Identification and characterization of
MAGO
and
Y14
genes
in
Hevea brasiliensis
Zi-Ping Yang
1,2, Hui-Liang Li
1, Dong Guo
1and Shi-Qing Peng
1,2,# 1Key Laboratory of Biology and Genetic Resources of Tropical Crops, Ministry of Agriculture, Institute of
Tropical Bioscience and Biotechnology, Chinese Academy of Tropical Agricultural Sciences, Haikou, China
2
College of Agriculture, Hainan University, Haikou, China
Abstract
Mago nashi (MAGO) and Y14 proteins are highly conserved among eukaryotes. In this study, we identified two MAGO (designated as HbMAGO1 andHbMAGO2) and two Y14 (designated as HbY14a and HbY14b) genes in the rubber tree (Hevea brasiliensis) genome annotation. Multiple amino acid sequence alignments predicted that HbMAGO and HbY14 proteins are structurally similar to homologous proteins from other species. Tissue-specific ex-pression profiles showed that HbMAGO andHbY14 genes were expressed in at least one of the tissues (bark, flower, latex, leaf and root) examined. HbMAGOs and HbY14s were predominately located in the nucleus and were found to interact in yeast two-hybrid analysis (YTH) and bimolecular fluorescence complementation (BiFC) assays. HbMAGOs and HbY14s showed the highest transcription in latex and were regulated by ethylene and jasmonate. In-teraction between HbMAGO2 and gp91phox
(a large subunit of nicotinamide adenine dinucleotide phosphate) was identified using YTH and BiFC assays. These findings suggested that HbMAGO may be involved in the aggregation of rubber particles inH. brasiliensis.
Keywords: interaction,Hevea brasiliensis, Mago nashi, Y14 proteins
Received: December 24, 2014; Accepted: June 08, 2015.
Introduction
The exon junction complex (EJC) regulates post-transcriptional events that include mRNA intracellular ex-port, cytoplasmic localization, non-sense mediated mRNA decay (NMD) and translation enhancement in metazoans
(Park and Muench, 2007; Leeet al., 2009;
Ashton-Beau-cageet al., 2010; Roignant and Treisman, 2010; Boothby
and Wolniak, 2011; Mufarregeet al., 2011). The EJC is a
multiprotein complex assembled onto mRNAs as a conse-quence of splicing and is thought to provide a molecular link between splicing and post-splicing mRNA metabolism
20-24 bases upstream of mRNA exon-exon junctions (Leet
al., 2000, 2003; Tangeet al., 2004; Gehringet al., 2009).
Several of the EJC components have been identified and classified into two groups, namely, EJC “core” and “pe-ripheral” factors. Four proteins form the EJC core: MAGO (short form of MAGO NASHI and known as MAGOH in humans), Y14 (also known as Tsunagi or RBM8A), eIF4A/eIF4A3 and BTZ (short form of Barentsz, also
known as MLN51), of which MAGO and Y14 constitute a
stable heterodimeric complexin vitroandin vivo(Tangeet
al., 2004, 2005; Le and Seraphin, 2008). Additional
com-ponents of the EJC include the splicing factors SRm160, Pinin and RnpS1, the NMD factors Upf1, Upf2 and Upf3, the translation factors Skar and Pym, and the export factors UAP56 and REF/Aly (Roignant and Treisman, 2010). These proteins are considered to be peripheral EJC compo-nents that are transiently associated with the EJC core in the nucleus or cytoplasm, where they are involved in splicing, export, translation and NMD (Lejeune and Maquat, 2005;
Tangeet al., 2005).
The MAGO (meaning “no grandchildren’’ in Japa-nese) gene was first identified as a strict maternal effect
gene inDrosophila (Boswell et al., 1991; Newmark and
Boswell, 1994; Micklem et al., 1997; Newmark et al.,
1997). MAGO can interact with an RNA binding protein, Y14, to regulate cell growth and is expressed throughout
the organism (Zhaoet al., 1998, 2000; Leet al., 2001; Mohr
et al., 2001; Chenet al., 2007). Based on the crystal
struc-tures of theDrosophilaand human MAGO-Y14 complex,
MAGO and Y14 proteins are core components of the EJC and can form a stable heterodimer that strongly associates
with spliced mRNA (Lauet al., 2003; Shi and Xu, 2003).
Both MAGO and Y14 are evolutionarily highly conserved Send correspondence to Shi-Qing Peng. Key Laboratory of Biology
and Genetic Resources of Tropical Crops, Ministry of Agriculture, Institute of Tropical Bioscience and Biotechnology, Chinese Acad-emy of Tropical Agricultural Sciences, Haikou 571101, China. E-mail: shqpeng@163.com
#
proteins (Hachet and Ephrussi, 2001; Mohret al., 2001) and slow co-evolution of the two protein families is re-quired for the maintenance of their obligate
heterodime-rization mode (Gonget al., 2014). Comparative sequence
analysis reveals that MAGO is devoid of known structural motifs while Y14 contains a central RNA-binding domain
(Kataokaet al., 2000; Kimet al., 2001; Shi and Xu, 2003).
The crystal structure of the heterodimeric complexes, formed in the absence of RNA, shows that the RNA-binding motif is used for Y14/MAGO protein-protein inter-action and is not readily available for binding RNA
(Fri-bourget al., 2003; Lauet al., 2003; Shi and Xu, 2003).
In plants, MAGO proteins have been studied to
vary-ing degrees (Chenet al., 2007; Gong and He, 2014). The
orthologs of MAGO genes are linked to male fertility. For example, PFMAGO proteins interact with MADS-domain protein MPF2 and are responsible for male fertility and
ca-lyx development in Physalis (He et al., 2007). In
Arabidopsis,AtMAGO is required for pollen grain
devel-opment (Johnsonet al., 2004) and embryo development, as
well as meristem cell death events (Park et al., 2009).
MAGO is essential for spermatogenesis as shown by RNAi
knockdown ofMvMAGOinMarsilea(van der Weeleet al.,
2007; Boothby and Wolniak, 2011). MAGO genes are also involved in the development of other organs, such as lea-ves, stems, roots and seeds. The overexpression of
TcMAGO in transgenic tobacco plants results in longer
roots and a more complex root system (Chenet al., 2007).
InArabidopsis, RNAi-AtMAGOplants produce a greater number of leaves, short and fasciated stems, short lateral
roots and non-viable seeds (Parket al., 2009).OsMAGO
knockdown generated short rice plants with abnormal flowers (Gong and He, 2014). The function of MAGO as a component of the EJC is well-understood, and some func-tions of MAGO have been reported for other plants; how-ever, the existence of a duplicated MAGO gene in rubber trees is not widely known.
The rubber tree (Hevea brasiliensis) is the main
re-newable world-wide source of commercial natural rubber (NR), a biopolymer of high economic interest composed mainly of cis-1,4-polyisoprene. Latex is produced by lati-cifers after tapping and is a white cytoplasmic colloidal sus-pension containing mainly rubber particles, but also non-rubber particles, organelles, proteins and serum. In this study, the MAGO and Y14 gene families in rubber trees were characterized. To investigate their evolutionary rela-tionships and functions, we undertook structural and phylo-genetic analyses and examined the subcellular locations of these proteins. The expression profiles of MAGOs and Y14s in various organs and the response to ethylene (ET) and jasmonate (JA) were also studied. Furthermore, the in-teraction partners of HbMAGO were identified in latex and
the HbMAGO2 interaction with gp91phox(a large subunit of
nicotinamide adenine dinucleotide phosphate) was demon-strated using yeast two-hybrid analysis (YTH) and
bimo-lecular fluorescence complementation (BiFC) assays. Our results indicate that HbMAGO may be involved in the ag-gregation of natural rubber in rubber trees.
Materials and Methods
Plant materials
Hevea brasiliensiscultivar RY7-33-97 was planted on the experimental farm of the Chinese Academy of Trop-ical Agriculture Sciences, Hainan, China. The shoots were treated with 0.5% ethylene (ET) or 0.1% jasmonic acid (JA), as described by Hao and Wu (2000). Latex samples were collected 1, 3, 6, 9, 24 and 48 h after treatment from 12 shoots for each interval and were immediately stored at -80 °C for RNA extraction. Three independent biological replicates were done for each treatment. For latex RNA ex-traction, the latex was dropped directly into liquid nitrogen in an ice kettle. Rubber tree flowers, leaves and bark were washed with double-distilled water to remove latex and then immediately frozen in liquid nitrogen.
Database search and sequence conservation analysis of rubber tree MAGO and Y14 genes
A local whole genome shotgun database for rubber
tree was established by Yanget al.(2014). The full-length
cDNA of HbMAGO2 was obtained from a yeast
two-hybrid cDNA library of H. brasiliensis laticifers using
HbWRKY as the bait in the yeast two-hybrid assay (data unpublished). HbY14a was obtained from the yeast
two-hybrid cDNA library of H. brasiliensis laticifers using
HbMAGO2 as the bait. HbMAGO2 and HbY14a were used as queries in Blast searches of the rubber tree whole ge-nome shotgun database. The shotgun sequences that pro-duced the highest significant alignments judged by the scores and E-values were used for HMM-based gene struc-ture prediction (http://linux1.softberry.com/berry.phtml)
based on theArabidopsisand rubber tree databases. The
genomic DNA and cDNA sequences of four genes were amplified using appropriate primers (developed based on gene structure prediction) and sequenced.
Phylogenetic analysis and genomic structure
Analysis and comparison of the HbMAGO and HbY14 sequences were done using BLAST at the NCBI database. Clustal X (http://www.ebi.ac.uk/clustalw) was used for multiple alignments of the nucleic acid and amino acid sequences of HbMAGOs and HbY14s. A phylogenetic
tree was constructed with MEGA5.2
software (http://gsds.cbi.pku.edu.cn/) and the correspond-ing genomic DNA sequences that were extracted from the local whole genome shotgun database.
Transient protein expression
To determine the subcellular location of the proteins, full-length cDNAs were cloned (see Table 1 for primers) and inserted into the vector pCAMBIAC1302 driven by the 35S promoter. The resulting pCAMBIA1302 constructs were first transformed into the GV3101 strain of
Agrobacterium tumefaciens by electroporation (Gene Pulser Xcell, Bio-Rad, USA) and then introduced into on-ion epidermal cells by agroinfiltraton-ion using a water circu-lating vacuum pump (model SHZ-III B, Shanghai, China)
(Yanget al., 2000, 2014). The empty GFP vector was
infil-trated as a control. For the BiFC assay, the open reading
frames of HbMAGO2 and gp91phox (without their stop
codons) were subcloned into pSPYNE-35S (split YFP N-terminal fragment expression) or pSPYCE-35S (split YFP C-terminal fragment expression) vectors driven by the 35S promoter (see Table 1 for primers). The resulting pSPYNE and pSPYCE plasmids were transformed into the GV3101 strain and the YNE- and YCE-fused proteins were then co-expressed in onion epidermal cells by
agroinfil-tration (Walteret al., 2004; Yanget al., 2014).
Co-expres-sions of target genes and YFPN or YFPC were used as negative controls. The cells were dipped in 30% sucrose so-lution for 30 min to induce plasmolysis and the green fluo-rescent protein (GFP) or yellow fluofluo-rescent protein (YFP) signal was then examined with a confocal laser scanning microscope (Zeiss LSM510, Germany).
Expression analysis
Total RNA was extracted as described by Tanget al.
(2007). First-strand cDNA was synthesized using a
RevertAidTMfirst-strand cDNA synthesis kit (Fermentas,
Lithuania). qRT-PCR was done using the primers indicated in Table 1, with the rubber tree actin gene (GenBank HQ260674.1) being used as a reference gene. The qPCR fragments were amplified and sequenced to determine primer specificity. qRT-PCR was done using the fluores-cent dye SYBR-Green (Takara, China) and the melting curves of the amplification products were assessed using a Stratagene Mx3005P real-time thermal cycler (Agilent, America). The qRT-PCR conditions were as follows: 30 s at 95 °C for denaturation followed by 40 cycles of 5 s at 94 °C, 20 s at 56 °C and 20 s at 72 °C for amplification. An av-erage of three independent biological replicates was run for each time interval. The experimental results were reported
as the mean±SEM of three repli-cates. The data were
ana-lyzed with one-way ANOVA and the level of significance was set at p < 0.01 or p < 0.05 (Fisher’s protected least sig-nificant difference). All data analyses were done using Sta-tistical Product and Service Solutions software (SPSS,
version 16.0). Table
Yeast two-hybrid assays
For yeast mating, the open reading frame of HbMAGO2 was inserted into pGBKT7 vectors to create a
bait plasmid. The bait plasmid (pGBKT7-HbMAGO2) was
transformed into the yeast strain AH109 by the lithium ace-tate method, according to the Yeastmaker yeast transforma-tion system 2 user manual (Clontech); the yeast cells were subsequently grown on SD/-Trp medium for 3 days. The bait strain was co-incubated with the library strain to pro-mote fusion in 2YPDA liquid medium containing
kana-mycin (50mg/mL) and the intermixture was shaken slowly
at 30 °C. If zygotes were present, the mated culture was centrifuged and resuspended in 0.5YPDA/Kan liquid me-dium followed by plating on DDO/X/A (double dropout
medium: SD/-Trp/-Leu with X-a-Gal and aureobasidin A)
medium and incubation at 30 °C for 3-5 days. Plasmids
were rescued from yeast, transformed intoE. coliand the
DNA then purified. The interaction was confirmed by co-transforming the library plasmid and bait into the yeast strain AH109. The cDNA inserts were subsequently se-quenced to confirm their identity.
To confirm one-to-one interaction, the open reading
frame of HbMAGOs, HbY14s and gp91phoxwere inserted
into pGBKT7 or pGADT7 vectors to create bait or prey (primers listed in Table 1). The indicated combination of the bait and prey plasmids was co-transformed into yeast strain AH109 and the yeast cells were plated onto
QDO/X/A medium (quadruple dropout medium:
SD/-Trp/-Leu/-Ade/-His with X-a-Gal and aureobasidin
A).b-Galactosidase activity was measured with
o-nitro-phenyl-b-D-galactopyranoside (ONPG) as the substrate
ac-cording to the Yeast Protocols Handbook (no. PT3024-1, Clontech). The changes in absorbance were monitored at OD420nm. The experimental results were reported as the
means±SEM of three repli-cates. The data were analyzed
with one-way ANOVA and the level of significance was set at p < 0.01 or p < 0.05 (Fisher’s protected least significant difference). All data analyses were done using Statistical Product and Service Solutions software (SPSS, version 16.0).
Results
Identification and sequence conservation of rubber tree MAGO and Y14 genes
Two MAGO genes (designated as HbMAGO1 and
HbMAGO2) and two Y14 genes (designated asHbY14a
and HbY14b) were identified in rubber tree. The HbMAGO1/2 proteins had calculated molecular masses of 17.476 and 17.688 kDa and isoelectric points (pI) of 5.68 and 5.69 (Table 2). Alignment analysis indicated signifi-cant similarities among homologous proteins. The most striking feature of MAGO proteins was their highly con-served primary amino acid sequences (Figure 1A). The
HbMAGO1 protein shared 94%, 93%, 92% and 92% ami-no acid identity with the homologs TcMAGO, PtMAGO, CsMAGO and VvMAGO, respectively; the HbMAGO2 protein shared 94%, 91%, 92% and 91% amino acid iden-tity with the homologs of TcMAGO, PtMAGO, CsMAGO and VvMAGO, respectively. The secondary structure pre-diction of the MAGO protein revealed that it consisted of
six b-strands (b1-b6) and three a-helices (a1-a3) in a
b1-b2-b3-b4-a1-b5-b6-a2-a3 arrangement (Figure 1A).
The HbY14a/b proteins had calculated molecular masses of 21.737 kDa and 21.903 kDa and isoelectric points (pI) of 4.79 and 4.79 (Table 2).
The HbY14a protein shared 92%, 80%, 72%, 77%, 68% and 72% amino acid identity with the homologs of RcMAGO, PtaMAGO, PtbMAGO, CsiMAGO, MtMAGO and GmMAGO, while the HbY14b protein shared 91%, 80%, 71%, 75%, 67% and 69% amino acid identity with the
homologs of RcMAGO, PtaMAGO, PtbMAGO,
CsiMAGO, MtMAGO and GmMAGO. The Y14s protein
possessed sevenb-strands (b1-b7) and twoa-helices (a
1-a2) in ab1-b2-a1-b3-b4-b5-a2-b6-b7 arrangement
(Fig-ure 1B). Alignment of the Y14 proteins revealed a well-conserved RNA-binding domain (RBD) in the middle of the protein (Figure 1B). An exon-intron structure analysis revealed that HbMAGOs consisted of three introns and two exons, while the HbY14s had three introns and four exons, respectively (Figure 1C). Although, the differences in the cDNA sequences between two MAGOs or two Y14s were small, the differences in genomic DNA sequences were enormous.
Phylogenetic analysis of the rubber tree HbMAGO and HbY14 families
To obtain information about the evolutionary rela-tionships of HbMAGOs and HbY14s, phylogenetic analy-ses were done based on multiple sequence alignments of all transcribed proteins from these two gene families in plants. Phylogenetic analysis based on the alignments of MAGO family proteins showed that rubber trees and other dicots were clustered in same group (Figure 2A), whereas mem-bers of the Y14 family clustered with other dicots in a sin-gle group (Figure 2B). These analyses also revealed that the MAGO and Y14 genes were present in algae and that their differentiation accompanied that of the corresponding spe-cies.
Subcellular localization of HbMAGOs and HbY14s
MAGO and Y14 are nucleocytoplasmic shuttling proteins located predominantly in the nucleoplasm and
nuclear speckles (Micklemet al., 1997; Kataoka et al.,
2000; Mohret al., 2001). To confirm the subcellular
mi-croscope. HbMAGOs and HbY14s were targeted exclusively to the nucleus of the epidermal cells, whereas
the GFP control protein was distributed throughout the cells (Figure 3).
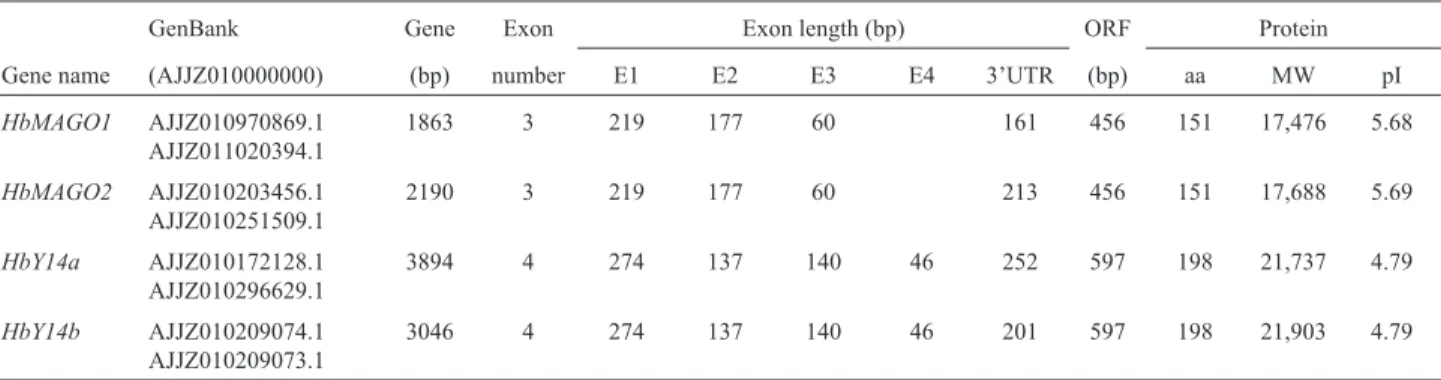
Table 2- Characterization of identified HbMAGO and HbY14 families.
GenBank Gene Exon Exon length (bp) ORF Protein Gene name (AJJZ010000000) (bp) number E1 E2 E3 E4 3’UTR (bp) aa MW pI
HbMAGO1 AJJZ010970869.1 AJJZ011020394.1
1863 3 219 177 60 161 456 151 17,476 5.68
HbMAGO2 AJJZ010203456.1 AJJZ010251509.1
2190 3 219 177 60 213 456 151 17,688 5.69
HbY14a AJJZ010172128.1 AJJZ010296629.1
3894 4 274 137 140 46 252 597 198 21,737 4.79
HbY14b AJJZ010209074.1 AJJZ010209073.1
3046 4 274 137 140 46 201 597 198 21,903 4.79
aa – amino acids, ORF – open reading frame.
Expression analysis ofHbMAGOsandHbY14sin different tissues
Real-time quantitative PCR was used to examine the expression patterns of HbMAGOs and HbY14s in bark, flowers, latex, leaves and roots. The expression profiles
showed that the four genes (two each for HbMAGO and HbY14) were transcribed in all of the tissues examined, with the highest transcription in latex; for HbMAGO1, the level of transcription in bark was similar to that seen in la-tex (Figure 4A).
Expression patterns ofHbMAGOsandHbY14s in latex respond to treatment with JA and ET
Since ET and JA have an important role as signaling molecules that regulate rubber biosynthesis in rubber trees
(Hao and Wu, 2000; Zeng et al., 2009), we examined
whether exposure to ET or JA could influence HbMAGO and HbY14 expression in latex (Figure 4B), despite the lack of evidence relating MAGOs and Y14s to hormone signal-ing pathways. HbMAGO1 was regulated by ET but not by JA, and HbMAGO2 was induced by JA but not by ET. Two HbY14s were induced by ET and JA in latex. Gene expres-sion increased significantly within 9 h and subsequently
de-creased when treated with the plant hormones, i.e.,
HbMAGO1 was influenced by ET, HbMAGO2 by JA, HbY14a by ET and JA, and HbY14b by JA; HbY14b ex-pression also increased significantly within 24 h and subse-quently decreased in response to ET.
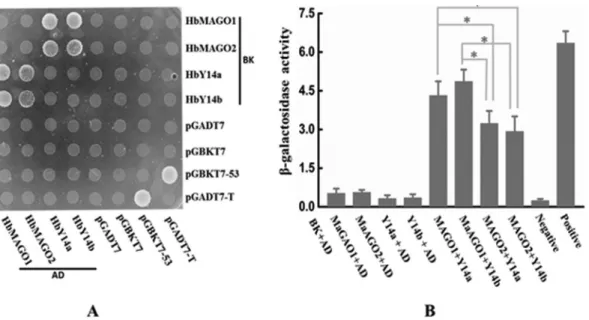
Interaction of HbMAGOs with HbY14s
As the core of EJC, MAGO and Y14 can form hetero-dimers. Figure 5A shows that HbMAGOs interacted with HbY14s in the YTH system. The interaction of HbMAGO1 with HbY14s was significantly greater than that of HbMAGO2 with HbY14s, as assessed by quantifying the
b-galactosidase activity (Figure 5B). This finding suggests
that HbMAGOl interacted specifically with HbY14s.
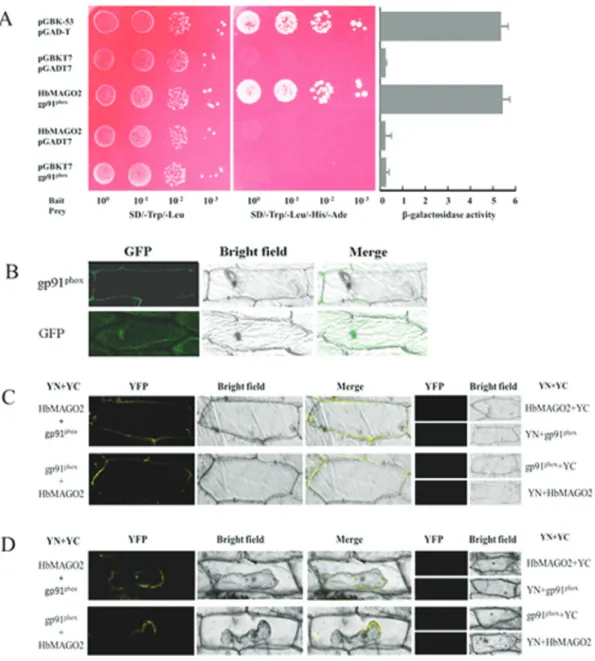
Interaction of HbMAGO2 with gp91phox
Using BK-MAGO as bait, we obtained gp91phoxas
one of the prey proteins screened from a two-hybrid latex
library (Figure 6A). The open reading frame of gp91phox
was cloned (GeneBank: AJJZ010935968.1). To confirm
the subcellular location of gp91phoxproteins, gp91phox:GFP
fusion proteins were transiently expressed in onion epider-mal cells. Confocal imaging of GFP fluorescence revealed
that the gp91phox:GFP fusion protein was located in the
cytomembrane (Figure 6B). The possible interaction
be-tween HbMAGO2 and gp91phox was examined using a
BiFC assay. A strong fluorescence signal was observed in the cytomembrane of epidermal cells under normal condi-tions (Figure 6C) and during plasmolysis (Figure 6D), but not with the negative controls. These data confirmed the
in-teraction between HbMAGO2 and gp91phox.
Discussion
There is increasing evidence that many factors in-volved in basic cellular processes in animals and plants are conserved. MAGO and Y14 proteins, which are important for cellular differentiation in animals, show marked se-quence conservation across a wide range of species (Chen
et al., 2007). The MAGO and Y14 proteins are encoded by
a small gene family in plants. The Arabidopsis genome
contains one MAGO and one Y14 gene (Park and Muench, 2007), whereas the rice genome contains two MAGO and two Y14 genes (Gong and He, 2014). We have identified Figure 3- Subcellular localization of HbMAGOs and HbY14s. Bottom
row of panels: fluorescence, bright field and merged fluorescence images of the GFP control. The other columns indicate the corresponding fluores-cence, bright field and merged fluorescence images of HbMAGOs and HbY14s.
Figure 4- Expression patterns ofHbMAGOsandHbY14sin different tis-sues (A) and the responses to treatment with jasmonic acid (JA) and ethyl-ene (ET) (B). Relative transcript abundances ofHbMAGOsandHbY14s
two MAGO and two Y14 genes in the rubber tree genome.
MAGO and Y14 proteins are highly conserved among eukaryotes, suggesting that the corresponding genes are es-sential for eukaryotes. Y14 proteins have a well-conserved RBD in the middle region. The RBD is a characteristic fea-ture of the ribonucleo-protein (RNP) family of RNA
bind-ing proteins and is evolutionarily conserved (Nagaiet al.,
1995). The homologs of Y14 that have these characteristics are known to be involved in RNA localization, mRNA
splicing and exon-exon junctions (Kimet al., 2001; Mohr
et al., 2001; Chuanget al., 2013). It has been suggested that HbY14 may also have these critical functions in plants. HbMAGOs and HbY14s shared high amino acid sequence identity with homologs from other species. Although MAGO and Y14 are highly conserved in eukaryotes, the clusters of MAGO and Y14 are easily distinguished be-tween dicots and other clades. The evidence from gene structure also supports this conclusion. The two MAGO genes in humans (MAGOH) consist of five exons with a conserved length and structure, but the coding sequence of
MAGOHB is longer than that of MAGOH (Singhet al.,
2013).
MAGO and Y14 are nucleocytoplasmic shuttling proteins located predominantly in the nucleoplasm and
nu-clear speckles (Micklemet al., 1997; Kataokaet al., 2000;
Mohr et al., 2001). In Taiwania cryptomerioides, TcMAGO-EGFP and TcY14-EGFP were detected at low levels in the cytoplasm at 20 h and 22 h. After 22 h, TcMAGO-EGFP was located only in the nuclei, whereas TcY14-EGFP was present in the nuclei and at low levels in
the cytoplasm (Chenet al., 2007). In rice, OsMAGO2 and
OsY14b showed identical distributions in cells and were lo-cated in the nuclei and cytoplasm. Surprisingly, OsY14a seemed to be uniquely located in the nuclei, in contrast to the established subcellular localization of these EJC sub-units (Gong and He, 2014). As shown here, HbMAGOs and HbY14s were located in the nuclei, in a manner similar to their homologs in other species.
In rubber trees, laticifers are tissues that are specifi-cally involved in the biosynthesis and storage of natural rubber, as well as in defense against pathogens. In this study, we found that HbMAGOs and HbY14s had a similar, constitutive expression pattern in different tissues of rubber tree. Four genes were transcribed in the tissues examined, with the highest transcription occurring in latex, a finding indicative of the vital role of these genes in laticifer cells. HbMAGO1 was regulated by ET but not by JA, and HbMAGO2 was induced by JA but not by ET. In contrast, two HbY14s were regulated by ET and JA in latex. The biosynthesis of natural rubber is enhanced in rubber trees by the endogenous accumulation and exogenous applica-tion of JA (Hao and Wu, 2000). Our findings suggest that the HbMAGO and HbY14 genes examined may regulate natural rubber biosynthesis in rubber tree laticifer cells via ET and JA signal transduction pathways. The differences in the levels of transcription of the genes in response to ET and JA also indicated functional differences in laticifer cells. This is the first demonstration that HbMAGO and HbY14 are linked to hormone signaling pathways. The pre-cise relationship will be investigated in future studies.
The MAGO gene was first identified as a strict
mater-nal effect gene inDrosophila, where a single point mutant
Figure 5- Protein interaction matrices for HbMAGO and HbY14 proteins (A) and quantification ofb-galactosidase activity (B). The combination of bait proteins (BD) and prey proteins (AD) is indicated. Interactions between HbMAGOs and HbY14s were detected in yeast and the same amounts of co-transformed yeast cells were grown in the highest stringent conditions (QDO/X/A medium). Transformants containing BK-53 and AD-T were used as positive controls and those containing only BK and AD or fused proteins with BK or AD only were used as negative controls (A). In panel (B),
in the MAGO locus gave rise to a grandchildless phenotype because of a defect in the correct cytoplasmic location of
oskar mRNA (Boswellet al., 1991; Newmark and Boswell,
1994; Micklemet al., 1997; Newmarket al., 1997). MAGO
always functions together with an RNA-binding protein,
known as Y14 inXenopus(Kataokaet al., 2000), RBM8A
in humans (Zhaoet al., 2000) and Tsunagi inDrosophila
(Mohret al., 2001); this protein shuttles between the
nu-cleus and cytoplasm (Hachet and Ephrussi, 2001; Kimet
al., 2001).
The MAGO-Y14 complex is the core of the EJC as-sembled on RNA 20 nucleotides upstream of exon-exon
junctions (Kataokaet al., 2001; Bonoet al., 2004).
Deter-mination of the crystal structure of the MAGO-Y14 com-plex showed that the MAGO-Y14 interaction was highly
specific and strongly conserved (Zhaoet al., 2000; Kataoka
et al., 2001; Leet al., 2001; Shi and Xu, 2003; Stroupeet
Figure 6- Functional analysis of HbMAGO2. Interaction of HbMAGO2 with gp91phoxin yeast (A), subcellular localization of gp91phox(B), and
bimolec-ular fluorescence complementation (BiFC) assays in plants (C,D). pGBK-HbMAGO2 (bait) and pGAD-gp91phox(prey) were co-transformed into AH109 yeast cells. Aliquots (10mL) of a 10 diluted yeast suspension culture co-transformed with bait and prey constructs was spotted onto SD/-Trp/-Leu and SD/-Trp/-Leu/-His/-Ade selection plates. Negative controls consisted of vector with only BK and AD or fused proteins with BK or AD. The intensity of the interaction was assessed by assayingb-galactosidase activity (A). Panel (B) shows the subcellular localization of gp91phox. The interaction between HbMAGO2 and gp91phoxwas confirmed using the BiFC assay (C). Epidermal cells were co-transformed with HbMAGO2 and gp91phoxproteins fused to
al., 2006). In plants, the MAGO-Y14 complex has also
been found in T. cryptomerioides and rice (Chen et al.,
2007; Gong and He, 2014). The functional barrier between the two protein families was not observed within dicots or duplicates of rice, but was observed between dicots and
monocot or plants and animals (Gonget al., 2014). The
high specificity and conservation of the MAGO-Y14 inter-action is clade-specific, and such co-evolution allows the interaction between these proteins to be maintained across large evolutionary time scales. In this work, we identified HbMAGO1/2 and HbY14a/b homologs derived from rub-ber tree latex and confirmed the interaction between HbMAGO and HbY14 using YTH analysis. Overall, our findings indicate that HbMAGO1/2 and HbY14a/b are evolutionarily highly conserved proteins with similar func-tions to their homologs.
In rubber trees, NADPH (nicotinamide adenine dinucleotide phosphate) oxidase is involved in the accumu-lation of reactive oxygen species (ROS) in tapping panel dryness (TPD), a physiological disorder characterized by the spontaneous drying up of the tapping cut that results in
an abnormally low yield or stoppage of latex flow (Jacobet
al., 1994; Chenet al., 2003; Venkatachalamet al., 2009).
ROS are involved in the coagulation of rubber particles that dramatically reduces natural rubber production. High ROS production in latex cells triggers oxidative stress leading to thein situcoagulation of rubber particles (Chrestinet al.,
1984; Liet al., 2010; Leclercqet al., 2012). ROS are also
important signaling molecules in plants.
ROS formation is one of the early physiological re-sponses of plant cells to biotic and abiotic stress, such as
wounding and pathogens (Bogreet al., 1997; Bolwellet al.,
2002). NADPH oxidase is the main source of ROS in plants
(Asaiet al., 2008). In animals, the NADPH oxidase
com-plex consists of two plasma membrane proteins, gp91phox
(phox for phagocyte oxidase) and p22phox. Cytosolic
regula-tory proteins p47phox, p67phox, p40phoxand Rac2, translocate
to the plasma membrane to form the active complex after
stimulation (Brandeset al., 2014). However, no homologs
of the p22phox, p67phox, p47phoxand p40phox regulators of
phagocyte NADPH oxidase were found in plants.
Plant NADPH oxidases are known as respiratory burst oxidase homologs (RBOHs) and are homologous to
the catalytic subunit (gp91phox) of mammalian NADPH
oxi-dases (Rodriguezet al., 2007; Suzukiet al., 2011). RBOHs
have been identified and characterized in several species,
includingArabidopsis thaliana(Kawarazakiet al., 2013),
potato (Chenet al., 2013), rice (Yoshieet al., 2005), wheat
(Yamauchi et al., 2014). In TPD trees, high levels of
NADPH oxidase activities lead to the release of O2-, a toxic
form of oxygen (Jacobet al., 1994). Cellular membranes
and especially lutoids, which contain coagulant factors in-volved in the aggregation of rubber particles, are damaged
by O2-(Chrestinet al., 1984; Liet al., 2010; Leclercqet al.,
2012).
In plants, MAGO can participate in biological
pro-cesses through protein-protein interactions. In Physalis,
MAGO interacts with a MADS-Domain protein that can regulate plant development through the formation of dimers and higher order complexes to influence male
fertil-ity and calyx development (Heet al., 2007); this finding
suggests that MAGO plays a role in plant biological pro-cesses independently of the EJC. Little is known about the function of HbMAGO mediated by interaction with target proteins in rubber trees. As shown here, the YTH and BiFC
assays indicated that HbMAGO2 interacted with gp91phox.
This finding suggests that HbMAGO2 may regulate NADPH oxidase-dependent oxidative bursts in rubber tree latex via protein-protein interactions.
Functional studies have shown that MAGO is in-volved in development of organization in multicellular or-ganisms. Over-expression or knockdown of MAGO results in phenotypic alterations that vary among plants, including visible longer roots and a more complex root system, a greater number of leaves, short and fasciated stems, short lateral roots and non-viable seeds, and short rice plants with
abnormal flowers (Chen et al., 2007; Park et al., 2009;
Gong and He, 2014). InDrosophila, the EJC controls the
splicing of mapk and other long intron-containing
tran-scripts and mitogen-activated protein kinase (MAPK) sig-naling depends on the regulation of MAPK levels by the
EJC (Ashton-Beaucageet al., 2010). During development
of the eye, MAPK is the primary functional target of MAGO (Roignant and Treisman, 2010). As core compo-nents of the EJC linked to MAPK signaling pathway, MAGOs can control animal cell or tissue development. Laticifer cells are some of the most important plant cells that continuously produce natural rubber and are arranged
as concentric sheaths in the phloem (Chenet al., 2000; Han
et al., 2000; Pickard, 2008). There are few reports on the
regulation of laticifer development in general (Tanet al.,
2014), and the development of laticifer cells in rubber trees remains poorly understood. Our findings suggest that MAGO may regulate the development of laticifer cells, al-though further experiments are need to confirm this sugges-tion.
Conclusions
Acknowledgments
This work was supported by the National Natural Sci-ence Foundation of China (grant no. 31170634) and Inno-vation Subject of Hainan province (grants nos. B201301 and Hyb2014-03).
References
Asai S, Ohta K and Yoshioka H (2008) MAPK signaling regulates nitric oxide and NADPH oxidase-dependent oxidative bursts inNicotiana benthamiana. Plant Cell 20:1390-1406. Ashton-Beaucage D, Udell CM, Lavoie H, Baril C, Lefrancois M, Chagnon P, Gendron P, Caron-Lizotte O, Bonneil E, Thi-bault P,et al.(2010) The exon junction complex controls the splicing ofmapkand other long intron-containing transcripts inDrosophila. Cell 143:251-262.
Bolwell GP, Bindschedler LV, Blee KA, Butt VS, Davies DR, Gardner SL, Gerrish C and Minibayeva F (2002) The apo-plastic oxidative burst in response to biotic stress in plants: a three-component system. J Exp Bot 53:1367-1376. Bogre L, Ligterink W, Meskiene I, Barker PJ, Heberle-Bors E,
Huskisson NS and Hirt H (1997) Wounding induces the rapid and transient activation of a specific MAP kinase path-way. Plant Cell 9:75-83.
Bono F, Ebert J, Unterholzner L, Güttler T, Izaurralde E and Conti E (2004) Molecular insights into the interaction of PYM with the Mago-Y14 core of the exon junction complex. EMBO Rep 5:304-310.
Boothby TC and Wolniak SM (2011) Masked mRNA is stored with aggregated nuclear speckles and its asymmetric redis-tribution requires a homolog of Mago nashi. BMC Cell Biol 12:45.
Boswell RE, Prout ME and Steichen JC (1991) Mutations in a
newly identified Drosophila melanogaster gene, mago
nashi, disrupt germ cell formation and result in the formation of mirror-image symmetrical double abdomen embryos. De-velopment 113:373-384.
Brandes RP, Weissmann N and Schroder K (2014) Nox family NADPH oxidases: molecular mechanisms of activation. Free Radical Biol Med 76c:208-226.
Chen DH, Ye HC, Li GF and Liu Y (2000) Advances in molecular biology of plant isoprenoid metabolic pathway. Acta Bot Sin 42:551-558.
Chen HJ, Huang CS, Huang GJ, Chow TJ and Lin YH (2013) NADPH oxidase inhibitor diphenyleneiodonium and re-duced glutathione mitigate ethephon-mediated leaf senes-cence, H2O2elevation and senescence-associated gene ex-pression in sweet potato (Ipomoea batatas). J Plant Physiol 170:1471-1483.
Chen SC, Peng SQ, Huang GX, Wu KX, Fu XH and Chen ZQ (2003) Association of decreased expression of a Myb tran-scription factor with the TPD (tapping panel dryness) syn-drome inHevea brasiliensis. Plant Mol Biol 51:51-58. Chen YR, Shaw JF, Chung MC and Chu FH (2007) Molecular
identification and characterization ofTcmagoandTcY14in
Taiwania (Taiwania cryptomerioides). Tree Physiol
27:1261-1271.
Chrestin H, Bangratz J, d’Auzac J and Jacob JL (1984) Role of the lutoidic tonoplast in the senescence and degeneration of the
laticifers of Hevea brasiliensis. Zeitschrift für Pflanzen-physiol 114:261-268.
Chuang TW, Chang WL, Lee KM and Tarn WY (2013) The RNA-binding protein Y14 inhibits mRNA decapping and modulates processing body formation. Mol Biol Cell 24:1-13.
Fribourg S, Gatfield D, Izaurralde E and Conti E (2003) A novel mode of RBD-protein recognition in the Y14-Mago com-plex. Nat Struct Biol 10:433-439.
Gehring NH, Lamprinaki S, Kulozik AE and Hentze MW (2009) Disassembly of exon junction complexes by PYM. Cell 137:536-548.
Gong PC, Zhao M and He CY (2014) Slow co-evolution of the MAGO and Y14 protein families is required for the mainte-nance of their obligate heterodimerization mode. PLoS One 9:e84842.
Gong PC and He CY (2014) Uncovering divergence of rice exon junction complex core heterodimer gene duplication reveals their essential role in growth, development, and reproduc-tion. Plant Physiol 165:1047-1061.
Hachet O and Ephrussi A (2001)DrosophilaY14 shuttles to the posterior of the oocyte and is required for oskarmRNA transport. Curr Biol 11:1666-1674.
Hao BZ and Wu JL (2000) Laticifer differentiation in Hevea brasiliensis: induction by exogenous jasmonic acid and lino-lenic acid. Ann Bot 85:37-43.
Han KH, Shin DH, Yang J, Kim IJ, Oh SK and Chow KS (2000) Genes expressed in the latex of Hevea brasiliensis. Tree Physiol 20:503-510.
He CY, Sommer H, Grosardt B, Huijser P and Saedler H (2007) PFMAGO, a MAGO NASHI-like factor, interacts with the MADS-domain protein MPF2 fromPhysalis floridana. Mol Biol Evol 24:1229-1241.
Jacob JL, Prevot JC and Lacrotte R (1994) Tapping panel dryness inHevea brasiliensis. Plant Rech Dev 1:15-21.
Johnson MA, von Besser K, Zhou Q, Smith E, Aux G, Patton D, Levin JZ and Preuss D (2004) Arabidopsis hapless muta-tions define essential gametophytic funcmuta-tions. Genetics 168:971-982.
Kataoka N, Diem MD, Kim VN, Yong J and Dreyfuss G (2001) Magoh, a human homolog ofDrosophilamago nashi pro-tein, is a component of the splicing-dependent exon-exon junction complex. EMBO J 20:6424-6433.
Kawarazaki T, Kimura S, Iizuka A, Hanamata S, Nibori H, Michi-kawa M, Imai A, Abe M, Kaya H and Kuchitsu K (2013) A low temperature-inducible protein AtSRC2 enhances the ROS-producing activity of NADPH oxidase AtRbohF. Biochim Biophys Acta Mol Cell Res 1833:2775-2780. Kataoka N, Yong J, Kim VN, Velazquez F, Perkinson RA, Wang
F and Dreyfuss G (2000) Pre-mRNA splicing imprints mRNA in the nucleus with a novel RNA-binding protein that persists in the cytoplasm. Mol Cell 6:673-682. Kim VN, Yong J, Kataoka N, Abel L, Diem MD and Dreyfuss G
(2001) The Y14 protein communicates to the cytoplasm the position of exon-exon junctions. EMBO J 20:2062-2068. Lau CK, Diem MD, Dreyfuss G and Van Duyne GD (2003)
Struc-ture of the Y14-Magoh core of the exon junction complex. Curr Biol 13:933-941.
Le HH, Nott A and Moore MJ (2003) How introns influence and enhance eukaryotic gene expression. Trends Biochem Sci 28:215-220.
Le HH, Izaurralde E, Maquat LE and Moore MJ (2000) The spliceosome deposits multiple proteins 20-24 nucleotides upstream of mRNA exon-exon junctions. EMBO J 19:6860-6869.
Le HH, Gatfield D, Braun IC, Forler D and Izaurralde E (2001) The protein Mago provides a link between splicing and mRNA localization. EMBO Rep 2:1119-1124.
Lee HC, Choe J, Chi SG and Kim YK (2009) Exon junction com-plex enhances translation of spliced mRNAs at multiple steps. Biochem Biophys Res Commun 384:334-340. Leclercq J, Martin F, Sanier C, Clement-Vidal A, Fabre D, Oliver
G, Lardet L, Ayar A, Peyramard M and Montoro P (2012) Over-expression of a cytosolic isoform of theHbCuZnSOD
gene inHevea brasiliensischanges its response to a water deficit. Plant Mol Biol 80:255-272.
Lejeune F and Maquat LE (2005) Mechanistic links between non-sense-mediated mRNA decay and pre-mRNA splicing in mammalian cells. Curr Opin Cell Biol 17:309-315. Li DJ, Deng Z, Chen CL, Xia ZH, Wu M, He P and Chen SC
(2010) Identification and characterization of genes associ-ated with tapping panel dryness fromHevea brasiliensis la-tex using suppression subtractive hybridization. BMC Plant Biol 10:140.
Micklem DR, Dasgupta R, Elliott H, Gergely F, Davidson C, Brand A, Gonzalez-Reyes A and St Johnston D (1997) The
mago nashi gene is required for the polarisation of the oocyte and the formation of perpendicular axes in
Drosophila. Curr Biol 7:468-478.
Mohr SE, Dillon ST and Boswell RE (2001) The RNA-binding protein Tsunagi interacts with Mago Nashi to establish po-larity and localizeoskarmRNA duringDrosophila ooge-nesis. Gene Dev 15:2886-2899.
Mufarrege EF, Gonzalez DH and Curi GC (2011) Functional in-terconnections ofArabidopsisexon junction complex pro-teins and genes at multiple steps of gene expression. J Exp Bot 62:5025-5036.
Nagai K, Oubridge C, Ito N, Avis J and Evans P (1995) The RNP domain: a sequence-specific RNA-binding domain involved in processing and transport of RNA. Trends Biochem Sci 20:235-240.
Newmark PA and Boswell RE (1994) Themago nashilocus en-codes an essential product required for germ plasm assem-bly inDrosophila. Development 120:1303-1313.
Newmark PA, Mohr SE, Gong L and Boswell RE (1997)mago
nashimediates the posterior follicle cell-to-oocyte signal to
organize axis formation in Drosophila. Development
124:3197-3207.
Park NI and Muench DG (2007) Biochemical and cellular charac-terization of the plant ortholog of PYM, a protein that inter-acts with the exon junction complex core proteins Mago and Y14. Planta 225:625-639.
Park NI, Yeung EC and Muench DG (2009) Mago Nashi is in-volved in meristem organization, pollen formation, and seed development inArabidopsis. Plant Sci 176:461-469. Pickard WF (2008) Laticifers and secretory ducts: two other tube
systems in plants. New Phytol 177:877-888.
Rodriguez AA, Ramiro Lascano H, Bustos D and Taleisnik E (2007) Salinity-induced decrease in NADPH oxidase
activ-ity in the maize leaf blade elongation zone. J Plant Physiol 164:223-230.
Roignant JY and Treisman JE (2010) Exon junction complex sub-units are required to spliceDrosophila MAP kinase, a large heterochromatic gene. Cell 143:238-250.
Shi H and Xu RM (2003) Crystal structure of theDrosophila
Mago nashi-Y14 complex. Genes Dev 17:971-976. Singh KK, Wachsmuth L, Kulozik AE and Gehring NH (2013)
Two mammalian MAGOH genes contribute to exon junc-tion complex composijunc-tion and nonsense-mediated decay. RNA Biol 10:1291-1298.
Stroupe ME, Tange TO, Thomas DR, Moore MJ and Grigorieff N (2006) The three-dimensional architecture of the EJC core. J Mol Biol 360:743-749.
Suzuki N, Miller G, Morales J, Shulaev V, Torres MA and Mittler R (2011) Respiratory burst oxidases: the engines of ROS signaling. Curr Opin Plant Biol 14:691-699.
Tan DG, Sun XP and Zhang JM (2014) Age-dependent and jasmonic acid-induced laticifer-cell differentiation in anther callus cultures of rubber tree. Planta 240:337-344.
Tang CR, Qi JY, Li H, Zhang CL and Wang YK (2007) A conve-nient and efficient protocol for isolating high-quality RNA from latex of Hevea brasiliensis (para rubber tree). J Biochem Biophys Meth 70:749-754.
Tange TO, Nott A and Moore MJ (2004) The ever-increasing complexities of the exon junction complex. Curr Opin Cell Biol 16:279-284.
Tange TO, Shibuya T, Jurica MS and Moore MJ (2005) Biochem-ical analysis of the EJC reveals two new factors and a stable tetrameric protein core. RNA 11:1869-1883.
van der Weele CM, Tsai CW and Wolniak SM (2007) Mago nashi is essential for spermatogenesis inMarsilea. Mol Biol Cell 18:3711-3722.
Venkatachalam P, Thulaseedharan A and Raghothama K (2009) Molecular identification and characterization of a gene asso-ciated with the onset of tapping panel dryness (TPD) syn-drome in rubber tree (Hevea brasiliensis Muell.) by mRNA differential display. Mol Biotechnol 41:42-52.
Walter M, Chaban C, Schutze K, Batistic O, Weckermann K, Nake C, Blazevic D, Grefen C, Schumacher K, Oecking C,
et al.(2004) Visualization of protein interactions in living plant cells using bimolecular fluorescence complemen-tation. Plant J 40:428-438.
Yamauchi T, Watanabe K, Fukazawa A, Mori H, Abe F, Kawa-guchi K, Oyanagi A and Nakazono M (2014) Ethylene and reactive oxygen species are involved in root aerenchyma formation and adaptation of wheat seedlings to oxygen-deficient conditions. J Exp Bot 65:261-273.
Yang YN, Li RG and Qi M (2000)In vivoanalysis of plant pro-moters and transcription factors by agroinfiltration of to-bacco leaves. Plant J 22:543-551.
Yang ZP, Li HL, Guo D, Tang X and Peng SQ (2014) Identifica-tion and characterizaIdentifica-tion of the 14-3-3 gene family inHevea brasiliensis. Plant Physiol Biochem 80:121-127.
Yoshie Y, Goto K, Takai R, Iwano M, Takayama S, Isogai A and Che FS (2005) Function of the rice gp91phox homologs
OsrbohAandOsrbohEgenes in ROS-dependent plant im-mune responses. Plant Biotechnol 22:127-135.
regula-tion of the rubber biosynthesis inHevea brasiliensis. Plant Sci 176:602-607.
Zhao XF, Colaizzo-Anas T, Nowak NJ, Shows TB, Elliott RW and Aplan PD (1998) The mammalian homologue of mago nashi encodes a serum-inducible protein. Genomics 47:319-322.
Zhao XF, Nowak NJ, Shows TB and Aplan PD (2000) MAGOH interacts with a novel RNA-binding protein. Genomics 63:145-148.
Internet Resources
Softberry platform for HMM-based gene structure prediction, http://linux1.softberry.com/berry.phtml
Multiple alignment software Clustal X,
http://www.ebi.ac.uk/clustalw
MEGA5.2 software for phylogenetic tree construction,
http://www.megasoftware.net/
GSDS software for comparison of cDNA sequences,
http://gsds.cbi.pku.edu.cn/
Associate Editor: Marcia Pinheiro Margis