www.elsevier.es/ai
Allergologia
et
immunopathologia
SociedadEspa˜nola deInmunolog´ıaCl´ınica,
Alergolog´ıayAsmaPedi´atrica
ORIGINAL
ARTICLE
Non-steroidal
anti-inflammatory
drug
hypersensitivity
in
children
C.
Alves
a,∗,
A.M.
Romeira
a,
C.
Abreu
b,
P.
Carreiro-Martins
a,c,
E.
Gomes
b,
P.
Leiria-Pinto
a,caDonaEstefâniaHospital,CentroHospitalardeLisboaCentral,RuaJacintaMarto,1169-045Lisbon,Portugal bCentroHospitalardoPorto,LargoProf.AbelSalazar,4099-001Oporto,Portugal
cCEDOC,RespiratoryResearchGroup,NovaMedicalSchool,CampodosMártiresdaPátria,130,1169-056Lisbon,Portugal
Received13January2016;accepted1April2016 Availableonline27July2016
KEYWORDS Allergy; Children; Drugprovocation test; Hypersensitivity; NSAID Abstract
Introduction:There are rather few publications about hypersensitivity reactions to non-steroidal anti-inflammatorydrugs (NSAID) inthepaediatric age.In this study,we aimedto assessthefrequencyofconfirmedNSAIDhypersensitivityinchildrenwithapreviousreported reactiontoNSAID inordertoinvestigate therole ofthedrug provocationtest (DPT)inthe diagnosticworkupandtoexplorethefactorsassociatedwithconfirmedNSAIDhypersensitivity.
Methods:Weconductedaretrospectiveanalysisoftheclinicalfilesfromeverypatientunder 18yearsoldwhoattendedtwoPortuguesepaediatricallergyoutpatientclinics,fromJanuary 2009toAugust2014,duetoasuspectedNSAIDhypersensitivity.
Results:Weincluded119patients,withamedianageofnineyears(P25---P75:5---14).Ibuprofen
wasthecommonestimplicatedNSAIDinthepatients’reports(n=94---79%).AfterDPT,NSAID hypersensitivitywasconfirmedinnine(7.6%)patients,excludedin93(78.2%)andwas incon-clusivein17(14.3%).Inthemajority(n=95---79.8%),thereactionoccurredinthefirst24hafter intake.Eighty-fourpatients(70.6%)reportedonlycutaneousmanifestationsand18(15.1%)had systemicsymptoms.AnaphylaxisrepresentedarelativerisktoNSAIDhypersensitivity confirma-tion.Noassociationwasfoundforatopyandthenumberofpreviousreactions.
Conclusion:In ourstudy,NSAID hypersensitivitywas confirmedinasmall proportionofthe patients with a previous reported reaction. Ibuprofen was the most implicated drug with urticaria/angio-oedemaasthecommonestmanifestation.Anaphylaxiswasassociatedwith con-firmeddrughypersensitivity.Thedrugprovocationtestwasessentialtoestablishthediagnosis. ©2016SEICAP.PublishedbyElsevierEspa˜na,S.L.U.Allrightsreserved.
∗Correspondingauthor.
E-mailaddress:catia@catia-alves.net(C.Alves).
http://dx.doi.org/10.1016/j.aller.2016.04.004
Introduction
Non-steroidalanti-inflammatorydrugs(NSAID)areawidely
used group of medications withantipyretic and analgesic
properties. This leads to frequent reports of
hypersensi-tivity reactions to NSAIDs (NSAID-H) by adults as well as
children.Thereportedprevalenceofhypersensitivity
reac-tionstoNSAIDsinthegeneralpopulationis0.3%inadults,
withsimilarresultsinchildren.1InPortugal,theprevalence
ofself-reportedNSAID-Hwas0.5%amongchildrenattending
day-carecentres.2
Apreviousstudy,whichwasperformedinPortugalamong
children,showedthat,althoughadversedrugreactionsare
frequently described in this population, after a thorough
investigation,atruedrughypersensitivitywillbeconfirmed
inonlyafewcases.3
In patients evaluated due to a possible NSAID-H, the
investigation confirmed this suspicion in a minority of
them.4,5 The drug provocation test (DPT) withthe culprit
drug is considered the gold standard to diagnose NSAID
hypersensitivity.6,7Theprevalenceofimmunologically
medi-atedreactionstoNSAIDsrangesfrom0.1%to3.6%.8
There are rather few studies in the paediatric
popu-lation regarding NSAID hypersensitivity reactions. Atopy,
immediate-typereactions or respiratorysymptomsas
pre-viousmanifestationshavebeenidentifiedasriskfactorsby
someauthors.5
The aim of our study was to assess the frequency of
confirmed NSAIDhypersensitivity in childrenwith a
previ-ousreportedreaction toNSAID,toinvestigate theroleof
the drug provocation test (DPT) in the diagnostic workup
andtoexplorethefactorsassociatedwithconfirmedNSAID
hypersensitivity.
Methods
Studydesign,settingandparticipants
We conductedaretrospective analysisof theclinical files
fromevery patient under18 years oldwho attendedtwo
PortugueseImmunoallergyDepartments(oneinLisbonand
anotherinOporto),fromJanuary2009toAugust2014,due
toasuspectedNSAIDhypersensitivity.
The participating centres were the Immunoallergy
DepartmentofDonaEstefâniaHospital,CHLC,inLisbonand
theImmunoallergyDepartmentof MariaPiaHospital,CHP,
inOporto,whicharethetwomainpublicpaediatricallergy
centresinPortugal.
Datacollection
Datawasretrospectivelycollectedbyastandardised
ques-tionnaire in order to gather information about NSAID
reactions and diagnostic test results. Inboth allergy
cen-tres, the usual diagnostic workup includes a first clinical
assessmentofthereportedreactionfollowedbyaDPT.
The standardised questionnaire comprised questions
regarding the chronology of the reaction (acute --- if the
reactionoccurredinthefirst24haftertheintake;delayed
--- if it occurred after 24h),7,8 implicated drugs, clinical
presentation,numberofpreviousreactions,ageatthetime
of the first reaction, drug provocation test result, atopic
status(definedasatleastonepositiveskinpricktest---SPT
---to aero/foodallergens) and previous diagnosis of
aller-gicdiseases(asthma,atopiceczema,allergicrhinitis,food
allergyandchronicurticaria).Anaphylaxiswasdefinedasa
severe,life-threateninggeneralisedorsystemic
hypersensi-tivityreaction,accordingtotheliterature.9
Drugprovocationtest
Iftherewasnocontraindication,7,8,10NSAIDprovocationtest
wasperformedtoconfirmorexcludethepresenceof
hyper-sensitivityandtoclassifythereaction.
OpenDPTs wereconducted byan experiencedallergist
according to the recommendations of the ENDA
(Euro-peanAcademyofAllergologyandClinicalImmunologyDrug
Hypersensitivity Interest Group).10 The diagnostic
work-up included single-blind, placebo-controlled DPT if the
reportedsymptomsweresubjectiveoriftheopenDPT
per-formed ended in an unclear result. Incremental doses of
thedrugwereadministratedatintervalsof30---60min,
stop-pingassoonasthefirstobjectivesymptomsoccurredorat
theendoftheintakeofthedefineddosesoftheprotocol.
Thefirstadministrationwasusually1/10of the
therapeu-ticdose, according to the weightand age, and the total
cumulativedosewassimilartotheusualmaximalindividual
intakedose (adjusted toweight and age).Symptoms and
vitalsigns weremonitored before, duringand at the end
oftheDPT.Threehourswastheminimalperiodof
surveil-lanceafterthelastdrugadministration.Written informed
consentwasobtainedfromtheparentsorguardiansbefore
theprocedure.
TheDPTwasconsideredpositiveifitreproducedthe
pre-viousreportedreactionorelicitedobjectivemanifestations.
ANSAID-Hconfirmation wasassumed whenthepatient
hadapositiveDPTwiththeimplicatedoralternativeNSAID.
ANSAID-HwasexcludediftheDPTwiththeimplicateddrug
wasnegative.Theinvestigationwasconsideredinconclusive
whenDPTwasnegativewiththealternativeNSAIDbutaDPT
withtheculpritNSAID wasnotperformed (in thiscase, a
singleNSAID-Hcannotbeexcluded),orwhenaDPTwasnot
performedatall(duetotheparent’srefusal).
Statisticalanalysis
Anexploratoryanalysisofthevariablesofinterest(gender,
age,presenceofatopyandallergicconditions,typeofdrug,
manifestationsandchronology,NSAIDtolerance)wascarried
out.TheMann---WhitneyUtestwasusedforcomparisonsof
differencesbetweenmedians.Achi-squaredtest orFisher
ExactTestwasusedtocomparethecategoricalvariables.
Thelevelofsignificanceconsideredwas˛=0.05.Statistical
analysiswasperformedusingIBMSPSSStatisticsVersion23®
(NewYork,USA).
Results
Weincluded119children,51.3%boys,withamedianageof
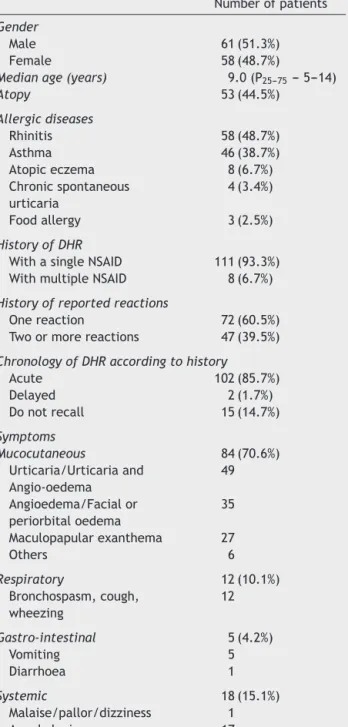
Table1 Demographiccharacteristicsofpatientsand clin-icalmanifestationsofthereportedreaction.
Numberofpatients
Gender
Male 61(51.3%)
Female 58(48.7%)
Medianage(years) 9.0(P25---75---5---14)
Atopy 53(44.5%) Allergicdiseases Rhinitis 58(48.7%) Asthma 46(38.7%) Atopiceczema 8(6.7%) Chronicspontaneous urticaria 4(3.4%) Foodallergy 3(2.5%) HistoryofDHR
WithasingleNSAID 111(93.3%) WithmultipleNSAID 8(6.7%)
Historyofreportedreactions
Onereaction 72(60.5%) Twoormorereactions 47(39.5%)
ChronologyofDHRaccordingtohistory
Acute 102(85.7%) Delayed 2(1.7%) Donotrecall 15(14.7%) Symptoms Mucocutaneous 84(70.6%) Urticaria/Urticariaand Angio-oedema 49 Angioedema/Facialor periorbitaloedema 35 Maculopapularexanthema 27 Others 6 Respiratory 12(10.1%) Bronchospasm,cough, wheezing 12 Gastro-intestinal 5(4.2%) Vomiting 5 Diarrhoea 1 Systemic 18(15.1%) Malaise/pallor/dizziness 1 Anaphylaxis 17
DHR---drughypersensitivityreaction;NSAID---non-steroidal anti-inflammatorydrug.
60.5%hadapreviousdiagnosisofallergicdiseases. Accord-ingtotheclinicalreports,111patients(93.3%)describeda reactiontoasingleNSAID(Table1).
Priortotheinvestigation,70(58.8%)patientshadproven
tolerability to at least one alternative NSAID (which was
verifiedafterthereportedreaction--- thepatienttookan
alternativeNSAIDafterthereportedreactionbutprevious
tothisinvestigation):60(85.7%)referredtoleranceto
para-cetamol,eight(11.4%)toacetylsalicylic acid(ASA), three
(4.3%) to metamizole, two (2.9%) to ibuprofen and one
(1.4%)tonimesulide.
Table2 NumberofpatientsandsuspectedNSAIDinvolved. ImplicatedNSAID Numberofpatients
Ibuprofen 94 Paracetamol 20 ASA 7 Nimesulide 4 Metamizol 1 Etoricoxib 1 Ketorolac 1
NSAID---non-steroidalanti-inflammatorydrugs;ASA--- acetylsal-icylicacid.
Inoursample,47(39.5%)patientsreportedtwoormore previousreactions(41withthesamedrugandsixwith dif-ferentNSAID)(Table1).
Parentsreported a previous adverse reaction toother
drugclassin24(20%)ofthechildren,mostlytoantibiotics
(n=17).
At the time of the first reported reaction, 84 (70.6%)
patientshadonly mucocutaneousmanifestationsafterthe
intake of the implicated NSAID, 18 (15.1%) had systemic
symptoms(17anaphylaxis),12(10.1%)hadisolated
respira-torymanifestationsandfive(4.2%)referredgastrointestinal
symptoms(Table1).
ThemostcommonimplicatedNSAIDinthepatients’
reac-tionreportswasibuprofen(N=94;79%)(Table2).
Thecharacteristics ofthefirstreactionand theresults
oftheinvestigationperformedinthepatientswhoreported
anaphylaxisaredetailedinTable3.
TheDPTdiagramispresentedinFig.1.Inoursample,99
(83.2%)patientswerechallengedwiththeimplicatedNSAID
(positiveinfiveDPT)and19(16%)withanalternativeNSAID
duetoseverityofthereactions/patientrefusal(positivein
four).OnepatientrefusedtheDPTwiththeimplicateddrug
ashealreadyhadaknowntolerancetoanalternativeNSAID.
ThepresenceofNSAIDhypersensitivitywasconfirmedin
ninepatients (7.6%)(Table4),excluded in93 (78.2%)and
inconclusivein17(14.3%)patients.
Oftheninepatientswithproven NSAID-H,seven hada
previous diagnosis of an allergicdisease (fiveatopic,
sen-sitised to house dust mites). Of the 93 patients without
NSAID-H,30hadconfirmedsensitisationtohousedustmites.
Patientswith confirmed hypersensitivity were older at
the time of the first reaction (p=0.008) than patients in
which NSAID-H was excluded. A reported history of
ana-phylaxiswasassociatedwithconfirmedNSAID-H(p=0.029)
(RR=5.48,95%CI1.69---17.8,p=0.0047).Nostatistically
sig-nificantdifferenceswerefoundforasthma,urticaria,atopy
andanumberofpreviousreactions.However,patientswith
asthmaorrhinitispresentedahigherfrequencyofconfirmed
NSAID-H(11.9%versus6.7%forasthmaand14%versus3.8%
forrhinitis)thanthosewithout allergicrespiratorydisease
(Table5).
Discussion
Inthepresentstudy,wefounda7.6%frequencyof
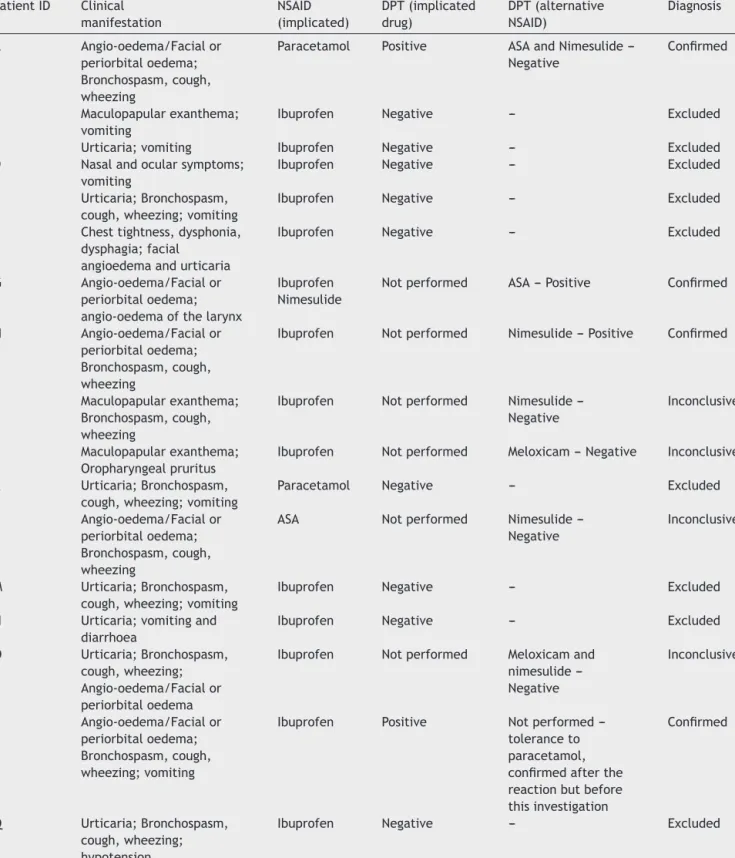
Table3 Clinicalmanifestationsofthereportedreaction,implicateddruganddrugprovocationtestsresultsintheanaphylaxis cases. PatientID Clinical manifestation NSAID (implicated) DPT(implicated drug) DPT(alternative NSAID) Diagnosis A Angio-oedema/Facialor periorbitaloedema; Bronchospasm,cough, wheezing
Paracetamol Positive ASAandNimesulide ---Negative
Confirmed
B Maculopapularexanthema; vomiting
Ibuprofen Negative --- Excluded
C Urticaria;vomiting Ibuprofen Negative --- Excluded
D Nasalandocularsymptoms; vomiting
Ibuprofen Negative --- Excluded
E Urticaria;Bronchospasm, cough,wheezing;vomiting
Ibuprofen Negative --- Excluded
F Chesttightness,dysphonia, dysphagia;facial
angioedemaandurticaria
Ibuprofen Negative --- Excluded
G Angio-oedema/Facialor periorbitaloedema; angio-oedemaofthelarynx
Ibuprofen Nimesulide
Notperformed ASA---Positive Confirmed
H Angio-oedema/Facialor periorbitaloedema; Bronchospasm,cough, wheezing
Ibuprofen Notperformed Nimesulide---Positive Confirmed
I Maculopapularexanthema; Bronchospasm,cough, wheezing
Ibuprofen Notperformed Nimesulide ---Negative
Inconclusive
J Maculopapularexanthema; Oropharyngealpruritus
Ibuprofen Notperformed Meloxicam---Negative Inconclusive K Urticaria;Bronchospasm,
cough,wheezing;vomiting
Paracetamol Negative --- Excluded
L Angio-oedema/Facialor periorbitaloedema; Bronchospasm,cough, wheezing
ASA Notperformed Nimesulide ---Negative
Inconclusive
M Urticaria;Bronchospasm, cough,wheezing;vomiting
Ibuprofen Negative --- Excluded
N Urticaria;vomitingand diarrhoea
Ibuprofen Negative --- Excluded
O Urticaria;Bronchospasm, cough,wheezing; Angio-oedema/Facialor periorbitaloedema
Ibuprofen Notperformed Meloxicamand nimesulide ---Negative Inconclusive P Angio-oedema/Facialor periorbitaloedema; Bronchospasm,cough, wheezing;vomiting
Ibuprofen Positive Notperformed ---toleranceto paracetamol, confirmedafterthe reactionbutbefore thisinvestigation
Confirmed
Q Urticaria;Bronchospasm, cough,wheezing; hypotension
Ibuprofen Negative --- Excluded
NSAID---non-steroidalanti-inflammatorydrug;ASA---acetylsalicylicacid;DPT---drugprovocationtest.
Totalcumulativedoseswere:ibuprofen10mg/kguntil600mg,paracetamol15mg/kguntil1000mg,nimesulide100mgandASA20mg/kg until1000mg.
99 patients DPT with the culprit
NSAID DPT positive in 5 patients DPT negative in 93 patients Investigation inconclusive in 1 patient(DPT inconclusive) 19 patients DPT with an alternative NSAID DPT positive in4 patients Investigation inconclusive in 15 patients (DPT inconclusive) 1 patient refused the DPT 119 Patients
Figure1 Drugprovocationtestdiagram.DPT---drugprovocationtest.
Table4 CharacterisationofthepatientswithconfirmedNSAID-H. PatientID Ageatthe
firstreaction Implicated NSAID Typeof reaction Numberof previous reactions Manifestation NSAIDDPT DPT manifestation A 12yrs Male
5yrs Paracetamol Acute ≥4 Anaphylaxis Culprit Bronchospasmand nasalsymptoms G 17yrs Male 13yrs Ibuprofen Nimesulide
Acute 2 Anaphylaxis Alternative---ASA Facial angio-oedema H
11yrs Male
10yrs Ibuprofen Acute 1 Anaphylaxis Alternative ---Nimesulide Facialand periorbital angio-oedema P 14yrs Male
14yrs Ibuprofen Acute 1 Anaphylaxis Culprit Periorbital angio-oedemaand ocularpruritus R
7yrs Female
6yrs Ibuprofen Acute 1 Urticaria; Angio-oedema/Facialor periorbital oedema
Culprit Urticariaand periorbital oedema S
13yrs Female
11yrs Ibuprofen Acute 1 Urticaria Alternative ---Nimesulide Hypotensionand dizziness T 8yrs Male
7yrs Nimesulide Acute 1 Urticaria; Angio-oedema/Facialor periorbital oedema Alternative ---Paracetamol Urticaria,facial angio-oedema, bronchospasm, coughand wheezing U 13yrs Female
13yrs Ketorolac Acute 1
Angio-oedema/Facialor periorbital oedema; Maculopapular exanthema
Culprit Facialand periorbital angio-oedema V 8yrs Male 6yrs Ibuprofen Paracetamol
Notclarified ≥4
Angio-oedema/Facialor periorbital oedema Culprit (paracetamol) Labial angio-oedema
Table5 CharacterisationofthepatientsthathadconfirmedandexcludedNSAID-H. ConfirmedNSAID-H patients(N=9) ExcludedNSAID-H patients(N=93) p-Value
Meanageatthefirstreaction(years)a 9.4 5.5 0.008
Gender Female(n=47) 3(6.4%) 44(93.6%) 0.657 Male(n=55) 6(10.9%) 49(89.1%) Anaphylaxisb With(n=13) 4(30.8%) 9(69.2%) 0.029 Without(n=89) 5(5.6%) 84(94.4%) Atopy With(n=44) 5(11.4%) 39(88.6%) 0.430 Without(n=58) 4(6.9%) 54(93.1%) Asthma With(n-42) 5(11.9%) 37(88.1%) 0.360 Without(n=60) 4(6.7%) 56(93.3%) Rhinitis With(n=50) 7(14%) 43(86%) 0.140 Without(n=52) 2(3.8%) 50(96.2%) Urticaria With(n=4) 0 4(100%) 0.530 Without(n=98) 9(9.2%) 89(90.8%) Numberofprevious reactions 1(n=61) 6(9.8%) 55(90.2%) 0.660 ≥2(n=41) 3(7.3%) 38(92.7%)
a FourpatientsinwhomNSAID-Hwasexcludeddidnotrecalltheageatthetimeofthefirstreaction. b ReportedtotheimplicatedNSAID.
urticaria and angio-oedema were the commonest clinical
manifestations.
ChildrenoftenhaveahistoryofNSAIDreactions,
partic-ularly toibuprofen. According totheliterature,in 86%of
thesinglereactorsandin56%ofcross-reactors,aNSAID-H
isnotconfirmed.6Itisbelievedthatconcomitantconditions
suchas infections, fever, or theuse of other drugs might
playaroleinthepathophysiologyofthereaction,eliciting
symptomsinthesepatients.6,8
A well-documented clinical history is essential for the
correctdiagnosisofNSAID-Handshouldincludeadescription
ofthesymptoms,timeintervalbetweendrugadministration
and the symptoms onset, implicated drugs, drugs
toler-atedafterthereaction,routeofadministration,numberof
episodesandunderlyingdiseases.8However,adiagnosisof
NSAID-Hsupportedsolelybytheclinicalhistorymayleadto
overdiagnosis.4
Ahistoryofrepeatedpreviousreactionswasreportedto
predictafutureresponsetoNSAID.7Inourstudy,similarlyto
thestudybyYilmazetal.6thenumberofpreviousreactions
wasnotassociatedwithapositiveDPTresult.
Itwassuggestedthatibuprofencouldbeusedtodetect
thecross-reactivetypeofNSAID-Hinchildren.6Accordingto
ourresults,ibuprofenwasthemostfrequentreported
cul-pritinsuspectedreactionsandthecommonestdruginvolved
inchallenge-provenNSAID-H.ThisisinlinewithZambonino
etal.1andGuveniretal.4Thisispartiallyexplainedbecause
in thepaediatric age theprescription of NSAIDs is largely
restrictedtoafewdrugs,namely ibuprofenand
paraceta-mol.
During the investigation of a suspected NSAID-H, it is
important tocheck for paracetamoltolerance in all
chil-drenwithcross-intolerancetoNSAIDsbecausetherearefew
medicationsapprovedforthetreatmentoffeveror
inflam-mationatyoungerages.1Ithasbeenshownthatreactivity
toparacetamolamongNSAID-Hpatientsrangesfrom0%to
25%.8,11Inthepresentstudy,2outof9patients(22%)with
confirmedNSAID-Hreactedtoparacetamol.
In this work, asfound by other authors,1,12,13 urticaria
andangio-oedemawerethemost frequentmanifestations
reportedby the patients, with facialangio-oedema, with
orwithoutgeneralisedurticaria,asthemostcommon
clin-icalpresentation.8,11 In the majority of the patients with
cutaneousmanifestations(94.0%),NSAID-Hwasconfirmedin
onlyfivepatients.Thelowfrequencyofconfirmeddiagnosis
hasbeen reportedby other authors,suggesting that even
in patients with a history of urticaria/angio-oedema, the
diagnosticprocedurecouldsafely startwiththeoffending
NSAID.14
Anaphylaxisisusuallyconsideredacontraindicationfor
DPTbecauseoftheriskassociated.IntheworkofZambonino
etal.1theDPTwiththeculpritdrugwasnotperformedin
patientswhoreportedanaphylaxis.However,inthestudyof
Yilmazetal.6areportofanaphylaxiswasnotassociatedwith
theDPTresult.Inourwork,17patientsreportedanaphylaxis
astheclinicalmanifestation.However,thehypersensitivity
wasonlyconfirmedinfour.TheseDPTwereperformedinthe
majority of anaphylaxis reported cases, asother possible
causes, such asviral infections or other drugs, couldnot
beruledout.Mostdrugchallengeswerenegative,although
thisclinicalpresentationwasassociatedwithmorepositive
resultsthanhavebeendescribedintheliterature.7
Inwhat concernsatopy, severalstudies have shown an
association between NSAID-H, allergic diseases (asthma,
rhinitis)andatopysensitisationinchildrenandadults,which
has been considered a significant risk factor for NSAID-H
inyoung children.1,11,15---17 An association particularly with
house dust mite sensitisation has been described.18 The
exactmechanism remains unclear.15,17 However,this
asso-ciationwasnotfoundbyotherauthors.4,14
Inourstudy,patientswithconfirmedNSAID-Hpresented
ahigherfrequencyofasthmaandrhinitisthanpatientswith
excludedNSAID-H.Almosthalf(44.5%)ofourpatientswere
atopic.Nevertheless, thesedifferences were not
statisti-callysignificantpossiblyduetothelownumberofpositive
In our sample, genders were equally distributed (58
girls and 61 boys). However, in the nine patients with
NSAID-H,sixweremale.Thesedataareconsistentwith
pre-viousreports.11 Themalepreponderancemaybeexplained
consideringtheassociation ofNSAID-Hwithatopyandthe
somewhatincreasedprevalenceofatopicdiseasesinboys.11
Regardingthemedianageatthetimeof thefirst
reac-tion,patientswithconfirmedNSAID-Hwereolderthanthose
forwhichthediagnosiswasexcluded.Thisdifference,which
waspreviouslydescribed,19couldberelatedtothefactthat
youngerchildren aremore susceptibleto viralinfections,
causingskineruptionsandleadingtoadeceptivediagnosis
ofNSAID-H.
DPTisthegoldstandardfor theNSAID-Hdiagnosis,7,8,10
andanegativeresultshouldbereassuringtothe patient.
Nonetheless, patients frequently continue to avoid the
tested NSAID after a negative result,20 emphasising the
importanceofaneffectivecommunicationbetweenpatients
and physicians. There are few published studies about
NSAID-Hin children.Forthisreason,weconsiderthatthe
present study provides additional information about this
topic,reinforcingtheneedtoundergoaninvestigationbya
DPTwiththeimplicatedNSAID.
Conclusion
Wefound a low frequency (7.6%) oftrue NSAID-H in
chil-drenwithapreviousNSAIDreaction.Ibuprofenwasthemost
frequentlyimplicateddrugandurticariaandangio-oedema
were the commonest clinical manifestation. Anaphylaxis
was a relative risk to confirmed drug hypersensitivity.
Despitethis,inthefaceofreportedanaphylaxis,theclinical
historymightnotbesufficienttoestablishafinaldiagnosis
ofNSAID-H.Thedrugprovocationtestprovedtobeessential
intheNSAID-Hdiagnosisworkupandshouldbeconsidered,
evenwhensystemicsymptomsarereported.
Ethical
disclosures
Confidentialityofdata.Theauthorsdeclarethattheyhave
followedthe protocols of theirwork centre onthe
publi-cation of patient data and that all the patients included
inthestudyhavereceivedsufficientinformationandhave
giventheirinformedconsentinwritingtoparticipateinthat
study.
Righttoprivacyandinformedconsent.Theauthorshave
obtained the informed consent of the patients and/or
subjectsmentionedinthearticle.Theauthorfor
correspon-denceisinpossessionofthisdocument.
Protection of human and animal subjects.The authors
declarethatnoexperimentswereperformedonhumansor
animalsforthisinvestigation.
Conflicts
of
interest
Nonedeclared.
References
1.ZamboninoMA,TorresMJ,Mu˜nozC,Requena G, MayorgaC, PosadasT,etal.Drugprovocationtestsinthediagnosisof hyper-sensitivityreactionstonon-steroidalanti-inflammatorydrugsin children.PediatrAllergyImmunol.2013;24:151---9.
2.MartinsP,BeloJ,MarquesJ,PapoilaAL,CairesI,Araújo-Martins J,etal.Reporteddrugallergyamongchildrenattendingday carecenters.ActaMedPort.2014;27:444---9.
3.RebeloGomesE,FonsecaJ,AraújoL, DemolyP.Drugallergy claimsinchildren:fromselfreportingtoconfirmeddiagnosis. ClinExpAllergy.2008;38:191---8.
4.GuvenirH,MisirliogluE,VezirE,ToyranM,GinisT,CivelekE, etal.Nonsteroidalanti-inflamatorydrughypersensitivityamong children.AllergyAsthmaProc.2015;36:386---93.
5.CavkaytarO,YilmazEA,KaraatmacaB,BuyuktiryakiB,Sackesen C,SekerelBE,etal.Differentphenotypesofnon-steroidal anti-inflammatorydrughypersensitivity duringchildhood.IntArch AllergyImmunol.2015;167:211---21.
6.YilmazO,ErtoyKaragolIH,BakirtasA,TopalE,CelikGE, Demir-soyMS,etal.Challenge-provennonsteroidalanti-inflammatory drug hypersensitivity in children. Allergy. 2013;68: 1555---61.
7.KowalskiML, Asero R, Bavbek S, Blanca M, Blanca-Lopez N, BochenekG,etal.Classificationandpracticalapproachtothe diagnosisandmanagementofhypersensitivitytononsteroidal anti-inflammatorydrugs.Allergy.2013;68:1219---32.
8.Ortega N, Do˜na I, Moreno E, Audicana MT, Barasona MJ, Berges-Gimero MP, et al. Practical guidelines for diagnosing hypersensitivity reactions to nonsteroidal anti-inflammatory drugs.JInvestigAllergolClinImmunol.2014;24:308---23.
9.VivoloAunM,BlancaM,SabinoGarroL,RosimeireRibeiroM, KalilJ, AbilioMottaA, etal.Nonsteroidalanti-inflammatory drugsaremajorcausesofdrug-inducedanaphylaxis.JAllergy ClinImmunolPract.2014;2:414---20.
10.AbererW, BircherA, Romano A,Blanca M,Campi P, Fernan-dez J, et al. Drug provocation testing in the diagnosis of drughypersensitivityreactions:generalconsiderations.Allergy. 2003;58:854---63.
11.KidonMI,KangLW,ChinWC,HoonLS,HugoVB.Nonsteroidal anti-inflammatorydrughypersensitivityinpreschoolchildren. AllergyAsthmaClinImmunol.2007;3:114---22.
12.Blanca-Lopez N, Torres MJ, Do˜na I, Campo P, Rondón C, Seoane Reula ME, et al. Value of the clinical his-tory in the diagnosis of urticaria/angioedema induced by NSAIDs with cross-intolerance. Clin Exp Allergy. 2013;43: 85---91.
13.KidonMI, KangLW, ChinCW, HoonLS, See Y, Goh A, et al. Early presentation with angioedema and urticaria in cross-reactive hypersensitivity to non-steroidal anti-inflammatory drugs among young, Asian, atopic children. Pediatrics. 2005;116:e675---80.
14.Zisa G, Riccobono F, Bommarito L, D’Antonio C, Cala-mari AM, Poppa M, et al. Provocation tests with the offending nonsteroidal anti-inflammatory drugs in patients with urticaria/angioedema reactions. Allergy Asthma Proc. 2012;33:421---6.
15.AyusoP,Blanca-LopezN,Do˜naI,TorresMJ,Gueant-Rodrıguez RM, Canto G, et al. Advanced phenotyping in hypersensi-tivity drug reactions to NSAIDs. Clin Exp Allergy. 2013;43: 1097---109.
16.Asero R. Single NSAID hypersensitivity is associated with atopic status. Eur Ann Allergy Clin Immunol. 2015;47: 48---53.
17.Sánchez-BorgesM,Capriles-HulettA.Atopyisariskfactorfor non-steroidal anti-inflammatory drug sensitivity. Ann Allergy AsthmaImmunol.2000;84:101---6.
18.Sánchez-Borges M, Fernández-Caldas E, Capriles-Hulett A, Caballero-Fonseca F. Mite-induced inflammation: more than allergy.AllergyRhinol.2012;3:e25---9.
19.RubioM,BousquetP-J,GomesE,RomanoA,DemolyP.Results ofdrughypersensitivityevaluationsinalargegroupofchildren andadults.ClinExpAllergy.2012;42:123---30.
20.BommaritoL,ZisaG,RiccobonoF,VillaE,D’AntonioC, Cala-mari AM, etal. Avoidance ofnonsteroidal anti-inflammatory drugsafternegativeprovocationtestinurticaria/angioedema reactions: real world experience. Allergy Asthma Proc. 2014;35:303---6.