U R O L O G Y - O R I G I N A L P A P E R
Urodynamic effects of the combination of tamsulosin
and daily tadalafil in men with lower urinary tract
symptoms secondary to benign prostatic hyperplasia:
a randomized, placebo-controlled clinical trial
Rommel Prata Regadas•Ricardo Reges•Joa˜o Batista Gadelha Cerqueira•
Daniel Gabrielle Sucupira•Iatagan Rocha Josino •Emmanuel Almeida Nogueira•
Francisco Vagnaldo F. Jamacaru•Manoel Odorico de Moraes •Lu´cio Fla´vio Gonzaga Silva
Received: 27 August 2012 / Accepted: 10 October 2012 / Published online: 30 October 2012 ÓSpringer Science+Business Media Dordrecht 2012
Abstract
Objectives To evaluate the effect of association of tamsulosin/tadalafil taken daily compared with tam-sulosin/placebo in the lower urinary tract with urody-namic study (UDS).
Methods All patients underwent baseline UDS before randomization to tamsulosin 0.4 mg/tadalafil 5 mg (Group 1;n=20) or tamsulosin 0.4 mg/placebo (Group 2; n =20) once daily for 30 days. End-of-study UDS were performed on completion of the treatment period. The primary end point was to demonstrate changes in urodynamic variables in the voiding phase, detrusor pressure at maximum flow (PdetQmax), and maximum flow rate (Qmax), from baseline to week four.
Results The primary outcome measure of this clinical trial, PdetQmax, showed a significant reduction in tamsulosin/tadalafil group (13±17.0) compared to tamsulosin/placebo (-1.2±14.35) group (P=0.03). Qmax increased in both groups, tamsulosin/tadalafil
(1.0 ±2.4) and tamsulosin/placebo (1.4±2.4), but the difference was not significant between treatment groups (P=0.65). Total IPSS, storage, and voiding sub-score improved significantly in tamsulosin/tadala-fil compared with tamsulosin/placebo group.
Conclusions The association of tamsulosin/tadalafil reduces detrusor pressure at maximum flow without changing the maximum flow rate during micturition and significantly improves lower urinary tract symp-toms compared with the isolated use of tamsulosin.
Keywords Benign prostatic hyperplasia TamsulosinTadalafilLower urinary tract symptomsUrodynamic
Introduction
Benign prostatic hyperplasia (BPH) is the most common disease of the urinary tract in elderly patients [1]. Clinical BPH represents a constellation of signs and symptoms that develop in the male population in association with aging and prostatic enlargement presumably caused by bladder outlet obstruction (BOO) [2]. The presence of lower urinary tract symptoms (LUTS) has traditionally been associated with prostatic enlargement; however, LUTS is not specific for BPH. Elderly men may have a variety of pathological processes of the lower urinary tract with
R. P. RegadasR. Reges (&)J. B. G. Cerqueira
D. G. SucupiraI. R. JosinoE. A. Nogueira
L. F. G. Silva
Division of Urology, Universidade Federal do Ceara, 280, Antonele Bezerra st. Ap 1501, Meireles, Fortaleza, Ceara 60160-070, Brazil
e-mail: consultoriodeurologia@gmail.com
F. V. F. JamacaruM. O. de Moraes
Pharmacology Department,
similar symptoms. Therefore, the etiology of LUTS is complex and possibly multifactorial [3].
The erectile dysfunction (ED) is also a highly prevalent disease in elderly patients. Recent evidence suggests a strong association between severity of LUTS and ED [4]. It was observed that patients with ED and LUTS treated with phosphodiesterase (PDE) inhibitors improve erection and LUTS [5]. Therefore, the rational use of PDE inhibitors in the treatment of LUTS/BPH initiated from these population studies. As a conse-quence of this finding, there was great interest in defining the role of PDE inhibitors in the treatment of LUTS/ BPH. Therefore, researches with high level of evidence have emerged that clearly shows improvement in LUTS after treatment with PDE inhibitors [6–8].
According to the American Urological Association (AUA) guidelines,a-adrenergic blockers are consid-ered as the most effective monotherapy for the treatment of LUTS secondary to BPH [9]. On the other hand, PDE5 inhibitors are the first-line treatment for erectile dysfunction. Because of the strong asso-ciation between BPH/LUTS and erectile dysfunction, the coprescription of PDE5 inhibitors anda -adrener-gic blockers is likely to increase.
More recently, organ bath studies have shown that combininga-adrenergic blockers with PDE inhibitors reduces the adrenergic tone in the prostate and cavernous smooth muscle [10]. However, only few studies have assessed the urodynamic effect of this combination in the lower urinary tract.
Therefore, the aim of this study was to compare the effect of combination of tamsulosin/tadalafil taken daily with tamsulosin/placebo in the lower urinary tract with urodynamic study (UDS).
Methods
A randomized, double-blind, placebo-controlled study was done from October 2010 to September 2011.
Men with at least 45 years old complaining for BPH/LUTS were evaluated with pressure flow study (PFS) and the International Prostatic Symptoms Score (IPSS). Only patients with bladder outlet obstruction index (BOOI) greater than 20 and IPSS score greater than 14 were recruited in this study.
Exclusion criteria were suspect or confirmed pros-tate cancer, LUTS not related to BPH, hypotension, retinitis pigmentosa, use of 5a-reductase inhibitor in
the last 6 months, use ofa-blockers, anticholinergics or PDE inhibitors in the last month, surgery of the prostate, urethra and bladder, presence of neurological disease, urinary retention, bladder stones, use of nitrates, cardiovascular, hepatic or renal insufficiency. All patients were initially evaluated with clinical history, physical examination, including rectal exam-ination, and basic neurological examexam-ination, urine analysis, culture, PSA, IPSS, transabdominal ultra-sound (prostate volume), and UDS.
All patients underwent baseline UDS before ran-domization to tamsulosin 0.4 mg/tadalafil 5 mg (Group 1) or tamsulosin 0.4 mg/placebo (Group 2) once daily for 30 days. End-of-study UDS were performed on completion of the treatment period.
UDS was performed based on the International Continence Society testing guidelines [11]. UDS assessed detrusor pressure at maximum flow (PdetQ-max), maximum flow (Qmax) during voiding, BOOI (calculated as pdetQmax–2Qmax), and detrusor over-activity (assessed as incidence).
This study was approved by the local research ethics committee and all patients signed an informed consent. The primary end point was to observe changes in urodynamic variables of the voiding phase, PdetQ-max, and QPdetQ-max, from baseline to week four. The secondary end point of this study was to demonstrate improvement in the IPSS.
For the analysis of quantitative data, the mean and standard deviation were evaluated. The normality of distribution was initially verified with the Kolmogo-rov–Smirnov test. The parametric variables were analyzed by Student’s t test unpaired for intergroup comparison and paired for intragroup comparison; the nonparametric variables were evaluated with the Mann–Whitney and Wilcoxon tests, respectively. All statistical tests were 2-sided and were evaluated at the 0.05 level of significance.
Randomization strata included baseline BOOI category (unobstructed—BOOI less than 20, equivo-cal—BOOI 20–40, obstructed—BOOI greater than 40) and baseline LUTS severity (severe—I-PSS 20 or greater, moderate—I-PSS 8–19, and mild lower than 8). Data are presented as mean ±SD for baseline characteristics and mean±SE for 4-week change from baseline data.
Results
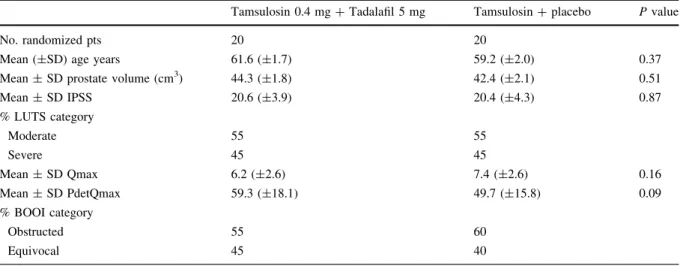
Patient demographics and baseline characteristics were homogeneous across groups at baseline (Table1).
A total of 40 men were randomized to receive tamsulosin 0.4 mg/tadalafil 5 mg (n=20) or tamsul-osin 0.4 mg/placebo (n=20) once daily for 4 weeks. Detrusor overactivity (DO) in the filling phase was observed in 12 (60 %) patients in tamsulosin/tadalafil and eight (40 %) in tamsulosin/placebo. After treat-ment, the DO disappeared in seven (58.3 %) of patients in Group 1 and three (37.5 %) in Group 2 (P=0.64).
The mean change of PdetQmax from baseline to end point was-13 (±17.0) in tamsulosin/tadalafil and was-1.22 (±14.3) in tamsulosin/placebo. Comparing the groups, PdetQmax decreases significantly in tamsulosin/tadalafil group (P=0.03; Fig.1).
The mean change of Qmax from baseline to end point was 1.05 (±0.5) in tamsulosin/tadalafil and was 1.22 (±0.5) in tamsulosin/placebo. No significant difference was observed in Qmax between the treat-ment groups (P=0.65; Fig.2).
Significant decrease was observed in the tamsulo-sin/tadalafil group in total IPSS (P=0.01), IPSS storage (P=0.05), and voiding sub-score (P =0.01) compared with tamsulosin/placebo (Table2).
Only one patient in tamsulosin/tadalafil group had significant myalgia who relived during treatment. The combination of tamsulosin/tadalafil was well toler-ated, and hypotension, dizziness, or any cardiovascu-lar side effect was not observed.
Table 1 Patients demographic and baseline characteristics
Tamsulosin 0.4 mg?Tadalafil 5 mg Tamsulosin?placebo Pvalue
No. randomized pts 20 20
Mean (±SD) age years 61.6 (±1.7) 59.2 (±2.0) 0.37
Mean±SD prostate volume (cm3) 44.3 (±1.8) 42.4 (±2.1) 0.51
Mean±SD IPSS 20.6 (±3.9) 20.4 (±4.3) 0.87
% LUTS category
Moderate 55 55
Severe 45 45
Mean±SD Qmax 6.2 (±2.6) 7.4 (±2.6) 0.16
Mean±SD PdetQmax 59.3 (±18.1) 49.7 (±15.8) 0.09
% BOOI category
Obstructed 55 60
Equivocal 45 40
Change PdetQmax
-40 -30 -20 -10 0
*
Tamsulosin/Tadalafil Tamsulosin/Placebo
cmH2O
Fig. 1 Changes in the detrusor pressure at maximum flow
(PdetQmax) after 4 weeks treatment. Results are expressed as mean±SD (n=20). *P=0.03 compared with tamsulosin/ placebo group
Change Qmax
0 1 2 3 4 5
Tamsulosin/Tadalafil Tamsulosin/Placebo
ml/second
Fig. 2 Changes in the maximum flow (Qmax) after 4 weeks
Discussion
The primary end point of this study was to demonstrate changes in urodynamic variables (PdetQmax and Qmax).
In this study, the combination of tamsulosin/ tadalafil decreased significantly the PdetQmax com-pared with tamsulosin/placebo. However, no signifi-cant change was observed in Qmax between the treatments groups.
There are some studies evaluating changes in free flow in patients taken PDE5i to treat LUTS/BPH [6–8]. In a randomized, double-blind, crossover study, the combination of tamsulosin 0.4 mg/day and tadalafil 20 mg/day improved the IPSS and IPSS-Quality of Life (QoL) significantly when compared to isolated use of tamsulosin, but the improvement in Qmax was similar [12]. In accord with these authors, in our study combining tadalafil with tamsulosin also did not change Qmax compared with tamsulosin/placebo.
Recently, a dose-finding study by Roehrborn et al. assessed 1058 men with BPH/LUTS who received randomly placebo or tadalafil (2.5, 5, 10 or 20 mg). The IPSS improved significantly for all doses com-pared to placebo, but there was no improvement in Qmax. The authors concluded that 5 mg tadalafil once daily may provide a positive risk–benefit profile [7].
In vitro studies demonstrated that combination of both PDE5 inhibitors and a-blockers resulted in an enhanced relaxant effect on human prostate and bladder neck smooth muscle compared with single agent [10,13]. Therefore, the effect related to PDE5
inhibitors in combination witha-blockers may lead to synergistic benefit explaining the marked drop in PdetQmax observed in our study. As previously known, PdetQmax reflects outlet resistance during micturition. So, when the urethra opens widely (Nitric Oxide) with an unaltered flow (Q), little Pdet is needed to achieve the work necessary to empty the bladder [14]. Therefore, probably the low voiding pressure found in patients using tamsulosin/tadalafil does equate with decreased urethral resistance.
The exact mechanism through which PDE inhibi-tors alleviate BPH–LUTS remains unclear [15]. The pathophysiological relationship between ED and LUTS is not clear yet, but there are several theories to explain it. The candidate mechanisms include pelvic atherosclerosis, autonomic hyperactivity, the calcium-independent Rho-kinase activation pathway, and reduced nitric oxide (NO) levels. It is likely that there is an overlap between the roles of each of these candidate mechanisms, and an ultimate effect leading to smooth muscle relaxation in prostatic, bladder neck, or erectile tissues appears to be crucial. Probably, the hypothesis of the reduction of NO is the best explanation.
Increased smooth muscle tension plays a central role in LUTS pathophysiology. The NO/cyclic gua-nosine monophosphate (cGMP) pathway is one of the major regulators of smooth muscle contractility [16]. Nitric oxide can activate guanylate cyclase, the enzyme that produces cGMP. The accumulation of intracellular cGMP triggers a cascade, leading to decreased intracellular calcium level and subsequent
Table 2 Change in pressure flow urodynamic and International Prostatic Symptoms of Score parameters from baseline to end point
Variables Tamsulosin/Tadalafil
(Group 1)
Tamsulosin/placebo (Group 2)
Difference of change (Group 1–Group 2)
Pvalue
Mean±SD Baseline (20)
Mean±SD Change (20)
Mean±SD Baseline (20)
Mean±SD Change (20)
Mean±SE Change (20)
Pressure flow parameters
PdetQmax (cm H2O) 59.3 (±18.1) -13 (±17.0) 49.7 (±15.8) -1.2 (±14.35) -11.7 (±5.2) 0.03
Qmax (ml/s) 6.2 (±2.6) 1.0 (±2.4) 7.4 (±2.61) 1.4 (±2.4) -0.3 (±0.7) 0.65
BOOI 53.1 (±27.6) -16.8 (±18.9) 35.1 (±16.6) -4.3 (±13.7) -12.5 (±5.5) 0.02
International Prostate Symptoms Score
Total score 20.6 (±3.9) -9.75 (±5.1) 20.4 (±4.3) -6.0 (±3.6) -3.7 (±1.4) 0.01
Filling sub-score 7.3 (±3.2) -3.9 (±3.1) 5.7 (±3.4) -1.5 (±1.7) -2.3 (±0.8) 0.05
relaxation of smooth muscle cells (SMCs). And the amount of cGMP results from the balance between production (NO) and degradation that is made by PDE inhibitors that are enzymes that can hydrolyze and inactivate cyclic nucleotides. It is known that NO is involved in the relaxation of the detrusor, bladder neck, urethra, and prostate [17,18].
The significant improvements in total IPSS, stor-age, voiding sub-score, and absence of serious side effect demonstrated combining tamsulosin with tad-alafil compared with tamsulosin/placebo in this study are other potential benefits of this combination to treat patients with BPH/LUTS.
The short-term follow-up and small number of patients are limitations of this study; therefore, studies with longer follow-up and greater number of partic-ipants would be needed to confirm these results.
In conclusion, the combination of tamsulosin/ tadalafil reduces detrusor pressure at maximum flow without changing the maximum flow rate during micturition and significantly improves lower urinary tract symptoms compared with the isolated use of tamsulosin.
Acknowledgments Fundac¸a˜o Cearense de Apoio ao
Desen-volvimento Cientı´fico e Tecnolo´gico—FUNCAP and National Counsel of Technological and Scientific Development—CNPq.
References
1. Filippi S, Morelli A, Sandner P et al (2007) Characterization and functional role of androgen-dependent PDE5 activity in the bladder. Endocrinology 148(3):1019–1029
2. Shapiro E, Lepor H (1995) Pathophysiology of clinical benign prostatic hyperplasia. Urol Clin N Am 22(2):285–290 3. Andersson KE, de Groat WC, McVary KT et al (2011) Tad-alafil for the treatment of lower urinary tract symptoms sec-ondary to benign prostatic hyperplasia: pathophysiology and mechanism(s) of action. Neurourol Urodyn 30(3):292–301 4. Boyle P, Robertson C, Mazzetta C et al (2003) The
asso-ciation between lower urinary tract symptoms and erectile dysfunction in four centres: the UrEpik study. BJU Int 92(7):719–725
5. Sairam K, Kulinskaya E, McNicholas TA et al (2002) Sil-denafil influences lower urinary tract symptoms. BJU Int 90(9):836–839
6. McVary KT, Monnig W, Camps JL Jr et al (2007) Sildenafil citrate improves erectile function and urinary symptoms in men with erectile dysfunction and lower urinary tract symptoms associated with benign prostatic hyperplasia: a randomized, double-blind trial. J Urol 177(3):1071–1077 7. Roehrborn CG, McVary KT, Elion-Mboussa A et al (2008)
Tadalafil administered once daily for lower urinary tract symptoms secondary to benign prostatic hyperplasia: a dose finding study. J Urol 180(4):1228–1234
8. Stief CG, Porst H, Neuser D et al (2008) A randomised, placebo-controlled study to assess the efficacy of twice-daily vardenafil in the treatment of lower urinary tract symptoms secondary to benign prostatic hyperplasia. Eur Urol 53(6):1236–1244
9. AUA guideline on management of benign prostatic hyper-plasia (2003). Chapter 1: diagnosis and treatment recom-mendations. J Urol 170(2 Pt 1):530–547
10. Oger S, Behr-Roussel D, Gorny D et al (2009) Combination of doxazosin and sildenafil exerts an additive relaxing effect compared with each compound alone on human cavernosal and prostatic tissue. J Sex Med 6(3):836–847
11. Schafer W, Abrams P, Liao L et al (2002) Good urodynamic practices: uroflowmetry, filling cystometry, and pressure-flow studies. Neurourol Urodyn 21(3):261–274
12. Bechara A, Romano S, Casabe A et al (2008) Comparative efficacy assessment of tamsulosin vs. tamsulosin plus tad-alafil in the treatment of LUTS/BPH. Pilot study. J Sex Med 5(9):2170–2178
13. Oger S, Behr-Roussel D, Gorny D et al (2010) Combination of alfuzosin and tadalafil exerts an additive relaxant effect on human detrusor and prostatic tissues in vitro. Eur Urol 57(4):699–707
14. Griffiths DJ (1973) The mechanics of the urethra and of micturition. Br J Urol 45(5):497–507
15. Dmochowski R, Roehrborn C, Klise S et al (2010) Urody-namic effects of once daily tadalafil in men with lower urinary tract symptoms secondary to clinical benign pros-tatic hyperplasia: a randomized, placebo controlled 12-week clinical trial. J Urol 183(3):1092–1097
16. Kedia GT, Uckert S, Jonas U et al (2008) The nitric oxide pathway in the human prostate: clinical implications in men with lower urinary tract symptoms. World J Urol 26(6): 603–609
17. Monica FZ, Reges R, Cohen D et al (2011) Long-term administration of BAY 41–2272 prevents bladder dys-function in nitric oxide-deficient rats. Neurourol Urodyn 30(3):456–460