ww w . r e u m a t o l o g i a . c o m . b r
REVISTA
BRASILEIRA
DE
REUMATOLOGIA
Original
article
Obesity
is
the
main
determinant
of
insulin
resistance
more
than
the
circulating
pro-inflammatory
cytokines
levels
in
rheumatoid
arthritis
patients
Jesus
Castillo-Hernandez
a,∗,♦,
Martha
Imelda
Maldonado-Cervantes
a,♦,
Juan
Pablo
Reyes
a,
Nuria
Pati ˜no-Marin
b,
Enrique
Maldonado-Cervantes
a,
Claudia
Solorzano-Rodriguez
a,
Esperanza
de
la
Cruz
Mendoza
c,
Brenda
Alvarado-Sanchez
daLaboratoriodeBiomedicina,UnidadAcadémicaMultidisciplinariaZonaMedia,UniversidadAutónomadeSanLuisPotosí,SanLuís Potosí,México
bLaboratoriodeInvestigaciónClínica,FacultaddeEstomatología,UniversidadAutónomadeSanLuisPotosí,SanLuísPotosí,México cLaboratoriodeMedicinaNuclear,FacultaddeMedicina,UniversidadAutónomadeSanLuisPotosí,SanLuísPotosí,México
dLaboratoriodeBiomedicina,UnidadAcadémicaMultidisciplinariaZonaHuasteca,UniversidadAutónomadeSanLuisPotosí,SanLuís Potosí,México
a
r
t
i
c
l
e
i
n
f
o
Articlehistory:
Received2March2016
Accepted25October2016
Availableonline15February2017
Keywords:
Insulinresistance
Obesity
Rheumatoidarthritis
TNF-␣
a
b
s
t
r
a
c
t
Background:SystemicblockadeofTNF-␣inRheumatoid arthritiswithinsulinresistance
seemstoproducemoreimprovementininsulinsensitivityinnormalweightpatientswith
RheumatoidarthritisthaninobesepatientswithRheumatoid arthritis,suggestingthat
systemic-inflammationandobesityareindependentriskfactorsforinsulinresistancein
Rheumatoidarthritispatients.
Objectives:Toevaluate theinsulin resistance in:normal weight patientswith
Rheuma-toidarthritis,overweightpatientswithRheumatoidarthritis,obeseRheumatoidarthritis
patients,andmatchedcontrolsubjectswithnormalweightandobesity;anditsassociation
withmajorcytokinesinvolvedinthepathogenesisofthedisease.
Methods:Assessmentsincluded:bodymassindex,insulinresistancebyHomeostasisModel
Assessment,ELISAmethod,andenzymaticcolorimetricassay.
Results:Outstandingresultsfromthesestudiesinclude:(1)InRheumatoidarthritispatients,
insulinresistancewaswellcorrelatedwithbodymassindex,butnotwithlevelsofserum
cytokines.In fact,levelsofcytokines weresimilarin allRheumatoid arthritispatients,
regardlessofbeingobese,overweightornormalweight(2)Insulinresistancewas
signif-icantlyhigherinRheumatoidarthritiswithnormalweightthaninnormalweight(3)No
significantdifferencewasobservedbetweeninsulinresistancesofRheumatoidarthritis
withobesityandobesity(4)Asexpected,levelsofcirculatingcytokinesweresignificantly
higherinRheumatoidarthritispatientsthaninobesity.
∗ Correspondingauthor.
E-mail:jesus.castillo@uaslp.mx(J.Castillo-Hernandez).
♦ Thesetwoauthorscontributedequallytothiswork.
http://dx.doi.org/10.1016/j.rbre.2017.01.008
2255-5021/© 2017 Published by Elsevier Editora Ltda. This is an open access article under the CC BY-NC-ND license (http://
Conclusions: ObesityappearstobeadominantconditionaboveinflammationtoproduceIR
inRApatients.ThedissociationoftheinflammationandobesitycomponentstoproduceIR
suggeststheneedofanindependenttherapeuticstrategyinobesepatientswithRA.
©2017PublishedbyElsevierEditoraLtda.ThisisanopenaccessarticleundertheCC
BY-NC-NDlicense(http://creativecommons.org/licenses/by-nc-nd/4.0/).
A
obesidade
é
um
determinante
da
resistência
à
insulina
mais
importante
do
que
os
níveis
circulantes
de
citocinas
pró-inflamatórias
em
pacientes
com
artrite
reumatoide
Palavras-chave:
Resistênciaàinsulina
Obesidade
Artritereumatoide
TNF-␣
r
e
s
u
m
o
Introduc¸ão: Obloqueio sistêmicodoFatordeNecroseTumoral-␣(TNF-␣)nosindivíduos
comartritereumatoide(AR)comresistênciaàinsulina(RI)pareceproduzirmaismelhoria
nasensibilidadeàinsulinaempacientescomARcompesonormaldoqueempacientes
obesoscomAR.Issosugerequeainflamac¸ãosistêmicaeaobesidadesãofatoresderisco
independentesparaaRIempacientescomAR.
Objetivos: AvaliararesistênciaàinsulinaempacientescompesonormalcomAR(AR-PN),
pacientescomsobrepesocomAR(AR-SP),pacientescomARobesos(AR-OB)eindivíduos
controlecompesonormal(PN)eobesidade(OB)pareados;eaassociac¸ãocomasprincipais
citocinasenvolvidasnapatogênesedadoenc¸a.
Métodos: Asavaliac¸õesincluíram:índicedemassacorporal(IMC),resistênciaàinsulina
comomodelodeavaliac¸ãodahomeostase(Homa-IR),métodoElisaeensaiocolorimétrico
enzimático.
Resultados: Osresultadosmarcantesdopresenteestudoincluíram:(1)Empacientescom
AR,aRIestavabemcorrelacionadacomoÍndicedeMassaCorporal(quantomaioroIMC,
maioraRI),masnãocomosníveisséricosdecitocinas.Naverdade,osníveisdecitocinas
eramsemelhantesemtodosospacientescomAR,independentementedeseremobesos,
comsobrepesooupesonormal.(2)ARIfoisignificativamentemaiornogrupoAR-PNdoque
nogrupoPN.(3)Nãohouvediferenc¸aestatisticamentesignificativaentreaRIdepacientes
AR-OBeOB.(4)Comoesperado,osníveiscirculantesdecitocinasforamsignificativamente
maioresempacientescomARdoqueemOB.
Conclusões: Aobesidadepareceserumacondic¸ãomaisimportantedoqueainflamac¸ãoem
produzirRIempacientescomAR.Adissociac¸ãodoscomponentesdainflamac¸ãoeda
obesi-dadenaproduc¸ãodeRIsugereanecessidadedeumaestratégiaterapêuticaindependente
empacientesobesoscomAR.
©2017PublicadoporElsevierEditoraLtda.Este ´eumartigoOpenAccesssobuma
licenc¸aCCBY-NC-ND(http://creativecommons.org/licenses/by-nc-nd/4.0/).
Introduction
Rheumatoidarthritis(RA)isachronic,systemic,
inflamma-tory disorder of unknown etiology; manifested mostly by
jointinflammation.Thisinflammatoryprocessinvolves
mul-tiplefactors.LymphocytesproducecytokinessuchasTumor
Necrosis Factor-␣ (TNF-␣), IL-6 and IL-1, which results in
recruitment of several other immune cells to the affected
site.Whenuncontrolled,thismayresultinjointdestruction
anddeformity.1Therefore,theseincapacitatingconsequences
fully justify the therapeutic use of drugs such as
selec-tiveinhibitors ofTNF-␣, IL-6 and IL-1, aimed atreducing
structuraldamage.1–3 These cytokinesaremainlyproduced
bymacrophages, monocytes and T-cells, but they are also
produced by different kinds of non-immune cells,
includ-ing adipocytes, muscle cells and renal tubular cells.4–6 On
theotherhand,thesepro-inflammatorycytokinesareclosely
relatedwithinsulinresistance(IR),bydefinitionIRisknownas
adiminishedcellularresponsetoinsulin,moreover
compen-satoryhyperinsulinemiaisaclinicalfeaturewell-established
ofIR.ThegoldstandardforevaluatingIRisthe
euglycemic-hyperinsulinemicclamp,butthismethodisrisky,invasiveand
requiresmedicalintervention;howeverinmanypopulation
studieslikeours,IRisevaluatedwiththeHomeostasisModel
Assessment(HOMA-IR)whichestimatesthebasal
homeosta-sisthroughfastinglevelsofglucoseandinsulinandhasahigh
correlationwiththegoldstandard.7
Atthecellularlevel, insulinsignalingisimpairedinthe
main insulin-sensitiveorgans, particularly skeletal muscle,
liver,heartandadiposetissue8;thiscellularphenomenonis
stronglyrelatedtoagreatmyriadofmetabolicabnormalities
as obesity, diabetes mellitus, hyperlipidemia,
cardiovascu-lar disease, hypertension, cancer, syndrome of polycystic
ovarian and inflammatory disease. Increased serine
phos-phorylationoftheinsulin-receptorsubstrate(IRS-1and2)by
variousstimuliincludinginflammatorycytokinesisthe
cytokineshavebeenshowntodecreasethetyrosinekinase
activityoftheinsulinreceptor.9 Severalstudiesprovide
evi-denceforTNF-␣playingimportantrolesinvarious aspects
of the metabolic syndrome, including obesity-induced IR.
Furthermore,itisknownthatpatientswithIRhavehigh
cir-culatinglevelsofTNF-␣.10,11
Obesity,alow-gradechronicinflammatorycondition,has
beenconsideredanimportantriskfactortodevelopIR.
Adi-posetissue,particularlythevisceral,isnowrecognizedasthe
primarycontributor tothe IRsyndrome.12 TNF-␣ and IL-6,
whichareexpressedandsecretedbythehumanadipose
tis-sue,showincreasedplasmalevelsinhumanobesity,which
arereducedinobesesubjectsafterweightloss.Infact,plasma
levelsofthesecytokinesare correlatedwiththebodymass
index(BMI).TNF-␣hasalsobeenproposedasalinkbetween
obesityandIR.12,13Indeed,IRandobesityareprevalentinRA
patients.14
Recent studies suggest that systemic TNF-␣ blockade
(usinginfliximab,etanerceptandadalimumab)andIL-6
recep-torantagonist(usingtocilizumab)mayimproveIRinpatients
withRA.3,15 Nonetheless, it hasbeen reported that
benefi-cialeffectoninsulinsensitivityismainlyobservedinnormal
weightbut notinobeseRApatients,16 mostlikelybecause
anti-inflammatorytherapyactsuponthe“inflammatory
com-ponent”(pro-inflammatorycytokines)ratherthan uponthe
obesitycomponentsinthesepatients.
Hence,thepresentstudyaimsatevaluatingthe
relation-shipbetweenIRandplasmaticconcentrationsofTNF-␣,IL-6
andIL-1inRApatientswithandwithoutobesitytoevaluate
theobesityroleasariskfactorforIRinRApatients.
Methods
Studyparticipants
Thisstudywasconductedundertheguidelinesofthe
dec-larationofHelsinkioftheWorldMedicalAssociation,which
establishestheethicalprinciplesformedicalresearch
involv-ing humans. All participants gave informed consent. We
recruited59adultsubjectsamong19–70yearsold.
RApatientsinclude27femalefulfillingtheAmerican
Col-legeofRheumatology1987criteriaand withactivedisease
admittedtothehospitalGeneralandtheMexicanInstituteof
SocialSecurityofRioverdecity,stateofSanLuisPotosi;
Mex-ico.TotalRApatients(RAw/woOB)weredividedinto3groups:
7RApatients with normalweight (RA NW), 7RApatients
withoverweight (RAOW),and 13 RApatientswithobesity
(RAOB);RAgroupswerematchedbyage,genderandyears
afterdiagnosiseventhoughtheywereundertreatmentwith
methotrexate(MTX),glucocorticoids(GCs),andnon-steroidal
anti-inflammatorydrugs(NSAIDs). Inclusion criteriaforRA
patientswereconfirmeddiagnosisofactiveRA(assessedby
TheDiseaseActivityScore-28Joints,DAS28>3.2),never
hav-ingselectivebiologictreatmentanti-TNF-␣ oranti-IL-6,nor
anti-IL-1. Exclusion criteria were patients with a history
of allergies, infections, diabetes or any other systemic
ill-ness.RAw/wo OBand OBgroups were similarinage and
gender.
Sixteen normal weight subjects without RA (NW) were
includeasnegativecontrolforIR,and16non-diabeticobese
womenwithoutRA(OB)aspositivecontrolforIR.
Anthropometricsandclinicaldata
Bodyweightwasobtainedbyusingaweighingscalewith
par-ticipantswearinglightclothingandnofootwear.Heightwas
measuredbyusingastadiometer,withnofootwear.BMIwas
calculatedbydividingthesubject’sweightinkilogramsbythe
squaredheightinsquaredmeters.TheBMIclassifications
cor-respondtotheoneproposedbytheWorldHealthOrganization
(WHO)andwere asfollows:normalweightrange18.5–24.9,
overweight25.0–29.9andobese≥30.0(kg/m2).Waist
circum-ferencewasmeasuredusingananthropometrictapemeasure.
Themeasurementwastakenatthesmallestpointof
circum-ferencebetweentheiliaccrestandtheribcage.Also,arterial
bloodpressurewasobtainedbyindirectmethodusinga
man-ualcuffandsphygmomanometer.
Analysesofsamples
Venous blood samples were collected after an overnight
fast(8h)between7:00a.m.and9:00a.m.bytrained
person-nelusingidentical,standardizedprotocols,intonon-treated
vacutainers.Serumwasseparatedfromwholebloodby
cen-trifugation (10min at 3000rpm) and stored at −20◦C for
later analysis. The serum was used to determine the
fol-lowing parameters:glucose, insulin,triglycerides(TG), total
cholesterol (TC), HDL-c, TNF-␣, IL-6 and IL-1. Serum
glu-cosewas measuredbyglucoseoxidasemethod,astandard
enzymaticcolorimetric assay(SpinreactS.A.Spain).Serum
TC,high-densitylipoproteincholesterol(HDL-C)andTGwere
measured by enzymatic colorimetric assay using a
semi-automaticchemistryanalyser(Spinlab,Spain).Low-density
lipoprotein cholesterol(LDL-C) was calculatedaccording to
the Friedewald formula. Serum insulinwas determined by
chemiluminescent micro-particle immunoassay (IMMULITE
1000 system). The HOMA-IR was used to evaluate IR in
patientsandcontrols.[Fastinginsulin(IU/mL)×fasting
glu-cose(mg/dL)/405].ItestablishedthepresenceofIRtostudy
subjectswhenHOMA-IRwas>2.5.7SerumsTNF-␣,IL-6and
IL-1weremeasuredbyELISAmethod.TheELISAkitswere
purchasedfromInvitrogenCorporationandtheprotocolused
inthisstudywaspermanufacturer’sinstructions.Thecolor
producedbytheenzymaticreactionwasmeasuredat450nm
onanAwarenessStatFax303PlateReader.
Statistical
analysis
Alldatawereexpressedasmean±SEM.ShapiroWilktestwere
assessedfornormality. Thecomparisonofmeans between
groupswasmadeusing:(1)one-wayANOVAwithTukey
post-test for parametric data and the Kruskal–Wallis test with
Dunnspost-testwhendatawerenon-parametricforthe
anal-ysisofthethreemaingroupsNW,OBandRAw/woOB.(2)
unpairedttestforanalysisbetweenOBvs.RAOB;RANWvs.
Table1–ClinicalandmetabolicparametersofpatientswithRA.
NW OB RANW RAOW RAOB
(n=16) (n=16) (n=7) (n=7) (n=13)
Age(years) 29.0±2.35 40.94±3.51 51.33±5.34a 40.43±3.82 45.31±3.45
Gender(female%) 50 100 100 100 100
Diseaseduration(years) – – 7.0±1.7 5.57±1.7 7.55±1.64
Bodyweight(kg) 61.28±2.28 82.38±3.2 52.14±2.53 65.57±2.56 81.35±2.60b
BMI(kg/m2) 22.12±0.63 34.19±1.17 22.53±0.91 27.96±0.62 34.12±0.98b
WC(cm) 81.05±1.77 106.8±3.12 88.14±4.33 95.57±2.47 107.5±2.61b
SBP(mmHg) 99.23±2.39 121.3±4.16 118.6±4.0 116.7±4.2 123.6±3.63 DBP(mmHg) 64.62±1.43 79.25±2.13 75.71±3.68a 78.33±4.77 82.27±3.39
Insulin(IU/mL) 4.22±0.60 20.96±2.22 10.96±2.84a 11.96±2.09 23.27±4.35b
Glucose(mg/dL) 93.59±2.73 93.34±3.81 88.57±4.89 105.93±9.15 100.5±4.88 Triglycerides(mg/dL) 84.19±9.53 162.2±18.68 125.6±18.22a 168.7±31.62 123.9±16.41
TC(mg/dL) 147.0±7.26 166.2±5.19 166.6±9.55 153.3±9.53 160.4±11.81 HDL-C(mg/dL) 57.88±3.50 44.5±2.09a 42.14±2.53a 43.29±5.19 39.57±3.80
LDL-C(mg/dL) 72.29±7.47 89.25±5.67 99.31±8.04 76.26±6.07 89.20±11.74 LDL-C/HDL-C 1.36±0.16 2.10±0.19 2.44±0.29a 1.98±0.33 2.60±0.29
MTX(%) – – 42.8(3/7) 57.1(4/7) 38.4(5/13)
GCs(%) – – 57.1(4/7) 42.8(3/7) 53.8(7/13)
NSAIDs(%) – – 86.7(6/7) 71.4(5/7) 53.8(7/13)
Dataareexpressedasmean±SEMunlessotherwiseindicated,tocomparethedispersalmeansanunpairedttestwasusedforanalysisbetween OBvs.RAOB,RANWvs.RAOBandRANWvs.NW.Statisticalsignificancewassetatp<0.05.
a vs.NW.
b vs.RANW.
OnlycomparisonsbetweenNWvs.RANW,RANWvs.RAOBandOBvs.RAOBwereshown.
BMI,bodymassindex(calculatedasweightinkilogramsdividedbythesquareofheightinmeters),theBMIclassificationscorrespondtothe oneproposedbytheWorldHealthOrganization(WHO)andwereasfollows:normalweightrange18.5–24.9,overweight25.0–29.9andobese
≥30.0(kg/m2);NW,subjectsnormalweight;OB,subjectswithobesity;RA,patientswithRheumatoidarthritis;RAw/woOB,totalRApatients withorwithoutobesity;RANW,RApatientswithnormalweight;RAOW,RApatientswithover-weight;RAOB,RApatientswithobesity; WC,Waistcircumference;SBP,systolicbloodpressure;DBP,diastolicbloodpressure;TC,totalcholesterol;HDL-C,high-densitylipoprotein cholesterol;LDL-C,low-densitylipoproteincholesterol;LDL-c/HDL-c,cardiovascularriskindex;MTX,methotrexate;GCs,glucocorticoids; NSAIDs,non-steroidalanti-inflammatorydrugs.
measuredwithSpearman’srfornonparametricdata.
Statisti-calsignificancewassetatp<0.05.
Results
Demographicandclinicaldata
Atotalof59consecutiveparticipantswereenrolled.The
clin-ical and demographic characteristics were summarized in
Table1. There was nodifference in thefollowing
parame-ters:bodyweight,BMIandwaistcircumferenceamongnormal
weightgroups(NWvs.RANW),orbetweenobesegroups(OB
vs.RAOB).Asexpected,therewerestatisticalsignificant
dif-ferencesbetweenRANW,RAOWandRAOBgroupsinthese
parameters(p=0.0001).Insulinlevelsweresignificantlyhigher
inRANWcomparedagainstNW(p=0.003).Ontheotherhand,
the RANW had significantlylower insulinvalues than RA
OB(p=0.03);insulinlevelsbetweenOBandRAOBwerenot
significantlydifferent.TheserumTGlevelsweresignificantly
higherinRANWvs.NW(p=0.02),butnotamongRAsubgroups
orbetweenRAOBvs.OB.LDL-C/HDL-Cindexwashigherin
RANWvs. NW (p=0.001),but notamongRAsubgroupsor
betweenRAOBvs.OB.Ofthe27patientswithRA,44%were
undertreatmentwithMTX;alittlemorethan50%withGCs
andthemostfrequenttreatmentweretheNSAIDs(66%).
TheIRinRApatientsisprincipallyassociatedwithBMI
butnottoserumlevelsofTNF-˛
Using thehomeostaticmodel(HOMA-IR),theIRstatuswas
assessedin59subjectsthatintegratedthethreemainstudy
groups and aHOMA-IR value ≥2.5 was accepted to define
thepresenceofIR.7TheresultsshowthatRAw/woOBand
OBgroups havesimilarvaluesofHOMA-IR (4.32±0.72 and
4.86±0.56 respectively),about 4.5–5 timeshigher than NW
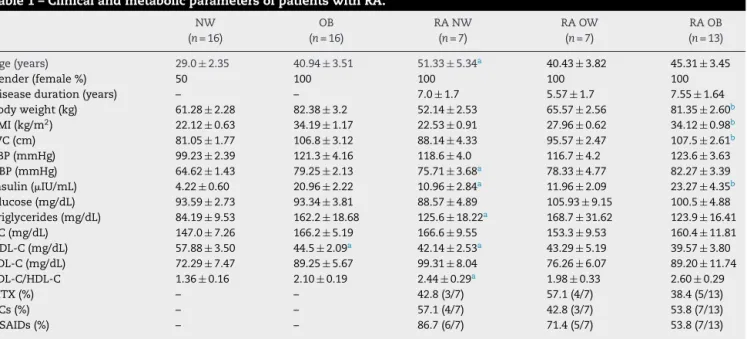
(0.97±0.13)(Fig.1A).
TheHOMA-IRvalueincreased,asBMIwashigher:RANW
(2.39±0.61),RAOW(3.15±0.64)andRAOB(6.0±1.31,p=0.01
vs.RANW)(Fig.1B).Interestingly,RANW showeda
signif-icantlyhigherHOMA-IRthan NW(2.39±0.61vs.0.97±0.13;
p=0.008) (Fig. 1B). As expected, the correlation analysis
showed a strong association between BMI and HOMA-IR
(Spearman r=0.6, p=0.0008) in RApatients (Fig. 1C).
Addi-tionally, serum TNF-␣ levels were significantly elevated in
RA w/wo OB (14.22±3.08pg/mL) compared to NW and OB
(4.66±0.74pg/mLand 6.12±1.01pg/mL;p=0.01and p=0.05
respectively), around 3 and 2 times higher respectively
(Fig. 2A). Furthermore,itwas foundthat serumTNF-␣
lev-els ofRAOBwere significantlyhigherthanOB(14.87±5.36
vs. 6.12±1.0pg/mL; p=0.02). Also, RA NW showed higher
serumTNF-␣levelsthanNW(11.7±3.22vs.4.66±0.74pg/mL;
NW
NW
RA NW RA OW RA OB
OB RA w/wo OB 0
2 4 6 8
∗∗∗
∗∗
Hom
a
IR
OB
0 2 4 6 8
∗
Φ
Homa IR
0 10 20 30 40 50
0 5 10 15
20 r=0.6056
p=0.0008
BMI
Homa-IR
B
A
C
Fig.1–BMIcorrelatedwithHOMA-IRinRApatients.Dataareexpressedasmean±SEM.(A)TheKruskalWallistestwith Dunnet’spost-testwasapplied.(B)Unpairedttestwasmade.(C)PositivecorrelationbetweenBMIandHOMA-IRinRA patientswasfound,Spearmancorrelationtestwasmadeforthisdataanalysis.Statisticalsignificancewassetatp<0.05. InsulinresistancewasdeterminedbyHOMAIR(homeostasismodelassessment),BMI,bodymassindex.TheBMI
classificationscorrespondtotheoneproposedbytheWorldHealthOrganization(WHO)andwereasfollows:normalweight range18.5–24.9,overweight25.0–29.9andobese≥30.0(kg/m2);NW,subjectsnormalweight(n=16);OB,subjectswith obesity(n=16);RA,patientswithRheumatoidarthritis;RAw/woOB,totalRApatientswithorwithoutobesity(n=27);RA NW,RApatientswithnormalweight(n=7);RAOW,RApatientswithoverweight(n=7);RAOB,RApatientswithobesity (n=13).
*vs.NW;vs.RANW.
NW OB
NW
RA w/wo OB 0
5 10 15 20
∗
ϕT
N
F-
α
(pg/m
l)
T
N
F-
α
(pg/m
l)
OB
R
A NW
RA OW RA OB 0
5 10 15 20 25
ϕ
∗
B
A
Fig.2–EnhancedserumamountsofTNF-␣aredissociatedofBMIinRApatients.Dataareexpressedasmean±SEM;to
comparethemeansdispersionwereused:(A)Kruskal–WallistestwithDunns’spost-testforno-parametricdata.(B) ComparisonsRANWvs.NWandRAOBvs.OBweremadebyunpairedttest.Also,statisticaldifferencesbetweenRA subgroupswerenotfound.Statisticalsignificancewassetatp<0.05.
TheBMIclassificationscorrespondtotheoneproposedbytheWorldHealthOrganization(WHO)andwereasfollows: normalweightrange18.5–24.9,overweight25.0–29.9andobese≥30.0(kg/m2);NW,subjectsnormalweight(n=16);OB, subjectswithobesity(n=16);RA,patientswithRheumatoidarthritis;RAw/woOB,totalRApatientswithorwithoutobesity (n=27);RANW,RApatientswithnormalweight(n=7);RAOW,RApatientswithoverweight(n=7);RAOB,RApatientswith obesity(n=13).
NW OB RA wo/w OB 0
5 10 15 20
∗∗∗
ϕIL
-6
(
pg/m
l)
NW OB
RA NW RA OW RA OB 0
10 20
30
∗
ϕ
∗
ϕIL-6 (pg/m
l)
B
A
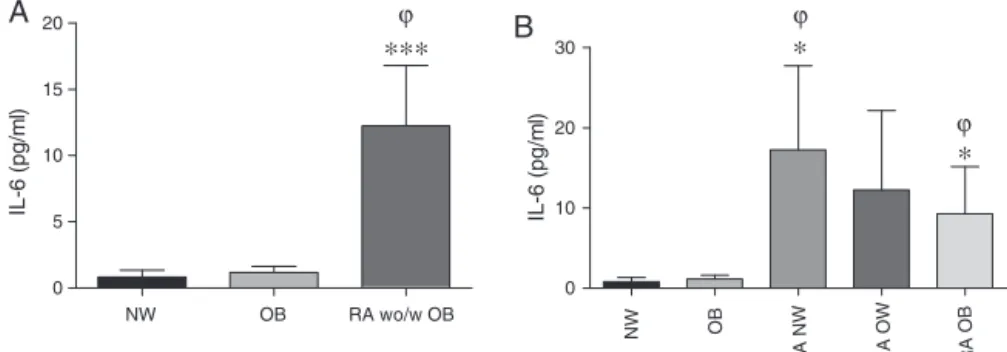
Fig.3–IncreasedserumlevelsofIL-6werenotassociatedtoBMIinRApatients.Dataareexpressedasmean±SEM.(A) IncreasedserumlevelsofIL-6inRApatientswerefound;Kruskal–WallistestwithDunns’spost-testwasapplied.(B) ComparisonsRANWvs.NWandRAOBvs.OBweremadebyunpairedttest.Also,statisticaldifferencesbetweenRA subgroupswerenotfound.Statisticalsignificancewassetatp<0.05.
TheBMIclassificationscorrespondtotheoneproposedbytheWorldHealthOrganization(WHO)andwereasfollows: normalweightrange18.5–24.9,overweight25.0–29.9andobese≥30.0(kg/m2);NW,subjectsnormalweight(n=16);OB, subjectswithobesity(n=16);RA,patientswithRheumatoidarthritis;RAw/woOB,totalRApatientswithorwithoutobesity (n=27);RANW,RApatientswithnormalweight(n=7);RAOW,RApatientswithover-weight(n=7);RAOB,RApatientswith obesity(n=13).
*vs.control;vs.OB.
correlateneitherwithBMInorwithHOMA-IRbySpearman’s
rtest.
SerumlevelsofIL-6areincreasedinRApatientsbutare
notassociatedwithHOMA-IR
TheevaluationofserumlevelsofIL-6clearlyshowsthatthey
weresignificantlyhigherinRAw/woOB(12.25±4.55pg/mL)
comparedwithNWandOB(0.81±0.52and1.15±0.48pg/mL;
p<0.001andp<0.05respectively),almost15–10timeshigher
thanNWandOBrespectively(Fig.3A).SerumIL-6levelsin
RANWweresignificantlyhigherthaninNW(17.27±10.47vs.
0.81±0.52pg/mL;p=0.01)andIL-6levelsinRAOBwere
sig-nificantlyhigherthaninOB(9.30±5.85vs.1.15±0.48pg/mL;
p=0.03)(Fig.3B).Interestingly,inRApatients,weobserveda
tendency–thoughnotsignificant-regardtothehigherBMI,
thelowerIL-6 levels.CorrelationanalysisbetweenIL-6and
BMI,wasnotsignificant(Spearmanr=−0.1540,p=0.45;data
notshown).Also,correlationanalysisbetweenIL-6levelsand
HOMA-IR was not significant (Spearman r=−0.21, p=0.28;
datanotshown).
EvaluationofplasmalevelsofIL-1ˇ
PlasmalevelsofIL-1werealsoevaluatedinRANW,RAOW
andRAOB(1.52±0.34,1.72±0.18,1.51±0.20pg/mL
respec-tively), these were similar to those found in OB and NW
(1.74±0.16,1.56±0.16pg/mLrespectively)(datanotshown).
AllsubgroupsofRAshowednodifferencesbetweenthemin
IL-1levels.NocorrelationwasfoundbetweenIL-1levelsand
HOMA-IRinRApatients(datanotshown).
Discussion
ThepurposeofthisstudywastoevaluateIRinRApatients
with and without obesity and its association with major
cytokines involvedin thepathogenesis ofthe disease.Our
studyindicatesthatobesityisthemaindeterminantofIRin
RA.Furthermore,thedegreeofIRobservedinobesesubjects
(RAOBorOB)isnotattainableevenwithhighserum
concen-trationsofthecytokinesinvolvedintheinflammatoryprocess
(RANW).Theseconclusionsarebasedonthefollowing
obser-vations:(1)RANW,RAOWandRAOBhavesimilarlevelsof
pro-inflammatorycytokinesandthesearesignificantlyhigher
thaninOB(Figs.2Band3B);(2)HOMA-IRbetweenOBandRA
OBwerenotdifferent(Fig.1B);(3)Despite similarhigh
lev-elsofpro-inflammatorycytokinesamongRAOBandRANW
patients,thislatterhadsignificantlylowerHOMA-IR(Fig.1B);
(4)HOMA-IRwassignificantlyhigherinRANWthaninNW
(Fig.1B);(5)HOMA-IRwascorrelatedwithBMIbutnotwith
pro-inflammatorycytokinelevelsinRA(Fig.1C).
Weselected27female patientswithactiveRAwithand
withoutobesityofwhichonly12patients(44%)weretreated
withMTX,indosesof7.5–10mgperweek.Nopatienthasused
biologicDMARDstherapyinourstudy(Table1).MTXisafolic
acidantagonistdrug,whosemaineffectisthoughttocome
fromtheinhibitionofenzymesinvolvedinpurinesynthesis
leading tothe accumulationofadenosine and thus
inhibi-tingtheTcell activation.17 MTXapparently doesnotaffect
the TNF-␣levelsinRAbut IL-6andIL-1levelsare indeed
affected.18,19 We observed that the main treatment in RA
patientsincludedinourstudywasdiclofenac,anon-selective
cyclooxygenase inhibitor (66%) followed by corticosteroids
(≈51%).Recentstudiesshowthattreatmentwithhighdoses
ofintraperitonealdiclofenac(500g/mL)doesnotaffectlevels
ofserumTNF-␣andIL-6inananimalmodeloffever.20
Addi-tionally,othershaveshownthatNSAIDshaveaslighteffect
decreasingIL-1levels,21whilecirculatingandlocallevelsof
IL-6remainunaffected.22Ontheotherhand,treatmentwith
lowdosesofGCs(<7.5mgofprednisone),asreceivedbysome
RApatientsinourstudy doesnotseemtoaffect thelevels
ThebasalTNF-␣amountsinourpatientsweresimilartothose
foundbyCharlesandco-workersinRApatients(14.21±3.08
vs. 15.5pg/mL)25 and by Penesová and co-workers in lean
patients treated with low-doses of prednisone or
equiva-lent(11.70±3.22vs.13.6±53.6pg/mL).23Hence,itseemsthat
treatmentsreceivedbyourRApatientsdidnothavea
signifi-cantinfluenceoncirculatinglevelsofTNF-␣andIL-6.
Obesity,IRand inflammationare closelyrelated. Infact
thepro-inflammatorycytokinesareoftenelevatedinsubjects
withIR,a“hallmark”ofthemetabolicsyndrome(MS),aswell
aschronicinflammation.2,11SomeauthorsagreethatTNF-␣,
IL-6andIL-1promoteIR;ithasevenbeenproposedthatTNF-␣
actsastheprincipallinkbetweenobesityandIR.12,13Atthis
point,itisworthmentioningthatwedidnotobservea
sig-nificantdifferenceinTNF-␣andIL-6levelsbetweenNWand
OBgroups(Figs.2and3,respectively),eventhoughthereis
atendency,asexpected,fortheselevelstobehigherinthe
OBgroup;thisisdiscussedbelow.Furthermore,observational
studieshaveshownthatRAisassociatedwithanincreased
prevalenceoftheMSandinflammatorymarkers.26
addition-ally,increasedHOMA-IRhasbeenfoundinRApatients.27
Althoughthe role ofpro-inflammatory cytokines inthe
pathogenesis of RA is clear, as evidenced by the
benefi-cialdamage-preventingeffectsofanti-rheumaticagents as
etanercept and infliximab (anti-TNF-␣ agents), tocilizumab
(IL-6Rblocker)andanakinra(recombinanthumanIL-1
recep-torantagonist),3,17,28,29theirmetabolicrolesarestillunclear.
Forexample:inhibitionofTNF-␣resultsinimprovedinsulin
sensitivityinRApatients,3,30–32thissuggeststhatTNF-␣plays
animportantroleinthedevelopmentofIRinRApatients,this
isconsistentwithasimilarroleofTNF-␣inIRdevelopmentin
thecasesofobesityandMS.10,11Incontrast,however,recent
studiescastdoubtontheimportanceofcirculatingTNF-␣inIR
development.Forexamplearecentstudyshowsthat1yearof
systemicblockadeofTNF-␣inagroupofsixteenRApatients
didnothaveasignificant impactonIRstatus.33 Thesame
wasobservedin56MSpatientstreatedwithetanerceptfor
4weeks.34Theresultsofthepresentworkmayhelpto
rec-oncilethesecontrastingreports,sincecomparisonofRANW
withNWsuggeststhatTNF-␣mayplayamodestrole–
com-paredtoobesity–inIRdevelopmentinRApatients,butother
obesity-linkedfactorsaremorecrucialinthedetermination
ofIR.Therefore,theseadditionalfactorsshouldbetakeninto
account,andmayexplainthediscrepanciesamongdifferent
reportsinregardtoIRinRApatients.
InthisstudyweevaluatedtheIRbyHOMA-IRinagroup
ofRApatientswithandwithoutobesity.Wefoundincreased
HOMA-IRvaluesin RAOB,similar tothe valuesofthe OB
group(Fig.1B).Nonetheless,thecomparisonofserum
TNF-␣andIL-6levelsbetweenthesetwogroupswassignificantly
higherintheRAOB(Figs.2Band3B).Thisfirstobservation
seemstodissociate therelationshipbetweeninflammation
andIR.Insupportofthislattercamethefollowing
observa-tion:thegroupofRANWshowedlowerHOMA-IRthantheRA
OB(Fig.1B),eventhoughbothgroupshadsimilarhighlevels
ofTNF-␣andIL-6(Figs.2Band3B).Thisstronglysuggeststhat
highcirculatinglevelsofpro-inflammatorycytokinesarenot
necessarilyassociatedwithahighdegreeofIRinRApatients.
Indeed we found no correlation between circulating levels
ofTNF-␣ and IL-6 vs. HOMA-IR (datanot shown), but BMI
doescorrelatewithHOMA-IRinRA(Fig.1C).Thisis
consis-tentwiththefindingsintherecentstudyofPenesováetal.
where theyanalyzed the relationship between the
inflam-matory component and glucose metabolism ina group of
RApatientsfreeofmetabolicriskfactorssuchasobesityor
endocrine disturbances,whichmay overlapwiththe effect
ofinflammationon insulinsensitivity;no relationshipwas
foundbetweenhighcirculatingpro-inflammatorycytokines
and metabolicparameters.23 Itislikelythatthe circulating
inflammatorycomponentisnotthemaindeterminantofIR
in RA.35 Indeed, althoughin our study we found that the
valueofHOMA-IRandserumlevelsofTNF-␣andIL-6were
significantly higherinRANW than NW (bothgroups were
freeoftraditionalmetabolicfactorrisks),HOMA-IRwas
sig-nificantlyhigherinRAOBcomparedwithRANWinspiteof
havingsimilarhighinflammatorycytokinelevels.This
indi-cates that IRdeterminationinRApatients could havetwo
independentcomponents: inflammationand obesity.
More-over,obesityplaysamainroleaboveinflammationtoproduce
IR.Insupportofthis,astudyshowedthatbeneficialeffectsof
anti-TNF-␣ therapyinregardtoinsulinsensitivity
improve-ment can be observed onlyin normal weight RA patients
withIRbutnotinobese RApatientswithIR,despite
treat-mentreducedtheinflammatoryactivityofthediseaseinthe
sameextentinallRApatients.16Again,thiscanbeexplained
because,thesystemicblockadeofTNF-␣inRApatientshas
agreaterimpactonIRassociatedtotheinflammatory
com-ponent(pro-inflammatorycytokines),butnotontheobesity
components.Manyothermoleculesnamedadipokines,such
as adiponectin, resistin,leptin, and retinolbindingprotein
4(RBP4),whichareproducedbyfattissue,arerelatedtoIR
inducedbyobesity.Theymediatetheregulationofmultiple
organsandtissuessuchastheskeletalmuscle,the
cardiovas-cularsystemandthepancreas.36,37Interestinglyandcontrary
tootherreports,wedidnotfindincreasedserumlevelsof
pro-inflammatorycytokines(TNF-␣andIL-6)(Fig.3A)intheOB
group,althoughstrongIRwasfoundintheOBgroupin
com-parison withthe NW.Itcannotberuledout thattheeffect
ofpro-inflammatorycytokineswouldbepredominantlylocal
ratherthansystemictomodulateotherindirecteffectsin
adi-posetissue.38,39Forexample,TNF-␣stimulatestheexpression
ofmediatorsinfatcells,suchasFFAs(freefattyacids)and
lep-tin,whichmightinduceIRinotherorgans.40Particularly,the
availabilityand utilizationofFFAsiswidelyacceptedasan
indirectmechanismthatcontributestothedevelopmentofIR
inskeletalmuscle.41
WithregardtoIL-6,apleiotropiccytokinewithawiderange
ofbiologicalactivitieswithapivotalroleinthe
physiopathol-ogyofRAwhichisfoundinabundanceinthesynovialfluid
andserumofpatientswithRA,42thisisconsistentwithour
findings, sinceall RA groupsshowed elevated levels of
IL-6incomparisonwithNW (Fig.3A).SerumlevelsofIL-6in
ourpatientsweresimilartothosefoundinotherstudies.18,23
However,unlikeotherreportsindicatingapositivecorrelation
betweenIL-6levelsandthedegreeofobesity,43wefoundthat
thelevelsofTNF-␣andIL-6intheOBgroupwerenot
signif-icantlyincreasedincomparisonwiththeNWgroup(Fig.3A),
eventhoughatendencyforthelevelstobemodestlyhigher
in OBis present.In fact, this lackofsignificance hasalso
cytokinesinblood,39anditisarguedthatitisattissuelevel
wherehigher levels are usually found in the obese, while
serumlevelsmayremaininsomecaseswithoutnoticeable
dif-ferencesbetweenNWandOB.Overthelastdecade,ithasbeen
reported, that IL-6 hasdual effects onmetabolic disorders
andthecontrolofbodyweight.44Weobservedaninteresting
tendency-thoughnotreachingsignificancelevels–inregard
tothehighertheBMIthelowertheserumlevelsofIL-6inRA
patients.EvidencebyTekayaetal.andvanderHelm-vanMil
etal.showedthatobesityandBMIhaveprotectiveeffectson
theamountofjointdestruction,diseaseprogression45,46and
diseaseseverity(ahighBMIisassociatedwithalesssevere
diseaseoutcomeinanti-CCP-positivepatientswithRA).This
protectiveeffectofobesitycouldbemediatedbyadecrease
inIL-6 in obese patients withRA.47 A study conducted in
2009byT. Ruge and co-workers foundthat IL-6 correlated
inverselywithBMIinpatientswithhyperglycaemia.48 This
couldexplainthetendencytoinversecorrelationbetweenBMI
andIL-6levelsthatweobservedinRApatients.Limitations
inourstudy regardingsamplesizepreventusfrom further
exploringIL-6levels and BMIinthese patients; weneed to
repeatthisapproachwithagreatersample.Ingeneral,more
studiesarenecessarytoclarifytheroleofIL-6inobesepatients
withRA.
Ontheotherhand,IL-1isapotentinflammatory
medi-ator in RA, reported as cytokine actively involved in the
progressionofthediseasebyactivationofosteoclastsinthe
joints.AlthoughIL-1playsakeyrole,theserumlevelIL-1
was almost undetectable in our study. It is well
acknowl-edgedthatthis cytokineispresent inthejoints inRA,but
it is difficult tomeasure in serum.25 Besides, as we
men-tionedabove,thetreatmentusedbyourpatientsislikelyto
decreaseIL-1levels.Therefore, thiscouldexplainthevery
lowlevelsofthiscytokineinourpatients(datanotshown).
PlasmalevelsofIL-1havebeenshowntodecreaseinpatients
treatedwithMTXandprednisolone.19,24 Nevertheless,there
isevidencethat therapywith GCssuchas dexamethasone
destabilizesthemRNAofIL-1␣andIL-1inadose
depend-entmannerinhumanmonocytesbytwomechanisms:(1)by
inhibitingtranscription ofIL-1 geneand (2)bydecreasing
the stability of mRNA IL-1,49 reducing the plasma IL-1
concentrationonanimalmodelsandproductioninprimary
culturesofhumanadipocytes.50SerumlevelsofIL-1donot
correlate withHOMA-IR (p=0.0853) inthis study (data not
shown).
Thesamplesizeisthemainlimitation ofourstudy.We
hadalimitednumberofindividualsthatfulfilledourinclusion
criteria.However,similarpublishedstudieshavealsoshown
thesamelimitations.16
Themainconclusionofthis studyisthat obesityisthe
maindeterminant ofIRin RApatients. Thisconclusion is
basedoncomparisonsbetweenthe OBgroup(positive
con-trolofIR)andthethreedifferentRAgroups(RANW,RAOW,
RAOB)whichwerematchedbyageandgender.
Asecondlimitationinourstudyisrelatedtothefactthat
theNW groupwasnotmatchedneitherbygendernorage
withtherestofthegroups.Thus,theobservationthat
HOMA-IRvaluesweresignificantlylargerinRANWcomparedtoNW
shouldbetakenwithcaution.Nevertheless,asanargument
supportingthatthislimitationmay notbedecisive,several
studiesindicatethatmetabolicalterationssuchasIRand
glu-coseintolerancearegenderindependent.51,52
In ourstudy,the RANW grouphasan averageage
sig-nificantly larger than NW group, and this may be related
toage-dependentvariationsinglucoseandinsulin.Inturn,
this age-relatedeffectmayaffectHOMA-IRdeterminations,
independentlyofinflammationandBMI.Eventhoughthere
is an age-dependent reduction in basal insulin release in
non-diabetic subjects, this fact is not reflected in fasting
insulinserumlevels.Theexplanationforthisobservationis
related tothe fact that insulinclearing isalso reduced(to
thesame extentinmenandwomen),sothiskeepsinsulin
serumlevelsnormal,despitetheage-dependentreductionin
insulinrelease.52Hence,eventhoughwecannotruleout
age-dependent alterationsinbasal insulinrelease inourstudy
groups,wehaveobservedthattheRANWgrouphasfasting
seruminsulinlevelsevenhigherthantheNWgroup.These
higherlevelsintheRANWgroupcouldbeexplainedby:(1)
areductionininsulinclearinginfastingconditionswhichis
notaccompaniedbyage-relatedreductionininsulinrelease.
However,thisisunlikely,becausereductionsinclearingand
inreleasegenerallyoccuratthesametime.52(2)IRthatisnot
linkedtoobesitybuttoinflammation.Thisisinagreement
withtheconclusionsofourstudy;thosegroupswiththe
high-estHOMA-IRvaluesalsohavehigherinsulinlevels,keeping
glucoselevelsveryclosetothenormalvalues(Table1).Inthis
way,significantlyhigherHOMA-IRvaluesaredeterminedby
obesitythanbyinflammation.
Inconclusion,theseresultsindicatethatobesityisamajor
determinantofIRinRApatients,moredeterminantthanthe
circulatinginflammatorycomponents,consideredintermsof
TNF␣,IL-6andIL-1levels.Additionally,thehigherIRinRA
NWincomparisontonormalweightsubjects(NW)appears
tobesolelyexplainedbytheimpactofinflammatory
compo-nents.Sincethemainconclusionofourstudyisthatobesity
plays a dominant role over the inflammation in IR in RA
patients,cliniciansshouldmuchemphasizetheimportanceof
weightcontrolintheirpatients,inordertoavoidundesirable
andpotentiallyseverecomplicationsderivedfromunhealthy
weightgain.
Funding
ThisworkwassupportedbyGrantPROMEP/103.5/11/8623and
C12-FAI-03-90.90.
Conflicts
of
interest
Theauthorsdeclarenoconflictsofinterest.
Acknowledgments
The authors are gratefulfor excellent technical assistance
byundergraduatestudentsMelissaBadilloReyes,Alejandro
Martinez-Mendez,DomitilaMendez,SayraOlveraandSandra
Don-Gonzalez. As well asFamily Medicine Unit 9of IMSS,
of Rioverde, Mexico; by valuable medical assistance with
Rheumatoidarthritispatients.
r
e
f
e
r
e
n
c
e
s
1. WolfeF.Thenaturalhistoryofrheumatoidarthritis.J RheumatolSuppl.1996;44:13–22.
2. BradleyJR.TNF-mediatedinflammatorydisease.JPathol. 2008;214:149–60.
3. SerioloB,FerroneC,CutoloM.Longtermanti-tumornecrosis factor-alphatreatmentinpatientswithrefractory
rheumatoidarthritis.JRheumatol.2008;35:355–7.
4. GimenoRE,KlamanLD.Adiposetissueasanactiveendocrine organ:recentadvances.CurrOpinPharmacol.2005;5:122–8.
5. SaghizadehM,OngJM,GarveyWT,HenryPR,KernPA.The expressionofTNF-␣byhumanmuscle.JClinInvest. 1996;97:1111–6.
6. Maldonado-CervantesMI,GaliciaOG,Moreno-JaimeB, Zapata-MoralesJR,Montoya-ContrerasA,Bautista-PerezR, etal.AutocrinemodulationofglucosetransporterSGLT2by IL-6andTNF-␣inLLC-PK1cells.JPhysiolBiochem. 2012;68:411–20.
7. MatthewsDR,HoskerJP,RudenskiAS,NaylorBA,TreacherDF, TurnerRC.Homeostasismodelassessment:insulinresistance andbeta-cellfunctionfromfastingplasmaglucoseand insulinconcentrationsinman.Diabetologia.1985;28:412–9.
8. Olivares-ReyesJA,Arellano-PlancarteA,Castillo-Hernandez JR.AngiotensinIIandthedevelopmentofinsulinresistance: implicationsfordiabetes.MolCellEndocrinol.
2009;302:128–39.
9. MollerDE.PotentialroleofTNF-alphainthepathogenesisof insulinresistanceandtype2diabetes.TrendsEndocrinol Metab.2000;11:212–7.
10.MiyazakiY,PipekR,MandarinoLJ,DeFronzoRA.Tumor necrosisfactor␣andinsulinresistanceinobesetype2 diabeticpatients.IntJObesRelatMetabDisord.2003;27:88–94.
11.OlsonNC,CallasPW,HanleyAJG,FestaA,HaffnerSM, WagenknechtLE,etal.CirculatinglevelsofTNF-␣are associatedwithimpairedglucosetolerance,increasedinsulin resistance,andethnicity:theInsulinResistance
AtherosclerosisStudy.JClinEndocrinolMetab. 2012;97:1032–40.
12.DandonaP,AljadaA,BandyopadhyayA.Inflammation:the linkbetweeninsulinresistance,obesity,anddiabetes.Trends Immunol.2004;25:4–7.
13.Nieto-VazquezI,Fernandez-VeledoS,KramerDK,
Vila-BedmarR,Garcia-GuerraL,LorenzoM.Insulinresistance associatedtoobesity:thelinkTNF-alpha.ArchPhysiol Biochem.2008;114:183–94.
14.Stavropoulos-KalinoglouA,MetsiosGS,KoutedakisY,Kitas GD.Obesityinrheumatoidarthritis.Rheumatology. 2011;50:450–62.
15.SmolenJS,Martinez-AvilaJC,AletahaD.Tocilizumabinhibits progressionofjointdamageinrheumatoidarthritis
irrespectiveofitsanti-inflammatoryeffects:disassociationof thelinkbetweeninflammationanddestruction.AnnRheum Dis.2012;71:687–93.
16.Stavropoulos-KalinoglouA,MetsiosGS,PanoulasVF, NightingaleP,KoutedakisY,KitasGD.Anti-tumournecrosis factoralphatherapyimprovesinsulinsensitivityin normal-weightbutnotinobesepatientswithrheumatoid arthritis.ArthritisResTher.2012;14:R160.
17.KumarP,BanikS.Pharmacotherapyoptionsinrheumatoid arthritis.ClinMedInsightsArthritisMusculoskeletDisord. 2013;6:35–43.
18.NishinaN,KanekoY,KamedaH,KuwanaM,TakeuchiT. ReductionofplasmaIL-6butnotTNF-␣bymethotrexatein patientswithearlyrheumatoidarthritis:apotential biomarkerforradiographicprogression.ClinRheumatol. 2013;32:1661–6.
19.BarreraP,HaagsmaCJ,BoerboomsAM,Van-RielPLCM,Borm GF,VandePutteLBA,etal.Effectofmethotrexatealoneorin combinationwithsulphasalazineontheproductionand circulatingconcentrationsofcytokinesandtheirantagonists. Longitudinalevaluationinpatientswithrheumatoid arthritis.BrJRheumatol.1995;34:747–55.
20.GreisA,MurgottJ,RafalzikS,GerstbergerR,HübschleT,Roth J.Characterizationofthefebrileresponseinducedby fibroblast-stimulatinglipopeptide-1inguineapigs.AmJ PhysiolRegulIntegrCompPhysiol.2007;293:R152–61.
21.PelletierJP,CloutierJM,Martel-PelletierJ.Invitroeffectsof NSAIDsandcorticosteroidsonthesynthesisandsecretionof interleukin1byhumanosteoarthriticsynovialmembranes. AgentsActions.1993;39:181–93.
22.RothJ,HübschleT,PehlU,RossG,GerstbergerR.Influenceof systemictreatmentwithcyclooxygenaseinhibitorson lipopolysaccharide-inducedfeverandcirculatinglevelsof cytokinesandcortisolinguinea-pigs.PflugersArch. 2002;443:411–7.
23.PenesováA,RádikováZ,VlˇcekM,KerlikJ,LukáˇcJ,Rovensk ´yJ, etal.Chronicinflammationandlow-doseglucocorticoid effectsonglucosemetabolisminpremenopausalfemales withrheumatoidarthritisfreeofconventionalmetabolicrisk factors.PhysiolRes.2013;62:75–83.
24.UeharaA,KohdaH,SekiyaC,TakasugiY,NamikiM. Inhibitionofinterleukin-1betareleasefromculturedhuman peripheralbloodmononuclearcellsbyprednisolone. Experientia.1989;45:166–7.
25.CharlesP,ElliottMJ,DavisD,PotterA,KaldenJR,AntoniC, etal.Regulationofcytokines,cytokineinhibitors,and acute-phaseproteinsfollowinganti-TNF-alphatherapyin rheumatoidarthritis.JImmunol.1999;163:1521–8.
26.RostomS,MengatM,LahlouR,HariA,BahiriR, Hajjaj-HassouniN.Metabolicsyndromeinrheumatoid arthritis:casecontrolstudy.BMCMusculoskeletDisord. 2013;14:147.
27.ChungCP,OeserA,SolusJF,GebretsadikT,ShintaniA,Avalos I,etal.Inflammationassociatedinsulinresistance:
differentialeffectsinrheumatoidarthritisandsystemic lupuserythematosusdefinepotentialmechanisms.Arthritis Rheum.2008;58:2105–12.
28.ScherJU.Monotherapyinrheumatoidarthritis.BullHospJt Dis.2013;71:204–7.
29.CohenS,HurdE,CushJ,SchiffM,WeinblattME,MorelandLW, etal.TreatmentofRheumatoidArthritiswithAnakinra,a recombinanthumaninterleukin-1receptorantagonist,in combinationwithmethotrexateresultsofa
twenty-four-week,multicenter,randomized,double-blind, placebo-controlledtrial.ArthritisRheum.2002;46:614–24.
30.SerioloB,PaolinoS,FerroneC,CutoloM.Effectsofetanercept orinfliximabtreatmentonlipidprofileandinsulinresistance inpatientswithrefractoryrheumatoidarthritis.Clin Rheumatol.2007;26:1799–800.
31.Lai-ShanT,TomlinsonB,ChuTT,LiTK,LiEK.ImpactofTNF inhibitiononinsulinresistanceandlipidslevelsinpatients withrheumatoidarthritis.ClinRheumatol.2007;26:1495–8.
32.StagakisI,BertsiasG,KarvounarisS,KavousanakiM,VirlaD, RaptopoulouA,etal.Anti-tumornecrosisfactortherapy improvesinsulinresistance,betacellfunctionandinsulin signalinginactiverheumatoidarthritispatientswithhigh insulinresistance.ArthritisResTher.2013;14:1–11.
ofTNF-␣doesnotimproveinsulinresistanceinhumans. HormMetabRes.2011;43:801–8.
34.BernsteinLE,BerryJ,KimS,CanavanB,GrinspoonSK.Effects ofetanerceptinpatientswiththemetabolicsyndrome.Arch InternMed.2006;166:902–8.
35.AltomonteJ,HarbaranS,RichterA,DongH.Fatdepotspecific expressionofadiponectinisimpairedinZuckerfattyrats. Metabolism.2003;52:958–63.
36.RomachoT,ElsenM,RöhrbornD,EckelJ.Adiposetissueand itsroleinorgancrosstalk.ActaPhysiol.2014;210:733–53.
37.FangP,ShiM,YuM,GuoL,BoP,ZhangZ.Endogenous peptidesasriskmarkerstoassessthedevelopmentofinsulin resistance.Peptides.2014;51:9–14.
38.ArnerP.Theadipocyteininsulinresistance:keymolecules andtheimpactofthethiazolidinediones.TrendsEndocrinol Metab.2003;14:137–45.
39.HotamisligilGS,ArnerP,CaroJF,AtkinsonRL,Spiegelman BM.Increasedadiposetissueexpressionoftumournecrosis factor-alphainhumanobesityandinsulinresistance.JClin Invest.1995;95:2409–15.
40.SethiJK,HotamisligilGS.TheroleofTNF-␣inadipocyte metabolism.SeminCellDevBiol.1999;10:19–29.
41.KraegenEW,CooneyGJ.Freefattyacidsandskeletalmuscle insulinresistance.CurrOpinLipidol.2008;19:235–41.
42.SrinivasanS,ChoyEH.TheroleofInterleukin6inthe pathophysiologyofrheumatoidarthritis.TherAdv MusculoskeletDis.2010;2:247–56.
43.KhaodhiarL,LingPR,BlackburnGL,BistrianBR.Serumlevels ofinterleukin-6andC-reactiveproteincorrelatewithbody massindexacrossthebroadrangeofobesity.JPEN. 2004;28:410–5.
44.WalleniusK,WalleniusV,SunterD,DicksonSL,JanssonJO. Intracerebroventricularinterleukin-6treatmentdecreases bodyfatinrats.BiochemBiophysResCommun.
2002;293:560–5.
45.TekayaR,SahliH,ZribiS,MahmoudI,BenHadjYahiaC, AbdelmoulaL,etal.Obesityhasaprotectiveeffecton radiographicjointdamageinrheumatoidarthritis.Tunis Med.2011;89:462–5.
46.vanderHelm-vanMilAH,vanderKooijSM,AllaartCF,Toes RE,HuizingaTW.Ahighbodymassindexhasaprotective effectontheamountofjointdestructioninsmalljointsin earlyrheumatoidarthritis.AnnRheumDis.2008;67: 769–74.
47.KaufmannJ,KielsteinV,KilianS,SteinG,HeinG.Relation betweenbodymassindexandradiologicalprogressionin patientswithrheumatoidarthritis.JRheumatol. 2003;30:2350–5.
48.RugeT,LocktonJA,RenstromF,LystigT,SukoninaV, SvenssonMK,etal.Acutehyperinsulinemiaraisesplasma interleukin-6inbothnondiabeticandtype2diabetes mellitussubjects,andthiseffectisinverselyassociatedwith bodymassindex.Metabolism.2009;58:860–6.
49.LeeSW,TsouAP,ChanH,ThomasJ,PetrieK,EuguiEM,etal. Glucocorticoidsselectivelyinhibitthetranscriptionofthe interleukin1geneanddecreasethestabilityofinterleukin 1mRNA.Immunology.1988;85:1204–8.
50.ZhangHH,KumarS,BarnettAH,EggoMC.Dexamethasone inhibitstumornecrosisfactor-␣inducedapoptosisand interleukin-1releaseinhumansubcutaneousadipocytes andpreadipocytes.JClinEndocrinolMetab.2001;86: 2817–25.
51.GarmendiaML,LeraL,SánchezH,UauyR,AlbalaC. Homeostasismodelassessment(HOMA)valuesinChilean elderlysubjects.RevMédChile.2009;137:1409–16.