Bothropoides insularis
venom cytotoxicity in renal tubular
epithelia cells
Clarissa P. Mello
a, Isabel C.O. Morais
b, Ramon R.P.P.B. Menezes
b,
Gustavo J.S. Pereira
c, Alba F.C. Torres
a, D
^
anya B. Lima
a, Ticiana P. Pereira
a,
Marcos H. Toyama
d, Helena S.A. Monteiro
b, Soraya S. Smaili
c,
Alice M.C. Martins
a,*aDepartment of Clinical and Toxicological Analysis, Federal University of Ceara, Fortaleza 60430370, Ceara, Brazil bDepartment of Physiology and Pharmacology, Faculty of Medicine, Federal University of Ceara, Fortaleza, Ceara, Brazil cDepartment of Pharmacology, Federal University of S~ao Paulo (UNIFESP/EPM), Brazil
d
Sao Vicente Unit, Paulista Coastal Campus, S~ ~ao Paulo State University (UNESP), S~ao Paulo, Brazil
a r t i c l e
i n f o
Article history:
Received 19 January 2014
Received in revised form 2 April 2014 Accepted 6 May 2014
Available online 27 May 2014
Keywords:
Bothropoides insularisvenom Nephrotoxicity
Cell death
a b s t r a c t
Bothropoides insularis (jararaca-ilhoa) is a native endemic snake limited to the specific region of Queimada Island, on S~ao Paulo coast. Several local and systemic effects have been described due to envenomation caused by it, such as edema, tissue necrosis, hemorrhage and acute renal failure. Our previous studies have shown thatBothropoides insularisvenom (BinsV) demonstrated important functional and morphologic alterations in rat isolated kidney, especially decrease in tubular electrolyte transport, osmotic clearance and tubular necrosis. In order to elucidate the direct nephrotoxicity mechanism, the aim of the present study was to investigateBinsV cytotoxicity effect on renal epithelial cells. The treatment withBinsV over MDCK culture decreased cell viability in all concentrations tested with IC50 of 9mg/mL.BinsV was able to induce membrane rupture and cell death with phosphati-dilserine externalization. Furthermore, BinsV induced ROS overproduction and mito-chondrial membrane potential collapse, as well as Bax translocation and caspases 3 and 7 expression. Therefore, these events might be responsible by BinsV-induced cell death caused by mitochondrial dysfunction and ROS overproduction in the direct cytotoxicity process.
©2014 Elsevier Ltd. All rights reserved.
1. Introduction
Snake venoms contain a mix of peptides and proteins, polyamides, histamines and alkaloids used in self-defense and predatory strategies (Oliveira Junior et al., 2013), which effectively perturb vital physiological systems, especially those related to movement, respiration and cir-culation (Cho and Manjunatha, 2011) causing local and systemic damage, including acute kidney injury, an
important component of systemic pathophysiology of en-venomation by Viperidae snakes. Acute kidney injury (AKI) induced by snake venom is a frequent complication of Bothropssnakebite, showing relevant morbidity and mor-tality (Sitprija, 2006; Sgrignolli et al., 2011).
The pathogenesis of renal injury in snakebites is com-plex and many factors are involved in the development of this condition (Sgrignolli et al., 2011). These various factors could be due to a number of specific injuries that occur at systemic and cellular levels. Hypovolemia, hypotension and hypoperfusion, associated to thromboembolic events, are commonly related to its appearance (Albuquerque et al.,
*Corresponding author. Tel.:þ55 85 33668263; fax:þ55 85 33668292. E-mail address:martinsalice@gmail.com(A.M.C. Martins).
Contents lists available atScienceDirect
Toxicon
j o u r n a l h o m e p a g e : w w w . e l s e v i e r . c o m / l o c a t e / t o x i c o n
http://dx.doi.org/10.1016/j.toxicon.2014.05.009 0041-0101/©2014 Elsevier Ltd. All rights reserved.
2013), leading to tubular cell damage. Additionally, it has been hypothesized that this tubular damage is also caused by direct action of toxin presents in bothropic venoms (De Morais et al., 2013). After the initial kidney injury, the organ develops a molecular response that determines cell fate. Some effects are identified in the cell undergoing renal failure: cells may undergo necrosis, apoptosis, and cell di-vision or behave indifferently to stress, with necrosis and apoptosis currently being the most often studied forms of cell death (Vieira, 2001).
TheBothropsgenus and the newly designated genera Bothriopsis,Bothrocophias,BothropoidesandRhinocerophis, which belong to the Viperidae family and are popularly known as pit vipers, have a wide distribution and corre-spond to the most important group of venomous snakes in number of species, population density and number of snakebites occurring in Brazil (Pinho and Pereira, 2001; Queiroz et al., 2008; Oliveira et al., 2010). Specific varia-tions in composition and toxicity of these venoms are usually associated with geographic origin, habitat, climatic variations, diet and age of these snakes (Furtado et al., 2006; Queiroz et al., 2008).
Bothropoides insularis (jararaca-ilhoa) is a native endemic snake limited to the specific region of Queimada Island, on S~ao Paulo coast (Braga et al., 2006, 2007;Valente et al., 2009; Sgrignolli et al., 2011). This venom has similar characteristics to otherBothropsandBothropoides, causing local damage and systemic lesions, with a fourteen-fold greater potency (Valente et al., 2009).
Compared with other species of the same genus, B.
insularis venom (BinsV) is still poorly studied. Tran-scriptomic and proteomic analyses have described that it consists mostly of fraction constituents, such as metal-loproteinases, bradykinin-potentiating peptides, C-type
lectins, serine proteases, phospholipases A2 (PLA2),
vascular endothelial growth factors, L- amino oxidases
(LAAO), cysteine secretory proteins, G10 proteins and
neurotrophic growth factor (Valente et al., 2009). Recently,
it was demonstrated thatBinsV changes kidney function
by promoting alterations in electrolyte transport and os-motic clearance, whereas morphological alterations in renal tubular cells have also been observed (Braga et al., 2006).
However, consideringBinsV important functional and
morphologic alterations in rat isolated kidney, there is scarce knowledge about cellular renal injury mechanism
induced by BinsV. In this context, this study aimed to
characterize thein vitrocytotoxic effect ofBinsV on Mad-ineDarby Canine Kidney Cells (MDCK).
2. Methods
2.1. Venom, chemicals and drugs
B. insularis venom (BinsV) was kindly donated by Dr. Marcos H. Toyama, Universidade Estadual Paulista
(UNESP). RPMI medium, Trifluorocarbonylcyanide
Phenylhydrazone (FCCP), (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) and Annexin V-FITC kit were purchased from SigmaeAldrich Chemical Co.
(St. Louis, MO, USA). 2,7-dichlorodihydrofluorescein
diacetate (DCFH-DA) and tetramethylrhodamine ethyl ester (TMRE) was purchased from Molecular Probes (Eugene, OR, USA). For assays, BinsV was diluted in sterile Phosphate-Buffered Saline (PBS), pH 7.4. Caspase 3 (#96615) and Caspase 7 (#9492) antibodies were acquired from Cell
Signaling (Danvers, Massachusetts, USA), and
a
-tubulinantibody (#T8203) was purchased from SigmaeAldrich
Chemical Co. (St. Louis, MO, USA).
2.2. Cell culture
MDCK cells were cultured at 37C and 5% of CO
2in RPMI 1640 medium, supplemented with 10% fetal bovine serum (FBS), 1% penicillin (10,000 IU/mL) and streptomycin (10 mg/mL). Before each experiment, cells were kept in medium without FBS for 24 h to obtain cells in the G0phase of cell cycle. Cells were plated at 105cells/mL and treated withBinsV in different concentrations to evaluate biolog-ical effects. This cell line maintains morphologbiolog-ical and functional characteristics of distal and/or collecting tubule cells, which allows studying the intracellular action mechanisms of bioactive substances (Collares-Buzato et al., 2002).
2.3. Cytotoxicity assays
2.3.1. MTT assay
Cell viability was assessed by
3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) reduction
assay. Briefly, after 24 h of treatment withBinsV, MTT was added (2.5 mg/mL) for 4 h. The solubilization of formazan crystals was performed with Sodium dodecyl sulphate (SDS) 10% in HCl 0.01N. After 17 h, absorbance at 570 nm
was performed in a microplate reader (Biochrom® Asys
Expert Plus). Cell viability was calculated in comparison with control group. The IC50(venom concentration able to inhibit 50% of cell growth) was determined by non-linear regression (Mosmann, 1983).
2.3.2. Lactate dehydrogenase (LDH) assay
In order to evaluate membrane damage induced by the venom, culture supernatant was collected after 24 h of incubation withBinsV, to determine Lactate Dehydro-genase (LDH) release, using a commercial kit (TOX 7, SigmaeAldrich®, St. Louis, USA). In this method, LDH present in samples causes the conversion of lactate into
pyruvate, with consequent reduction of NADþ
to
NADHþ
H. NADH is then used to convert iodotetrazolium
chloride (INT) into a colored product (Fotakis and
Timbrell, 2006).
2.4. Annexin V-FITC and propidium iodide (PI) staining
After 24 h,BinsV-treated cells were stained withfl uo-rescein isothiocyanate (FITC) conjugated to
annexinV/pro-pidium iodide (PI) according to the manufacturer's
instructions (AnnexinV/FITC Apoptosis Detection Kit, BD Pharmigen, CA, USA). The populations of annexinV PI viable cells and annexinVþ
apoptotic and annexinV PIþ
2.5. ROS intracellular measurement
Cytosolic ROS was measured using
2,7-dichlorodihy-drofluorescein diacetate (DCFH-DA) (Molecular Probes
Eugene, OR, USA), a dye that can readily enter cells and be cleaved by esterase to yield DCFH, a polar, nonfluorescent product. ROS in cells promotes the oxidation of DCFH to yield a fluorescent product, dichlorofluorescein. BinsV -treated cells were collected and then incubated with
DCFH-DA (10 uM, 30 min 37C). Tert-Butyl hydroperoxide
(TBHP) 5
m
M solution served as a positive control for the induction of ROS in cells. These samples were analyzed in a FACSCaliburflow cytometer.2.6. Mitochondrial membrane potentiality (
DJ
m) measurements2.6.1. Real time real space imaging
To evaluate the influence ofB. insularisvenom (BinsV)
(18
m
g/mL) onDJ
m, cells were cultured in coverslipscoated with poly-ornithine (0.01 mg/mL) at a density of
2 105 cells. Cells were incubated with TMRE (20 nM,
15 min, 37C) (Invitrogen, Eugene Oregon, USA) a
poten-tiometric and cationic indicator dye that accumulates preferentially into the energized mitochondria. Before starting the experiments, cells were exposed to light for at least 5 min to ensure a stable baseline. Also, the experi-ments were performed in the presence of the dye to reduce the decrease influorescence due to photobleaching (Smaili and Russell, 1999). TMREfluorescence (548 nm excitation and 585 nm emission) was acquired using a Nikon TE 300
inverted fluorescence microscope (Nikon, Osaka, Japan)
coupled to a high resolution cooled CCD camera (CoolSnap, Roper Scientific Inc., New Jersey, USA).
2.6.2. Flow cytometry
The (
DJ
mt) was measured in control and MDCK-treated cells after 12 h (105) byflow cytometry using 488 nm exci-tation and 670 nm emission. These samples were collected in FACS tubes (BD Biosciences Discovery Labware, MA, USA)and stained with TMRE (20 nM) for 20 min at 37C.
Tri-fluorocarbonylcyanide Phenylhydrazone (FCCP 5
m
M) wasused as positive control for mitochondrial depolarization.
Log scale fluorescence histograms (105 events) were
analyzed for median relativefluorescent unit (RFU) intensity using FACSCaliburflow cytometer and Cell Quest software.
2.7. GFP-bax punctuation
MDCK-cells were transfected with GFP-Bax (0.5
m
g/m
L) using FuGENE 6 Transfection Reagent (Roche Applied Sci-ence, Indianapolis, USA) according to manufacturer's in-structions for 24 h. Experiments were performed using high-resolutionfluorescence microscopy in Real-time real-space experiments. MDCK overexpressing GFP-Bax were stimulated withBinsV (18m
g/mL) during 5 h.2.8. Flow cytometry for detection of caspase-3 activity
The extent of caspase-3 activation in MDCK cells treated withBinsV (IC50) for 24 h was detected byflow cytometry
analysis using the active caspase-3 monoclonal
anti-body, which specifically recognizes the active form of
caspase-3. Briefly, cells were washed twice in PBS, fixed using 2% paraformaldehyde for 30 min and then per-meabilized using PBS with saponine (0.01%) and BSA (1%) for 30 min at room temperature. Cells were stained with anti-active caspase-3 antibody for 1 h at room temperature in the dark. Following incubation with the antibody, cells were washed in wash buffer, resuspended in PBS and
analyzed in a FACSCalibur flow cytometer. Doxorrubicin
(10
m
M) was used as positive control.2.9. Immunoblotting
Whole cell extracts were obtained by lysing cells in a
buffer containing 25 mM TriseHCl pH7.4, 1 mM EDTA,
1 mM EGTA, 1% SDS, protease and phosphatase inhibitors. Protein concentration was determined by the BCA protein assay. Cell lysates were resolved by SDS-PAGE, transferred to nitrocellulose membranes, blocked with 5% non-fat milk, washed with 0.1% Tween/PBS and incubated overnight with a specific primary antibody. Membranes were washed and probed with the appropriate secondary antibody con-jugated with horseradish peroxidase for enhanced chem-iluminescence detection (Amersham Pharmacia Biotech).
2.10. Statistical analysis
Experiments were performed in triplicate (n¼3), and
data were expressed as mean±SEM. Data were analyzed
using ANOVA with Bonferroni or Dunett post-test and significance was set atp<0.05.
3. Results and discussion
3.1. Cytotoxic effect of theB. insularisvenom on MDCK cells
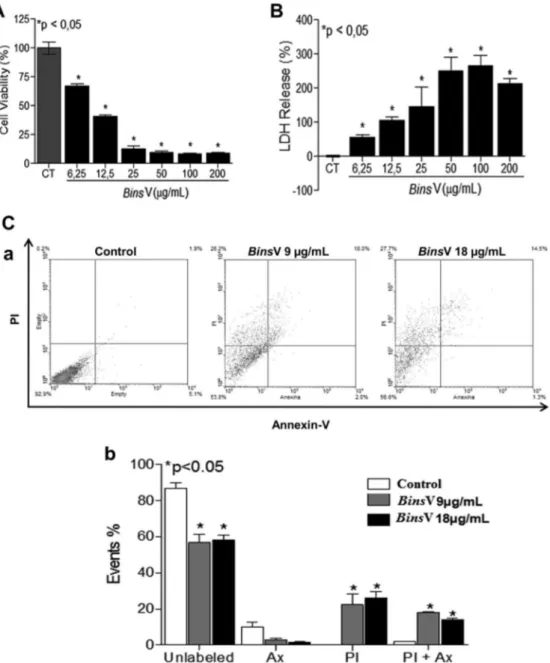
First, the cytotoxicity effect ofBinsV was assessed through MTT assay: this treatment after 24 h was able to reduce cell viability at all tested concentrations (200; 100; 50; 25; 12.5 and 6.25
m
g/mL) with an IC50of 9m
g/mL (Fig. 1A).BinsV induced an apparent membrane rupture, as demonstrated by LDH-release assay, as significant release of LDH enzyme was observed in all concentrations studied, mainly in higher concentrations in relation to untreated cells (Fig. 1B). Annexin-PI loading cell analysis demonstrated an increase mainly in PIþcells at the concentrations of 9 and 18
m
g/mL, as well as Annexin VþPIþ
cells, suggesting the participation of late apoptosis and necrosis onBinsV-induced cell death (Fig 1C, aeb). Therefore, this result indicates thatBinsV causes cell membrane alterations, suggesting a lytic effect ofBinsV
on MDCK cells. Bothrops venom cytotoxicity was also
the cytotoxicity induced by snake venoms. The identification of cytotoxicity mechanism of these substances is important to understand snakebite pathophysiology and its systemic complications (Lewis and Garcia, 2003).
Necrosis is a type of cell death in which cells undergo stimuli, such as mechanical injury and hypoxia, resulting in increased cell volume, chromatin aggregation, disruption of cytoplasm, loss of plasma membrane integrity and there-fore cell rupture. In necrosis changes occur in mitochondria
first, whereas changes in nucleus are much less common
(Cruchten and Broeck, 2002; Zong and Thompson, 2006; Kroemer et al., 2010). In kidney cells, cell death by necro-sis results from the combined effects of a number of biochemical pathways, such as severe depletion of cellular energy reserves (ATP), an increase in cytosolic Ca2þ
, ROS generation and activation of several enzymes, including phospholipases, proteases and endonucleases (Rana et al., 2001; Mchugh and Turina, 2006; Boujrad et al., 2007). Fig. 1.Cytotoxic effect ofBothropoides insularisvenom on MDCK cells. (A) MDCK cells were treated with different concentrations ofB. insularisvenom for 24 h and were evaluated by the reduction in the 3-(4,5-Dimethylthiazol-2-Yl)-2,5-Diphenyltetrazolium Bromide (MTT) salt in cells underBinsV concentration-curve after 24 h of treatment. (B) Percentage release of the lactate dehydrogenase enzyme of MDCK cells treated under aBinsV concentration-curve treatment. (C) Cell death was measured by annexin V and PI staining and detected byflow cytometry. MDCK cells were treated with two different concentrations (IC50e 2IC50) of
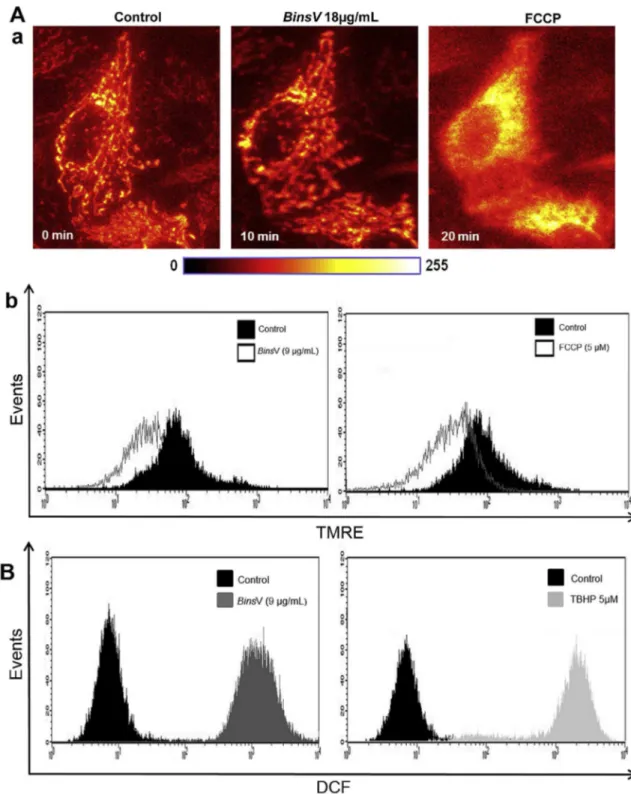
Fig. 2.Intracellular Mitochondrial membrane potentiality (DJm) and Reactive Oxygen Species (ROS)BinsV -regulated. (A) Alterations inDJmwas measured by
TMRE staining (20 nM, 20 min at 37
C) and detected byfluorescence microscopy orflow cytometry. (a)BinsV (2IC5018mg/mL) caused a decrease inDJm
indicated by the dissipation of TMRE dye from mitochondria. FCCP (5mM) was used as positive control measured byfluorescence microscopy. MDCK image cells loaded with TMRE were detected before and after the addition ofBinsV, using a cooled CCD camera with a delay of 6 s between them (40objective). (b) MDCK cells were treated with (IC50, 9mg/mL) ofBinsV for 12 h. Displacement of the TMRE Fluorescence to the left when compared with negative control, shows the decrease in
3.2. Intracellular mitochondrial membrane potentiality (
DJ
m) and reactive oxygen species (ROS)To verify the effects ofBinsV on mitochondrial trans-membrane potential (
DJ
m), thefluorescence intensity of mitochondrial specific probe, tetramethylrhodamine ethylester (TMRE), was measured, which indicates the
DJ
musingflow cytometer (9
m
g/mL, 24 h) or in real-timeex-periments using fluorescence microscopy (18
m
g/mL,10 min).
DJ
mwas measured before and after the addition ofBinsV followed by positive control, FCCP (5m
M). In the present study,BinsV caused a decrease in TMREfluores-cence, indicating that the
DJ
m rapidly reduced whenor 24 h of stimulus (Fig. 2A, a-b, respectively). It is believed that the loss of mitochondrial membrane potential is due to the opening of the permeability transition pore, a mito-chondrial megachannel that is strongly affected by oxida-tive stress conditions. Mitochondria might also be a primary target for the damaging effects of ROS (Wang et al., 2013;Bernardi et al., 2001).
Next, we decided to test the involvement of reactive oxygen species on cell death signalingBinsV -induced. To evaluate ROS levels afterBinsV treatment on MDCK cells, they were assessed by flow cytometry through a specific
fluorescent dye, DCFH-DA, which leads to enhancedfl
uo-rescent intensity following the generation of intracellular reactive metabolites. We showed that the amounts of ROS in all treatment groups were significantly increased compared with untreated control group, as observed by right disloca-tion of thefluorescence peak in the histogram (Fig. 2B); the concentration 9
m
g/mL produced the highest level of ROS in comparison with the untreated control group. It has beenshown in several studies using Bothrops moojeni and
Bothrops alternatusvenoms on MDCK cells, which induced necrosis and possibly through the ROS actions ( Collares-Buzzato et al., 2002; Nascimento et al., 2007).
3.3. Bax translocation and caspase-3 and -7 activities
Since the change in mitochondrial membrane potential and ROS increase can be associated with apoptosis and necrosis, in order to further clarify the BinsV -mediated nephrotoxic effect, experiments using GFP-Bax transfected cells were performed to investigate the involvement of the mitochondrial pathway. Proteins of the Bcl-2 family (Bax
and Bcl-2) play an important role in initiating
mitochondrion-dependent apoptosis. Bax is a
pro-apoptotic protein that resides in the cytoplasm as inactive monomer in healthy cells. Upon apoptotic stimuli, Bax undergoes conformational activation, leading to its
trans-location to mitochondria. The formation of BaxeBax
homodimers creates pores in the mitochondrial mem-brane, allowing the release of apoptogenic factors (Qi et al., 2011). Our results showed that treatment of MDCK cells that express GFP-Bax withBinsV (18
m
g/mL) resulted in Bax translocation into the mitochondria within 5 h (Fig. 3A), suggesting that Bax probably starts the mechanism involved inBinsV -induced cell death.Therefore caspases 3 and 7 are key proteases required for the occurrence of apoptosis (Wu et al., 2012). This
experiment was performed to confirm whether caspases
are activated after treatment withBinsV. We measured the changes in caspases 3 and 7 activity in MDCK cells. As shown inFig. 3B and C,BinsV (18
m
g/mL) treatment induced activation of caspase 3 and caspase 7 on MDCK cells. These data indicate that apoptosis is involved in BinsV-induced cell death in these cells. Furthermore other cell death mechanisms cannot be excluded, as such as autophagy. This pathway is related to mitochondrial dysfunction as a pro-tective mechanism (Kroemmer et al., 2011).Therefore, this is evidence thatBinsV-induced cell death involves an increase in ROS production, which may contribute to the potential loss of mitochondrial and translocation of Bax. Other studies using MDCK cells have
shown thatBothropsvenoms are cytotoxic to renal cells by mechanisms that involve increase in ROS production (Collares-Buzato et al., 2002; Nascimento et al., 2007), mitochondrial depolarization and increases in cytosolic Ca2þ(De Morais et al., 2013). In summary, we have reported
that B. insularisvenom induced MDCK death, which in-volves an increase in ROS production and mitochondrial dysfunction, with participation of necrotic and apoptotic mechanisms. The elucidation of these mechanisms allows interference in some points of their signaling pathways and helps the development of drugs and more effective thera-peutic strategies.
Acknowledgments
The authors would like to thank Hanako Hirata for the manuscript review. This project was supported by grants from Coordenaç~ao de Aperfeiçoamento do Ensino Superior (CAPES), Conselho Nacional de Desenvolvimento Científico e Tecnologico (CNPq), Fundaç~ao do Amparo a Pesquisa do Estado de S~ao Paulo (FAPESP) and Fundaç~ao Cearense de
Apoio ao Desenvolvimento Cientifico e Tecnologico do
Estado do Ceara (FUNCAP).
Conflict of interest
The authors declare that they have no conflicts of
interest.
References
Albuquerque, L.M.M., Jacinto, C., Silva Júnior, G.B., Lima, J.B., Veras, M.S.B., Daher, Daher, E.F., 2013. Acute kidney injury caused by Crotalus and Bothrops snake venom: a review of epideology, clinical manifestation and treatment. Rev. Inst. Med. Trop. S. Paulo 55, 295e301.
Bernardi, P., Petronilli, V., Di LISA, F., Forte, M., 2001. A mitochondrial perspective on cell death. Trends Biochem. Sci. Oxon. 26, 112e117.
Boujrad, H., Gubkina, O., Robert, N., Krantic, S., Susin, S.A., 2007. AIF-mediated programmed necrosis: a highly regulated way to die. Cell Cycle 21, 2612e2619.
Braga, M.D.M., Martins, A.M.C., Amora, D.N., De Menezes, D.B., Toyama, M.H., Toyama, D.O., Marangoni, S., Barbosa, P.S.F., Alves, R.D., Fonteles, M.C., Monteiro, H.S.A., 2006. Purification and biological ef-fect of C-type lectin isolated from Bothropoides insularis venom. Toxicon 7, 859e867.
Braga, M.D., Costa Martins, A.M., Alves, C.D., de Menezes, D.B., Martins, R.D., Ferreira Barbosa, P.S., de Sousa Oliveira, I.M., Toyama, M.H., Toyama, D.O., Dos Santos Diz Filho, E.B., Ramos Fagundes, F.H., Fonteles, M.C., Azul Monteiro, H.S., 2007. Purification and biological effects of L-amino acid oxidase isolated from Bothro-poides insularisvenom. Toxicon 51, 199e207.
Collares-Buzato, C.B., Le Sueur, L.P., Cruz-Hofling, M.A., 2002. Impairment of the cell-to-matrix adhesion and cytotoxicity induced byBothrops moojenisnake venom in cultured renal tubular epithelia. Toxicol. Appl. Pharmacol. 181, 124e132.
Cruchten, S.V., Broeck, W.V.D., 2002. Morphological and biochemical as-pects of apoptosis, oncosis and necrosis. Anat. Histol. Embryol. 31, 214e223.
De Morais, I.C., Torres, A.F., Pereira, G.J., Pereira, T.P., Pessoa Bezerra de Menezes, R.R., Mello, C.P., Coelho Jorge, A.R., Binda, A.H., Toyama, M.H., Monteiro, H.S., Smaili, S.S., Martins, A.M., 2013.
Bothrops leucurus venom induces nephrotoxicity in the isolated perfused kidney and cultured renal tubular epithelia. Toxicon 61, 38e46.
Fotakis, G., Timbrell, J.A., 2006. In vitro cytotoxicity assays: comparison of LDH, neutral red, MTT and protein assay in hepatoma cell lines following exposure to cadmium chloride. Toxicol. Lett. 160, 171e177.
Jorge, R.J., Martins, A.M., Morais, I.C., Ximenes, R.M., Rodrigues, F.A., Soares, B.M., Evangelista, J.S., Toyama, M.H., Martins, A.M., Moraes Filho, M.O., Monteiro, H,S., 2011. In vitro studies on Bothrops venom cytotoxic effect on tumor cells. J. Exp. Ther. Oncol. 9, 249e253.
Kroemer, G., Marino, G., Levine, B., 2010. Autophagy and the integrated~ stress response. Mol. Cell 22, 280e293.
Lewis, R.J., Garcia, M.L., 2003. Therapeutic potencial of venom peptides. Nat. Rev. Drug Discov. 2, 790e802.
Mchugh, P., Turina, M., 2006. Apoptosis and necrosis: a review for sur-geons. Sur. Infect. (Larchmt) 7, 53e68.
Mosmann, T., 1983. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J. Immunol. Methods 65, 55e63.
Nascimento, J.M., Franchi, G.C.J.R., Nowill, A.E., Collares-Buzato, C.B., Hyslop, S., 2007. Cytoskeletal rearrangement and cell death induced byBothrops alternatussnake venom in cultured Madin-Darby canine kidney cells. Biochem. Cell Biol. 5, 591e605.
Oliveira, F.N., Brito, M.T., Morais, I.C., Fook, S.M., Albuquerque, H.N., 2010. Accidents caused by Bothrops and Bothropoides in the State of Par-aiba: epidemiological and clinical aspects. Rev. Soc. Bras. Med. Trop. 43, 662e667.
Oliveira Junior, N.G., Silva Cardoso, M.H., Franco, O.L., 2013. Snake Venom: attractive antimicrobial proteinaceous compounds for therapeutic purposes. Cell. Mol. Sci. 70, 4645e4658.
Pinho, F.M.O., Pereira, I.D., 2001. Ofidismo. Rev. Assoc. Med. Bras. 47, 24e29.
Queiroz, G.P., Pessoa, L.A., Portaro, F.C.V., Furtado, M.F.D., Tambourgi, D.V., 2008. Interspecific variation in venom composition and toxicity of Brazilian snakes fromBothropsgenus. Toxicon 52, 842e851.
Rana, A., Sathianara, P., Lieberthal, W., 2001. Role of apoptosis on renal tubular cells in acute renal failure: therapeutic implications. Apoptosis 6, 83e102.
Sgrignolli, L.R., Mendes, G.E.F., Carlos, C.P., Burdmann, E.A., 2011. Acute kidney injury caused byBothropssnake venom. Nephron. Clin. Pract. 119, 131e137.
Shankland, S.J., Wolf, G., 2000. Cell cycle regulatory proteins in renal disease: role in hypertrophy, proliferation, and apoptosis. Am. J. Physiol. Ren. Physiol. 278, 515e529.
Sitprija, V., 2006. Snakebite nephropathy. Nephrology 11, 442e448.
Smaili, S.S., Russell, J.T., 1999. Permeability transition pore regulates both mitochondrial membrane potential and agonist-evoked Caþ2 signals in oligodendrocyte progenitors. Cell Calcium 26, 121e130.
Valente, R.H., Guimar~aes, P.R., Junqueira, M., Neves-Ferreira, A.G., Soares, M.R., Chapeaurouge, A., Trugilho, M.R., Leon, I.R., Rocha, S.L., Oliveira-Carvalho, A.L., Wermelinger, L.S., Dutra, D.L., Le~ao, L.I., Jun-queira-De-Azevedo, I.L., Ho, P.L., Zingali, R.B., Perales, J., Domont, G.B., 2009. Bothropoides insularis venomics: a proteomic analysis supported by transcriptomic-generated sequence data. J. Proteomics 72, 241e255.
Vieira, J.M., 2001. Atualizaç~ao em insufici^encia renal: expressao g~ ^enica na insufici^encia renal aguda (IRA). J. Bras. Nefrol. 23, 230e233.
Wang, T., He, Q., Xiao, L., Wang, Q., Zhang, B., Wang, B., Liu, G., Zheng, J., Yu, B., Zhang, L., 2013. Mitochondrial dysfunction contributes to the cytotoxicity induced by tentacle extract from the jellyfishCyanea capillatain rat renal tubular ephitelial NRK-52E cells. Toxicon 74, 1e7.
Wu, W., Liu, P., Li, J., 2012. Necroptosis: an emerging form of programmed cell death. Crit. Rev. Oncol. Hematol. 82, 249e258.