Clinical report
Bothrops leucurus
venom induces nephrotoxicity in the isolated perfused
kidney and cultured renal tubular epithelia
Isabel Cristina Oliveira de Morais
a, Alba Fabíola Costa Torres
b, Gustavo José da Silva Pereira
c,
Ticiana Praciano Pereira
b, Ramon Róseo de Paula Pessoa Bezerra de Menezes
a,
Clarissa Perdigão Mello
b, Antonio Rafael Coelho Jorge
a, Alexandre Havt Bindá
a,
Marcos Hikari Toyama
d, Helena Serra Azul Monteiro
a, Soraya Soubhi Smaili
c,
Alice Maria Costa Martins
b,*aDepartment of Physiology and Pharmacology, Faculty of Medicine, Federal University of Ceará, Fortaleza, Ceará, Brazil bDepartment of Clinical and Toxicological Analysis, Federal University of Ceará, Fortaleza, Ceará, Brazil
cDepartment of Pharmacology, Federal University of São Paulo (UNIFESP/EPM), São Paulo, Brazil dSão Vicente Unit, Paulista Coastal Campus, Paulista State University (UNESP), São Paulo, Brazil
a r t i c l e
i n f o
Article history: Received 1 May 2012
Received in revised form 8 October 2012 Accepted 10 October 2012
Available online 2 November 2012
Keywords: Bothrops leucurus Nephrotoxicity Renal tubular epithelia
a b s t r a c t
Bites from snake (Bothropsgenus) cause local tissue damage and systemic complications, which include alterations such as hemostatic system and acute renal failure (ARF). Recent studies suggest that ARF pathogenesis in snakebite envenomation is multifactorial and involves hemodynamic disturbances, immunologic reactions and direct nephrotoxicity. The aim of the work was to investigate the effects of theBothrops leucurusvenom (BlV) in the renal perfusion system and in cultured renal tubular cells of the type MDCK (Madin–Darby Canine kidney).BlV (10mg/mL) reduced the perfusion pressure at 90 and 120 min. The renal vascular resistance (RVR) decreased at 120 min of perfusion. The effect on urinaryflow (UF) and glomerularfiltration rate (GFR) started 30 min afterBlV infusion, was transient and returned to normal at 120 min of perfusion. It was also observed a decrease on percentual tubular transport of sodium (%TNaþ) at 120 min and of chloride (%TCl ) at 60 and 90 min. The treatment withBlV caused decrease in cell viability to the lowest concentration tested with an IC50of 1.25mg/mL. Flow cytometry with annexin V and propidium iodide showed that cell death occurred predominantly by necrosis. However, a cell death process may involve apoptosis in lower concentrations.BlV treatment (1.25mg/mL) led to significant depolariza-tion of the mitochondrial membrane potential and, indeed, we found an increase in the expression of cell death genes in the lower concentrations tested. The venom also evoked an increase in the cytosolic Ca2þin a concentration dependent manner, indicating that Ca2þmay participate in the venom ofB. leucuruseffect. The characterization of the effects in the isolated kidney and renal tubular cells gives strong evidences that the acute renal failure induced by this venom is a result of the direct nephrotoxicity which may involve the cell death mechanism.
Published by Elsevier Ltd.
1. Introduction
Snake venoms consist of complex mixture of active substances, mainly peptides and protein, which interfere in various biological processes (Sitprija and Sitprija, 2012).
*Corresponding author. Tel.:þ55 85 33668263; fax:þ55 85 33668292. E-mail address:martinsalice@gmail.com(A.M.C. Martins).
Contents lists available atSciVerse ScienceDirect
Toxicon
j o u r n a l h o m e p a g e : w w w . e l s e v i e r . c o m / l o c a t e / t o x i c o n
0041-0101/$–see front matter Published by Elsevier Ltd.
http://dx.doi.org/10.1016/j.toxicon.2012.10.005
Envenoming following snakebite is a health problem in the tropical regions of the world, especially in Latin America, Africa and Asia. In Central and South America, most ophidic accidents are produced by Viperidae of whom 90% are caused by snakes of theBothropsgenus (Duque et al., 2007).
Bothrops venom presents proteolytic, coagulative and hemorrhagic actions (Oliveira et al., 2010). Bothrops spp. envenomation may cause local tissue damage in the form of bleeding, swelling, myonecrosis, severe pain and systemic effects, alterations to the hemostatic system and acute renal failure (Gomes et al., 2009). These effects that are mediated by toxins that act synergistically or individually such as metalloproteinases, (myotoxic) phospholipases A2, phosphodiesterases, L-amino acid oxidase and enzymes affecting the coagulation cascade might also be involved (Acosta et al., 2010).
The main cause of death following bites by Bothrops
species is acute renal failure (ARF) (Castro et al., 2004). Several studies have been conducted to understand the mechanisms by which the venom leads to ARF. Some authors attribute crucial role to the venom induced hemodynamic changes and renal ischemia (Chaves et al., 1989; Da Cruz-Höfling et al., 2001), glomerular fibrin deposition (Rezende et al., 1989), release of vasoactive substances (Havt et al., 2001). Recent studies have reported a directBothrops venom-induced tubular and glomerular injury (Castro et al., 2004;Nascimento et al., 2007).
TheBothrops leucurushas a limited range of distribution and is found in the Atlantic forest areas of the northeast of Brazil, including the States of Ceara and Bahia to Espirito Santo in the southeast. The venom ofB. leucurushas not been studied in as much detail. The biological effects due envenomation have similar profile to that observed in other
Bothrops, ie coagulant activity, hemorrhagic, fibrinolytic, and renal failurein vivo(Sanchez et al., 1992).
In order to elucidate the mechanism of direct nephro-toxicity, the aim of the present work was to investigate the effect ofB. leucurusvenom on the isolated perfused kidney and the cytotoxicity of venom on renal epithelial cells.
2. Material and methods
2.1. Venom, chemicals and drugs
B. leucurusvenom was kindly donated by Dr. Marcos H. Toyama, Paulista State University (UNESP), RPMI medium, albumin, inulin, poly-ornithine, phenylhydrazone (FCCP), MTT, Annexin V-FITC kit were purchased from Sigma Chemical Co. (St.Louis, MO, USA). Hoechst 33342, Fura-2-AM and tetramethylrhodamine ethyl ester (TMRE) were purchased from Molecular Probes (Eugene, OR, USA).
2.2. Kidney perfusion
Adult male Wistar rats (260–320 g) were fasted for 24 h with free access to water. The rats were anesthetized with sodium pentobarbitone (50 mg/kg, i.p.) and after careful dissection of the right kidney; the right renal artery was cannulated via the mesenteric artery without interrupting the bloodflow as described byBowman (1970). The perfu-sionfluid consisted of a modified Krebs–Henseleit solution
(MKHS) of the following composition (in mmol/L): 114.00 NaCl, 4.96 KCl, 1.24 KH2PO4, 0.5 MgSO4$7H2O, 2.10 CaCl2and 24.99 NaHCO3. Bovine serum albumin (BSA 6 g%; fraction V), urea (0.075 g), inulin (0.075 g) and glucose (0.15 g) were added to the solution, resulting in afinal perfusate volume of 100 mL. The pH was adjusted to 7.4. In each experiment, 100 mL of MKHS were recirculated for 120 min. The perfusion pressure (PP) was measured at the tip of the stainless steel cannula in the renal artery. Samples of urine and perfusionfluid were collected at 10 min intervals for analysis of the sodium, potassium and chloride levels by ion-selective electrodes (Rapid chem 744, Bayer Diagnostic, UK); inulin, as described byWalser et al. (1955)and
modi-fied byFonteles et al. (1983); and osmolality, which was measured in vapor pressure osmometer (Wescor 5100C, USA). The venom ofB. leucurus(BlV) (10
mg/mL) was added
to the system 30 min after the beginning of each perfusion. The perfusion pressure (PP), renal vascular resistance (RVR), urinary flow (UF), glomerular filtration rate (GFR), the percentage of sodium (%TNaþ), potassium (%TKþ
) and chloride (%TCl ) tubular transport were determined (Martinez-Maldonado and Opva-Stitzer, 1978). The results were compared to the internal control group, at 30 min early in each experiment (n¼5). The experimental proce-dures were conducted according to guidelines for the care and use of laboratory animals as approved by the Ethical Committee (79/08) from Federal University of Ceara.
2.3. Cell culture
Epithelial Madin–Darby Canine Kidney (MDCK) was cultivated in RPMI 1640 medium (MEM) supplemented with 10% fetal bovine serum, 1% penicillin (10 000 IU/mL), and streptomycin (10 mg/mL). For each experiment cells were removed and incubated with trypsin-EDTA (0.25/0.02% v/v) at 37C at about 5 min. After this, the cells were counted in
a Neubauer chamber and suspended in culture medium (1105cells) and 24 h later used for the experiments.
2.4. Cytotoxicity assay
Cell viability was assessed by MTT (4,5-dimetilazil-2-il)-2,5 diphenyl tetrazolium) assay as described byMosmann (1983). The MDCK cells are plated in 96-well plates at a density of 105 cells and treated with different concen-trations ofBlV (50, 25, 12.5, 6.25, 3.12, 1.56
mg/mL). After
24 h of treatment, the cells were incubated with 0.5 mg of MTT/mL for 4 h. The formazan crystals that resulted from MTT reduction were dissolved by adding SDS (10%) to each well followed by incubation for 17 h. The absorbance was read at 570 nm in a microplate reader, and cell viability was calculated by comparing the resulting absorbances with the mean absorbance of the control wells (without venom, considered to be 100% viable).2.5. Annexin V-FITC and propidium iodide (PI) staining
Phosphatidyl serine exposure was determined by the Annexin V (BD PharmingenÔ) binding usingflow cytom-etry. MDCK cells (105) were incubated withBlV for 24 h, cells were washed twice with PBS, resuspended in the
working solution of 5
mg/mL propidium iodide and annexin
V (0.25mg/mL), incubated for 15 min at 37
C. Allproce-dures were carried out according to the manufacturer’s instructions. (FACS, BD, New Jersey, USA). Cells were analyzed onflow cytometer (105events) using CellQuest software.
2.6. Evaluation of the expression of genes involved in cell death by apoptosis
Differential gene expression of caspase 3 and caspase 8 were assayed using iQ5 Real Time PCR Detection System (Bio-Rad). MDCK cells were grown in 24-well plates at a concentration of 105 cells/mL. After 24 h, theBlV was added in the concentrations of 1, 1.25 and 1.5
mg/mL. Total
RNA was isolated from each sample using the RNeasy Mini kit (Qiagen) and automation equipment for Qiacube. The cDNA was synthesized by a reverse transcriptase reaction using a SuperScriptÔ III Reverse Transcriptase System (Invitrogen, USA). Negative samples were also tested, with the cDNA being replaced with autoclaved Milli-Q water and control positive was tested with doxorubicin (3.12mg/mL).
The PCR conditions were as follows: an initial denaturation period of 3 min at 95C, followed by 40 cycles for gene amplification. Each cycle consisted of an initial denatur-ation phase of 30 s at 95C, followed by an annealing phase
of 30 s at 60C and an extension phase of 45 s at 72C. The
samples were then subjected to an extension step of 3 min at 72C. Gene expression was obtained by applying the
mathematical method ofPfaffl(2001).
2.7. Ca2þmeasurements
To evaluate Ca2þ
signaling, MDKC cells plated on cover slips were loaded with 3
mM Fura-2AM for 30 min
microscopy buffer containing (mM): 130 NaCl, 5.36 KCl, 0.8 MgSO4, 1 Na2HPO4, 25 glucose, 20 HEPES, pH 7.3. Fura-2fluorescence was acquired using alternate excitation
filters of 340 and 380 nm and 505 nm emission and Ca2þ
measurements were evaluated under a high resolution
fluorescence microscopy (Nikon TE 300; Nikon, Osaka, Japan) coupled to a cooled CCD camera (CoolSnap, Roper Scientific Inc., NJ). Images were acquired in BioIP software (Anderson Eng, Delaware, USA). Basal Ca2þ levels were
considered to be thefirst 20 images beforeBlV addition. Increases in fluorescence after venom addition were expressed as percentage of fluorescence ratio (340/380) values, normalized from the basalfluorescence.
2.8.
D
J
m measurementsTo check the influence ofBlV (1.25
mg/mL) in the
D
J
m, cells were cultured in coverslips coated with poly-ornithine (0.01 mg/mL) in a density of 2105cells. Cells were incu-bated with TMRE (50 nM, 37C, 20 min), a potentiometricand cationic indicator dye that accumulates preferentially into the energized mitochondria in regular microscopy buffer as described above. In general, before starting the experiments, the cells were exposed to light for at least 5 min to ensure a stable baseline. Also, the experiments were performed in the presence of the dye to diminish the
decrease influorescence due to photobleaching (Smaili and Russell, 1999). TMREfluorescence (548 nm excitation and 585 nm emission) was acquired using a Nikon TE 300 inverted fluorescence microscope (Nikon, Osaka, Japan) coupled to a high resolution cooled CCD camera (CoolSnap, Roper Scientific Inc., New Jersey, USA).
3. Results
3.1. Effects of theBothrops leucurusvenom in the isolated rat kidney
The effect on perfusion pressure started 60 min after of the venom infusion, persisted till the end of the experiment (PP;Fig. 1A). A decrease in renal vascular resistance (RVR; Fig. 1B) was also observed. The effect on urinaryflow (UF) and glomerularfiltration rate (GFR) started 30 min afterBlV infusion (Fig. 1C and D), was transient and returned to normal at 120 min of perfusion. The percent of sodium tubular transport (%TNaþ
) was reduced at 90 min and percent of chloride tubular transport (%TCl ) at 60 and 90 min of perfusion (Fig. 1E and F). There were no significant differences in the percent of potassium tubular transport after the infusion of BlV when compared in control group (%TKþ
CT¼69.134.14; %TKþ60¼69.046.6; %TKþ
90¼71.844.21; %TKþ
12069.946.86).
3.2. Cytotoxic effect of theBothrops leucurusvenom on MDCK cells
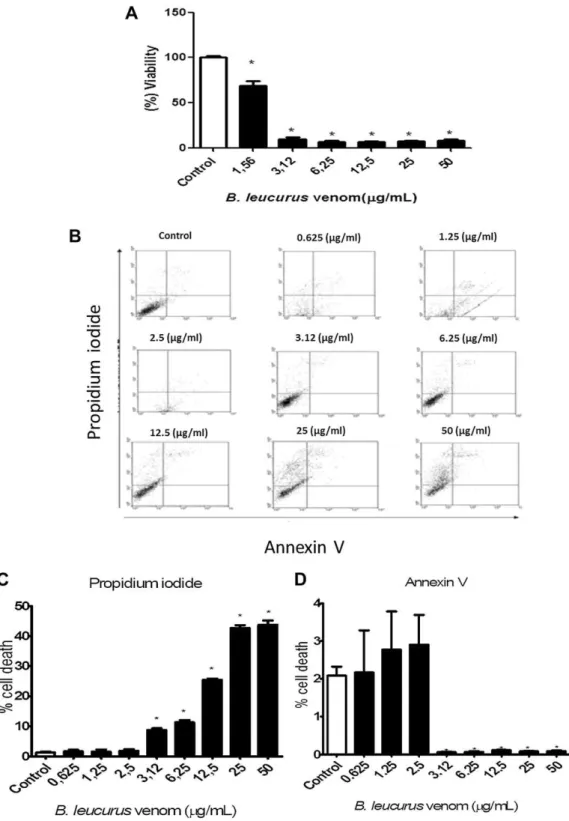
Cytotoxity was availablefirstly by assay MTT, treatment with BlV caused decrease in cell viability to the lowest concentration tested (1.56
mg/mL) with an IC50
of 1.25mg/
mL (Fig. 2A). Flow cytometric analysis showed that the necrotic cell population increased significantly in a concentration-dependent fashion after 24 h exposure, with little percentage of apoptotic cells only in lower concentrations (Fig. 2B–D). Taken together, these results show that the necrosis is predominant but there is apoptosis at low doses, what is important, because it can be related to a more specific effect of the venom, while necrosis which is a type of uncontrolled cell death, appears only with higher doses.3.3. Expression of genes involved in apoptotic cell death
For this purpose MDCK cells were treated withBlV and genes considered markers of apoptosis were selected such ascaspase 3andcaspase 8. Data show that there was an increase incaspase 3expression observed with the lowest concentration tested (1
mg/mL) (
Fig. 3A). As shown in Fig. 3B, the expression of caspase 8 after treatment of MDCK cells with the venom ofB. leucurusat concentration of 1mg/mL increased indicating the possible involvement in
cell death pathway mediated by venom ofB. leucurus.3.4. Ca2þmeasurements
For Ca2þ
measurements the cells MDCK were loaded with the cytosolic Ca2þ
dye, Fura-2, which is a ratiometric dye.Fig. 4A shows MDCK cells loaded with Fura-2 before
and after the addition of 2.5
mg/mL of
BlV.Fig. 4B illustrates the representative recording of the increase in intracellular Ca2þinduced byBlV (2.5
mg/mL). The average increase in
cytosolic Ca2þ were represented in Fig. 4C, where it isshown that at the lower concentrations the BlV caused a slight increase in cytosolic Ca2þ
which is further increased with 2.5
mg/mL. Thus, the increment in the concentration
evokes higher Ca2þresponses which might be related tothe increase in toxicity.
3.5.
D
J
m measurementsAnalyses of the
D
J
m were performed in MDCK cells treated with the venom ofB. leucurusin 1.25mg/mL (IC
50). MDCK cells were loaded with TMRE (50 nM) as described above.D
J
m measurements were performed in real-time experiments before and after the addition ofBlV followed by FCCP (5mM) (
Fig. 5A), which was used to induce a rapidand complete depolarization of the
D
J
m which can be used as a calibration on the analysis of the location.Fig. 5B shows representative traces ofBlV induced mitochondria depolarization followed by the effect of FCCP. At the end of each experiment, fluorescence of each individual mito-chondrion was extracted and analyzed by using the region of interest (ROI) tool. The analyzes of the total mitochondria showed that the addition ofBlV (1.25mg/mL) resulted in
57.6% of the mitochondria analyzed that showed depolar-ization, a 20.8% of the mitochondria that hyperpolarized and 21.5% of the organelles that did not respond (Fig. 5C). The results represent the mean of at least three experi-ments total of 309 mitochondria analyzed.4. Discussion
The etiology of acute renal failure in humans and experimental animals induced byBothrops venom is still
Fig. 1.Effects ofBothrops leucurusvenom (10mg/mL) on perfusion pressure (A), renal vascular resistance (B), urinaryflow (C), glomerularfiltration rate (D), sodium (E) and chloride (F) tubular transport percent. Data are expressed as meanS.E.M fromfive different animals. *p<0.05 compared to the corresponding control group for each interval. (ANOVA and Bonferroni test). The venom was added to the system 30 min after the beginning of each perfusion.
Fig. 2.Cytotoxic effect ofBothrops leucurusvenom on MDCK cells. (A) Viability of MDCK cells incubated with different concentration ofB. leucurusvenom for 24 h. Data are expressed as meanS.E.M of three independent experiments with six replicates (ANOVA and Dunnett test, *p<0.05). (B) Cell death was measured by annexin V and PI staining and detected byflow cytometry. MDCK cells were treated with different concentrations ofB. leucurusvenom for 24 h. On theflow cytometric scatter graphs, the left lower quadrant represents the remaining live cells. The right lower quadrant represents the population of early apoptotic cells. The right upper quadrant represents the accumulation of late apoptotic cells. (C and D) Percentage of necrosis and apoptosis of MDCK cells usingfluorescence labeling with propidium iodide (PI) and Annexin V. Data are representative of three independent experiments and express as meanSD (ANOVA and Dunnett test, *p<0.05).
not well understood. Some researchers assure the impor-tance of the direct action of venom components on renal tubules (Castro et al., 2004) and renal epithelial cells (Nascimento et al., 2007), as well as an indirect action through the release of endogenous mediators such as cytokines, peptides (bradykinin and natriuretic peptides), nitric oxide and arachidonic acid metabolites (Havt et al., 2001;Barbosa et al., 2006).
The kidney is generally considered to be a major route of venom elimination from the body and the accumulation of venom in renal tissue may cause morphological damage and renal dysfunction that could interfere with venom elimination (Mello et al., 2010).
To investigate the effect of the venom in the kidney without interference of systemic factors, we used perfusion in the rat kidney. In the present study, we observed a decrease in perfusion pressure, renal vascular resistance, urinary flow and glomerular filtration rate as well as a decrease in sodium transport and chloride after the kidney was perfused by the venom. This agrees withfi nd-ings for other snake venoms of the same genus that is toxic to kidney. In the isolated kidney model, it has been demonstrated thatBothrops marajoensis(Evangelista et al.,
2010),Bothrops insularis(Braga et al., 2006),Bothrops jar-araca (Monteiro and Fonteles, 1999) and Bothrops jarar-acussu (Havt et al., 2001) venoms decrease perfusion pressure, renal vascular resistance, urinary flow, and glomerularfiltration rate.
We evaluated the cytotoxic potential of venom using culture of renal tubular cells (MDCK). MDCK cells constitute a very-well-established cell line with morphological and functional characteristics similar to the cells of the col-lecting duct and/or mammalian distal tubule and have been extensively employed in the investigation of a variety of cellular processes, including epithelial transport and cellular response to toxic agents (Collares-Buzato et al., 2002).
To determine whether the venom ofB. leucuruswas able to induce cell death, we first used the MTT assay. The results showed that the venom is highly toxic to cells, significantly reducing cell viability at all concentrations studied. Bothrops venom has enzymes that can directly cause cellular injury. Metalloprotease can cause proteolysis of the extracellular matrix and disrupts cell-matrix, phos-pholipase A2 can cause membrane injury and tubular necrosis (Sitprija, 2006). Several authors agree that the proteolytic action of Bothrops venom has an important cytotoxic effect in various cell types (Damico et al., 2007; Gutierrez and Lomonte, 1995) and it has been shown that the venom ofB. leucurushas a strong proteolytic activity (Prianti et al., 2003) and may cause deleterious effects on renal tubular epithelium. This could also cause the loss of the transport function of renal epithelial cells, thus reducing the transport of electrolytes such as sodium and chloride as observed in this study.
In MDCK cells, theflow cytometry analysis showed that the percentage of necrosis increased significantly in a dose-dependent manner, although apoptosis was observed in lower concentrations. A number of cytotoxic agents have been reported to induce apoptosis and necrosis depending on the intensity of their deleterious effects on cells. Therefore, the predominant type of cytotoxic response observed, i.e. apoptosis or necrosis, depends on the venom concentration and, consequently, on the extent of plasma membrane injury (Mora et al., 2006). Other studies using MDCK cells have shown thatBothropsvenoms are cytotoxic to renal cells by mechanisms that involve extensive disruption of the cytoskeleton and necrotic cell death (Collares-Buzato et al., 2002;Nascimento et al., 2007).
Necrotic and apoptotic cell death are characterized by biochemical and morphological differences. Necrosis is characterized by the irreversible swelling of the cytoplasm and its organelle, loss of membrane integrity results in cell lysis and the release of noxious cellular constituents. Apoptosis is characterized as a controlled type of cell death with different signaling pathways that can involve caspase activation (extrinsic pathways) or Bax overexpression and transactivation (intrinsic pathway). These biochemical routes can lead to the cell shrinkage, chromatin conden-sation, DNA fragmentation, formation of the apoptotic bodies which might be engulfed by neighboring cells or macrophages (Schrader et al., 2010;Orrenius et al., 2003).
There is a growing discussion on the definition of the different apoptotic signaling pathways. However, there are
Fig. 3.Gene expression evaluation of cell death. (A) Relative expression of caspase 3 gene; and (B) relative expression of caspase 8 gene in MDCK cells treated with different concentrations ofBothrops leucurusvenom for 24 h. doxorubicin (3.12mg/mL) was used as positive control. Data are represen-tative of three independent experiments and express the mean SD (ANOVA and Bonferroni test, *p<0.05).
at least two types of apoptosis that were more stablished in the past few years. The first one is mediated by death receptors, such as Fas or tumor necrosis factor (TNF) receptor, was defined as extrinsic pathway, which induces the cleavage of procaspase 8 to caspase 8 and then the subsequent activation of downstream caspases (caspases 3, 6 and 7). The second pathway, defined was intrinsic pathway, involves different stress signals that may involve proapoptotic protein Bax overexpression and trans-activation which inserts into mitochondria membranes and evokes the release of cytochrome c and latter the caspase 9 and caspase 3 activation (Chien et al., 2008).
To continue the characterization of the mechanism of cytotoxicity, experiments were performed to check the expression of marker genes associated with apoptotic pathways. The present study, in concentration which showed apoptosis, the expression of bax gene is not increased (data not shown), on the other hand the
expression ofcaspase 3andcaspase 8are increased, which indicate a possible involvement in extrinsic pathway in lower concentrations of the venom used.
The mitochondrial membrane potential
D
J
m has been identified as an important hallmark associated with apoptotic cell death and a decrease inD
J
m which might be associated with a loss of mitochondrial integrity and function (Chien et al., 2008;Smaili et al., 2003). Therefore, we decided to test the influence ofBlV in theD
J
m and the treatment of MDCK cells with 1.25mg/mL (CI50) of
BlV promoted the mitochondrial depolarization in majority of the mitochondria investigated. Since the change inD
J
m and apoptosis (Nascimento et al., 2007;Smaili et al., 2003) can be associated with increases in cytosolic Ca2þwe havealso looked to the Ca2þ changes induced by the venom.
Indeed, our results showed that there is an increase in cytosolic Ca2þ
in MDCK cells after addition BlV. Some authors have demonstrated the presence of myotoxins and
Fig. 4.Cytosolic calcium mobilization byBothrops leucurusvenom. The images show the MDCK cells loaded with FURA-2 before and after stimulation with 2.5mg/ mL ofB. leucurusvenom (A). (B) Representative traces of the cytosolic Ca2þ
increase induced by 2.5mg/mL of theB. leucurusvenom. (C) Histogram representing the maximum effects of increased cytosolic Ca2þ
in relation to the baseline (considered normalized to zero) in MDCK cells treated with different concentrations of B. leucurusvenom. All data are expressed as mean of normalizedfluorescence ratioSD from at least 3 different experiments (ANOVA and Tukey test, *p<0.05). Scale bars: 20mm.
cytotoxins within the venom of otherBothropsspecies. The toxic effects may be mediated by formation of nonspecific ionic pores at the plasma membrane leading to an influx of Ca2þ
, mainly due to venom-containing phospholipase A2 (Gutierrez and Lomonte, 1995). In fact, increases in Ca2þ
might be involved in apoptosis or necrosis depending on the cytosolic concentration and the ability of cells to handle the increases in Ca2þ
concentration either by transporting the ion to the extracellular medium or storing it at the intact organelles (Hirata et al., 2006, 2012). Therefore, gradual and constant elevations in concentration represent a stress to cell functions which may result in cell death.
In conclusion, the B. leucurus venom caused nephro-toxicity in kidney isolated and induced cell death on cultured MCDK cells. At lower concentrations the venom induced death by apoptosis, which involves increased expression of caspases. On the other hand at higher concentrations indicate necrosis. The ion Ca2þ
signaling and mitochondria are involved in cell damage, and it could
be implicated in both modalities of death. In fact apoptosis and necrosis may co-exist under the same stimulus and the interference with certain signaling pathway might result in cell killing by different mechanism. Thesefindings may be important aspects of the process of nephrotoxicity medi-ated byB. leucurusvenom.
Ethical statement
The experiments follows the methodology recom-mended by the international ethical standards of the scientific committee of our university.
Acknowledgments
The authors thank Dr Hanako Hirata for manuscript revision. This work was supported by grants from the Conselho Nacional de Desenvolvimento Científico e Tecnológico.
Fig. 5.Bothrops leucurusvenom induces a decrease inDJm. MDCK cells loaded with TMRE 50 nM for 15 min at 37C, before and after the addition ofB. leucurus venom (A). The images were acquired using a cooled CCD camera with a delay of 6 s between them (40objective).B. leucurusvenom caused a decrease inDJm indicated by the dissipation of TMRE dye from mitochondria. (B) Changes inDJm resulting from stimulation withB. leucurus(1.25mg/mL) followed by FCCP (5mM) measured byfluorescence microscopy. (C) Histogram shows the percentage of mitochondria that depolarized, hyperpolarized or presented no response afterB. leucurusexposure. Majority of mitochondria analyzed showed depolarization. Data are representative of three independent experiments and express the meanSD (ANOVA, Tukey test, *p<0.05). Scale bars: 10mm.
Conflict of interest
The authors declare that there are no conflicts of interest.
References
Acosta, A.R., Sanchez, E.E., Marquez, A., Carvajal, Z., Salazar, A.M., Girón, M.E., Estrella, A., Gil, A., Guerrero, B., 2010. Hemostatic prop-erties of Venezuelan Bothrops snake venoms with special reference to Bothrops isabelaevenom. Toxicon 56, 926–935.
Barbosa, P.S., Havt, A., Faco, P.E., Sousa, T.M., Bezerra, I.S., Fonteles, M.C., Toyama, M.H., Marangoni, S., Novello, J.C., Monteiro, H.S., 2006. Renal toxicity ofBothrops moojenisnake venom and its main myotoxins. Toxicon 40, 1427–1435.
Bowman, R.H., 1970. Gluconeogenesis in the isolated perfused rat kidney. J.Biol. Chem. 245, 1604–1612.
Braga, M.D.M., Martins, A.M.C., Alves, C.D., Meneses, D.B., Martins, R.D., Barbosa, P.S.F., Oliveira, I.M.S., Toyama, M.H., Toyama, D.O., Diz Filho, E.B.S., Fagundes, F.H.R., Fonteles, M.C., Monteiro, H.S.A., 2006. Purification and renal effects of phospholipase A2 isolated from Bothrops insularisvenom. Toxicon 51, 181–190.
Castro, I., Burdmann, E.A., Seguro, A.C., Yu, L., 2004.Bothropsvenom induces direct renal tubular injury: role for lipid peroxidation and prevention by antivenom. Toxicon 43, 833–839.
Chaves, F., Gutiérrez, J.M., Lomonte, B., Cerdas, L., 1989. Histopathological and biochemical alterations induced by intramuscular injection of Bothrops asper(terciopelo) venom in mouse. Toxicon 27, 1085–1093. Chien, C.M., Yang, S.H., Yang, C.C., Chang, L.S., Lin, S.R., 2008. Cardiotoxin III induces c-jun Nterminal kinase-dependent apoptosis in HL-60 human leukaemia cells. Cell Biochem. Funct. 26, 111–118.
Collares-Buzato, C.B., de Paula Le Sueur, L., da Cruz-Hofling, M.A., 2002. Impairment of the cell-to-matrix adhesion and cytotoxicity induced byBothrops moojenisnake venom in cultured renal tubular epithelia. Toxicol. Appl. Pharmacol. 181, 124–132.
Da Cruz-Höfling, M.A., Paronetto, C.C., Cogo, J.C., Rodrigues-Simioni, L., D’Abreu, A.C., 2001. Histopathological changes in avian kidney caused byBothrops insularis(jararaca ilhoa) venom and a phospholipase A2-containing fraction. Histol. Histopathol. 16, 185–195.
Damico, D.C.S., Nascimento, J.M., Lomonte, B., Ponce-Soto, L.A., Joazeiro, P.P., Novello, J.C., Marangonia, S., Collares-Buzato, C.B., 2007. Cytotoxicity ofLachesis muta mutasnake (bushmaster) venom and its purified basic phospholipase A2(LmTX-I) in cultured cells. Toxicon 49, 678–692.
Duque, J., Sánchez, A., Fierro, L., Garzón, S., Castaño, R., 2007. Venenos de serpientes y moléculas antiveneno. Rev. Acad. Colomb. Cienc. 31,109–137. Evangelista, I.L., Martins, A.M., Nascimento, N.R., Havt, A., Evangelista, J.S., de Norões, T.B., Toyama, M.H., Diz-Filho, E.B., Toyama, de O., Fonteles, M.C., Monteiro, H.S.A., 2010. Renal and cardiovascular effects ofBothrops marajoensisvenom and phospholipase A2. Toxicon 55, 1061–1070.
Fonteles, M.C., Cohen, J.J., Black, A.J., Wertheim, S.J., 1983. Support of renal kidney function by long-chain fatty acids derived from renal tissue. Am. J. Phys. 244, 235–246.
Gomes, M.S.R., Mendes, M.M., Oliveira, F., Andrade, R.M., Bernardes, C.P., Hamacuchi, A., Alcantara, T.M., Soares, A.M., Rodrigues, V.M., Homsi-Brandeburgo, M.I., 2009. BthMP: a new weakly hemorrhagic metal-loproteinase fromBothrops moojenisnake venom. Toxicon 53, 24–32. Gutierrez, J.M., Lomonte, B., 1995. Phospholipase A2 myotoxins from
Bothropssnake venoms. Toxicon 33, 1405–1424.
Havt, A., Fonteles, M.C., Monteiro, H.S.A., 2001. The renal effects of Bothrops jararacussuvenom and the role of PLA2and PAF blockers. Toxicon 39, 1841–1846.
Hirata, H., Machado, L.S., Okuno, C.S., Lopes, G.S., Smaili, S.S., 2006. Apoptotic effect of ethanol is potentiated by caffeine-induced calcium release in rat astrocytes. Neurosci. Lett. 393, 136–140.
Hirata, H., Lopes, G.S., Jurkiewicz, A., Garcez-do-Carmo, L., Smaili, S.S., 2012. Bcl-2 modulates endoplasmic reticulum and mitochondrial calcium stores in PC12 cells. Neurochem. Res. 37 (2), 238–243. Martinez-Maldonado, M., Opava-Stitzer, R., 1978. Free water clearance
curves during saline, mannitol, glucose and urea diuresis in the rat. J Physiol. 280, 487–497.
Mello, S.M., Linardi, A., Rennó, A.L., Tarsitano, C.A.B., Pereira, E.M., Hyslop, S., 2010. Renal kinetics ofBothrops alternatus(Urutu) snake venom in rats. Toxicon 55, 470–480.
Monteiro, H.S.A., Fonteles, M.C., 1999. The effect of Bothrops jararaca venom on rat kidney after short-term exposure: preliminary results. Pharmacol. Toxicol. 85, 198–200.
Mora, R., Maldonado, A., Valverde, B., Gutiérrez, J.M., 2006. Calcium plays a key role in the effects induced by a snake venom Lys49 phospho-lipase A2homologue on a lymphoblastoid cell line. Toxicon 47 (1), 75–86.
Mosmann, T., 1983. Rapid colorimetric assay for cellular growth and survival: application to proliferation and citotoxicity. J. Immunol. Methods 65, 55–63.
Nascimento, J.M., Franchi Jr., G.C., Nowill, A.E., Collares-Buzato, C.B., Hyslop, S., 2007. Cytoskeletal rearrangement and cell death induced byBothrops alternatussnake venom in cultured Madin–Darby canine kidney cells. Biochem. Cell Biol. 85, 591–605.
Oliveira, F.N., Brito, M.T., Morais, I.C.O., Fook, S.M.L., Albuquerque, H.N., 2010. Accidents caused byBothropsandBothropoidesin the State of Paraiba: epidemiological and clinical aspects. Rev. Soc. Bras. Med. Trop. 43 (6), 1–6.
Orrenius, S., Zhivotovsky, B., Nicotera, P., 2003. Regulation of cell death: the calcium-apoptosis link. Nat. Rev. Mol. Cell Biol. 4 (7), 552–565. Pfaffl, M.W., 2001. A new mathematical model for relative quantification
in real-time RT-PCR. Nucleic Acids Res. 29, 2002–2007.
Prianti Jr., A.C., Ribeiro, W., Lopes-Martins, R.A., Lira-Da-Silva, R.M., Prado-Franceschi, J., Rodrigues-Simioni, L., da Cruz-Höfling, M.A., Leite, G.B., Hyslop, S., Cogo, J.C., 2003. Effect ofBothrops leucurusvenom in chick biventer cervicis preparations. Toxicon 41 (5), 595–603.
Rezende, N.A., Amaral, C.F.S., Bambirra, E.A., Lachatt, J.J., Coimbra, T.M., 1989. Functional and histopathological renal changes induced in rats byBothrops jararacavenom. Braz. J. Med. Biol. Res. 22, 407–416. Sanchez, E.F., Gabriel, L.M., Gontijo, S., Gremski, L.H., Veiga, S.S.,
Evangelista, K.S., Eble, J.A., Richardson, M., 1992. Structural and functional characterization of a P-III metalloproteinase, leucurolysin-B, fromBothrops leucurusvenom. Arch. Biochem. Biophys. 468, 193–
204.
Schrader, K., Huai, J., Jöckel, L., Oberle, C., Borner, C., 2010. Non-caspase proteases: triggers or amplifiers of apoptosis? Cell Mol. Life Sci. 67 (10), 1607–1618.
Sitprija, V., 2006. Snakebite nephropathy. Nephrology 11, 442–448. Sitprija, V., Sitprija, S., 2012. Renal effects and injury induced by animal
toxins. Toxicon 60, 943–953.
Smaili, S.S., Russell, J.T., 1999. Permeability transition pore regulates both mitochondrial membrane potential and agonist-evoked Caþ2signals in oligodendrocyte progenitors. Cell Calcium 26, 121–130. Smaili, S.S., Carvalho, A.C.P., Rosenstock, T.R., Sharpe, J.C., Youle, R.J., 2003.
Mitochondria, calcium and pro-apoptotic proteins as mediators in cell death signaling. Braz. J. Med. Biol. Res. 36, 183–190.
Walser, M., Davidson, D.G., Orloff, J., 1955. The renal clearance of alkali stable inulin. J. Clin. Invest. 34, 1520–1523.