w ww.e l s e v i e r . c o m / l o c a t e / b j p
Original
Article
Paniculatumoside
G,
a
new
C
21
steroidal
glycoside
from
Cynanchum
paniculatum
Hua
Gao,
Wei
Wang
∗,
Wenxi
Chu,
Kun
Liu,
Yang
Liu,
Xiaohong
Liu,
Huili
Yao,
Qi
Gao
SchoolofPharmacy,QingdaoUniversity,Qingdao,People’sRepublicofChina
a
r
t
i
c
l
e
i
n
f
o
Articlehistory:
Received16March2016 Accepted30June2016
Availableonline15September2016
Keywords:
Asclepiadaceae
Cynanchumpaniculatum
C21steroidalglycoside
NeocynapanogeninH 3-O-ˇ-d-oleandropyranoside
a
b
s
t
r
a
c
t
AnewC21steroidalglycoside,paniculatumosideG,togetherwithneocynapanogeninCisolatedforthe
firsttimefromthenaturalsourceandtwoknowncompoundswereisolatedandcharacterizedfromthe rootsandrhizomesofCynanchumpaniculatum(Bunge)Kitag.exH.Hara,Apocynaceae,acommonlyused TraditionalChineseMedicine.Onthebasisofspectroscopicanalysis,includingHR-ESI-MS,1Dand2D NMRspectraldata,thestructureofthenewC21steroidalglycosidewaselucidatedasneocynapanogenin
H3-O-ˇ-d-oleandropyranoside.
©2016SociedadeBrasileiradeFarmacognosia.PublishedbyElsevierEditoraLtda.Thisisanopen accessarticleundertheCCBY-NC-NDlicense(http://creativecommons.org/licenses/by-nc-nd/4.0/).
Introduction
Cynanchumpaniculatum(Bunge)Kitag.exH.Hara,Apocynaceae, a perennial herb native to east Asia, is commonly called ‘Xu ChangQing’inChinese,andhasbeenusedasa Traditional Chi-neseMedicinefor thetreatmentofperatodynia, gastroenteritis, venomoussnake bite,and ascites(Jiangand Li,1977).Previous phytochemicalinvestigationsonC.paniculatumhaverevealedthe presenceofphenolicderivatives,alkaloids,flavonoids, polysaccha-rides,triterpenoids,andC21steroidalglycosides(Niuetal.,2015;
Fuetal.,2015).Thereportedbioactivitiesoftheplantextractsand isolatedconstituentsincludeanti-adipogenic (Jangetal., 2014), neuroprotective(Weonetal.,2013),anti-tumor(Kimetal.,2012), anti-inflammatory,anti-nociceptive,sedative(Choiet al.,2006), araricidal (Kim et al., 2013a), and herpes simplex encephalitis inducingimpairmentpreventiveactivities(Lietal.,2012).Our pre-viousphytochemicalinvestigationonethanolextractofthissource resultedintheisolationofnineC21steroidalaglyconesand
glyco-sides(Chuetal.,2015).Inourcontinuingstudyonthissource,one newsteroidalglycoside(1)togetherwiththreeknowncompounds (2–4)wereisolatedandidentified.Itshouldbenotedthat com-pound2wasisolatedforthefirsttimefromthenaturalsource.
∗ Correspondingauthor.
E-mail:qddxwangwei@qdu.edu.cn(W.Wang).
TheirstructureswereelucidatedbydetailedinterpretationofNMR andMSdata.
Materialsandmethods
Generalexperimentalprocedures
OpticalrotationsweremeasuredbyusingaJASCOP-1020 auto-maticdigitalpolarimeter(JASCOCorporation,Tokyo,Japan).The
NMR spectral datawererecorded ona BrukerAV-500FT-NMR
(500MHzfor1Hand125MHzfor13C)inC
5D5N,usingvisualC5D5N
resonances(1Hı7.21,7.58,and8.73,13Cı123.5,135.5,and149.0)
for internalreference. Allchemical shifts (ı) aregiven in ppm.
HR-ESI-MSandESI-MSwereobtainedwitha BrukermicroTOFQ
massspectrometer(BrukerDaltonics,Bremen,Germany).Column
chromatographywasperformedwithmacroporousresinHPD100
(CangzhouBonAdsorberTechnologyCo.,Ltd,Cangzhou,China)and RP-18reversed-phasesilicagel(S-50mm,YMC,Kyoto,Japan).TLC analysiswascarriedoutonpre-coatedTLCplateswithsilicagel RP-1860F254 (Merck,Darmstadt,Germany,0.25mm).Detection
wasachievedbysprayingwith10%H2SO4inMeOHfollowedby
heating.PreparativeHPLCwasperformedonaNP7005Cpump con-nectedwithaSHODEXRI-102detector(ShokoScientificCo.,Ltd,
Tokohama,Japan),usingMegresODScolumn(250mm×10mm,
i.d.,5m,HanbangSci.&Tech.,Haian,China).HPLC-gradeMeOH waspurchasedfromMerck.HPLC-gradewaterwaspurifiedusing
http://dx.doi.org/10.1016/j.bjp.2016.06.010
aMilli-Qsystem(millipore,Boston,MA,USA).Allsolventsusedfor thechromatographicseparationsweredistilledbeforeuse.
Plantmaterial
The roots and rhizomes of Cynanchum paniculatum (Bunge)
Kitag.exH.Hara,Apocynaceae,wereobtainedinJingde Pharma-ceuticalCompany,Bozhou,AnhuiProvinceofChina,andidentified byProf.BaominFeng,DalianUniversity,China.Avoucher speci-men(CPXCQ-2014-03)wasdepositedattheCollegeofPharmacy, QingdaoUniversity,China.
Extractionandisolation
Theroots andrhizomesofC.paniculatum(10kg)werereflux extractedtwicewith90%ethanol for1.5hand thesolventwas evaporatedunderreducedpressuretogiveanEtOHextract(1.5kg). TheEtOHextract(1.2kg)wasdissolvedwithwaterandsubjected
tocolumnchromatographyonHPD-100macroporousresin and
elutedwithEtOH-H2O(0:100,30:70,70:30,and95:5),successively.
Thefractionelutedwith70%ethanol(100g)waschromatographed overaD941macroporousresincolumn,elutingwith95%ethanol andatotalof15gresiduewascollected.Theresiduewas chro-matographed further on a RP-C18 silica gel and eluted with a
gradient increasing MeOH (30–80%) in water to give sixteen
subfractions (Fr.C1–C16) on the basis of TLC analyses. Fr.C14 waspurified bypreparativeHPLCusingMeOH/H2O(60:40)ata
flow rate of 2ml/min (Megres C18 column, 250mm×10.0mm, 5m)toyieldcompound1(4.91mg,tR=41.0min).Compound2
(5.60mg,tR=16.0min)and compound 3(8.25mg, tR=60.0min)
wereobtainedfromFr.C13andFr.C12bypreparativeHPLC(Megres C18 column, 250mm×10.0mm, 5m; flow rate, 2.0ml/min)
employing MeOH/H2O (55:45) and MeOH/H2O (52:48) as the
mobilephase,respectively.Thefractionelutedwith95%ethanol (10g)wasseparatedchromatographicallyonaRP-C18silicagelto
getfivesubfractions(Fr.C1′–C5′)onthebasisofTLCanalysis.Fr.C4′
wasisolatedbypreparativeHPLCusingMeOH/H2O(60:40)ata
flowrateof1.6ml/min(MegresC18column,250mm×10.0mm, 5m)toyieldcompound4(62.29mg,tR=140min).
Spectraldata
NeocynapanogeninH3-O-ˇ-d-oleandropyranoside(1):An amor-phouspowder;[␣]D25+45.7(c0.01,MeOH);1H-(C5D5N,500MHz)
and 13C-NMR (C
5D5N, 125MHz) see Table 1; HR-ESI-MS m/z
573.2667[M+Na]+(calcdforC
29H42NaO10,573.2676).
NeocynapanogeninC(2):Anamorphouspowder;[␣]D25−65.4
(c 0.01, MeOH); 1H- (C
5D5N, 500MHz) and 13C-NMR (C5D5N,
125MHz)seeTable2;HR-ESI-MSm/z399.1783[M+Na]+(calcdfor
C21H28NaO6,399.1784).
Resultsanddiscussion
Compound1wasobtainedaswhiteamorphouspowder,and
showed positive Liebermann–Burchard and Keller–Kiliani reac-tions,suggestingittobeasteroidalglycosidewitha2-deoxysugar moiety(Zhuetal.,1999).Itsmolecularformulawasdeterminedas C29H42O10onthebasisofpositiveHR-ESI-MSadduction[M+Na]+at m/z573.2667(calcdforC29H42NaO10:573.2676),whichwas
fur-thersupportedbythe1H-and13C-NMRspectraldata(Table1).
The13C-NMRand DEPTspectrarevealed29 carbonsignals due
tofivemethylcarbons,sixmethylenecarbons,thirteenmethine
carbons, and five nonprotonated carbons, of which 22 carbons
wereassignedtotheaglyconepartincludingtwotertiarymethyl carbons(ıC20.6 and 24.3),onemethoxylcarbon (ıC55.0),one
oxygenatedmethylenecarbon(ıC70.4),fouroxygenatedmethine
carbons(ıC70.0,78.1,84.8,and104.3),fourolefiniccarbons(ıC
120.4,131.0,139.4and142.3),oneacetaliccarbon(ıC114.6),and
onecarbonylcarbon(ıC179.3),whichexhibitedthecharacteristics
of13,14:14,15-disecopregnane-type steroidalglycoside.The1
H-NMRspectrumoftheaglyconemoietyshowedtwoangularmethyl protonsatıH1.09(3H,s)and1.73(3H,s),twogeminalcoupled
oxygenated-methyleneprotonsatıH4.14(1H,dd,J=10.0,4.8Hz) and4.42(1H,dd,J=9.9,7.4Hz),fouroxygen-substitutedmethine protonsatıH3.69(1H,m),4.02(1H,ddd,J=12.6,9.0,4.6Hz),5.62
(1H,s),and5.74(1H,ddd,J=8.1,7.4,4.8Hz),togetherwithtwo olefinicprotonsatıH5.43(1H,m)and5.47(1H,m).Inaddition,
onemethoxygroupresonatedatıH3.50(3H,s)wasobservedin
Table1
1H-NMRand13C-NMRspectraldataofcompound1(500and125MHz,C
5D5N,ıppm,JinHz).
Position 1 PaniculatumosideAa
ıH ıC ıC
Aglycone
1˛ 1.40(t,J=12.2Hz) 45.5(t) 37.2(t)
1ˇ 2.42(dd,J=13.0,4.6Hz)
2 4.02(ddd,J=12.6,9.0,4.6Hz) 70.0(d) 29.7(t)
3 3.69(m) 84.8(d) 77.0(d)
4˛ 2.65(m) 37.5(t) 39.1(t)
4ˇ 2.59(m)
5 – 139.4(s) 140.3(s)
6 5.43(m) 120.4(d) 120.1(d)
7˛ 2.62(m) 29.2(t) 30.4(t)
7ˇ 2.50(m)
8 2.47(m) 40.9(d) 41.4(d)
9 2.13(td,J=11.4,5.2Hz) 51.9(d) 52.1(d)
10 – 38.6(s) 37.8(s)
11˛ 2.55(m) 30.4(t) 30.3(t)
11ˇ 2.29(ddd,J=11.9,7.4,4.4Hz)
12 5.47(m) 131.0(d) 133.2(d)
13 – 142.3(s) 139.4(s)
14 – 179.3(s) 179.4(s)
15˛ 4.42(dd,J=9.9,7.4Hz) 70.4(t) 70.5(t)
15ˇ 4.14(dd,J=10.0,4.8Hz)
16 5.74(ddd,J=8.1,7.4,4.8Hz) 78.1(d) 78.0(d)
17 3.29(d,J=8.1Hz) 56.1(d) 56.0(d)
18 5.62(s) 104.3(d) 107.3(d)
19 1.09(s) 20.6(q) 19.6(q)
20 – 114.6(s) 115.1(s)
21 1.73(s) 24.3(q) 24.3(q)
18-OCH3 3.50(s) 55.0(q)
Sugar
1′(Ole) 4.84(dd,J=9.8,1.8Hz) 99.3(d) 98.3(d)
2′˛ 2.59(m) 37.3(t) 37.5(t)
2′ˇ 1.78(ddd,J=12.0,9.8,4.5Hz)
3′ 3.51(m) 81.5(d) 81.7(d)
4′ 3.46(m) 76.1(d) 76.5(d)
5′ 3.65(m) 73.1(d) 72.9(d)
6′ 1.56(d,J=6.1Hz) 18.4(q) 18.8(q)
3′-OCH3 3.49(s) 57.1(q) 57.1(q)
aDatafromLietal.(2004).
aglyconespectraldataof1withthoseofneocynapanogeninC,the aglyconeofpaniculatumosideB(Lietal.,2004),themain differ-enceswerethepresenceofsignalforanadditionalmethoxyl(ıH/C
3.50/55.0)andthechangesofthechemicalshiftsinC-1(+8.2ppm), C-2(+39.7ppm),andC-3(+7.7ppm),aswellasinC-18(+5.6ppm) andC-13(−3.4ppm)intheNMRspectraof1.Theaglyconemoiety
ofcompound 1wastherefore proposedtobea
2-hydroxyl-18-methoxylderivativeofneocynapanogeninC,whichwereproved bytheHMBCcorrelationsfromıH1.40and2.42(H-1)toıC70.0
(C-2),84.8(C-3),139.4(C-5),38.6(C-10),20.6(C-19),fromıH2.59 and2.65(H-4)toıC70.0(C-2),84.8(C-3),139.4(C-5),120.4(C-6),
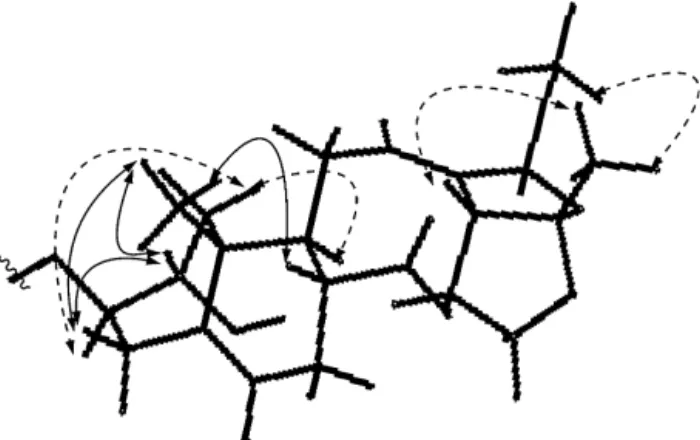
38.6(C-10),andfromıH3.50(18-OCH3)toıC104.3(C-18)(Fig.1).
Therelativeconfigurationoftheaglyconewaselucidatedbythe NOESYspectrumandthevicinalproton-protoncouplingconstant. ThecouplingconstantbetweenH-2andH-3(9.0Hz)wastypicalfor
Fig.1.KeyHMBCcorrelationsofcompound1.
trans-diaxialprotons,indicatingthatbothoxygenatedsubstituents wereequatorial.Observed1,3-diaxialNOEcorrelationsfor H-2/H-4,H-2/H-19,H-4/H-19andH-1␣/H-3(Fig.2)furthersupported the-orientationofH-2and␣-orientationofH-3andrevealedthe chairconformationoftheAring.Thetrans-diaxialrelationshipof H-8andH-9,namely,the-orientationofH-8and␣-orientation ofH-9,wassuggestedbythesplittingpatternofH-9(td,J=11.4, 5.2Hz)andtheNOESYcorrelationsforH-8/H-19andH-1␣/H-9(Bai etal.,2005).Inaddition,theNOEcorrelationfromthemethoxyl groupatC-18toH3-21confirmedthemethoxylgroupatC-18as ˛-orientation.Thusthestructurefortheaglyconeofcompound1
wasdeducedandatrivialnameneocynapanogeninHwasassigned. Protonsignalswerealsoassignedtoonesecondarymethylgroup atıH1.56(d,J=6.1Hz),onemethoxylgroupatıH 3.49(s),and
oneanomericprotonatıH4.84(dd,J=9.8,1.8Hz),whose
mul-tiplicities suggestedthepresenceof one2,6-dideoxy-sugarin a saccharidechainand-configurationofthehexoseunit.The13C
NMRandDEPTdataindicatedtheexistenceofone oleandropyra-nosylunit.ItwasconfirmedbytheobservedDQFCOSYandHMBC correlations.Forthedeoxysugars,sinceonlyd-formauthentic sam-plescouldbeobtained,theirabsoluteconfigurationscouldnotbe assignedbyGCanalysis,butdeterminedtobed-formsby compar-isonoftheir13C-NMRspectroscopicdatawiththosereporteddata.
Themostsignificantdifferencesinthe13C-NMRdatabetweend
-andl-configurationoleandropyranosylinvolvetheresonancesof C-2.ThechemicalshiftofC-2inthel-oleandropyranosylisless than35ppm,butthatofC-2inthed-oleandropyranosylappears above 36ppm. Therefore, the oleandropyranosyl unit of 1 was determinedtobed-configurationbasedonits13C-NMRchemical shiftofC-2at37.3ppm(Table1)(Lietal.,2004;Maetal.,2007; Yangetal.,2011;Kimetal.,2013b),anditslocationwas deter-minedtobeC-3bytheH-1′/C-3HMBCcorrelation(Fig.1).Thus,
thestructureof1wasfinallyestablishedasneocynapanogeninH 3-O-ˇ-d-oleandropyranoside.
Compound2wasobtainedaswhiteamorphouspowder,and
showed positive Liebermann–Burchard reaction. Its molecular
formulawasdeterminedasC21H28O6onthebasisofpositive
HR-ESI-MSadduction[M+Na]+atm/z399.1783(calcdforC
21H28NaO6:
399.1784),whichwasfurthersupportedbythe1H-NMRand13
C-NMRdata(Table2).The1H-NMRdatashowedtwoolefinicprotons
atıH5.34(1H,brd,J=4.6Hz)and5.55(1H,d,J=11.0Hz),three
oxygen-substitutedmethineprotonsatıH3.82(1H,m),5.77(1H,
ddd,J=8.1,7.7,5.2Hz),and6.33(1H,brd,J=6.0Hz),twogeminal coupledoxygenated-methyleneprotonsatıH4.16(1H,dd,J=9.8,
5.0Hz)and4.39(1H,dd,J=9.8,7.2Hz),twomethylsignalsatıH
1.04(3H,s)and1.84(3H,s).The13C-NMRspectrumshowed21
carbonsignals,includingtwotertiarymethylcarbons(ıC19.8and
25.0),anoxygenatedmethylenecarbon(ıC70.0),threeoxygenated
Table2
1H-NMRand13C-NMRspectraldataofcompound2(500and125MHz,C
5D5N,ı
ppm,JinHz).
Position ıH ıC
1 1.17(m) 37.6(t)
1.83(m)
2 1.74(m) 32.5(t)
2.08(m)
3 3.82(m) 70.7(d)
4 2.54(m) 43.1(t)
2.62(m)
5 – 141.1(s)
6 5.34(brd,J=4.6Hz) 119.4(d)
7 2.58(m) 29.1(t)
2.90(q,J=12.2Hz)
8 2.52(m) 41.6(d)
9 2.08(m) 52.2(d)
10 – 37.7(s)
11 2.27(m) 30.4(t)
2.51(m)
12 5.55(d,J=11.0Hz) 130.2(d)
13 – 145.5(s)
14 – 179.6(s)
15 4.16(dd,J=9.8,5.0Hz) 70.0(t)
4.39(dd,J=9.8,7.2Hz)
16 5.77(ddd,J=8.1,7.7,5.2Hz) 78.3(d)
17 3.38(d,J=8.1Hz) 56.8(d)
18 6.33(brd,J=6.0Hz) 98.7(d)
19 1.04(s) 19.8(q)
20 – 113.6(s)
21 1.84(s) 25.0(q)
methinecarbons(ıC70.7,78.3,and98.7),fourolefiniccarbons(ıC 119.4,130.2,141.1and145.5),anacetaliccarbon(ıC113.6),anda carbonylcarbon(ıC179.6),whichexhibitedthecharacteristicsof 13,14:14,15-disecopregnane-typesteroidalglycoside.Comparison ofthespectraldataof2withthoseofpaniculatumosideB,anewC21 steroidalglycosideisolatedfromthedriedrootofC.paniculatum(Li etal.,2004),thechangesofthechemicalshiftsinC-2(+2.5ppm), C-3(−6.4ppm),C-4(+4.0ppm)showedthatithasnolinkageofthe sugarmoietyattheC-3hydroxylgroupoftheaglycone.Thus,the structureof2wasestablishedasneocynapanogeninC,the agly-coneofpaniculatumosideB.Itshouldbenotedthatcompound2
wasisolatedforthefirsttimefromthenaturalsource.
Compounds3and4wereidentifiedbycomparingthe1H-and 13C-NMR,aswellasMSspectrawiththosereportedinthe
liter-atures.TheyweredeterminedtobecynapanosideA(3)(Sugama etal.,1986)andcynatratosideA(4)(Zhangetal.,1985).
Authors’contributions
HG,WXC,HLY,andQGperformedtheextraction,isolation,and elucidation of theconstituents. KL, YL,and XHL contributed to checkingandconfirmingalloftheproceduresoftheisolationand identification.WWdesignedthestudy,supervisedthelaboratory work,andcontributedtocriticalreadingofthemanuscript.Allthe authorshavereadthefinalmanuscriptandapprovedthe submis-sion.
Conflictsofinterest
Theauthorsdeclarenoconflictsofinterest.
Acknowledgments
This projectwassupported by theNational Natural Science
FoundationofChinaunderGrant81273396;ShandongProvince
HigherEducationalScienceandTechnologyProgramunderGrant J15LM12.
References
Bai, H., Li, W., Koike,K., Satou, T., Chen,Y.J., Nikaido,T., 2005. Cynanosides A–J,tennovelpregnaneglycosidesfromCynanchumatratum.Tetrahedron61, 5797–5811.
Choi,J.H.,Jung,B.H.,Kang,O.H.,Choi,H.J.,Park,P.S.,Cho,S.H.,Kim,Y.C.,Sohn,D.H., Park,H.,Lee,J.H.,Kwon,D.Y.,2006.Theanti-inflammatoryandanti-nociceptive effectsofethylacetatefractionofcynanchipaniculatiradix.Biol.Pharm.Bull. 29,971–975.
Chu,W.X.,Liu,X.H.,Liu,K.,Huo,L.N.,Yao,H.L.,Gao,Q.,Gao,H.,Wang,W.,2015. ChemicalconstituentsfromactivefractioninrootsandrhizomesofCynanchum paniculatumwithreversalactivityofmultidrugresistance.Chin.Tradit.Herbal Drugs18,2674–2679.
Fu,M.,Wang,D.Y.,Hu,X.,Guo,M.Q.,2015.ChemicalconstituentsfromCynanchum paniculatum.J.Chin.Med.Mater.38,97–100.
Jang,E.J.,Kim,H.K.,Jeong,H.,Lee,Y.S.,Jeong,M.G.,Bae,S.J.,Kim,S.,Lee,S.K.,Hwang, E.S.,2014.Anti-adipogenicactivityofthenaturallyoccurring phenanthroin-dolizidinealkaloidantofineviadirectsuppressionofPPAR␥expression.Chem. Biodivers.11,962–969.
Jiang,Y.,Li,B.T.,1977.Angiospermae,Dicotyledonae,Apocynaceaeand Asclepi-adaceae.FloraofChinaEditorialCommitteeFloraofChina,vol.63.SciencePress, Beijing,pp.351–353.
Kim,C.S.,Oh,J.Y.,Choi,S.U.,Lee,K.R.,2013b.Chemicalconstituentsfromtherootsof Cynanchumpaniculatumandtheircytotoxicactivity.Carbohydr.Res.381,1–5. Kim,E.H.,Min,H.Y.,Chung,H.J.,Song,J.,Park,H.J.,Kim,S.,Lee,S.K.,2012.
Anti-proliferativeactivity and suppressionof P-glycoproteinby (−)-antofine,a naturalphenanthroindolizidinealkaloid,inpaclitaxel-resistanthumanlung cancercells.FoodChem.Toxicol.50,1060–1065.
Kim,M.G.,Yang,J.Y.,Lee,H.S.,2013a.Acaricidalpotentialsofactiveproperties isolatedfromCynanchumpaniculatumandacaricidalchangesbyintroducing functionalradicals.J.Agric.FoodChem.61,7568–7573.
Li,X.F.,Guo,Y.J.,Zhang,D.M.,Chen,Z.,Wei,X.,Li, Y.H.,Zhang,S.L.,Tao,J.Y., Dong,J.H.,Mei,Y.W.,Li,L.L.,Zhao,L.,2012.Protectiveactivityoftheethanol extractofCynanchumpaniculatum(Bunge)Kitagawaontreatingherpessimplex encephalitis.Int.J.Immunopathol.Pharmacol.25,259–266.
Ma,X.X.,Jiang,F.T.,Yang,Q.X.,Liu,X.H.,Zhang,Y.J.,Yang,C.R.,2007.Newpregnane glycosidesfromtherootsofCynanchumotophyllum.Steroids72,778–786. Niu,Y.L.,Chen,X.,Wu,Y.,Jiang,H.Q.,Zhang,X.L.,Li,E.T.,Li,Y.Y.,Zhou,H.L.,Liu,J.G.,
Wang,D.Y.,2015.ChemicalconstituentsfromCynanchumpaniculatum(Bunge) Kitag.Biochem.Syst.Ecol.61,139–142.
Sugama,K.,Hayashi,K.,Mitsuhashi,H.,Kaneko,K.,1986. Studiesonthe con-stituentsofAsclepiadaceaeplants.LXVI.Thestructuresofthreenewglycosides, cynapanosideA,B,andC,fromtheChinesedrugXU-ChangQing,Cynanchum paniculatumKitagawa.Chem.Pharm.Bull.34,4500–4507.
Weon,J.B.,Ko, H.J.,Ma,C.J.,2013. Theamelioratingeffectsof 2,3-dihydroxy-4-methoxyacetophenone on scopolamine-induced memory impairment in mice and its neuroprotective activity. Bioorg. Med. Chem. Lett. 23, 6732–6736.
Yang,Q.X.,Ge,Y.C.,Huang,X.Y.,Sun,Q.Y.,2011.CynanauriculosideC–E,threenew antidepressantpregnaneglycosidesfromCynanchumauriculatum.Phytochem. Lett.4,170–175.
Zhang,Z.X.,Zhou,J.,Hayashi,K.,Mitsuhashi,H.,1985.Studiesontheconstituentsof Asclepiadaceaeplants.LVIII.Theconstituentsoffiveglycosides, cynatratoside-A,-B,-C,-D,and–E,fromtheChinesedrugPai-Wei,CynanchumatratumBunge Chem.Pharm.Bull.33,1507–1514.
Zhu,N.Q.,Wang,M.F.,Kikuzaki,H.,Nakatani,N.,Ho,C.T.,1999.TwoC21-steroidal