutinelyscreenedforTORSCH
(Toxoplasmosis,Rubella, Syphilis,Cytomegalovirus,
Hepati-tisandHIV).36,37Thoseclinically,epidemiologicallysuspected
oftoxoplasmosisandhavingpositiveIgMorlowIgGavidity
foranti-T.gondiiantibodiesunderwentamniocentesisforPCR
testinginamnioticfluid.Beforecollectionpregnantwomen
wereinformedoftheprocedureandrequiredtosigninformed
consent forms. Thenewborns ofthese mothers with
sus-pectedorconfirmedfetalinfectionwereadmittedandtreated
attheHighRiskPediatricServiceandFetalMedicineService.
MothersandnewbornsweretreatedbyDrLígiaCJFSpegiorin’s
group.
T.gondiiantigenandserologicaldiagnosis
T.gondiilysateantigenandELISAwereperformedexactlyas
describedbefore.19,38,39
DNApurification
BLsamples(5mL)werepreparedasdescribedbefore.28Plasma
sampleswereseparatedbycentrifugationandusedin
sero-logical diagnosis. Pellets were mixed with three times the
volumeofabuffercontaining150-mMammoniumchlorate,
1-mM potassiumbicarbonate, 0.1-mM EDTA, pH 7.3,
incu-batedfor 15min atroom temperature undermild shaking
andcentrifuged(2500g/10min).AFsamples(10mL)were
cen-trifuged(2500g/10min)andthe supernatantwasdiscarded.
Blood pellets, containingonlynuclei cells, AFpackedcells
and crushed tachyzoites (for control and standard curves)
weredigestedfor10minat56◦CwithproteinaseK(20g)in
200LofALbuffer(Qiagen).AUfragments(5sections of
4-m-thickFFPE)weredissolvedinxylene(1mL),incubatedfor
30sec,centrifugedtwice(8000g/1min)andsupernatantswere
removed.Pelletsweremixedwith1mLethanol,centrifuged
(8000g/1min)andincubatedfor10min,atroomtemperature
forcompletexyleneevaporation.40
DNAmoleculeswereextractedfromBL,AF,andtachyzoites
byQIAampDNAMiniKit(Qiagen);andfromAU,byQIAamp
DNA FFPETissue kit (Qiagen).Theprotocols were followed
according to the manufacturer’s instructions in a Robotic
workstationforautomatedpurificationofDNA(QIAcube,
Qia-gen).DNAmoleculesfromCSFsamples(3mL)wereextracted
asdescribed before.41 DNA concentrationsand puritywere
determinedbytheratioofO.D.at260and280nminNanoDrop
ND1000.ForuseinPCR,sampleswithhighDNA
concentra-tions(assomeBLandAU)weredilutedwithultra-purewater
untilconcentration100ng/L.
cPCRtargetfortoxoplasmosisandinternalcontrol
T. gondiiwas identifiedbycPCR usingthe primer pair
B22-B23,31designedtoamplifya115-bpampliconfromtheB1gene
astarget.Thereactionswererunfollowingthesame
condi-tions describedelsewhere.42 AbsenceofPCRinhibitorswas
verified usingahousekeepinggene thatamplifieda140bp
fragment of the human -globulin gene.43 Each
amplifica-tion run containedtwo negativecontrols(ultra-pure water
andnegativeDNAfortoxoplasmosis)andonepositive(DNA
extractedfromtachyzoites).Afterthermalcycles,PCR
prod-uctswereelectrophoresedin2%agarosegelandstainedwith
ethidiumbromideandvisualizedunderUVillumination.
qPCRtargetfortoxoplasmosisandinternalcontrol
The performance of two different primer sets for qPCR
was analyzed. The first (B1) amplified a 71-bp
frag-ment of the B1 gene as template,29,35 which has 35
copies in the genome and is conserved in different
parasite strains.31 The design included the forward
(5′-GAAAGCCATGAGGCACTCCA-3′) and reverse (5′
-TTCACCCGGACCGTTTAGC-3′) primers; and a hybridization
probe (5′-CGGGCGAGTAGCACCTGAGGAGATACA-3′) labeled
with FAM (6-carboxyfluorescein) and BHQ1 (Black Hole
Quencher1)atthe5′and3′ends,respectively.
The second (REP-529) amplified a 112-bp of the highly
repetitivesequenceREP-529(GenbankAF487550),23,29 which
has200–300copiesinT.gondiigenome.30Thedesignincluded
theforward(5′-AGAGACACCGGAATGCGATCT-3′)andreverse
(5′-TTCGTCCAAGCCTCCGACT-3′)primers;andthe
hybridiza-tionprobe(5′-TCGTGGTGATGGCGGAGAGAATTGA-3′)labeled
with FAMand BHQ1.The designand location in the gene
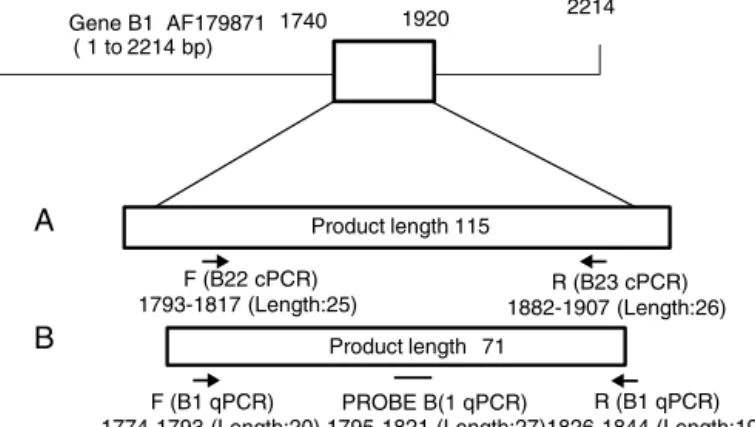
of each primer pair is described in Fig. 1. Both molecular
markersequenceswerepreviouslystandardizedinour
labora-toryusingserialdilutionsofDNAfromtachyzoites(RHstrain)
astemplate.ToconfirmtheabsenceofPCRinhibitors,aprimer
settoaEukaryotic18SrRNAgene(GenBankaccessioncode
X03205.1),purchasedfromAppliedBiosystemswasusedas
housekeepinggene.Thereactionswereperformedinfinal
vol-umeof20L.DNAsamples(3LofDNAuntil100ng/L),DNA
control(5ng/L)orDNAforstandardcurve(3Lforeachpoint)
wereaddedtoreactionmixturecontaining10Lof2XTaqMan
UniversalPCRMasterMix.Next,1.25Lof“AssayMix”(18M
offorward and reverse primersand 5M ofthe hydrolysis
probe).AmplificationswereperformedinanApplied
Biosys-tems7500Real-timePCRSystemusingthefollowingthermal
profile:2min,50◦C,and95◦Cfor10min.Next,40cycleswere
Gene B1 AF179871 ( 1 to2214 bp) 1
2214 1920
1740
F (B22 cPCR) 1793-1817 (Length:25)
R (B23 cPCR) 1882-1907 (Length:26)
F (B1 qPCR)
1774-1793 (Length:20) 1795-1821 (Length:27)1826-1844 (Length:19) R (B1 qPCR) PROBE B(1 qPCR)
Product length 115
Product length 71
REP 529 AF487550 ( 1 to404 bp)
1 270 390 404
Productlength112
F (REP-529 qPCR) PROBE (REP-529 qPCR) R (REP-529 qPCR) 270-290 (Length:21) 310-334 (Length:25) 363-381 (Length:19)
A
C
B
Fig.1–Designandgeneslocationofprimersetsusedinthisstudy.(A)cPCR(B22-B23),(B)qPCRB1,and(C)qPCRREP-529. Theanalysesweredoneinhttps://www.ncbi.nlm.nih.gov/tools/primer-blast/primertool.cgi?ctgtime=1486921082&jobkey=
1N4LxE9DQutl1VjQVbB84i-rbdACuHbNAw&CheckStatus=CheckforB22/B23;
https://www.ncbi.nlm.nih.gov/tools/primer-blast/primertool.cgi?ctgtime=1486924834&jobkey=l51I5ugp5YHCu3W-eN5RjALFQL4v1lujLgfor(B1),andhttps://www.ncbi.
nlm.nih.gov/tools/primer-blast/primertool.cgi?ctgtime=1487009304&jobkey=ycMWuJUKmKKmAidBf0sr3mPZ1S9SaAUw&
CheckStatus=CheckforREP-529.TheschemeswerebasedonLinetal.46
Standardcurveanalysis
Two T.gondiistandardcurves usingREP-529 and B1primer
setswereconstructedinnineconcentrationsofDNAobtained
from 1×107 tachyzoites(in triplicate).Initialconcentration
(1×107tachyzoites)was35ng/Landserialdilutionsranged
upto10−1tachyzoites(3.5fg).Thecyclethresholdvalues(CT)
wereplottedasmean(triplicate)againstthestandardcurve
valuestodeterminethedetectionlimitofbothprimersets.
Parasite concentrationswere determined afterthe
calcula-tionofthelinearregressionequation(y=ax+b),wherey=CT;
a=curveslope(slope);x=parasitenumber;andb=wherethe
curveintersectsy-axis(yintercept).44
Qualityassurance
IneachPCRrun,ablankcontrolwasusedconsistingof
DNA-freewaterplusthePCRmix.Separateroomswereusedfor
(i)DNAextraction;(ii)PCRmixandprimerpreparation;(iii)
addingDNAfromclinicalsamplesandpositivecontrol;and
(iv)post-PCRagarosegelelectrophoresisanalysisincaseof
thecPCR.DNAsampleswereassayedintriplicateand,atleast
twicetodeterminereproducibility.ThequalityofDNA
sam-plesstockedfornine yearsand retrospectivelyre-analyzed
with qPCR was confirmed by the positivity using the 18S
primerset.
Dataanalysis
ThediscordantresultsinqPCR werere-analyzed,usingthe
sameprotocols,atleasttwice.Sensitivitiesandspecificitiesof
bothprimersetsinclinicalsamplesweredetermined
consid-ering the true diagnosis (clinicaland laboratory diagnoses)
andwerecalculatedas:(i)percentofsensitivity=ratiooftrue
positives/truepositives+falsenegatives×100;(ii)percentof
specificity=ratiooftruenegatives/(truenegatives+false
pos-itives)×100. Reproducibilitycalculationswere done exactly
asdescribedbefore.45Thepercentagesofconcordantindices
werecalculatedasthefollowingratio:(numberofconcordant
results)/(number total of samples)×100. Linear regressions
wereconstructedfromthestandardcurvesforREP-529and
B1andCTmeancomparisonofbothprimerpairs.Bothcurves
werestatisticallyanalyzedusinganunequal-variancet-test
basedonacriticalvalueofp≤0.05.Allcalculationswererun
inGraphPadPrism6.0software.
Results
REP-529andB1testedinT.gondiitachyzoites
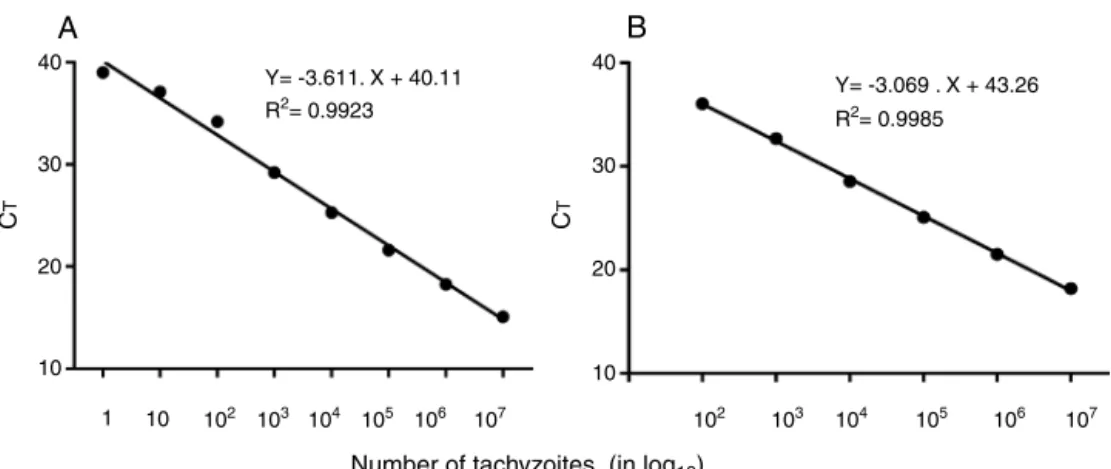
The first step was to evaluate the reportable range of the
reactionthatwascalculatedusingDNAextractfrom
A
40 40
30 30
20
C
T
C
T
20
10 10
Y= -3.069 . X + 43.26 Y= -3.611. X + 40.11
R2= 0.9985 R2= 0.9923
10
Number of tachyzoites (in log10)
102 103 104 105 106 107 102 103 104 105 106 107 1
B
Fig.2–StandardcurveofT.gondii,tachyzoitesusingREP-529(A)andB1(B)primersets,respectivelyusingthehydrolysis probeFAMdye-labeled.Resultsareshownasmeancyclethreshold(CT)obtainedfromtriplicateofeachDNAconcentration.
Standardcurveanalysiswasdonein10-foldserialdilutionsofDNAextractedfromtachyzoites,atinitialconcentrationof 35ng/L(1×107tachyzoites).ForREP-529,R2=0.9923andB1,R2=0.9985.
Table1–SummaryofretrospectiveofqPCRanalysisemployingREP-529andB1primersetsin515DNAsampleswith resultsinclinicaldiagnosis,ELISA,andcPCR(B22-B23).
Clinicalsamples Results
Serologicaldiagnosis(ELISA) n cPCR(B22-B23) qPCR(REP-529) qPCR(B1)
Negative Positive Negative Positive % Negative Positive %
Negative 122 122 0 121 1 99.2a 122 0 100a
Positive 393 247 146b 253 140 95.9b 281 122 83.6
Total 515 369 146 374 141 – 403 122 –
a Percentofspecificitywascalculatedasratiooftruenegatives/truenegatives+falsepositives × 100(clinicaldiagnosisandELISAresultswere
consideredgoldstandardmethod− 122patientswithouttoxoplasmosis).
b Percentofsensitivitywascalculatedasratiooftruepositives/truepositives+falsenegatives× 100(clinicaldiagnosisandcPCRwereconsidered
goldstandardmethod).
Theresulting standardcurveshowedthe detectionlimitof
eachcurve.R2=0.9923forREP-529and0.9985forB1showed
highlinearityamongthevariables.46Theminimumdetection
limit(inCT)was39.28(correspondingtooneparasite)for
REP-529, higherthan the 37.18(corresponding to100 parasites)
forB1.
REP-529andB1qPCRtestedinBrazilianclinicalsamples
Thefirst515DNAsamples(collecteduntil2015)hadresultsof
clinicaldiagnosis,ELISAandcPCR(B22-B23).TheseDNA
sam-pleswereusedtotestREP-529andB1inqPCR,andresultsare
showninTable1.DNAsamplesof122patientshadnegative
serological,molecular,andclinicaldiagnosis (without
toxo-plasmosis).REP-529andB1providedspecificitiesof99.2%and
100%,respectively.Allsampleswerenegativewhen
consider-ingresultsofatleastoneprimerset.
Table1alsoshowsPCRresultsof393clinicalsampleswith
positiveELISA.PositiveIgG antibodies anti-T.gondiiare not
necessarilyindicative ofasymptomaticinfection, whereas
detectionoftachyzoiteDNA inbiologicalfluidsascerebral,
ocular, congenital or disseminating forms can
character-izeasymptomaticinfection.19,40,42,47,48Thus,sensitivitiesof
REP-529 and B1 were 95.9% and 83.6%, respectively were
calculatedconsideringcPCRandclinicaldiagnosis(146
sam-ples,whichincluded94patientswithcerebraltoxoplasmosis,
10newbornswithcongenitalinfection,21patientswith
ocu-lartoxoplasmosis,sixpregnantwomenwithtoxoplasmosis,
and15postmortempatientswithseveredisseminated
toxo-plasmosis)(Table2).REP-529amplified140/146sampleswith
averageCTof31.71±0.36.B1amplified122/146,withaverage
CTof32.12±0.38.
Intheprospectivestudy(2016),atotalof292clinical
sam-pleswereanalyzedbyREP-529andB1.AsshowninTable2,
among807clinical samples,217(26.89%) had positivePCR,
atleast oneprimer set anda confirmedclinical diagnosis.
These included: (i) 30.94% (125/404) patients with cerebral
toxoplasmosis; (ii) 12.80% (16/125) newbornswith
congeni-tal alterations; (iii) 18.18%(12/66)ofpregnant womenwith
acutetoxoplasmosiswithT.gondiiDNAinAForBL;(iv)23.83%
(46/193)patientswithocularalterationsandT.gondiiDNAin
BL;and (v)94.74%(18/19)postmortempatientswithsevere
disseminatedtoxoplasmosis.
Table3describestheperformanceofprimersetsand
clin-icalcharacteristicsof217positivesamples.REP-529amplified
T.gondiiDNAin97.23%(211/217)ofpositivesamples,andB1
Table2–ClinicalandPCRresultsofthe807clinicalsamplesanalyzed.
Toxoplasmosis(clinical form)
Totalnumberof clinicalsamples
analyzed
Positivesamplesclinical,molecular,and serologicaldiagnosis
%Positivity (bothstudies)
Retrospectivestudy (total–515samples)
Prospectivestudy (total–292)
Bothstudies(total– 807samples)
Cerebral 404 94 31 125 30.94
Congenital 125 10 6 16 12.80
Gestational 66 6 6 12 18.18
Ocular 193 21 25 46 23.83
Disseminated (postmortem)
19 15 3 18 94.74
Total 807 146 71 217 26.89
Table3–Characteristicsofclinicalsamples,sensitivitiesofREP-529andB1consideringthe217positivesamples.
Clinicalform(n) Sample Totala REP-529 B1
Positive % Positive %
Cerebral(125) CSF 49 49 100 41 83.67
BL 76 72 94.74 62 81.58
Congenital(16) CSF 3 3 100 2 66.67
BL 13 13 100 9 69.23
Gestational(12) AF 8 8 100 5 62.50
BL 4 4 100 3 75.00
Ocular(46) BL 46 44 95.65 36 78.26
Disseminated(18) AU 18 18 100 13 72.22
Total 217 211 97.23 171 78.80
a Atleastoneprimersetpositive.
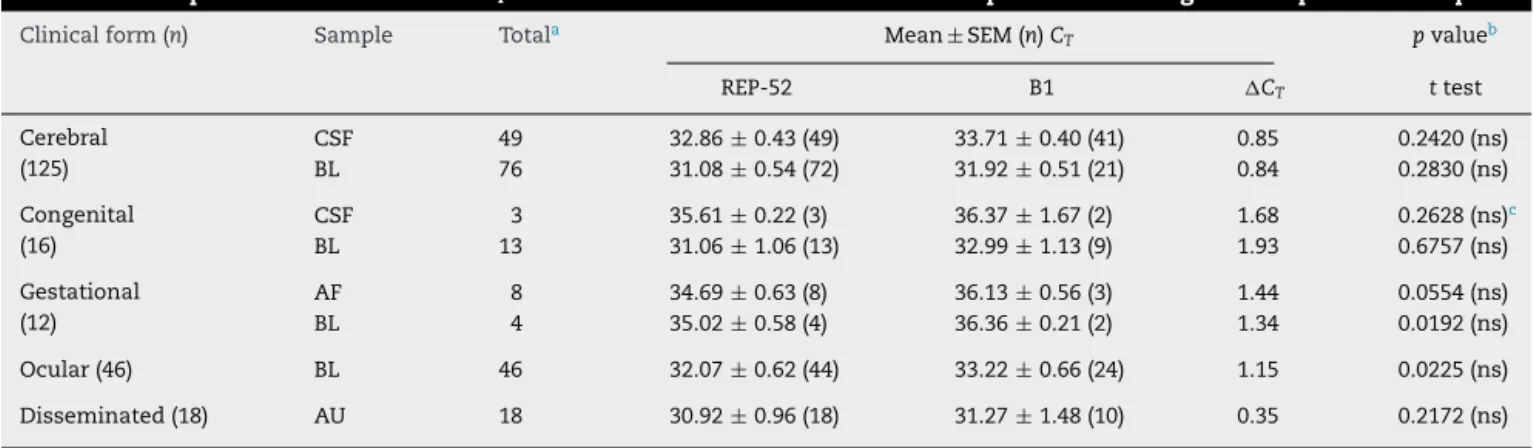
Table4–ComparisonbetweenmeanCTvaluesobtainedwithREP-529andB1qPCRconsideringthe217positivesamples.
Clinicalform(n) Sample Totala Mean± SEM(n)CT pvalueb
REP-52 B1 CT ttest
Cerebral (125)
CSF 49 32.86± 0.43(49) 33.71± 0.40(41) 0.85 0.2420(ns)
BL 76 31.08± 0.54(72) 31.92± 0.51(21) 0.84 0.2830(ns)
Congenital (16)
CSF 3 35.61± 0.22(3) 36.37± 1.67(2) 1.68 0.2628(ns)c
BL 13 31.06± 1.06(13) 32.99± 1.13(9) 1.93 0.6757(ns)
Gestational (12)
AF 8 34.69± 0.63(8) 36.13± 0.56(3) 1.44 0.0554(ns)
BL 4 35.02± 0.58(4) 36.36± 0.21(2) 1.34 0.0192(ns)
Ocular(46) BL 46 32.07± 0.62(44) 33.22± 0.66(24) 1.15 0.0225(ns)
Disseminated(18) AU 18 30.92± 0.96(18) 31.27± 1.48(10) 0.35 0.2172(ns)
a SEM(standarderrorofthemean).
b Significantvalueatap<0.01.
c Non-significant.
AlthoughaverageCT ofREP-529islowerthanforB1,the
CT withboth primersets were notstatisticallydifferent in
positivesamples(Table4).
Theconcordanceindexamongtheprimer setswas
cal-culated basedon the positiveresults. Thetotal number of
sampleswasbasedontheresultsof515clinicalsampleswith
positive(146)andnegative(369)resultsincPCR(B22-B23)until
2015,and807clinicalsampleswithpositive(217)andnegative
(590)resultsinqPCR(REP529andB1)(2008–2016).Asshownin
Table5,concordanceofresultsamongthethreeprimersets
was83.56%.ConcordancebetweenB22-B23andREP-529was
95.89%; betweenB22-B23and B1 was 83.56%;and between
REP-529andB1,83.87%.
Discussion
Until 2015,molecular diagnosis in our laboratory included
cPCR using primer set B22-B23, which amplified a
Table5–ConcordanceindexbetweenthreemarkersfordetectingT.gondiiinclinicalsamples.
Periodofthestudy Primersets Clinicalsampleswith symptomatic toxoplasmosis
PCRresults
Concordanta Discordantb Concordance
indexc(%)
B22-B23/REP-529/B1 146 122 24 (83.56)
2008–2015 B22-B23/REP-529 146 140 06 (95.89)
B22-B23/B1 146 122 24 (83.56)
2008–2016 REP-529/B1 217 182 35 (83.87)
a Positiveinallmarkers.
b Negativeatleastonemarker.
c Percentsofconcordantindexwerecalculatedastheratio:(numberofconcordantresults)/(numbertotalofsamples)× 100.
Numbertotalofsampleswasbasedonresultsof515clinicalsampleswithpositive(146)andnegative(369)resultsincPCR(B22-B23)until
2015;and807clinicalsampleswithpositive(217)andnegative(590)resultsinqPCR(REP529andB1)(2008–2016).
anddetectsDNAofasingleorganismdirectlyfromacrude
celllysateor10parasitesinthepresenceof100,000human
leukocytes.31 Highsensitivityand specificity ofthisprimer
setwerepreviouslyreportedinCSFandBLsamplescollected
fromAIDSpatientsandfromthosewithoculartoxoplasmosis
in our setting.19,41,42 Thus, to include qPCR in molecular
diagnosis,wepreviouslyevaluatedtheperformanceofqPCR
todetectT.gondiiDNAtestingtwoprimersets.23,29,35
Thefirstexperimentswereaimedtodeterminethe
mini-mumdetectionlimitcurve(inCT)ofeachprimerset.REP-529
wasmore sensitivetodetect T. gondiiDNAthan B1,which
were39.28(correspondingtooneparasite)and37.18
(corre-spondingto100parasites),respectively.Similarresultswere
showninotherstudy33 andtheycouldbeattributedatthe
number of repeated copies in T. gondiigenome (B1gene –
35copiesand529bpsequence200–300copies).33Infact,the
REP-529sequenceismoreabundantinnumberofrepetitions
thantheB1.30 Despitethesedifferencesofsensitivity,good
reproducibility(100%)wasshownwithbothprimersets,since
similarresultswere presentedinthereplicas(in triplicate)
andindifferentreactions(samesampleanalyzedondifferent
days).
Next,REP-529andB1wereretrospectivelyevaluatedusing
515DNAextractedfrom differentclinicalsamplescollected
fromBrazilianpatientswithknownclinicalandlaboratorial
diagnoses.Thesensitivityandspecificityofdiagnostictests
were assessed on optimal working conditions. To improve
the PCR sensitivity, the clinical samples were processed
rapidlywithin48h ofcollectionto preventTaqpolymerase
inhibition.42,49ConsideringthevariationoftheparasiteDNA
concentration, allsamples usedinthis study were
quanti-fiedafterextraction(sinceconcentrationsupto100ng/Lcan
inhibitthereaction)andwerecheckedfortheDNAinhibitors
byamplifyingahumanhousekeepinggene.Thismainquality
controlpreventednegativeresultsduetolowDNAquality.50
ThequalityofDNAsamplesstockedforuptonineyearsand
retrospectivelyre-analyzedwithqPCRwasconfirmedby
posi-tivityusingthe18Sprimerset.Theseresultswereessentialto
verifyiflong-termstorageat−20◦Cafterinitialdiagnosishad
notalteredDNA.Inaddition,alldiscordantresultsbetween
thethreemarkerswererepeated(intriplicate)atleasttwice.
Onlyone DNA (0.8%)sample had positiveqPCR in
REP-529from the122patientswithouttoxoplasmosis(theyhad
negativeELISA,cPCR,andclinicaldiagnosis).Similarresults
havebeenshowninotherstudies.28,51Thus,bothprimersets
providedhighspecificities(99.2%and100%).
Fromthe393clinicalsampleswithpositiveELISA,146had
positivecPCRandthesepatientshadsymptomaticinfection,
according to the clinical diagnosis. Considering both cPCR
andclinicaldiagnosisREP-529andB1sensitivitieswere95.9%
and83.6%,respectively.ThemeansofCTforREP-529andB1
were31.71±0.36and32.12±0.38,respectively.AlthoughDNA
samples stockedup tonine years were checkedusing two
humanhousekeepinggenes(-globulinand18Sprimersets),
six(2.8%)werefalsenegativesamplesdeterminedbyREP-529
and24(16.4%)byB1inthefirstpartofthestudy(retrospective).
Theseresultscouldbeattributedto:(i)degradationofT.gondii
DNAbythelong-termstorageat−20◦C;(ii)absenceofsuch
sequencesinsomeofthebiologicalsamplesasmentionedby
others51;and(iii)humanDNAcouldinhibitPCRwhen
target-ingtheB1gene,whichoccursin35copiesinT.gondiigenomeor
in529bpsequence,whichoccursinmorecopies(200–300).30,33
Despitethat,theseresultsconfirmthatREP-529was
consider-ablybetterthanB1indetectingT.gondiiDNA.Thesedatahave
beenconfirmedbyotherstudies.29,32,33,52
When only the qPCR for both primer sets were tested
inmoleculardiagnosis,the reactionsweredoneusingDNA
extractedrecently.ComparisonofREP-529 andB1
perform-anceswasalsoprospectivelyanalyzedin292clinicalsamples
collected in2016 (Table 2). Thus, atotal of 807DNA
sam-pleswereanalyzedretrospectivelyandprospectively.Ofthem,
217 (26.9%) had positive PCR, at least one primer set and
clinicaldiagnosisconfirmedassymptomatictoxoplasmosis.
REP-529amplifiedT.gondiiDNAin211(97.23%)clinical
sam-ples, whereas B1 amplified only 78.80% of them. Positive
resultsdeterminedbyREP-529in18AUbrainsampleswere
also confirmed by parasite visualization by
immunohisto-chemistryinbraintissues.However,fiveofthemhadnegative
resultswhentheB1wasused.Althoughtheseanalyseswere
done inBrazilianclinicalsamples,theyconfirmfindingsof
otherstudiesconductedinEuropeanclinicalsamplesaiming
thatthosefromrepeatregionsweremoresensitivethanthose
fromtheB1gene.26,29,33,52
Theconcordanceindex betweenthreeprimersets
(B22-B23/REP-529/B1)variedfrom83.56%to95.89%.Althoughthe
B22-B23and REP-529genesamplifydifferentT.gondiigenic
regions,bothhadalmostthesamepowerindetectingT.gondii.
Ontheotherhand,theconcordanceindicesbetweenB1and
REP-529 or B22-B23 were 83.87 and 83.56respectively. The
combinationofB1withotherprimersetsdidnotincreasePCR
sensitivity,sincenosamplewerepositiveforB1andnegative
forREP-529.Similartheresultshavebeenpreviousreported.52
Despite B1 and B22-B23 amplify the same B1 gene region
(Fig. 1), they presented different sensitivities. Although T.
gondiigenotypingwasnotdeterminedinthe217patientswith
positive PCR, probably, different Brazilian genotypes could
becirculating amongthesepatients, asalready showed by
others.53,54Onesmallconfirmationofthesedatawasverified
bythegenotypingof15ofthe18AUbrainsamplesusedhere.
Amongthem,sixgenotypesweredeterminedinourprevious
study.40OneofthemwasToxoDB#11,previouslyidentifiedin
differentBrazilianinfectednewbornsanddomesticanimals.54
TheotherfivegenotypesidentifiedwereTgHuDis1,TgHuDis3
andTgHuDis5,TgHuDis2andTgHuDis4.40AlthoughREP529
hasbeenregardedwitharegionwithalargevariationinits
copynumberindifferentT.gondiistrains,52theseresultscan
suggestthatindependentoftheinfectingstrain,REP-529can
besatisfactorilyusedformoleculardiagnosisofsymptomatic
toxoplasmosis.
Inconclusion,thiscomparativestudy,whichevaluated
sev-eralsamplesinaBrazilianreferrallaboratoryconcludedthat
REP-529hadbetterperformancethanB1one.These
observa-tionswereconcludedafterusingcaseswithdefinedclinical
diagnosis,ELISA,andcPCR.
Ethics
approval
and
consent
to
participate
TheEthicsCommitteeofallinvolvedInstitutions approved
theentirestudy,whichwasperformedfollowingthe
recom-mendationsofthesameCommittee(CONEP-IAL/SESnumber:
815489).
Consent
to
publish
Allauthors revisedthe manuscript,approvedthefinal
ver-sionsubmitted,published,andagreedtobeaccountableforall
aspectsoftheworkinensuringthatquestionsrelatedtothe
accuracyorintegrityofanypartoftheworkareappropriately
investigatedandresolved.
Funding
ThisstudywasfundedbygrantsfromFAPESP(Fundac¸ãode
AmparoàPesquisadoEstadodeSaoPaulo,Brazil)
2014/09496-1 to VLP-C, 2013/10050-5 to MP, 2013/15879-8 to FHAM,
2013/25650-8to LCM, 2014/01706-7to MNF;by CNPq
(Con-selhoNacionaldeDesenvolvimentoCientíficoeTecnológico)
toVLP-C301369/2015-1.
Authors’
contributions
VL Pereira-Chioccola, LM Camilo and CS Meira-Strejevitch
designed the study and experiments; performed the data
analysis,interpretedthedata.VLPereira-Chioccolawrotethe
manuscript.LMCamiloandRGavaperformedthelaboratorial
experiments(DNAisolation,cPCR,qPCR,ELISAanddata
anal-ysis).JEVidalcriticallyrevisedthemanuscriptanddetermined
theclinicaldiagnosisforpatientswithcerebraland
dissem-inatedtoxoplasmosis.CCBrandãodeMattos,LCMattos,FB
Frederico,RCSiqueira,MPreviato,APBarbosa,FHAMurata,
MNFerreira,LCJFSpegiorin,DMUBarbosa,FSGonc¸alves,CM
Dias, MW Catelan D. Cavallini performed the inclusion of
patients withocular, congenital, and gestational
toxoplas-mosis,samplecollection,developtheclinicaldiagnosis and
evaluationinFAMERP.
Conflicts
of
interest
Theauthorsdeclarenoconflictsofinterest.
Acknowledgements
JimHessonofAcademicEnglishSolutionsproofreadthepaper
(http://academicenglishsolutions.com/AES/home.html).
r
e
f
e
r
e
n
c
e
s
1.DubeyJP.AdvancesinthelifecycleofToxoplasmagondii.IntJ Parasitol.1998;7:1019–24.
2.MontoyaJG,LiesenfeldO.Toxoplasmosis.Lancet. 2004;63:1965–76.
3.HillDE,ChirukandothS,DubeyJP.Biologyandepidemiology ofToxoplasmagondiiinmanandanimals.AnimHealthRes Rev.2005;6:41–61.
4.GlasnerPD,SilveiraC,Kruszon-MoranD,etal.Anunusually highprevalenceofoculartoxoplasmosisinsouthernBrazil. AmJOphthalmol.1992;114:136–44.
5.BossiP,BricaireF.Severeacutedisseminatedtoxoplasmosis. Lancet.2004;364:579.
6.Pereira-ChioccolaVL,VidalJE,SuC.Toxoplasmagondii infectionandcerebraltoxoplasmosisinHIV-infected patients.FutureMicrobiol.2009;4:1363–79.
7.Elbez-RubinsteinA,AjzenbergD,DardéML,etal.Congenital toxoplasmosisandreinfectionduringpregnancy:casereport, straincharacterization,experimentalmodelofreinfection, andreview.JInfectDis.2009;199:280–5.
8.CarmeB,BissuelF,AjzenbergD,etal.Severeacquired
toxoplasmosisinimmunocompetentadultpatientsinFrench
Guiana.JClinMicrobiol.2002;40:4037–44.
9.DelairE,LatkanP,NobleAG,RabiahP,McLeodR,BrezinA. Clinicalmanifestationsofoculartoxoplasmosis.Ocul
ImmunolInflamm.2011;19:91–102.
10.OlariuTR,RemingtonJS,McLeodR,AlamA,MontoyaJG. SeverecongenitaltoxoplasmosisintheUnitedStates:clinical andserologicfindingsinuntreatedinfants.PediatrInfectDis. 2011;30:1056–61.
12.FerreiraAI,DeMattosCC,FredericoFB,etal.Riskfactorsfor oculartoxoplasmosisinBrazil.EpidemiolInfect.
2014;142:142–8.
13.PreviatoM,FredericoFB,MurataFH,etal.ABrazilianreport usingserologicalandmoleculardiagnosistomonitoring acuteoculartoxoplasmosis.BMCResNotes.2015;8:746.
14.HoffmannC.Opportunisticinfections.In:HoffmannC, RockstrohJK,KampsBS,editors.HIVmedicineflying publisher[Online].2005,www.HIVMedicine.com.
15.VidalJE,HernandezAV,deOliveiraAC,DauarRF,BarbosaSPJr, FocacciaR.CerebraltoxoplasmosisinHIV-positivepatientsin Brazil:clinicalfeaturesandpredictorsoftreatmentresponse intheHAARTera.AIDSPatientCareSTDS.2005;19:840–8.
16.CarruthersVB,SuzukiY.EffectsofToxoplasmagondiiinfection onthebrain.SchizophrBull.2007;33:745–51.
17.VidalJE,OliveiraAC.AIDS-relatedcerebraltoxoplasmosisin SãoPauloState,Brazil:markedimprovementsinthehighly activeantiretroviraltherapy-erabutthechallengescontinue. BrazJInfectDis.2013;17:379–80.
18.HernandezAV,ThotaP,PellegrinoD,etal.Asystematic reviewandmeta-analysisoftherelativeefficacyandsafetyof treatmentregimensforHIV-associatedcerebral
toxoplasmosis:istrimethoprim-sulfamethoxazoleareal option?HIVMed.2017;18:115–24.
19.MattosCC,MeiraCS,FerreiraAI,etal.Contributionof laboratorymethodsindiagnosingclinicallysuspectedocular toxoplasmosisinBrazilianpatients.DiagnMicrobiolInfect Dis.2011;70:362–6.
20.PomaresC,MontoyaJG.Laboratorydiagnosisofcongenital toxoplasmosis.JClinMicrobiol.2016;54:2448–54.
21.GilbertRE,FreemanK,LagoEG,etal.EuropeanMulticentre
StudyonCongenitalToxoplasmosis(EMSCOT).Ocular
sequelaeofcongenitaltoxoplasmosisinBrazilcompared withEurope.PLoSNeglTropDis.2008;2:e277.
22.OkayTS,YamamotoL,OliveiraLC,ManuliER,AndradeJunior HF,DelNegroGM.Significantperformancevariationamong PCRsystemsindiagnosingcongenitaltoxoplasmosisinSão Paulo,Brazil:analysisof467amnioticfluidsamples.Clinics. 2009;641:171–6.
23.Robert-GangneuxF,DupretzP,YvenouC,etal.Clinical relevanceofplacentaexaminationforthediagnosisof congenitaltoxoplasmosis.PediatrInfectDisJ.2010;29:33–8.
24.TeixeiraLE,KanunfreKA,ShimokawaPT,etal.The performanceoffourmolecularmethodsforthelaboratory diagnosisofcongenitaltoxoplasmosisinamnioticfluid samples.RevSocBrasMedTrop.2013;46:584–8.
25.CostaJM,PautasC,ErnaultP,FouletF,CordonnierC,Bretagne S.Real-timePCRfordiagnosisandfollow-upofToxoplasma reactivationafterallogeneicstemcelltransplantationusing fluorescenceresonanceenergytransferhybridizationprobes. JClinMicrobiol.2000;38:2929–32.
26.ChabbertE,LachaudL,CrobuL,BastienP.Comparisonoftwo widelyusedPCRprimersystemsfordetectionofToxoplasma inamnioticfluid,bloodandtissues.JClinMicrobiol. 2004;42:1719–22.
27.CalderaroA,PiccoloG,GorriniC,etal.Comparisonbetween twoReal-timePCRassaysandanested-PCRforthedetection ofToxoplasmagondii.ActaBiomed.2006;77:75–80.
28.MesquitaRT,ZieglerAP,HiramotoRM,VidalJE,
Pereira-ChioccolaVL.Real-timequantitativePCRincerebral toxoplasmosisdiagnosisofBrazilianhuman
immunodeficiencyvirus-infectedpatients.JMedMicrobiol. 2010;59:641–7.
29.BelazS,GangneuxJP,DupretzP,GuiguenC,Robert-Gangneux F.A10-yearretrospectivecomparisonoftwotarget
sequences,REP-529andB1,forToxoplasmagondiidetectionby quantitativePCR.JClinMicrobiol.2015;53:1294–300.
30.HomanWL,VercammenbM,DeBraekeleerJ,VerschuerenH.
Identificationofa200-to300-foldrepetitive529bpDNA
fragmentinToxoplasmagondii,anditsusefordiagnosticand quantitativePCR.IntJParasitol.2000;30:69–75.
31.BurgJL,GroverCM,PoulettyP,BoothroydJC.Directand sensitivedetectionofapathogenicprotozoan,Toxoplasma gondii,bypolymerasechainreaction.JClinMicrobiol. 1989;27:1787–92.
32.CassaingS,BessièresMH,BerryA,BerrebiA,FabreR, MagnavalJF.Comparisonbetweentwoamplificationsetsfor moleculardiagnosisoftoxoplasmosisbyreal-timePCR.JClin Microbiol.2006;44:720–4.
33.EdvinssonB,LappalainenM,EvengårdB,ESCMIDStudy GroupforToxoplasmosis.Real-timePCRtargetinga529-bp repeatelementfordiagnosisoftoxoplasmosis.ClinMicrobiol Infect.2006;12:131–6.
34.AjzenbergD,LamauryI,DemarM,etal.Performancetesting ofPCRassayinbloodsamplesforthediagnosisof
toxoplasmicencephalitisinAIDSpatientsfromtheFrench DepartmentsofAmericaandgeneticdiversityofToxoplasma gondii:aprospectiveandmulticentricstudy.PLoSNeglTrop Dis.2016;10:e0004790.
35.FekkarA,BodaghiB,TouafekF,LeHoangP,MazierD,ParisL. Comparisonofimmunoblotting,calculationofthe
Goldmann-Witmercoefficient,andreal-timePCRusing aqueoushumorsamplesfordiagnosisofocular toxoplasmosis.JClinMicrobiol.2008;46:1965–7.
36.Gonc¸alvesMA,MattosCC,SpegiorinLC,OlianiDC,OlianiAH, MattosLC.Seropositivityratesfortoxoplasmosis,rubella, syphilis,cytomegalovirus,hepatitisandHIVamongpregnant womenreceivingcareatapublichealthservice,SãoPaulo state,Brazil.BrazJInfectDis.2010;14:601–5.
37.MurataFHA,FerreiraMN,CamargoNS,etal.Frequencyofthe anti-ToxoplasmagondiiantibodiesclassIgA,IgMandIgGin high-riskpregnancywomen,Brazil.RevSocBrasMedTrop. 2016;49:512–4.
38.MeiraCS,Costa-SilvaTA,VidalJE,FerreiraIM,HiramotoRM, Pereira-ChioccolaVL.Useoftheserumreactivityagainst Toxoplasmagondiiexcreted-secretedantigensincerebral
toxoplasmosisdiagnosisinhumanimmunodeficiency
virus-infectedpatients.JMedMicrobiol.2008;57:845–50.
39.Costa-SilvaTA,MeiraCS,Frazzatti-GallinaN,
Pereira-ChioccolaVL.Toxoplasmagondiiantigens:recovery analysisoftachyzoitescultivatedinVerocellmaintainedin serumfreemedium.ExpParasitol.2012;130:463–9.
40.BastosdaSilvaI,BatistaTP,MartinesRB,etal.Genotypingof Toxoplasmagondii:DNAextractionfromformalin-fixed paraffin-embeddedautopsytissuesfromAIDSpatientswho diedbyseveredisseminatedtoxoplasmosis.ExpParasitol. 2016;165:16–21.
41.VidalJE,ColomboFA,deOliveiraAC,FocacciaR,
Pereira-ChioccolaVL.PCRassayusingcerebrospinalfluidfor diagnosisofcerebraltoxoplasmosisinBrazilianAIDS patients.JClinMicrobiol.2004;42:4765–8.
42.ColomboFA,VidalJE,PenalvadeOliveiraAC,etal.Diagnosis ofcerebraltoxoplasmosisinAIDSpatientsinBrazil:
importanceofmolecularandimmunologicalmethodsusing
peripheralbloodsamples.JClinMicrobiol.2005;43: 5044–7.
43.LeeCN,CavanaghHM,LoST,NgCS.Humanpapillomavirus
infectioninnon-neoplasticuterinecervicaldiseaseinHong Kong.BritJBiomedSci.2001;58:85–91.
44.BustinSA,BenesV,GarsonJA,etal.TheMIQEguidelines: minimuminformationforpublicationofquantitative real-timePCRexperiments.ClinChem.2009;55:611–22.
45.BartlettJW,FrostC.Reliability,repeatabilityand
reproducibility:analysisofmeasurementerrorsincontinuous variables.UltrasoundObstetGynecol.2008;31:466–75.
47.CostaJG,CarneiroAC,TavaresAT,etal.Real-timePCRasa prognostictoolforhumancongenitaltoxoplasmosis.JClin Microbiol.2013;51:2766–8.
48.RomandS,ChossonM,FranckJ,etal.Usefulnessof quantitativepolymerasechainreactioninamnioticfluidas earlyprognosticmarkeroffetalinfectionwithToxoplasma gondii.AmJObstetGynecol.2004;190:797–802.
49.LinMH,ChenTC,KuoTT,TsengCC,TsengCP.Real-timePCR forquantitativedetectionofToxoplasmagondii.JClin Microbiol.2000;38:4121–5.
50.Al-SoudWA,RadstromP.CapacityofninethermostableDNA polymerasestomediateDNAamplificationinthepresenceof PCR-inhibitingsamples.ApplEnvironMicrobiol.
1998;64:3748–53.
51.WahabT,EdvinssonB,PalmD,LindhJ.Comparisonofthe AF146527andB1repeatedelements,tworeal-timePCR
targetsusedfordetectionofToxoplasmagondii.JClin Microbiol.2010;48:591–2.
52.CostaJM,BretagneS.VariationofB1geneandAF146527 repeatelementcopynumbersaccordingtoToxoplasmagondii strainsassessedusingreal-timequantitativePCR.JClin Microbiol.2012;50:1452–4.
53.FerreiraIM,VidalJE,deMattosCC,etal.Toxoplasmagondii isolates:multilocusRFLP-PCRgenotypingfromhuman patientsinSaoPauloState,Brazilidentifieddistinct genotypes.ExpParasitol.2011;129:190–5.
54.CarneiroAC,AndradeGM,CostaJG,etal.Genetic