Bothropoides pauloensis
venom effects on isolated perfused kidney and
cultured renal tubular epithelial cells
Aline D. Marinho
a,*, Isabel C.O. Morais
a, D
^
anya B. Lima
b, Ant
^
onio R.C. Jorge
a,
Roberta J.B. Jorge
a, Ramon R.P.P.B. Menezes
a, Clarissa P. Mello
b, Gustavo J.S. Pereira
c,
Jo
ao A.M. Silveira
~
a, Marcos H. Toyama
d, Mar Orz
aez
e, Alice M.C. Martins
b,
Helena S.A. Monteiro
aaDepartment of Physiology and Pharmacology, School of Medicine, Federal University of Ceara, 60430-270, Fortaleza, Ceara, Brazil bDepartment of Clinical and Toxicological Analysis, Federal University of Ceara, Fortaleza, Ceara, Brazil
cDepartment of Pharmacology, Federal University of S~ao Paulo (UNIFESP/EPM), S~ao Paulo, Brazil dS~ao Vicente Unit, Paulista Coastal Campus, S~ao Paulo State University (UNESP), S~ao Paulo, Brazil eDepartment of Medicinal Chemistry, Centro de Investigacion Príncipe Felipe, Valencia, Spain
a r t i c l e
i n f o
Article history: Received 11 June 2015 Received in revised form 16 September 2015 Accepted 21 September 2015 Available online 26 September 2015
Keywords:
Bothropoides pauloensis Renal effects Cell death
a b s t r a c t
Snake envenomation (Bothropsgenus) is common in tropical countries and acute kidney injury is one of the complications observed in Bothrops snakebite with relevant morbidity and mortality. Here, we showed thatBothropoides pauloensisvenom (BpV) decreased cell viability (IC50 of 7.5mg/mL). Flow cytometry with annexin V and propidium iodide showed that cell death occurred predominantly by apoptosis and late apoptosis, through caspases 3 and 7 activation, mitochondrial membrane potential collapse and ROS overproduction.BpV reduced perfusion pressure, renal vascular resistance, urinary flow, glomerularfiltration rate, percentage of sodium, chloride or potassium tubular transportation. Thesefindings demonstrated thatBpV cytotoxicity on renal epithelial cells might be responsible for the nephrotoxicity observed in isolated kidney.
©2015 Published by Elsevier Ltd.
1. Introduction
The World Health Organization reports indicate that at least 421,000 envenomings and 20,000 deaths occur worldwide from snakebite each year. Africa, Asia and Latin America are the most affected geographical area (WHO, 2009). In Brazil, approximately 90% of snakebites reported to the Ministry of Health are caused by snakes of Bothrops and Bothropoides genus (Oliveira et al., 2010; Morais et al., 2013).
Accidents caused by the Bothrops genus in humans are typically associated with rapid edema formation, pain, ecchymosis, hemor-rhage, extensive cellulitis with skin necrosis, local myonecrosis and systemic hemolysis, severe coagulopathies, hypotensive shock and acute kidney injury (Gutierrez and Lomonte, 1995; Sgrignolli et al., 2011).
The Bothrops genera were recently reclassified and the species
Bothrops pauloensis was renamed as Bothropoides pauloensis
(Fenwick et al., 2009).B. pauloensisis a venomous snake, popularly known as“jararaca pintada”, which is found in the Brazilian terri-tory, more commonly in the southwest of the state of S~ao Paulo (Ferreira et al., 2013).B. pauloensisvenom (BpV) contains peptides and enzymes such metalloproteinases, PLA2s, and vasoactive (bradykinin-potentiating) peptides, which can explain the local and systemic effects observed in envenomations by B. pauloensis
(Rodrigues et al., 2012).
The kidney is highly susceptible to toxins due to its high vascularity. Acute kidney injury is an important complication of Bothrops accidents and is considered the main cause of death in patients that survive thefirst envenomation effects (Otero-Patino,~ 2009; Albuquerque et al., 2013).
Experimental studies made with Bothrops venom suggest a multifactorial pathogenesis for ARF, which includes the following mechanisms: renal damage resulting from hypovolemia and hypoperfusion secondary to cardiovascular disturbances, fibrin deposit in the glomerular capillaries leading to thrombotic micro-angiopathy and direct cytotoxic action of some venom components
*Corresponding author.
E-mail address:alinediogo_marinho@hotmail.com(A.D. Marinho).
Contents lists available atScienceDirect
Toxicon
j o u r n a l h o m e p a g e : w w w . e l s e v i e r . c o m / l o c a t e / t o x i c o n
http://dx.doi.org/10.1016/j.toxicon.2015.09.031 0041-0101/©2015 Published by Elsevier Ltd.
on the renal tubules (Gutierrez et al., 2009).
In order to elucidate the mechanism of direct nephrotoxicity, the aim of the present work was to investigate the effect ofB. pauloensis
venom on renal epithelial cells and isolated perfused kidney.
2. Methods
2.1. Cell culture, venom and chemical compounds
MadineDarby Canine Kidney (MDCK) epithelial cells were
cultivated in RPMI 1640 medium supplemented with 10% fetal bovine serum (FBS) (Sigma) at 37C and 5% CO
2.BpV was kindly
donated by Dr. Marcos H. Toyama, Paulista State University (UNESP), whereas DMEM medium, phenylhydrazone (FCCP), 2,7 dichlorodihydrofluorescein diacetate (DCFH-DA) kit and 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) were obtained from Sigma Chemical Co. (St. Louis, MO, USA), AnnexinV/FITC Apoptosis Detection Kit was obtained from BD Pharmigen (CA, USA) and tetramethylrhodamine ethyl ester (TMRE) was purchased from Molecular Probes (Eugene, OR, USA). The antibodies against Caspase 3 (#96615) and Caspase 7 (#9492) were obtained from Cell Signaling, while
a
-tubulin antibody (#T8203) was obtained from SigmaeAldrich.2.2. MTT assay
Mitochondrial functionality was measured by MTT colorimetric assay. MDCK cells were cultured in sterile 96-well microtiter plates
at a seeding density of 105cells/well. After seeding, cells were left to adhere to the plate overnight, and then were treated with different concentrations of BpV (100; 50; 25; 12.5; 6.25; 3.12
m
g/mL) and incubated at 37C for 24 h. MTT reagent (5 mg/mL in PBS) wasadded to each well and plates were further incubated for 4 h at 37C. Finally, the medium was removed and the precipitated
for-mazan crystals were dissolved in Sodium dodecyl sulfate (SDS) 10% in HCl 0.01 N. After 17 h, absorbance at 570 nm was performed in a microplate reader (Biochrom®
Asys Expert Plus). Cell viability was calculated in comparison with the control group. The IC50 (venom concentration able to inhibit 50% of cell growth) was determined by non-linear regression (Mosmann, 1983).
2.3. Lactate dehydrogenase (LDH) release
Cytotoxicity induced by BpV (7.5; 15; 25; 50
m
g/mL) was assessed by lactate dehydrogenase (LDH) leakage into the culture medium. After 12 h of treatment, cell supernatant was removed and reactive mixture was added for 30 min at room temperature under protection from light for determination of LDH release using the Promega kit (6179A). Plates were read at 440 nm on a Wallac 1420 workstation (Fotakis and Timbrell, 2006).2.4. Annexin V-FITC and propidium iodide (PI) staining
Cells incubated with different concentrations of BpV (15; 7.5
m
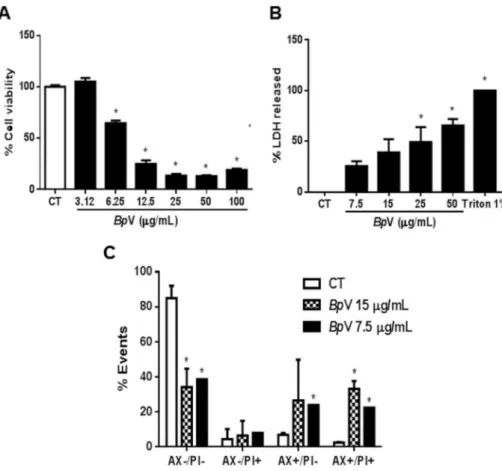
g/ mL) for 12 h were stained withfluorescein isothiocyanate (FITC)-conjugated to annexinV/propidium iodide (PI) and incubated forFig. 1.Cytotoxic effect ofBothropoides pauloensisvenom on MDCK cells. (A) MDCK cells were treated with different concentrations ofB. pauloensisvenom for 24 h and were evaluated by the reduction in the 3-(4,5-Dimethylthiazol-2-Yl)-2,5-Diphenyltetrazolium Bromide (MTT) salt in cells underBpV concentration-curve after 24 h of treatment. (B) Percentage release of the lactate dehydrogenase enzyme of MDCK cells treated under aBpV concentration-curve treatment. (C) Cell death was measured by annexin V and PI staining and detected byflow cytometry. MDCK cells were treated with two different concentrations (IC50e 2xIC50) ofBpV after 24 h. All data are expressed as mean±SEM of three independent experiments with six replicates (One-way analysis of variance and Dunnett post-test, *p<0.05).
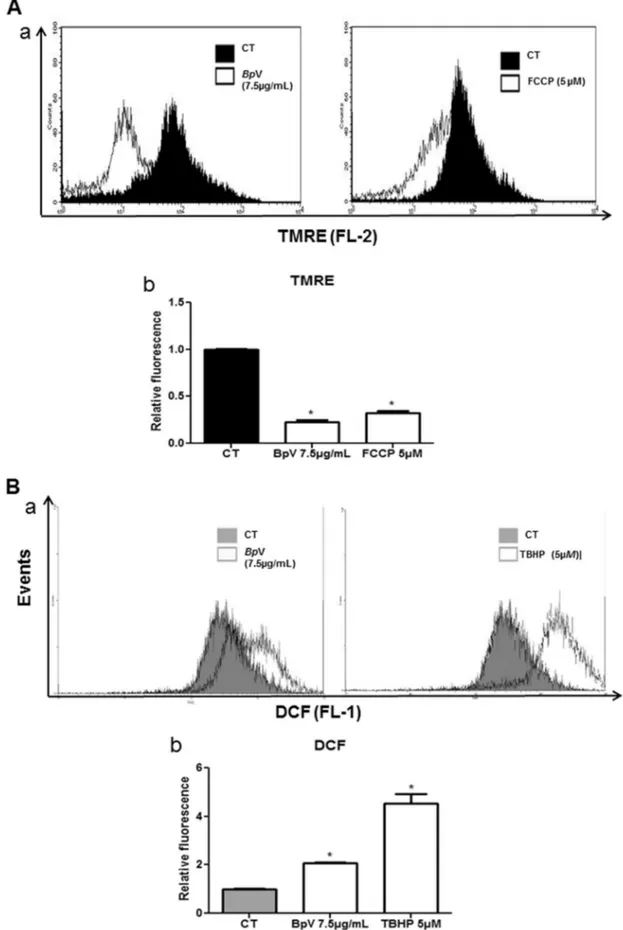
Fig. 2.Intracellular Mitochondrial membrane potentiality (DJm) andBpV -regulated Reactive Oxygen Species (ROS). (A) (a). Alterations inDJmwas measured by TMRE staining (20 nM,
20 min at 37C) and detected byflow cytometry. MDCK cells were treated with (IC
50, 7.5mg/mL) ofBpV for 12 h. Displacement of the TMRE Fluorescence to the left, when compared with
negative control, shows the decrease inDJm. FCCP (5mM) was used as positive control. (A) (b). Summarized data shows relativefluorescence of TMRE, expressed as the ratio of mean fluorescence relative of treated groups to that of untreated control. Data are means±S.E.M. of three replicates from three independent experiments and compared with sample without any treatment (One-way analysis of variance and Dunnett post-test, *p<0.05). (B) (a). IntracellularBpV-induced ROS generation (7.5mg/mL) in MDCK cells. After treatment with the
15 min. All procedures were carried out according to the manu-facturer's instructions. The populations of annexinV-PI viable cells and annexinVþ
apoptotic cells were evaluated byflow-cytometry. Data were collected in a FACS Calibur (Becton-Kickinson, Moun-tain View, Calif.) and analyzed using Cell Quest software (BectoneDickinson).
2.5.
DJ
m measurementsMDCK cells (105) were incubated withBpV (7.5
m
g/mL) for 12 h. After this period, the medium was collected and the cells were centrifuged at 500gfor 5 min. Adherent cells were washed in PBS three times and then detached with trypsin (2.5 mg/mL)e0.02%EDTA, followed by centrifugation at 500g for 5 min. Then, cell culture was incubated with 25 nM TMRE for 30 min at 37 C in the dark. The cells were then washed once in PBS and resuspended to a
final volume of 400
m
L and analyzed on FL2 byflow cytometry.2.6. Reactive oxygen species (ROS) intracellular measurement
Cytosolic ROS was measured using
2,7-dichlorodihydro-fluorescein diacetate (DCFH-DA) (Sigma, USA), which can readily enter cells and be cleaved by esterase to yield DCFH, a polar, nonfluorescent product. ROS in cells promotes the oxidation of DCFH to yield thefluorescent product, dichlorofluorescein (DCF). After cells were treated for the recommended period of time (BpV 7.5
m
g/mL for 12 h), they were washed with PBS twice, trypsinized, re-suspended, and then they were collected and incubated in PBS containing the reagent DCFH-DA (10 mMol/L) for 30 min at 37C.Blank controls were set, in which DCFH-DA incubation was omitted. After that, they were submitted toflow cytometric analysis using a FACScanflow cytometer. Tert-Butyl hydroperoxide (TBHP) solution served as a positive control for the induction of ROS in cells. The data based on the FL1 channel were analyzed with the CellQuest program.
2.7. Caspase 3/7 activity measurements
Cell extracts were prepared from 3. 105cells seeded in 6-well plates. Cells were treated withBpV (25; 15; 7.5
m
g/mL) for 12 h, and were scraped and washed with PBS. The cells were lysed in extraction buffer (50 mM PIPES, 50 mM KCl, 5 mM EDTA, 2 mM MgCl2, 2 mM DTT, supplemented with protease inhibitors). After 3freeze and thaw rounds, cell lysates were centrifuged at 14,000 rpm for 5 min, and supernatants were collected.
Quanti-fication of the total protein concentration was performed using the BCA protein assay (Thermo Scientific). Total protein (40
m
g) was mixed with 200m
L of caspase assay buffer (PBS, 10% glycerol, 0.1 mM EDTA, 2 mM DTT) containing 20m
M of Ac-DEVD-afc (Enzo Life Sciences) of the caspase-3/7 substrate. DVDase activity was continuously monitored following the release offluorescent afc at 37 C in a Wallac 1420 Workstation (l
exc ¼ 400 nm;l
em¼508 nm).2.8. Immunoblotting
Whole cell extracts were obtained by lysing cells in a buffer containing 25 mM TriseHCl pH 7.4, 1 mM EDTA, 1 mM EGTA, 1%
SDS, plus protease and phosphatase inhibitors. Protein
concentration was determined by the BCA protein assay. Cell ly-sates were resolved by SDS-PAGE, transferred to nitrocellulose membranes, blocked with 5% nonfat milk, washed with 0.1% Tween/PBS and incubated overnight with a specific primary anti-body. Membranes were washed and probed with the appropriate secondary antibody conjugated to horseradish peroxidase for enhanced chemiluminescence detection (Amersham Pharmacia Biotech).
2.9. Kidney perfusion
Adult male Wistar rats (260e320 g) were fasted for 24 h with
free water access. The rats were anesthetized with sodium pento-barbitone (50 mg/kg, i.p) and after careful dissection of the right kidney, the right renal artery was cannulated via the mesenteric artery without interrupting the bloodflow as described byBowman (1970). The perfusate consisted of a modified KrebseHenseleit
so-lution (MKHS) of the following composition (in mmol/L): 114.00 NaCl, 4.96 KCl, 1.24 KH2PO4, 0.5 MgSO4$7H2O, 2.10 CaCl2and 24.99
NaHCO3. Bovine serum albumin (BSA 6 g%; fraction V), urea
(0.075 g), inulin (0.075 g) and glucose (0.15 g) were added to the solution, resulting in afinal perfusate volume of 100 mL. The pH was adjusted to 7.4. In each experiment, 100 mL of MKHS were recirculated for 120 min. Perfusion pressure (PP) was measured at the tip of the stainless steel cannula in the renal artery. Samples of urine and perfusate were collected at 10-min intervals for analysis of sodium, potassium and chloride levels by ion-selective elec-trodes (Rapid chen 744, Bayer Diagnostic, UK); inulin, as described byWalser et al. (1955)and modified byFonteles et al. (1983); and osmolality, which was measured in a vapor pressure osmometer (Wescor 5100C, USA). BpV (3 and 10
m
g/mL) was added to the system 30 min after the beginning of each perfusion. The perfusion pressure (PP), renal vascular resistance (RVR), urinaryflow (UF), glomerular filtration rate (GFR) and the percentages of sodium (% TNaþ), potassium (%TKþ
) and chloride (%TCl) tubular transport were determined (Martinez-Maldonado and Opava-Stitzer, 1978). The results were compared to the internal control group, at 30 min early in each experiment (n¼6). This study was approved by the Ethics Committee on Animal Research (CEPA) of the Federal Uni-versity of Ceara (Permit Number: 79/08).
2.10. Statistical analysis
Cell experiments were performed in triplicate (n¼3) and data were expressed as mean±SEM. Data were analyzed using one-way analysis of variance (ANOVA) followed by Dunnett post-test and significance was set at *p<0.05. Six isolated perfused kidney ex-periments in each group were performed and data were expressed as mean ± SEM. Data were analyzed using ANOVA followed by Bonferroni post-test and significance was set at *p<0.05.
3. Results and discussion
3.1. B. pauloensis induces apoptosis and necrosis in MDCK cells
Previous studies showed that cell death plays an important role in the nephrotoxicity caused by snake venom from the Bothrops and Bothropoides genus (Collares-Buzato et al., 2002; Nascimento et al., 2007; Morais et al., 2013; Mello et al., 2014). In order to
venom for 12 h, cells were incubated with 5mM DCFH-DA for 30 min and then immediately submitted toflow cytometric analysis. ROS generation was expressed as a ratio of relative fluorescent intensity compared to the control group. One representative histogram of three independent experiments for each sample is presented. TBHP (5mM) was used as positive control. (B) (b). Summarized data shows intracellular ROS content, expressed as the ratio of meanfluorescence relative of treated groups to that of untreated control. Data are means±S.E.M. of three replicates from three independent experiments and compared with sample without any treatment (One-way analysis of variance and Dunnett post-test, *p<0.05).
study the direct nephrotoxicity mechanisms, cell death assays were performed using MDCK cells, a very-well established cell line employed in the investigation of a variety of cellular processes, including response to toxic agents and venoms (Collares-Buzato et al., 1994, 1998, 2002; Schwerdt et al., 2004; Peixoto and Collares-Buzato, 2005; Chaim et al., 2006; Chen et al., 2006; Nas-cimento et al., 2007; Damico et al., 2007; Ribeiro et al., 2007; Kusma et al., 2008).
Cytotoxicity wasfirst assessed by MTT assay and treatment with
BpV caused a decrease in cell viability from 6.25
m
g/mL concen-tration with an IC50 of 7.5m
g/mL (Fig. 1A). Based on this concen-tration, we chose the concentrations to perform the another assays. Cell injury was assessed by quantifying the damage or disruption of the plasma membrane through the release of cytoplasmic enzyme lactic dehydrogenase (LDH), used as a marker of cell injury.In vitrorelease of LDH from cells provides an accurate measure of mem-brane integrity and cell viability, irrespective of the type of cell death (Fotakis and Timbrell, 2006). The results indicated an apparent membrane rupture in MDCK cells at the highest con-centrations studied (Fig. 1B), showing an increase in LDH release. The predominant characteristics of one or the other type of cell death are usually determined by intensity and not by specificity of stimulus (Orrenius et al., 1992; Dypbukt et al., 1994; Bonfoco et al., 1995).
The different morphologies of apoptotic and necrotic cells can also be detected by analyzing their light-scattering properties in
flow cytometry (Krysko et al., 2011). In this study, the combination of Annexin-V and PI were used for cell staining to distinguish apoptotic from necrotic cell death. Annexin-PI loading cell analysis demonstrated an increase mainly in AXþ
/PI cells at the concen-trations of 7.5
m
g/mL, as well as AXþ/PIþ
cells in both concentrations (7.5 and 15
m
g/mL), suggesting the participation of apoptosis and late apoptosis onBpV-induced cell death (Fig. 1C), confirming the results showed in MTT and LDH assays.Snake venom causes apoptosis in a variety of cells (Suhr and Kim, 1996; Herkert et al., 2001; Araki et al., 2002; Taketo and Sonoshita, 2002; Shakhman et al., 2003; Yan et al., 2007; Georgieva et al., 2008) and this effect is mediated by various different components, such as metalloprotease, phospholipase A2 andL-amino acid oxidase (Stabeli et al., 2007;Tanjoni et al., 2005; Ren et al., 2006; Lee et al., 2014).
Several studies have confirmed the involvement of Bothrops and Bothropoides venom components in apoptosis (Díaz et al., 2005; Laing and Moura-da-Silva, 2005; Mora et al., 2006;Tanjoni et al., 2005; Alves et al., 2008; Jimenez et al., 2008; Morais et al., 2013; Mello et al., 2014).
3.2. BpV exposure induces mitochondrial depolarization in MDCK cells
Mitochondria plays a critical role in multiple cellular functions, including generation of ATP to supply cellular energy, storage of intracellular Ca2þ, and release of apoptotic factors (Wen et al., 2015). To determine whether the mitochondrial pathway was involved in cell death, we assessed the effect ofBpV mitochondrial transmembrane potential (
DJ
m). Thefluorescence intensity of the mitochondrial specific probe, tetramethylrhodamine ethyl ester (TMRE), was measured, which indicates theDJ
m using flow cytometry.DJ
m was measured before and after the addition ofBpV followed by positive control; FCCP (5m
M).BpV (7.5m
g/mL) caused a left dislocation in TMRE fluorescence after 12 h of treatment (Fig. 2A). It is believed that the loss of mitochondrial trans-membrane potential is due to the opening of the permeability transition pore, a mitochondrial megachannel, which is strongly affected by oxidative stress conditions (Mello et al., 2014).3.3. ROS generation involvement in cytotoxicity induced by BpV in MDCK cells
The mitochondria are a major site of generation of free radicals. It is now well established that the generation or addition of ROS can cause cell death either by apoptosis or necrosis, two distinct cell death pathways (Curtin et al., 2002). It has been shown that ROS generation plays a pivotal role in cell death induction by Bothrops venom (Collares-Buzato et al. 2002; Mello et al., 2014). The present study tested the involvement of reactive oxygen species on BpV-induced cell death signaling pathways.
Fig. 3.Activation of caspases 3 and 7 in MDCK cells. (A) Caspase-3/7 activity deter-mined in presence of thefluorogenic Ac-DEVD-afc substrate after treatment with Bothropoides pauloensisvenom (12 h). The data represent the mean±S.E.M of three independent experiments. (B) Protein expression: Immunoblotting analysis of acti-vated caspase-3 and caspase- 7 in MDCK cells after 24 h of treatment withBpV in different concentrations. Doxorubicin was used as a positive control (One-way analysis of variance and Dunnett post-test, *p<0.05).
Intracellular ROS generation was measured by flow cytometry following staining with dichlorodihydrofluorescein diacetate (DCFH-DA); a positive control treated with TBHP (5
m
M) was also used. We showed that the amounts of ROS in theBpV-treated MDCK-cells (7.5m
g/mL) showed significant right dislocation of thefluorescence peak in the histogram, when compared with the untreated control group (Fig. 2B).
3.4. B. pauloensis venom activates caspases 3 and 7 in MDCK cells
Apoptosis can be initiated in two basic ways, known as intrinsic or mitochondrial and extrinsic or death receptors (Schmitz et al., 2000; Ferri and Kroemer, 2001a, 2001b; Baetu and Hiscott, 2002). Both ways converge onto caspases to initiate cell death (Mondragon et al., 2008 ). Caspases 3 and 7 are key proteases
Fig. 4.BpV induced alterations in rat isolated kidney. Effects ofBothrops pauloensisvenom on perfusion pressure (A), vascular renal resistance (B), glomerular filtration rate (C), urinaryflow (D), sodium, potassium and chloride (E, F, G) tubular transport percentage.BpV was added to the system 30 min after the beginning of each perfusion. The data represent the mean±S.E.M of at least three independent experiments. *Significantly different from control group (One-way analysis of variance and Bonferroni post-test, *p<0.05).
required for this outcome (Wu et al., 2014). We measured the changes in caspases 3 and 7 activity in MDCK cells. As shown in Fig. 3A and B,BpV (15 and 25
m
g/mL) treatment induced activation of caspase 3 and caspase 7. These data indicate apoptotic involve-ment inBpV-induced cell death in these cells. Similar results were found for venoms of other Bothrops and Bothropoides genus snakes.Bothrops alternatus(Nascimento et al., 2007),Bothropoides insularis(Mello et al., 2014) andBothrops leucurus(Morais et al., 2013) also showed caspases 3/7 activation in MDCK cells.3.5. B. pauloensis venom induced alterations in rat isolated kidney
To investigate the effect of the venom in the kidney without interference of systemic factors, we used perfusion in the rat kid-ney. In the present study, we observed a decrease in all the analyzed parameters at both concentrations used (3 and 10
m
g/mL) when compared to control perfused kidneys. The infusion ofBpV (10m
g/ mL) significantly decreased the perfusion pressure (PP;Fig. 4A) and renal vascular resistance (RVR;Fig. 4B) at 90 and 120 min; however, at the lowest venom concentration (3m
g/mL), only renal vascular resistance decreased at 120 min of perfusion, when compared with the control group. At both assessed concentrations, urinary flow (UF;Fig. 4D) and glomerularfiltration rate (GFR;Fig. 4C) decreased at 90 and 120 min. At 3 and 10m
g/mL, the percentage of sodium (% TNa; Fig. 4E) and chloride tubular transport (%TCl; Fig. 4G) decreased significantly at 60, 90 and 120 min of perfusion. The percentage of potassium tubular transport (%TK;Fig. 4F) decreased at 60, 90 and 120 min at the highest concentration.Snake venoms of the same genus are toxic to the kidney. The effect of many Bothrops genus snakes venom has been assessed in the isolated kidney model, such asB. leucurus(Morais et al., 2013) andBothrops marajoensis(Evangelista et al., 2010). The presence of the venom reduced perfusion pressure, renal vascular resistance, urinaryflow, glomerularfiltration rate and sodium tubular trans-port. However,Bothrops moojeni(Barbosa et al., 2002) andBothrops jararacussu(Havt et al., 2001) venom, which showed some toxic effects similar to the other above mentioned examples, showed a diuresis effect as well.
Tubular epithelial cells are the main targets of acute injury kidney caused by venoms (Sitprija and Sitprija, 2012). The loss of tubular epithelial cell function induced byB. pauloensisvenom, via apoptosis, oxidative stress and mitochondrial dysfunction can explain some of the renal effects observed in this study, such as decrease on percentage tubular transport of sodium, potassium and chloride.
Our results showed direct toxicity ofBpV on renal cells and the alterations in rat isolated kidney. The elucidation of these cell death mechanisms will allow interfering with their signaling molecular pathways at some point and will contribute to the knowledge of kidney pathophysiology of envenomation.
Acknowledgments
This work was supported by grants provided by the Coor-denaç~ao de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), Conselho Nacional de Desenvolvimento Científico e Tecnologico (CNPq), Fundaç~ao de Amparo a Pesquisa do Estado do Ceara (FUNCAP) and Centro de Investigacion Príncipe Felipe, Valencia, Spain.
Transparency document
Transparency document related to this article can be found online athttp://dx.doi.org/10.1016/j.toxicon.2015.08.025.
References
Albuquerque, P.L., Jacinto, C.N., Silva Junior, G.B., Lima, J.B., Veras Mdo, S., Daher, E.F., 2013. Acute kidney injury caused by Crotalus and Bothrops snake venom: a review of epidemiology, clinical manifestations and treatment. Rev. Inst. Med. Trop. Sao Paulo 55, 295e301.
Alves, R.M., Antonucci, G.A., Paiva, H.H., Cintra, A.C., Franco, J.J., Mendonça-Franqueiro, E.P., Dorta, D.J., Giglio, J.R., Rosa, J.C., Fuly, A.L., Dias-Baruffi, M., Soares, A.M., Sampaio, S.V., 2008. Evidence of caspase-mediated apoptosis induced by l-amino acid oxidase isolated fromBothrops atroxsnake venom. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 151, 542e550.
Araki, S., Masuda, S., Maeda, H., Ying, M.J., Hayashi, H., 2002. Involvement of specific integrins in apoptosis induced by vascular apoptosis-inducing protein 1. Tox-icon 40, 535e542.
Baetu, T.M., Hiscott, J., 2002. On the TRAIL to apoptosis. Cytokine Growth Factor Rev. 13, 199e207 (Review).
Barbosa, P.S., Havt, A., Faco, P.E., Sousa, T.M., Bezerra, I.S., Fonteles, M.C., Toyama, M.H., Marangoni, S., Novello, J.C., Monteiro, H.S., 2002. Renal toxicity of Bothrops moojeni snake venom and its main myotoxins. Toxicon 40, 1427e1435.
Bonfoco, E., Krainc, D., Ankarcrona, M., Nicotera, P., Lipton, S.A., 1995. Apoptosis and necrosis: two distinct events induced, respectively, by mild and intense insults with N-methyl-D-aspartate or nitric oxide/superoxide in cortical cell cultures. Proc. Natl. Acad. Sci. U. S. A. 92, 7162e7166.
Bowman, R.H., 1970. Gluconeogenesis in the isolated perfused rat kidney. J. Biol. Chem. 245, 1604e1612.
Chaim, O.M., Sade, Y.B., da Silveira, R.B., Toma, L., Kalapothakis, E., Ch avez-Olortegui, C., Mangili, O.C., Gremski, W., von Dietrich, C.P., Nader, H.B., Sanches Veiga, S., 2006. Brown spider dermonecrotic toxin directly induces nephro-toxicity. Toxicol. Appl. Pharmacol. 211, 64e77.
Chen, W.C., Cheng, H.H., Huang, C.J., Chou, C.T., Liu, S.I., Chen, I.S., Hsu, S.S., Chang, H.T., Huang, J.K., Jan, C.R., 2006. Effect of riluzole on Ca2þmovement and cytotoxicity in Madin-Darby canine kidney cells. Hum. Exp. Toxicol. 25, 461e469.
Collares-Buzato, C.B., de Paula Le Sueur, L., da Cruz-Hofling, M.A., 2002. Impairment of the cell-to-matrix adhesion and cytotoxicity induced by Bothrops moojeni snake venom in cultured renal tubular epithelia. Toxicol. Appl. Pharmacol. 181, 124e132.
Collares-Buzato, C.B., Jepson, M.A., McEwan, G.T., Simmons, N.L., Hirst, B.H., 1994. Junctional uvomorulin/E-cadherin and phosphotyrosine-modified protein content are correlated with paracellular permeability in Madin-Darby canine kidney (MDCK) epithelia. Histochemistry 101, 185e194.
Collares-Buzato, C.B., Jepson, M.A., Simmons, N.L., Hirst, B.H., 1998. Increased tyrosine phosphorylation causes redistribution of adherens junction and tight junction proteins and perturbs paracellular barrier function in MDCK epithelia. Eur. J. Cell Biol. 76, 85e92.
Curtin, J.F., Donovan, M., Cotter, T.G., 2002. Regulation and measurement of oxidative stress in apoptosis. J. Immunol. Methods 265, 49e72.
Damico, D.C., Nascimento, J.M., Lomonte, B., Ponce-Soto, L.A., Joazeiro, P.P., Novello, J.C., Marangoni, S., Collares-Buzato, C.B., 2007. Cytotoxicity of Lachesis muta muta snake (bushmaster) venom and its purified basic phospholipase A2 (LmTX-I) in cultured cells. Toxicon 49, 678e692.
Díaz, C., Valverde, L., Brenes, O., Rucavado, A., Gutierrez, J.M., 2005. Characterization of events associated with apoptosis/anoikis induced by snake venom metal-loproteinase BaP1 on human endothelial cells. J. Cell. Biochem. 94, 520e528.
Dypbukt, J.M., Ankarcrona, M., Burkitt, M., Sjoholm, A., Str€ om, K., Orrenius, S.,€ Nicotera, P., 1994. Different prooxidant levels stimulate growth, trigger apoptosis, or produce necrosis of insulin-secreting RINm5F cells. The role of intracellular polyamines. J. Biol. Chem. 269, 30553e30560.
Evangelista, I.L., Martins, A.M., Nascimento, N.R., Havt, A., Evangelista, J.S., de Noroes, T.B., Toyama, M.H., Diz-Filho, E.B., Toyama, D.O., Fonteles, M.C.,~ Monteiro, H.S., 2010. Renal and cardiovascular effects of Bothrops marajoensis venom and phospholipase A2. Toxicon 55, 1061e1070.
Fenwick, A.M., Gutberlet, J.R.R., Evans, J.A., Parkinson, C.L., 2009. Morphological and molecular evidence for phylogeny and classification of South American pit-vipers, genera Bothrops, Bothriopsis, and Bothrocophias (Serpentes: Viperidae). Zool J Linn Soc. 156, 617e640.
Ferreira, F.B., Gomes, M.S., de Souza, D.L., Gimenes, S.N., Castanheira, L.E.,
Borges, M.H., Rodrigues, R.S., Yoneyama, K.A., Brandeburgo, M.I.,
Rodrigues, V.M., 2013. Molecular cloning and pharmacological properties of an acidic PLA2 from Bothrops pauloensis snake venom. Toxins Basel 5, 2403e2419.
Ferri, K.F., Kroemer, G., 2001a. Mitochondriaethe suicide organelles. Bioessays 23,
111e115. Review.
Ferri, K.F., Kroemer, G., 2001b. Organelle-specific initiation of cell death pathways. Nat. Cell Biol. 3, E255eE263. Review.
Fonteles, M.C., Cohen, J.J., Black, A.J., Wertheim, S.J., 1983. Support of renal kidney function by long-chain fatty acids derived from renal tissue. Am. J. Phys. 244, 235e246.
Fotakis, G., Timbrell, J.A., 2006. In vitro cytotoxicity assays: comparison of LDH, neutral red, MTT and protein assay in hepatoma cell lines following exposure to cadmium chloride. Toxicol. Lett. 160, 171e177.
Georgieva, D., Arni, R.K., Betzel, C., 2008. Proteome analysis of snake venom toxins: pharmacological insights. Expert Rev. Proteom. 5, 787e797.
Gutierrez, J.M., Escalante, T., Rucavado, A., 2009. Experimental pathophysiology of
systemic alterations induced by Bothrops asper snake venom. Toxicon 54, 976e987.
Gutierrez, J.M., Lomonte, B., 1995. Local pathological effects induced by Bothrops snake venoms. Mem. Inst. Butantan 33, 1405e1474.
Havt, A., Fonteles, M.C., Monteiro, H.S., 2001. The renal effects of Bothrops jarar-acussu venom and the role of PLA(2) and PAF blockers. Toxicon 39, 1841e1846.
Herkert, M., Shakhman, O., Schweins, E., Becker, C.M., 2001. Beta-bungarotoxin is a potent inducer of apoptosis in cultured rat neurons by receptor-mediated internalization. Eur. J. Neurosci. 14, 821e828.
Jimenez, V., Paredes, R., Sosa, M.A., Galanti, N., 2008. Natural programmed cell death in T. cruzi epimastigotes maintained in axenic cultures. J. Cell. Biochem. 105, 688e698.
Krysko, D.V., Kaczmarek, A., Krysko, O., Heyndrickx, L., Woznicki, J., Bogaert, P., Cauwels, A., Takahashi, N., Magez, S., Bachert, C., Vandenabeele, P., 2011. TLR-2 and TLR-9 are sensors of apoptosis in a mouse model of doxorubicin-induced acute inflammation. Cell Death Differ. 18, 1316e1325.
Kusma, J., Chaim, O.M., Wille, A.C., Ferrer, V.P., Sade, Y.B., Donatti, L., Gremski, W., Mangili, O.C., Veiga, S.S., 2008. Nephrotoxicity caused by brown spider venom phospholipase-D (dermonecrotic toxin) depends on catalytic activity. Biochimie 90, 1722e1736.
Laing, G.D., Moura-da-Silva, A.M., 2005. Jararhagin and its multiple effects on he-mostasis. Toxicon 45, 987e996. Review.
Lee, M.L., Fung, S.Y., Chung, I., Pailoor, J., Cheah, S.H., Tan, N.H., 2014. King cobra (Ophiophagus hannah) venom L-amino acid oxidase induces apoptosis in PC-3 cells and suppresses PC-3 solid tumor growth in a tumor xenograft mouse model. Int. J. Med. Sci. 6, 593e601.
Martinez-Maldonado, M., Opava-Stitzer, R., 1978. Free water clearance curves dur-ing saline, mannitol, glucose and urea diuresis in the rat. J. Physiol. 280, 487e497.
Mello, C.P., Morais, I.C., Menezes, R.R., Pereira, G.J., Torres, A.F., Lima, D.B., Pereira, T.P., Toyama, M.H., Monteiro, H.S., Smaili, S.S., Martins, A.M., 2014. Bothropoides insularis venom cytotoxicity in renal tubular epithelia cells. Toxicon 88, 107e114.
Mondragon, L., Orzaez, M., Sanclimens, G., Moure, A., Armin~an, A., Sepúlveda, P., Messeguer, A., Vicent, M.J., Perez-Paya, E., 2008. Modulation of cellular apoptosis with apoptotic protease-activating factor 1 (Apaf-1) inhibitors. J. Med. Chem. 3, 521e529.
Morais, I.C., Torres, A.F., Pereira, G.J., Pereira, T.P., Pessoa Bezerra de Menezes, R.R., Mello, C.P., Coelho Jorge, A.R., Binda, A.H., Toyama, M.H., Monteiro, H.S., Smaili, S.S., Martins, A.M., 2013. Bothrops leucurus venom induces nephrotox-icity in the isolated perfused kidney and cultured renal tubular epithelia. Tox-icon 61, 38e46.
Mora, R., Maldonado, A., Valverde, B., Gutierrez, J.M., 2006. Calcium plays a key role in the effects induced by a snake venom Lys49 phospholipase A2 homologue on a lymphoblastoid cell line. Toxicon 47, 75e86.
Mosmann, T., 1983. Rapid colorimetric assay for cellular growth and survival: application to proliferation and citotoxicity. J. Immunol. Methods 65, 55e63.
Nascimento, J.M., Franchi Jr., G.C., Nowill, A.E., Collares-Buzato, C.B., Hyslop, S., 2007. Cytoskeletal rearrangement and cell death induced by Bothrops alternatus snake venom in cultured MadineDarby canine kidney cells. Biochem. Cell Biol.
85, 591e605.
Oliveira, F.N., Brito, M.T., Morais, I.C.O., Fook, S.M.L., Albuquerque, H.N., 2010. Ac-cidents caused by Bothrops and Bothropoides in the State of Paraiba:epidemi-ological and clinical aspects. Rev. Soc. Bras. Med. Trop. 43, 1e6.
Orrenius, S., McCabe Jr., M.J., Nicotera, P., 1992. Ca(2þ)-dependent mechanisms of cytotoxicity and programmed cell death. Toxicol. Lett. 64e65, 357e364. Review.
Otero-Patino, R., 2009. Epidemiological, clinical and therapeutic aspects of~ Bothrops
asperbites. Toxicon 54, 998e1011.
Peixoto, E.B., Collares-Buzato, C.B., 2005. Protamine-induced epithelial barrier disruption involves rearrangement of cytoskeleton and decreased tight junction-associated protein expression in cultured MDCK strains. Cell Struct. Funct. 29, 165e178.
Ribeiro, R.O., Chaim, O.M., da Silveira, R.B., Gremski, L.H., Sade, Y.B., Paludo, K.S., Senff-Ribeiro, A., de Moura, J., Chavez-Olortegui, C., Gremski, W., Nader, H.B., Veiga, S.S., 2007. Biological and structural comparison of recombinant phos-pholipase D toxins from Loxosceles intermedia (brown spider) venom. Toxicon 50, 1162e1174.
Ren, A., Wang, S., Cai, W., Yang, G., Zhu, Y., Wu, X., Zhang, Y., 2006. Agkistin-s, a disintegrin domain, inhibits angiogenesis and induces BAECs apoptosis. J. Cell. Biochem. 99, 1517e1523.
Rodrigues, R.S., Boldrini-França, J., Fonseca, F.P., de la Torre, P., Henrique-Silva, F., Sanz, L., Calvete, J.J., Rodrigues, V.M., 2012. Combined snake venomics and venom gland transcriptomic analysis ofBothropoides pauloensis. J. Proteom. 75, 2707e2720.
Schmitz, I., Kirchhoff, S., Krammer, P.H., 2000. Regulation of death receptor medi-ated apoptosis pathways. Int. J. Biochem. Cell Biol. 32, 1123e1136. Review.
Schwerdt, G., Freudinger, R., Schuster, C., Silbernagl, S., Gekle, M., 2004. Inhibition of mitochondria and extracellular acidification enhance achratoxin A-induced apoptosis in renal collecting duct-derived MDCK-C7 cells. Cell. Physiol. Bio-chem. 14, 47e56.
Sgrignolli, L.R., Mendes, G.E.F., Carlos, C.P., Burdmann, E.A., 2011. Acute kidney injury caused by Bothrops snake venom. Nephron Clin. Pract. 119, 131e137.
Shakhman, O., Herkert, M., Rose, C., Humeny, A., Becker, C.M., 2003. Induction by beta-bungarotoxin of apoptosis in cultured hippocampal neurons is mediated by Ca(2þ)-dependent formation of reactive oxygen species. J. Neurochem. 87, 598e608.
Sitprija, V., Sitprija, S., 2012. Renal effects and injury induced by animal toxins. Toxicon 5, 943e953.
Stabeli, R.G., Sant'Ana, C.D., Ribeiro, P.H., Costa, T.R., Ticli, F.K., Pires, M.G., Nomizo, A., Albuquerque, S., Malta-Neto, N.R., Marins, M., Sampaio, S.V., Soares, A.M., 2007. Cytotoxic L-amino acid oxidase from Bothrops moojeni: biochemical and functional characterization. Int. J. Biol. Macromol. 41, 132e140.
Suhr, S.M., Kim, D.S., 1996. Identification of the snake venom substance that induces apoptosis. Biochem. Biophys. Res. Commun. 224, 134e139.
Taketo, M.M., Sonoshita, M., 2002. Phospolipase A2 and apoptosis. Biochim. Bio-phys. Acta 1585, 72e76.
Tanjoni, I., Weinlich, R., Della-Casa, M.S., Clissa, P.B., Saldanha-Gama, R.F., de Freitas, M.S., Barja-Fidalgo, C., Amarante-Mendes, G.P., Moura-da-Silva, A.M., 2005. Jararhagin, a snake venom metalloproteinase, induces a specialized form of apoptosis (anoikis) selective to endothelial cells. Apoptosis 10, 851e861.
Walser, M., Davidson, D.G., Orloff, J., 1955. The renal clearance of alkali stable inulin. J. Clin. Invest. 34, 1520e1523.
Wen, X., Zhou, J., Zhang, D., Li, J., Wang, Q., Feng, N., Zhu, H., Song, Y., Li, H., Bai, C., 2015. Denatonium inhibits growth and induces apoptosis of airway epithelial cells through mitochondrial signaling pathways. Respir. Res. 16, 13.
WHO, 2009. Neglected tropical diseases: snakebite. Available at.http://www.who. int/neglected_diseases/diseases/snakebites/en/index.html(accessed 31.06.15). Wu, H., Che, X., Zheng, Q., Wu, A., Pan, K., Shao, A., Wu, Q., Zhang, J., Hong, Y., 2014.
Caspases: a molecular switch node in the crosstalk between autophagy and apoptosis. Int. J. Biol. Sci 10, 1072e1083.
Yan, C.H., Yang, Y.P., Qin, Z.H., Gu, Z.L., Reid, P., Liang, Z.Q., 2007. Autophagy is involved in cytotoxic effects of crotoxin in human breast cancer cell line MCF-7 cells. Acta Pharmacol. Sin. 28, 540e548.