5-Fluorouracil induces inflammation and oxidative stress in the major
salivary glands affecting salivary flow and saliva composition
Luana E. Bomfin
a, Cíntia M. Braga
b, Thais A. Oliveira
b, Conceição S. Martins
b, Danielle A. Foschetti
b,
Ana A.Q.A. Santos
a, Deiziane V.S. Costa
b, Renata F.C. Leitão
b, Gerly A.C. Brito
a,b,c,⇑aDepartment of Medical Sciences, School of Medicine, Federal University of Ceará, Fortaleza, Ceará, Brazil
bPostgraduate Program in Morphofunctional Sciences, Department of Morphology, School of Medicine, Federal University of Ceará, Fortaleza, Ceará, Brazil cDepartment of Physiology and Pharmacology, School of Medicine, Federal University of Ceará, Fortaleza, Ceará, Brazil
a r t i c l e
i n f o
Article history:
Received 5 May 2017 Accepted 30 August 2017 Available online 1 September 2017
Keywords:
Xerostomia 5-Fluorouracil Major salivary glands Oxidative stress Inflammation
a b s t r a c t
This study aimed to elucidate the effect of 5-fluorouracil (5-FU) on the histological aspects of the major salivary glands, salivary flow and saliva composition using an established oral mucositis model in ham-sters. Oral mucositis was induced by two intraperitoneal administrations of 5-FU in two consecutive days (60 and 40 mg/kg), followed by cheek pouch mucosa scratch, on day 4. The Pilocarpine-stimulated sali-vary flow was measured 4 and 10 days after the first 5-FU injection. Salisali-vary glands were harvested for histopathological analysis, measurement of inflammatory cells, quantification of pro-inflammatory cytokines (TNF-aand IL-1b), investigation of cell death and cell proliferation. Oxidative stress and oxida-tive defense system were also investigated in the salivary gland tissues using MDA (malondialdehyde), nitrite, non-protein sulfhydryl groups (NP-SH), SOD (superoxide dismutase) and CAT (catalase). In addi-tion, the CAT and lysozyme activities and the IgA and SOD levels were evaluated in the saliva samples. 5-FU significantly reduced the pilocarpine-stimulated salivary flow rate on the 4th experimental day, associated with an increase in the SOD levels in saliva. Recovery of the salivary flow and SOD were observed on day 10, when an increase in the saliva lysozyme levels was detected. In addition, 5-FU pro-moted vacuolization in parotid (P) and periductal edema in submandibular (SM) gland, combined with an increase in the inflammatory cells influx, mostly observed on the 4th day in SM gland and on 4th and 10th days in P. Oxidative stress was found mostly on day 10 in SM, SL and P glands, associated with release of proinflammatory cytokines, observed in SM and SL glands, but not in P. 5-FU induces an inflam-matory response in the major salivary glands, most observed ten days after its first injection, which may contribute to the major salivary glands hypofunction, leading to alterations in the salivary flow rate and composition.
Ó2017 Elsevier Inc. All rights reserved.
1. Introduction
Oral mucositis is one of the most common adverse reactions of radiation therapy for head and neck cancers and chemotherapy, particularly associated with drugs affecting DNA synthesis. Patient-associated endogenous variables, treatment protocol, con-comitant chemo and radiotherapy can affect the course and the severity of this complication, leading to instability of oral health and quality of life[1–6]. Cancer therapies, especially head and neck radiation, may also induce xerostomia (dry mouth), other impor-tant oral symptoms, worsening of pain [2,7] and significantly
increasing the risk of oropharyngeal candidiasis [8] and dental decays[9]. It’s well established that xerostomia is induced mainly by the effect of localized radiation over the major salivary glands (submandibular, sublingual and parotid)[10,11], but recent reports show that chemotherapy, even when is not associated with radio-therapy, may also decrease the salivary flow and alters the saliva composition [2,4,12]. The mechanisms involved in this effect, however, were not clarified and merit further investigation.
5-Fluorouracil (5-FU) is a fluoropyrimidine commonly used in the treatment of a variety of cancers, including those of the ovary, breast, gastrointestinal tract and head and neck[13–17]. 5-FU acts as a thymidylate synthase (TYMS) inhibitor, which results in a thymidine monophosphate (dTMP) deficiency, one of the three nucleotides that form thymine[18]. This process also implicates in oxidative stress generation (OS) [19] and leads to impaired
http://dx.doi.org/10.1016/j.bcp.2017.08.024
0006-2952/Ó2017 Elsevier Inc. All rights reserved.
⇑Corresponding author at: Department of Morphology, Faculty of Medicine, Federal University of Ceará, Rua Delmiro Farias, Fortaleza 60430-170, CE, Brazil.
E-mail address:gerlybrito@hotmail.com(G.A.C. Brito).
Contents lists available atScienceDirect
Biochemical Pharmacology
DNA replication, genome damage and programmed cell death
[18,20,21]. Previous reports show that 5-FU can modify salivary
pH, ionic components, salivary enzymes levels and reduces salivary flow rate resulting in xerostomia in human[22–24]. It was also demonstrated that 5-FU induces oxidative stress in submandibular and sublingual glands by unknown mechanisms which were pre-vented by laser phototherapy[12].
The present study investigates the effects of 5-FU on the sali-vary flow rate and composition, associated with histological alter-ations of the major salivary glands, considering some aspects as immune-inflammatory cell infiltration, cell death, cell proliferation and oxidative stress, in hamsters.
2. Materials and methods
2.1. Animals
Golden hamsters, weighing 140–200 g, were housed in temperature-controlled rooms under 12-h light–dark cycles, and received water and foodad libitum. Surgical procedures and animal treatments were conducted in accordance with the Guidelines for Institutional and Animal Care and Use of Federal University of Ceará, Brazil. All procedures involving animals were approved by the Federal University of Ceará Committee for the ethical treat-ment of research animals (Protocol N°.43/2012).
2.2. 5-FU-induced oral mucositis experimental model
Oral mucositis was induced by two intraperitoneal (i.p.) admin-istration of 5-FU (Roche, Rio de Janeiro, Brazil) on the first and sec-ond days (60 and 40 mg/kg, respectively), based on an established oral mucositis model[25]. On day 4, under anesthesia with 2% xylazine (König laboratories S.A., 10 mg/kg; i.p.) and 10% ketamine (Syntec do Brasil Ltda., 200 mg/kg; i.p.), the right cheek pouch mucosa was irritated by superficial scratching to potentiate the oral mucositis. The scratching comprised dragging the tip of an 18-gauge needle, twice in a linear manner, across the everted cheek pouch. The animals were euthanized with an overdose of 2% xylazine and 10% ketamine (50/1000 mg/kg; i.p.) four or ten days after the first injection of 5-FU. Samples of the salivary glands were surgically removed and fixed in 10% neutral buffered forma-lin or stored at -80°C for specific assays.
2.3. Experimental groups
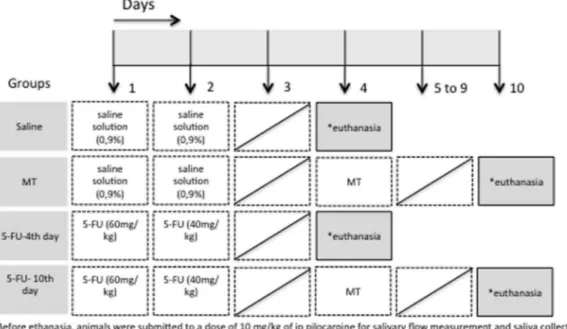
Hamsters were randomly divided into four groups: (1) saline group received intraperitoneal 0.9% saline solution on the 1st and 2nd experimental days to mimic 5-FU injection and was eutha-nized on day 4; (2) mechanical trauma (MT) group received intraperitoneal saline administration, was submitted to MT in the right cheek mucosa on the 4th day and was euthanized on day 10; (3) 5-FU-4th day group received 5-FU injections on the 1st and 2nd days (60 and 40 mg/kg i.p.) and was euthanized on day 4 (4) 5-FU-10th day group received 5-FU injections on the 1st and 2nd days (60 and 40 mg/kg i.p.), was submitted to MT in the right cheek mucosa on the 4th day and was euthanized on day 10. There were at least 6 animals assigned to each group (Fig. 1).
In another set of experiment, to assess the role of the oxidative stress on 5-FU-induced xerostomia, we evaluated the antioxidative effect of N-acetyl-cysteine (NAC; Sigma-Aldrich, St. Louis, USA), on the salivary flow rate and oxidative stress-related parameters. Hamsters were randomly divided into four groups, as described in the Fig. 8a. The treatment protocol was based on a previous report[26]. The dose of 20 mg/kg of NAC was chosen after a previ-ous pilot study performed in the current work, to evaluate different
doses (20, 200 and 1000 mg/kg) of NAC (data not shown). The mor-tality rate was higher in NAC 200 and NAC 1000 mg/kg groups.
2.3.1. Pilocarpine-stimulated salivary flow
On the day of euthanasia, on the 4th or 10th experimental day, under anesthesia (2% xylazine and 10% ketamine; 10/200 mg/kg; i.p.), the salivation was stimulated by a single intraperitoneal injec-tion of pilocarpine (Sigma-Aldrich, St. Louis, USA; 10 mg/kg). The saliva produced during the first 15 min after the pilocarpine injec-tion was dropped in previously weighted plastic tubes, which were reweighed after the saliva collection. The salivary flow was deter-mined by the difference between the two weightings and was expressed in ml/minute based on a previously described method
[25,27]. Then, the samples were centrifuged at 3000 rpm for
15 min. Aliquots containing 30
lL of supernatants were collected
and stored at 80°C to further investigate the lysozyme andcata-lase activities and to quantify the IgA and superoxide dismutase (SOD) levels.
2.4. Determination of lysozyme activity
Samples of pilocarpine stimulated saliva, collected on the 4th and 10th experimental days, were stored at 80°C to determine
the lysozyme activity (Lysozyme detection kit; Sigma-Aldrich, #LY0100). The results were shown as units of lysozyme/mL.
2.5. Quantification of IgA levels by ELISA
The concentration of IgA levels from pilocarpine stimulated sal-iva samples, collected on the 4th and 10th experimental days, was determined using the IgA detection kit (Genway, #GWB-1B12B0), according to the manufacturer’s instructions. The results were shown as ng/mL.
2.6. Histopathological analysis of major salivary glands
Following euthanasia, 4 or 10 days after the first 5-FU injection, tissue samples of each major salivary gland were collected and fixed in 10% neutral buffered formalin, dehydrated and embedded in paraffin. Sections of 5
lm thickness were obtained for
hematoxylin-eosin (HE) and Mallory trichrome stains and exam-ined under light microscopy. The HE staexam-ined sections were evalu-ated qualitatively on 6 specimens per group, 5 fields per slide, on 200 magnification, to investigate the vacuolization and edema,using a 0–3 score grade, in a blind manner, considering the inten-sity (absence, moderate or accentuated) of vacuolization and/or edema and the number of affected areas in the same slide. Inflam-matory cells were counted manually in the HE stained sections, considering 6 specimens per group and 10 fields per slide, on 1000 magnification. The effects of 5-FU on the vasculature of
the major salivary glands were investigated in the Mallory tri-chrome stained sections, which highlight the blood vessels. It was considered at least 5 specimens per group. For each of the major salivary glands, 4 lobes with a larger amount of blood vessels were analyzed, considering 4 fields per lobe on 400
magnifica-tion. The delimitation and measurements of blood vessels and cap-illaries area were performed from digital images captured using a digital camera coupled to a Leica microscope, and analyzed by LAS V3.7.0 software.
2.7. Measurement of the superoxide dismutase (SOD) activity
Samples of the major salivary glands and pilocarpine stimulated saliva were collected on the 4th and 10th experimental days and stored at 80°C until the assay, according to a previously
by measuring its capacity to inhibit the photochemical reduction of nitro-blue tetrazolium (NBT). In this assay, the photochemical reduction of riboflavin generates O2 that reduces the NBT to
pro-duce formazan salt, which absorbs at a wavelength of 560 nm. In the presence of SOD, the reduction of the NBT is inhibited because the enzyme converts the superoxide radical to peroxide. The results were expressed as the quantity of SOD necessary to inhibit the rate of reduction of the NBT by 50% in units of the enzyme per gram of protein. The tubes containing the resulting solution were exposed to fluorescent light bulbs (15 W) for 15 min and then read using a spectrophotometer at 560 nm. The results were expressed as uSOD/lg or
ll protein in tissue and saliva, respectively. The
sub-lingual and submandibular salivary glands were collected together, due to their anatomical closeness, and were homogenized in the same tube.2.8. Measurement of the catalase (CAT) activity
Samples of the major salivary glands and pilocarpine stimulated saliva were collected on the 4th and 10th experimental days and stored at 80°C until the assay, as described previously[29]. The
results were shown as mM/min/mg or mL of CAT for salivary tissue and saliva, respectively. It must be noted that the submandibular and sublingual samples were homogenized together, in the same tube.
2.9. Determination of nitrite levels by Griess reaction
Samples of the major salivary glands were collected on the 4th and 10th experimental days and stored at 80°C until the assay as
a previously described method[30]. The production of NO was determined indirectly by measuring the nitrite/nitrate levels based on Griess reaction from tissue homogenates. The submandibular and sublingual samples were collected and homogenized together, in the same tube. Briefly, 100
ll of tissue homogenate was
incu-bated with 100ll of the Griess reagent (1% sulfanilamide in 1%
H3PO4/0.1% N-(1-naphthyl)ethylenediamine dihydrochloride/1%H3PO4/distilled water, 1:1:1:1) at room temperature for 10 min.
The absorbance was measured at 560 nm in a microplate reader, and nitrite/nitrate concentrations were determined from standard nitrite curve generated using NaNO2. The results were presented as
NO2/NO3(mM).
2.10. Determination of malonaldehyde (MDA) levels
Samples of the major salivary glands were collected on the 4th and 10th experimental days and stored at 80°C until the assay
based on a previously described method[31]. Each sample was homogenized in 1.15% KCl. In a test tube was added 250
lL of
the homogenate, 1.5 mL of 1% phosphoric acid, 0.5 ml of thiobarbi-turic acid (0.6%), 2 mL ofn-butanol and the samples were pipetted into 96-well plate for reading. Readings were taken at 520 nm and 535 nm and the results of MDA were given by subtracting the first reading for the second. The absorbance obtained was plotted on a MDA standard curve, and the values were expressed as nmol/g of MDA of salivary tissue. The submandibular and sublingual samples were collected and homogenized together, in the same tube.2.11. Determination of non-protein sulfhydryl groups (NP-SH) levels
Samples of the major salivary glands were collected on the 4th and 10th experimental days and stored at 80°C until the assay.
The NP-SH levels were measured following the protocol previously described [32] for NP-SH, mainly represented by glutathione (GSH). Salivary gland tissues were homogenized in 1 ml of 0.02 M of EDTA. The submandibular and sublingual samples were collected and homogenized together, in the same tube. Aliquots of 400
ll of homogenate were added to 320
ll of distilled water
and 80ll of 50% trichloroacetic acid (TCA) for precipitation of
pro-teins. The microtubes were centrifuged (3000 rpm/15 min/4°C),400
ll of supernatant was added to 800
ll of 0.4 M Tris (pH 8.9),
20ll of DTNB and agitated for 3 min, immediately before reading.
The absorbance was measured at 412 nm. The results were expressed aslg de NPSH/100 mg of salivary gland tissue.
2.12. Quantification of pro-inflammatory cytokines (TNF-
a
and IL-1b) by ELISASamples of the major salivary glands were collected on the 4th and 10th experimental days, stored at 80°C until the interleukin
1-b(IL-1b; DuoSet ELISA Development kit, R&D systems, #DY501) and tumor necrosis factor-
a
(TNF-a
; DuoSet ELISA Development kit, R&D systems, #DY510) measurements. The samples of sub-mandibular and sublingual glands were collected and processed together. The concentrations of cytokines contained in each sample were measured using an enzyme-linked immunosorbent assay (ELISA), as described previously[33]. The results were expressed as pg/ml of IL-1bor TNF-a
.2.13. Determination of cell proliferation
Following euthanasia, 4 or 10 days after the first 5-FU injection, tissue samples of each major salivary gland were collected and fixed in 10% neutral buffered formalin, dehydrated and embedded in paraffin. Sections of 5
lm thickness were obtained for
immuno-histochemical staining for ki-67, a nuclear antigen present in pro-liferating cells, using the Envision+Dual Link System HRP method. Sections were incubated overnight (4°C) with polyclonal goatanti-rabbit primary antibody anti-Ki67 (SpringBioscience, #M3064) diluted 1:200 in antibody diluent (Dako #S0809). After washing, the slides were then incubated with Dako labeled polymer (Envi-sionFlex Dako #K4010) for 30 min. Negative control sections were processed simultaneously as described above but the primary
anti-body was replaced with antianti-body diluent (DAKO). Slides were counterstained with Harris hematoxylin, dehydrated in a graded series of ethanol, cleared in xylene and coverslipped. DAB-stained cells were manually counted in 10 fields per slide, on 400
magni-fication, considering at least 2 animals per group, for statistical analysis. The results were expressed as the average of K-i67positive cells/field.
2.14. Determination of cell death
Cell death was investigated using a TdT-mediated dUTP nick end-labeling (TUNEL) assay according to the manufacturer’s proto-col (ApopTagR, #S7101, Merck, Millipore). Firstly, tissue samples of each major salivary glands (4 or 10 days after the first 5-FU injec-tion) were collected and fixed in 10% neutral buffered formalin, dehydrated and embedded in paraffin. Sections of 5
lm thickness
were obtained from tissue samples of each major salivary glands. The paraffin sections were deparaffinized, rehydrated and incu-bated with 20 mg/mL proteinase K for 15 min at room tempera-ture. Endogenous peroxidases were blocked by treating with 3% (v/ v) hydrogen peroxide in PBS for 5 min at room temperature. After washing, the sections were then incubated in a humidified chamber at 37°C for 1 h with TdT buffer containing TdT enzymeand reaction buffer. Specimens were incubated for 10 min at room temperature with a stop/wash buffer and then incubated in the humidified chamber for 30 min with anti-digoxigenin peroxidase conjugate at room temperature. After PBS washes, the slides were covered with peroxidase substrate for color development and then
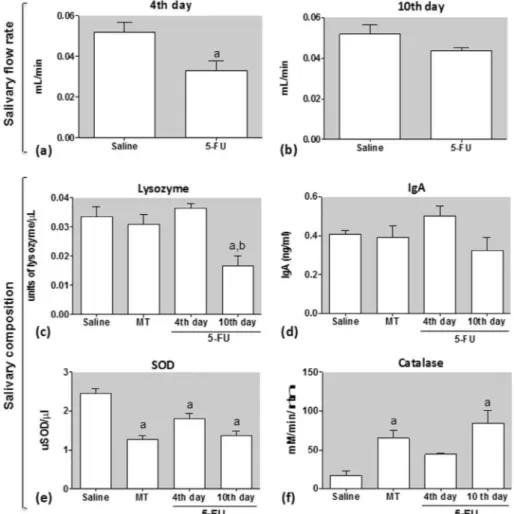
Fig. 2.Effect of 5-FU, observed on the 4th (a) and 10th (b) experimental days, on pilocarpine-stimulated salivary flow and saliva composition: lysozyme (c) and IgA levels (d), SOD (e) and catalase activities (f). Data represent the mean ± SEM of 6 animals per group.aDenotes values significantly different (p < 0.05) from saline group.bDenotes values
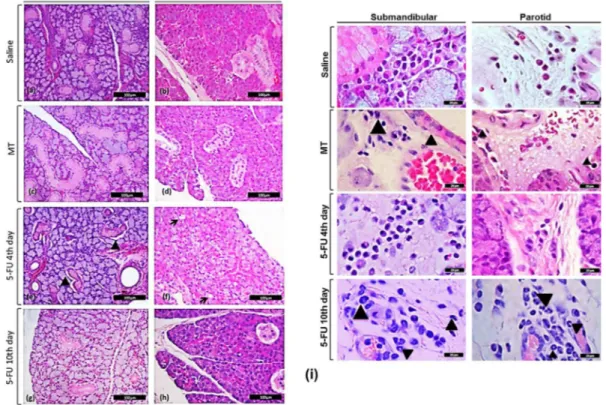
Fig. 3.Effect of 5-FU on the histopathological evaluation of submandibular (a, c, e, g) and parotid (b, d, f, h) glands. Arrowheads point to periductal edema on submandibular (e), arrows point to cell vacuolization on parotid gland (f) or neutrophil infiltrate (i). At least, 5 specimens per group were considered. Hematoxylin and eosin stain; Bar = 100mm (a–h) or 20mm (i).
Table 1
Score analysis of vacuolization and periductal edema in major salivary glands submitted to 5-FU.
Parameter Salivary glands Saline MT 5-FU
4th day 10th day Mean
Vacuolization SL 0 (0-1) 0 (0-0) 0 (0-1) 0 (0-0)
SM 0 (0-1) 0.5 (0-3) 1 (0-3) 1 (0-2)
P 0 (0-1) 1.5 (0-3) 2 (2-3)a 2 (1-2)
Periductal edema SL 0 (0-1) 0 (0-0) 0 (0-1) 0 (0-1)
SM 0 (0-1) 0 (0-2) 2 (2-3)a 0 (0-1)
P 2 (2-3) 0 (0-1) 1.5 (0-3) 1 (0-2)
aSenotes values significantly different (p < 0.05) from saline group. Data were analyzed using Kruskal-Wallis’s and Dunn tests. SL = sublingual; SM = submandibular;
P = parotid; MT = mechanical trauma; min = minimum; max = maximum.
Table 2
Mast cells, lymphocytes, plasma cells, macrophages and neutrophils quantification on 1000magnification.
Glands Groups Mast cells Lymphocytes Plasm cells Macrophages Neutrophils Mean ± SEM (cells/1000magnification field)
SL Saline 4.9 ± 0.2 0.4 ± 0.1 1.2 ± 0.2 0.1 ± 0.03 0.1 ± 0.1 MT 8.1 ± 0.9a 1 ± 0.3 0.3 ± 0.1 0.1 ± 0.03 0.4 ± 0.2
5FU 4th day 4.4 ± 0.3 1 ± 0.2 1 ± 0.4 0.2 ± 0.03 0.4 ± 0.1 5FU 10th day 4.1 ± 0.5b 1 ± 0.3 0.4 ± 0.01 0.2 ± 0 1.6 ± 0a,b
SM Saline 4.3 ± 0.3 1.9 ± 0.4 0.4 ± 0.1 0.04 ± 0.01 0.01 ± 0 MT 7.6 ± 0.5a 1.4 ± 0.3 0.6 ± 0.3 0.1 ± 0.06 0.9 ± 0.3a
5FU 4th day 7.4 ± 0.3a 5.8 ± 2a 0.3 ± 0.1 0.4 ± 0.2a 0.2 ± 0.2
5FU 10th day 8.4 ± 0.7a 0.7 ± 0.1 0.5 ± 0.1 0.05 ± 0.04 1.3 ± 0.2a
P Saline 7.3 ± 0.7 2.0 ± 0.5 0.1 ± 0.07 0.2 ± 0.1 0.04 ± 0.03 MT 12.2 ± 1.6a 1.7 ± 0.3 0.3 ± 0.1 0.01 ± 0 1.5 ± 0.6a
5FU 4th day 6.4 ± 0.4a,b 4.9 ± 0.5a 1.4 ± 0.5a 0.01 ± 0 0.2 ± 1.6
5FU 10th day 8.2 ± 0.7b 1.1 ± 0.2 0.1 ± 0.1 0.1 ± 0.04 3.2 ± 0.5a,b aDenotes values significantly different (p < 0.05) from saline group.
b Denotes values significantly different (p < 0.05) from MT group. Data were analyzed using Anova and Bonferroni’s test. SL = sublingual; SM = submandibular; P = parotid;
washed in three changes of dH2O and counterstained in 0.5% (w/v)
methyl green for 10 min at room temperature. TUNEL-positive cells were counted from digital images (400 magnification) of
at least ten different area of each section (from (4 animals per group). The results were expressed as the average of the TUNEL positive cells/field.
2.15. Statistical analysis
Data were presented as mean ± SEM. Analysis of variance (ANOVA) followed by Bonferroni’s test was performed to compare means and Kruskal-Wallis test followed by Dunn’s were used to compare medians, using GraphPrism 6 (GraphPad Software Inc., La Jolla, CA, USA). The level of significance adopted was 5% for all analysis.
3. Results
3.1. The effect of 5-FU on the salivary flow rate, saliva composition and oxidative stress in saliva
Salivary glands function was assessed by measuring salivary flow and saliva composition. A significant reduction in the pilocarpine-stimulated salivary flow rate was observed in the group 5-FU 4th day (Fig. 2a), but not 5-FU 10th day (Fig. 2b), when
compared with saline group, suggesting that 5-FU impacts the sali-vary flow rate at the beginning, but the saliva production achieves completely recovery 10 days after the first 5-FU injection, when its cytotoxicity effects is declining. It was also observed that 5-FU reduced the lysozyme activity 10 days after its first injection (p < 0.05) (Fig. 2c). It must be noted that no statistical difference was observed between the MT and saline groups. The levels of IgA, however, were not affected by 5-FU in none of the observed times. No significant difference was found between MT and saline group regarding the IgA levels (Fig. 3d).
The oxidative stress in the saliva samples was investigated by the evaluation of SOD levels and CAT activity, two endogenous enzymatic antioxidant mechanisms to counteract the effects of free radicals or to repair the resulting damages. A significant decrease in SOD levels was observed in the groups MT, 5-FU 4th day and 5-FU 10th day compared with saline group (Fig. 2e). This result was associated with a significant increase in the CAT activity in the groups MT and 5-FU 10th day, but not 5-FU 4th day, when compared to the saline group (Fig. 2f).
3.2. Effect of 5-FU on the histological aspects of the major salivary glands
Histological examination of the major salivary glands shows that 5-FU injection had adversely affected the histological
Fig. 4.Effect of 5-FU on the blood vessels area of sublingual (a), submandibular (b) and parotid (c) salivary glands and representative examples of this effect within sublingual (d–g), submandibular (f–k) and parotid (l–o) glands compared to saline (d, h, l) and mechanical trauma (MT) control groups (e, I, m). Data represent the mean ± SEM of 5 animals per group for each of the major salivary glands, 4 lobes with a larger amount of blood vessels were analyzed, considering 4 fields per lobe.aDenotes values
significantly different (p < 0.05) from saline group.bDenotes values significantly different (p < 0.05) from MT group. Data were analyzed using ANOVA and Bonferroni’s test.
architecture of both P and SM, but not SL gland. Vacuolization was observed in the acinar cells of the P gland of the 5-FU 4th day group (p < 0.05) (Table 1;Fig. 3f), while periductal edema was detected in the SM gland of the 5-FU 4th day group (p < 0.05) (Table 1;Fig. 3e). It must be noted that these alterations were not found in the MT group (Table 1; Fig. 3c–d) nor 5-FU 10th day group (Table 1;
Fig. 3g–h).Fig. 3a and b show the normal architecture of SM and P glands, respectively, observed in the saline group.
The histological effects of 5-FU, noted in the 5-FU 4th day group, are associated with an increased number of inflammatory cells (Table 2;Fig. 3i). More specifically, a greater number of mast cells, lymphocytes and macrophages was observed in the SM gland, 4 days after the first 5-FU injection, associated with an increase (p < 0.05) in the mast cells, lymphocytes and plasma cells in P gland, when compared to the saline group (Table 2). In addition, it was found an increased number of neutrophils (Fig. 3i) and mast cells in the SM and P glands of the 5-FU 10th day and MT groups, when compared with saline group. Increased number (p < 0.05) of mast cells was observed in the SM gland of MT and 5-FU 10th day groups and on the P of MT group, when compared to saline (Table 2).
Despite the lack of effect of 5-FU on the histological architecture of SL, it was observed an increase in the neutrophil infiltration to SL in the 5-FU 10th day group, but not 5-FU 4th day, compared with saline and MT groups (Table 2; Fig.3i). The mechanical trauma itself induced a significant increase in the mast cells number, observed in the SL, when compared with saline group (Table 2).
The analysis of the vasculature of the major salivary glands, investigated in Mallory’s stained sections, showed that 5-FU increased the blood vessels area of the SM gland, observed 10 days after its first injection, compared with saline and MT control groups (Fig. 4b). 5-FU-induced blood congestion was not observed in SL or P glands (Fig. 4a and c, respectively). It must be noted that no statistically significant differences were found in the blood ves-sels area between saline and MT groups in none of the major sali-vary glands. Fig. 4 also shows representative examples of the Mallory stained sections for each experimental groups (Fig. 4d–o).
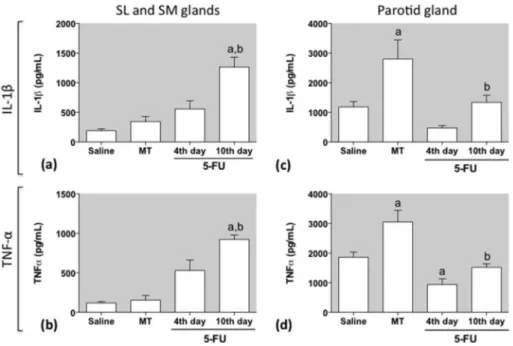
3.3. The effect of 5-FU on IL-1band TNF-
a
levelsFig. 5 shows a significant increase in the IL-1b and TNF-a
levels, observed in SL/SM glands tissues of the 5-FU 10th day group, but not in the 5-FU-4th day, compared with saline and MT control groups (p < 0.05) (Fig. 5a and b). The mechanical trauma itself did not have any effect on either IL-1b or TNF-a levels in SL/SM tissues since no significant difference was observed between MT and saline groups. In the P gland, on the other hand, IL-1band TNF-a levels were significantly higher in the MT group when compared with saline and 5-FU 10th day groups. 5-FU had an inhibitory effect on TNF-alevels, measured on the P gland, 4 days after its first injection, since it was observed a significant decrease in the TNF-alevels in 5-FU 4th day group compared to saline (Fig. 5d).
3.4. Oxidative stress in the major salivary glands
The tissue specific oxidative stress in the major salivary glands was investigated using some established biomarkers, such as nitrite/nitrate, MDA and NP-SH levels. In addition, the enzymatic antioxidant defenses were also investigated through the evaluation of CAT and SOD activities, determined in P and SM/SL tissues. The results indicate that 5-FU induced a significant oxidative stress in SM/SL and P tissues, mostly observed 10 days after its first injec-tion, detected by the activation of different anti-oxidative defense mechanisms: a significant increase in the nitrite/nitrate and MDA levels, associated with a decrease (p < 0.05) in NPSH, a established non-enzymatic antioxidant, were observed in the SM/SL tissues (Fig. 6a–c), while a significant increase in MDA levels, correlated with decreased (p < 0.05) NPSH levels and reduced CAT activity were observed in P tissue (Fig. 6g–i). Mechanical trauma itself induced a decrease (p < 0.05) in NPSH level and SOD activity in SM/SL tissues compared with saline group (Fig. 6c and e) and a decrease (p < 0.05) in NPSH level, CAT and SOD activities in P tis-sue, compared with saline group (Fig. 6h-j). No significant differ-ences in CAT activity were observed among the experimental
Fig. 5.Effect of 5-FU on the IL-1band TNF-alevels in the sublingual/submandibular (a, b) and parotid tissues (c, d). Data represent the mean ± SEM of 5 animals per group.
aDenotes values significantly different (p < 0.05) from saline group.bDenotes values significantly different (p < 0.05) from MT group. Data were analyzed using ANOVA and
groups in the SM/SL tissues (Fig. 6d). In P gland, an increase (p < 0.05) in the MDA level, associated with a significant decrease in NPSH level, was also observed in the 5-FU 4th day group com-pared with saline (Fig. 6g and h).
3.5. Effect of 5-FU on cell death and cell proliferation
5-FU induced cell death and cell proliferation, observed ten days after its first injection, in the SM tissues, since a significant
Fig. 6.Effect of 5-FU on the oxidative stress in the sublingual/submandibular (a–e) and parotid tissues (f–j) using the established biomarkers: nitrite/nitrate (a, f), malondialdehyde (MDA; b, g), non-protein thiols (NP-SH; c, h), catalase (d, i) and superoxide dismutase (SOD; e, j). Data represent the mean ± SEM of 5 animals per group.
aDenotes values significantly different (p < 0.05) from saline group.bDenotes values significantly different (p < 0.05) from MT group. Data were analyzed using ANOVA and
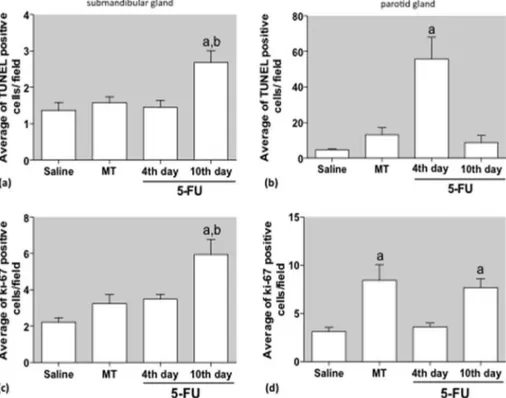
increase in the TUNEL- and Ki-67-positive cells were found in the tissues of the 5-FU 10th day group when compared with saline group (Fig. 7a and c). In the P gland, it was observed an increased number of TUNEL-positive cells in the 5-FU 4th day group com-pared with saline group (Fig. 7b) and a significant increased of the Ki-67 stained cells in the MT and 5-FU 10th day groups when compared with saline (Fig. 7d).
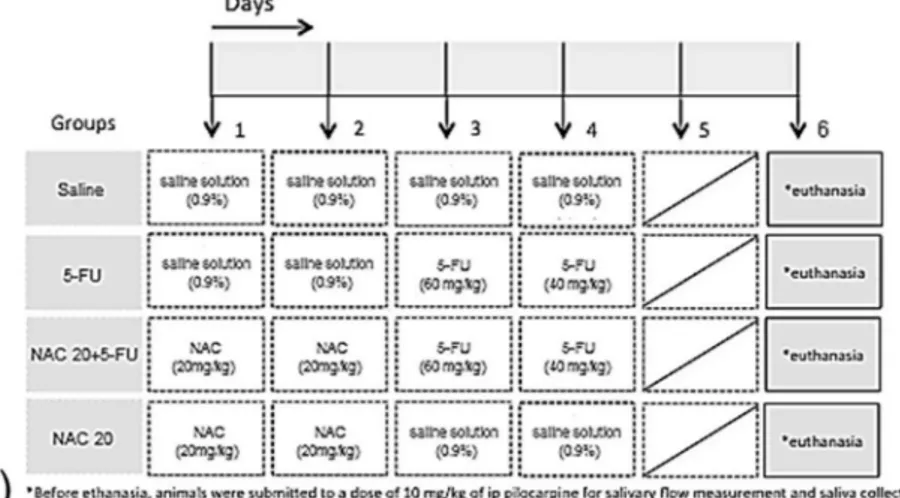
3.6. NAC reverts the 5-FU-induced hyposalivation and oxidative stress
To investigate the role of the oxidative stress on the 5-FU-induced hyposalivation, a group of hamsters were pretreated with the antioxidant N-acetylcysteine (NAC, 20 mg/kg; i.p.) for two days, once a day, before the first 5-FU injection (60 mg/kg,i.p.), and euthanized two days after the second 5-FU administration (40 mg/kg, i.p.), on day 6 (Fig. 8a). The administration of NAC pre-vented (p < 0.05) the 5-FU-induced xerostomia, as shown inFig. 8b. In addition, NAC prevented (p < 0.05) the reduction in SOD activity, induced by 5-FU, observed in the saliva (Fig. 8c) and also prevented the 5-FU-induced increase (p < 0.05) in the MDA concentration on parotid glands, when compared to the 5-FU group (Fig. 8d).
4. Discussion
The present study demonstrates that 5-FU can induce salivary gland damage, concomitantly with inflammation and oxidative stress, affecting salivary flow rate and saliva composition. Histopathological analysis showed tissue injuries such as vac-uolization on P gland and periductal edema on SM gland. In agree-ment with our data, vacuolization was observed in SM gland of rats submitted to experimental periodontitis, leading to apoptosis, mediated by the imbalance of oxidative and anti-oxidative stress and increased TNF-arelease[34]. Similarly, a previously published study[35] observed augmented vacuolization of acinar cells and
apoptosis in SM glands of mice treated with 15 Gy irradiation dose for 40 and 90 days.
In the present study, we showed that 5-FU impacts the salivary flow rate, evaluated 4 days after its first injection. Accordingly, pre-vious studies[24]have demonstrated a temporary reduction in the salivary flow during chemotherapy protocols that includes 5-FU
[24]. In the present work, the 5-FU-induced decrease in the salivary flow occurred concomitantly with periductal edema and increased numbers of mast cell, lymphocyte and macrophage in the SM glands. According to the literature[25], patients diagnosed with colon cancer treated with 5-FU and leucovorin had a substantial reduction in the stimulated and unstimulated salivary flow rate during the intermediate phase of the treatment. The salivary flow, however, was recovered 21 days after the end of the treatment. Accordingly, our study shows that the saliva production achieves completely recovery 10 days after the first 5-FU injection when its cytotoxicity effects are declining, suggesting a temporary effect of 5-FU on the major salivary glands. In addition, our results also suggest an association between 5-FU-induced xerostomia and gen-eration of oxidative stress, since the pretreatment of animals with NAC, previously to 5-FU administration, was able to prevent the oxidative stress-related parameters.
Additionally, our data show that 5-FU modifies the saliva com-position, decreasing lysozyme and superoxide dismutase (SOD) and increasing catalase (CAT). The immunoglobulin A (IgA) levels, however, were not affected by 5-FU. This finding is in agreement with Mazzeo et al. [25] but not with Janovic et al. [36], who observed a significant reduction in the IgA levels in the saliva of patients undergoing 5-FU treatment for metastatic tumors. In addi-tion, NAC pretreatment was able to improve the 5-FU-induced SOD activity alterations on the saliva.
We also demonstrated that 5-FU leads to oxidative stress in the major salivary glands with consequent generation of reactive oxy-gen species, indicated by increased MDA and decreased NPSH levels in SM and P glands. In accordance, it had been demonstrated
Fig. 7.Effect of 5-FU on cell death (a, b) and cell proliferation (c, d) in the submandibular (a, c) and parotid (b, d) tissues. Data represent mean ± SEM of 4 animals for TUNEL and 2 animals for ki-67 per group, and 10 fields per slide were analyzed on 400magnification.aDenotes values significantly different (p < 0.05) from saline group.bdenotes
that periodontal disease[37–39]induces oxidative stress. Canakci et al. [37] evaluated the oxidative stress in saliva samples of patients with periodontal disease. They verified that high levels of 8-OHdG and MDA and lower salivary antioxidant activities (SOD and glutathione peroxidase) seem to reflect increased oxygen radical activity during periodontal inflammation. In this regards, Trivedi et al. [39] also obtained increased MDA levels and decreased SOD, CAT and glutathione reductase activities in saliva samples. Furthermore, Almerich-Silla et al.[38]found high levels
of oxidative stress biomarkers (8-OHdG, MDA, glutathione peroxi-dase and total antioxidant capacity) associated with an increase in the periodontal bacteria species number in periodontal disease. This previous data support the hypothesis that oral microbiota changes could account for the increased catalase activity [3]
observed in the saliva of the MT and 5-FU groups.
Our results also demonstrated that 5-FU can induce oxidative stress in salivary gland tissues with an imbalance in free radicals and antioxidant enzymes that are produced to equilibrate the high
Fig. 8.NAC reverts the 5-FU-induced hyposalivation and the oxidative stress in parotid glands. (a) Study design: to investigate the effect of NAC on salivary flow rate and oxidative stress,animals submitted to 5-FU induced oral mucositis received intraperitoneal (i.p.) administration of NAC (NAC 20 + 5-FU), for 2 days, once a day, before the first injection of 5-FU. Hamsters from the Saline or 5-FU control groups received i.p. saline solution, instead of NAC, on days 1 and 2, followed by the i.p. administration of saline or 5-FU on the 4th day, respectively. The control group NAC 20, not subjected to oral mucositis, received two i.p. administration of NAC, on days 1 and 2, followed by i.p. administration of saline on days 3 and 4. Immediately before euthanasia, on day 6, animals received a single administration of Pilocarpine (10 mg/kg; i.p), used to stimulate normal saliva production. The saliva produced during the first 15 min after the pilocarpine injection was collected for the investigation of salivary flow rate (b) and SOD activity (c). In addition, parotid glands were collected for the determination of MDA concentrations (d). Graphs represent the mean ± SEM of 6 animals per group.aDenotes
values significantly different (p < 0.05) from saline group.**Denotes values significantly different (p < 0.05) from 5-FU group. Data were analyzed using ANOVA and
free radicals production and maintain homeostasis. Reactive oxy-gen species have been implicated in a range of diseases such as periodontal diseases[34], Sjogren syndrome[40] and radiation-induced salivary gland dysfunction[41]. Moreover, our data point to lipid peroxidation as a result of oxidative stress, leading to increased levels of malondialdehyde (MDA), and subsequent changes in the structure and permeability of cell membranes occa-sioning organelle damage followed by cell death [42,43]. The oxidative stress suggested by increased nitrite/nitrate and MDA levels and decreased NPSH concentrations in the SM gland on the 10th experimental day occurs concomitantly with cell death, detected by TUNEL and increased proinflammatory cytokines levels. On the other hand, the increase in the MDA levels and cell death were observed earlier in the parotid gland.
Moreover, we observed a decrease in the NPSH levels in all three major salivary glands and decreased SOD and CAT activities in P glands of 5-FU treated animals, suggesting that enzymatic antioxidant activities were not capable of controlling the oxidative stress in the salivary glands tissue. In accordance with our study, Campos et al.[12]evaluated the enzymatic activity in sublingual (SL) and submandibular (SM) glands of hamsters treated with 5-FU. The authors observed a significant reduction in the protein con-centration on the 10th experimental day, suggesting a detrimental effect of 5-FU on the acinar cells function with consequent damage to protein synthesis and release. Differently from our study, Cam-pos et al.[12]demonstrated that 5-FU injections caused a signifi-cant increase in CAT and peroxidase activities.
The present work also showed a significant increase in the blood vessels area of the SM gland induced by 5-FU on the 10th day, which may be a consequence of the increased release of nitric oxide. The participation of nitric oxide in the present study is sup-ported by the increase in the nitrite/nitrate levels observed in the SL/SM glands. It is well established that proinflammatory cytoki-nes, such as IL-6, IL-1band TNF-a, are potent inducers of iNOS in a wide variety of cell types, with consequent production of nitric oxide (NO). Accordingly, we showed that 5-FU induced a signifi-cant increase in the IL-1band TNF-alevels in SM and sublingual (SL) glands on the 10th experimental day, but not in P gland. This effect may be correlated with the neutrophil infiltration and increased number of mast cell observed in the SM gland tissue. Our findings suggest an association between inflammation and oxidative stress, mainly on SM gland, detected by increased levels of cytokines, nitrite/nitrate and MDA on the 10th experimental day. These data are consistent with Yao et al.[45]who demon-strated that intraperitoneal injection of lipopolysaccharide in mice induced the expression of mRNA of inflammatory cytokines such as IL-1b, IL-6 and TNF-ain the submandibular gland, but not in the parotid gland. These results reinforce our findings and strongly suggest that submandibular gland plays an important role as a defense system in the oral cavity, avoiding the entry of micro-organisms from the mouse.
Furthermore, Kamachi et al.[46]demonstrated that cytokines such as TNF-aand INF-cplay an important role regulating apopto-sis of acinar-ductal epithelial cells and salivary gland function in Sjogren syndrome patients. Accordingly, we found increased cell death in the salivary glands associated with decreased salivary flow. More recently, it was showed salivary glands hypofunction in rats submitted to experimental periodontitis, without any detectable morphological changes in the acinar structure[47].
On the 10th day, we also observed increased cell proliferation in SM and P glands of mice treated with 5-FU and in the P gland of mice subjected to mechanical trauma only (MT group). We specu-late that the increased epithelial cell proliferation may be respecu-lated to the healing process. In addition, an increased number of TUNEL positive cells was detected on the 4th day in the P gland, simulta-neously with increased cell proliferation in SM gland. In agreement
with our data, Xiang et al.[48]evaluated the protective effect of
a
1-adrenoreceptor activation on rat SM glands undergoingirradiation-induced damage and showed that irradiated SM gland had increased apoptosis and enhanced proliferation measured by proliferative cellular nuclei antigen (PCNA). When animals were treated with phenylephrine, apoptosis was decreased and prolifer-ation was further augmented compared to irradiated SM gland. Bralic et al.[49]also revealed a regeneration of SM gland tissue after irradiation measured by PCNA.
Although all three major salivary glands react towards 5-FU stimulus, SM and P glands were more affected and reacted differ-ently when exposed to 5-FU. Accordingly, Zalewska et al. [50]
observed oxidative stress in SM and P glands tissue, but not in the SM gland, of animals submitted to streptozotocin-induced diabetes.
In conclusion, our results suggest that 5-FU causes oxidative stress and inflammation, mainly observed in the SM gland, which leads to periductal edema and cell death, resulting in alterations in the salivary flow rate and composition. Antioxidants, such as NAC, demonstrated to be a good candidate to improve 5-FU-induced hyposalivation. The knowledge of the anticancer drug’s effect on the salivary glands will be able to contribute to the proper management of xerostomia and oral mucositis.
Conflict of interest
The authors declare no competing financial interest.
Author contribution statement
L.E.B. designed and performed all experiments, analyzed the data and wrote the manuscript. C.M.B., T.A.O., C.S.M., D.A.F., A.A. Q.A.A. and D.V.S.C. helped in the acquisition of the data. R.F.C.L. helped revise the manuscript. G.A.C.B. is the principal investigator for the grant and helped with the experimental design, supervised the project and helped write the manuscript.
Acknowledgements
The authors would like to thank Professor Gutencilda Colares de Vasconcelos, for her assistance in the histological assessment.
References
[1] A. Vissink, J.B. Mitchell, B.J. Baum, K.H. Limesand, S.B. Jensen, P.C. Fox, L.S. Elting, J.A. Langendijk, R.P. Coppes, M.E. Reyland, Clinical management of salivary gland hypofunction and xerostomia in head-and-neck cancer patients: successes and barriers, Int. J. Radiat. Oncol. Biol. Phys. (2010),http://dx.doi.org/ 10.1016/j.ijrobp.2010.06.052.
[2] S.B. Jensen, A.M.L. Pedersen, A. Vissink, E. Andersen, C.G. Brown, A.N. Davies, J. Dutilh, J.S. Fulton, L. Jankovic, N.N.F. Lopes, A.L.S. Mello, L.V. Muniz, C.A. Murdoch-Kinch, R.G. Nair, J.J. Napeñas, A. Nogueira-Rodrigues, D. Saunders, B. Stirling, I. Von Bültzingslöwen, D.S. Weikel, L.S. Elting, F.K.L. Spijkervet, M.T. Brennan, A systematic review of salivary gland hypofunction and xerostomia induced by cancer therapies: Prevalence, severity and impact on quality of life, Support. Care Cancer (2010),http://dx.doi.org/10.1007/s00520-010-0827-8. [3] M.A. Skeff, G.A.C. Brito, M.G. De Oliveira, C.M. Braga, M.M. Cavalcante, V.
Baldim, R.C. Holanda-Afonso, C.M. Silva-Boghossian, A.P. Colombo, R.A. Ribeiro, V. Moura-Neto, R.F.C. Leitão, S-nitrosoglutathione accelerates recovery from 5-fluorouracil-induced oral mucositis, PLoS One (2014), http://dx.doi.org/ 10.1371/journal.pone.0113378.
[4] Z.-H. Wang, C. Yan, Z.-Y. Zhang, C.-P. Zhang, H.-S. Hu, J. Kirwan, W.M. Mendenhall, Radiation-induced volume changes in parotid and submandibular glands in patients with head and neck cancer receiving postoperative radiotherapy: a longitudinal study, Laryngoscope (2009),http://dx.doi.org/ 10.1002/lary.20601.
[5] S. Al-Ansari, J.A.E.M. Zecha, A. Barasch, J. de Lange, F.R. Rozema, J.E. Raber-Durlacher, Oral mucositis induced by anticancer therapies, Curr. Oral Heal. Rep. (2015),http://dx.doi.org/10.1007/s40496-015-0069-4.
[7] J.B. Epstein, A.H.F. Tsang, D. Warkentin, J.A. Ship, The role of salivary function in modulating chemotherapy-induced oropharyngeal mucositis: A review of the literature, Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. (2002),
http://dx.doi.org/10.1067/moe.2002.126018.
[8]J.B. Epstein, M.M. Freilich, N.D. Le, Risk factors for oropharyngeal candidiasis in patients who receive radiation therapy for malignant conditions of the head and neck, Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. (1993). [9]A.V. Nieuw Amerongen, E.C. Veerman, Current therapies for xerostomia and
salivary gland hypofunction associated with cancer therapies. [Review] [45 refs], Support. Care Cancer (2003).
[10] K.E. Öhrn, Y.B. Wahlin, P.O. Sjödén, Oral status during radiotherapy and chemotherapy: A descriptive study of patient experiences and the occurrence of oral complications, Support. Care Cancer (2001),http://dx.doi.org/10.1007/ s005200000214.
[11] A. Trotti, L.A. Bellm, J.B. Epstein, D. Frame, H.J. Fuchs, C.K. Gwede, E. Komaroff, L. Nalysnyk, M.D. Zilberberg, Mucositis incidence, severity and associated outcomes in patients with head and neck cancer receiving radiotherapy with or without chemotherapy: a systematic literature review, Radiother. Oncol. (2003),http://dx.doi.org/10.1016/S0167-8140(02)00404-8.
[12] L. Campos, J. Nicolau, V.E. Arana-Chavez, A. Sim??es, Effect of laser phototherapy on enzymatic activity of salivary glands of hamsters treated with 5-fluorouracil, Photochem. Photobiol. (2013),http://dx.doi.org/10.1111/ php.12195.
[13]H. Watari, M. Hosaka, T. Mitamura, M. Moriwaki, Y. Ohba, Y. Todo, M. Takeda, Y. Ebina, N. Sakuragi, Weekly paclitaxel/5-fluorouracil followed by platinum retreatment for patients with recurrent ovarian cancer: a single institution experience, Eur. J. Gynaecol. Oncol. (2008).
[14] A. Gonçalves, J.Y. Pierga, J.M. Ferrero, M.A. Mouret-Reynier, T. Bachelot, R. Delva, M. Fabbro, F. Lerebours, J.P. Lotz, C. Linassier, N. Dohollou, J.C. Eymard, B. Leduc, J. Lemonnier, A.L. Martin, J.M. Boher, P. Viens, H. Roché, UNICANCER-PEGASE 07 study: a randomized phase III trial evaluating postoperative docetaxel-5FU regimen after neoadjuvant dose-intense chemotherapy for treatment of inflammatory breast cancer, Ann. Oncol. (2015),http://dx.doi. org/10.1093/annonc/mdv216.
[15] D. Kandioler, M. Mittlböck, S. Kappel, H. Puhalla, F. Herbst, C. Langner, B. Wolf, J. Tschmelitsch, W. Schippinger, G. Steger, F. Hofbauer, H. Samonigg, M. Gnant, B. Teleky, I. Kührer, TP53 mutational status and prediction of benefit from adjuvant 5-fluorouracil in stage III colon cancer patients, eBioMedicine (2015),
http://dx.doi.org/10.1016/j.ebiom.2015.06.003.
[16] A. Mahipal, D. Shibata, E. Siegel, G. Springett, K. Almhanna, W. Fulp, I. Williams-Elson, R. Kim, Phase I trial of combination of FOLFIRI and pasireotide, a somatostatin analogue, in advanced gastrointestinal malignancies, Invest. New Drugs. (2015),http://dx.doi.org/10.1007/s10637-015-0277-8.
[17] R. Kim, S. Hahn, J. Shin, C.-Y. Ock, M. Kim, B. Keam, T.M. Kim, D.-W. Kim, D.S. Heo, The effect of induction chemotherapy using docetaxel, cisplatin, and fluorouracil on survival in locally advanced head and neck squamous cell carcinoma: a meta-analysis, Cancer Res. Treat. (2015), http://dx.doi.org/ 10.4143/crt.2015.359.
[18] D.B. Longley, D. Harkin, P. Johnston, 5-Fluorouracil: mechanisms of action and clinical strategies, Nat. Rev. Cancer (2003),http://dx.doi.org/10.1038/nrc1074. [19] U. Ozer, K.W. Barbour, S.A. Clinton, F.G. Berger, Oxidative stress and response to thymidylate synthase-targeted antimetabolites, Mol. Pharmacol. (2015),
http://dx.doi.org/10.1124/mol.115.099614.
[20]F.G. Berger, S.H. Berger, Thymidylate synthase as a chemotherapeutic drug target: where are we after fifty years? Cancer Biol. Ther. (2006).
[21] K.W. Barbour, F.G. Berger, Cell death in response to antimetabolites directed at thymidylate synthase, Cancer Chemother. Pharmacol. (2008), http://dx.doi. org/10.1007/s00280-007-0461-4.
[22] S.B. Jensen, H.T. Mouridsen, J. Reibel, N. Brünner, B. Nauntofte, Adjuvant chemotherapy in breast cancer patients induces temporary salivary gland hypofunction, Oral Oncol. (2008), http://dx.doi.org/10.1016/j. oraloncology.2007.01.015.
[23] S.B. Jensen, H.T. Mouridsen, O.J. Bergmann, J. Reibel, N. Brünner, B. Nauntofte, Oral mucosal lesions, microbial changes, and taste disturbances induced by adjuvant chemotherapy in breast cancer patients, Oral Surgery, Oral Med. Oral Pathol. Oral Radiol. Endodontol. (2008), http://dx.doi.org/10.1016/j. tripleo.2008.04.003.
[24]M.A. Mazzeo, J.A. Linares, M.L. Campos, B.E. Busamia, C. Dubersarsky, M. Lavarda, G. Jarchum, A.B. Finkelberg, Oral signs of intravenous chemotherapy with 5- fluorouracil and leucovorin calcium in colon cancer treatment, Med. Oral Patol. Oral Cir. Bucal (2009).
[25] R.F.C. Leitão, R.A. Ribeiro, E.A.L. Bellaguarda, F.D.B. MacEdo, L.R. Silva, R.B. Oriá, M.L. Vale, F.Q. Cunha, G.A.C. Brito, Role of nitric oxide on pathogenesis of 5-fluorouracil induced experimental oral mucositis in hamster, Cancer Chemother. Pharmacol. (2007),http://dx.doi.org/10.1007/s00280-006-0301-y. [26]M. Konak, H. Cincik, E. Erkul, Z. Kucukodaci, A. Gungor, S. Ozdemir, E. Cekin, V. Arisan, M. Mutluoglu, M. Salihoglu, The protective effects of different treatments on rat salivary glands after radiotherapy, Eur. Arch. Otorhinolaryngol. (2016).
[27] M. Navazesh, S.K.S. Kumar, Measuring salivary flow: challenges and opportunities, J. Am. Dent. Assoc. (2008), http://dx.doi.org/10.14219/jada. archive.2008.0353.
[28] Y. Sun, L.W. Oberley, Y. Li, A simple method for clinical assay of superoxide dismutase, 1988.
[29] A.C. Maehly, B. Chance, The assay of catalases and peroxidases, Methods Biochem. Anal. (1954),http://dx.doi.org/10.1002/9780470110171.ch14. [30] L.C. Green, D.A. Wagner, J. Glogowski, P.L. Skipper, J.S. Wishnok, S.R.
Tannenbaum, Analysis of nitrate, nitrite, and [15N]nitrate in biological fluids, Anal. Biochem. (1982), http://dx.doi.org/10.1016/0003-2697(82) 90118-X.
[31] J.H. Lee, C.S. An, B.S. Yun, K.S. Kang, Y.A. Lee, S.M. Won, B.J. Gwag, S.I. Cho, K.B. Hahm, Prevention effects of ND-07, a novel drug candidate with a potent antioxidative action and anti-inflammatory action, in animal models of severe acute pancreatitis, Eur. J. Pharmacol. (2012), http://dx.doi.org/10.1016/j. ejphar.2012.04.048.
[32] J. Sedlak, R.H. Lindsay, Estimation of total, protein-bound, and nonprotein sulfhydryl groups in tissue with Ellman’s reagent, Anal. Biochem. (1968),
http://dx.doi.org/10.1016/0003-2697(68)90092-4.
[33] E.A. Bayer, M. Wilchek, The use of the avidin-biotin complex as a tool in molecular biology, Methods Biochem. Anal. (1980),http://dx.doi.org/10.1002/ 9780470110461.ch1.
[34] D. Ekuni, Y. Endo, K. Irie, T. Azuma, N. Tamaki, T. Tomofuji, M. Morita, Imbalance of oxidative/anti-oxidative status induced by periodontitis is involved in apoptosis of rat submandibular glands, Arch. Oral Biol. (2010),
http://dx.doi.org/10.1016/j.archoralbio.2009.11.013.
[35]M. Muhvic-Urek, M. Bralic, S. Curic, S. Pezelj-Ribaric, J. Borcic, J. Tomac, Imbalance between apoptosis and proliferation causes late radiation damage of salivary gland in mouse, Physiol. Res. (2006).
[36] L. Jankovic´, S. Jelic´, I. Filipovic´-Lješkovic´, Z. Ristovic´, Salivary immunoglobulins in cancer patients with chemotherapy-related oral mucosa damage, Eur. J. Cancer Part B Oral Oncol. (1995),http://dx.doi.org/10.1016/0964-1955(95) 00011-6.
[37]C.F. Canakci, Y. Cicek, A. Yildirim, U. Sezer, V. Canakci, Increased levels of 8-hydroxydeoxyguanosine and malondialdehyde and its relationship with antioxidant enzymes in saliva of periodontitis patients, Eur. J. Dent. (2009). [38] J.M. Almerich-Silla, J.M. Montiel-Company, S. Pastor, F. Serrano, M. Puig-Silla,
F. Dasí, Oxidative stress parameters in saliva and its association with periodontal disease and types of bacteria, Dis. Markers (2015),http://dx.doi. org/10.1155/2015/653537.
[39]S. Trivedi, N. Lal, A.A. Mahdi, B. Singh, S. Pandey, Association of salivary lipid peroxidation levels, antioxidant enzymes, and chronic periodontitis, Int. J. Periodontics Restorative Dent. (2015).
[40] G. Pagano, G. Castello, F.V. Pallardó, Sjøgren’s syndrome-associated oxidative stress and mitochondrial dysfunction: prospects for chemoprevention trials, Free Radical Res. (2013),http://dx.doi.org/10.3109/10715762.2012.748904. [41] N. Hanaue, I. Takeda, Y. Kizu, M. Tonogi, G. Yamane, Peroxynitrite formation in
radiation-induced salivary gland dysfunction in mice, Biomed. Res. (2007),
http://dx.doi.org/10.2220/biomedres.28.147.
[42]A.L. Ferreira, L.S. Matsubara, Free radicals: concepts, associated diseases, defense system and oxidative stress, Rev. Assoc. Med. Bras. (1997). [43] I.F.F. Benzie, Lipid peroxidation: a review of causes, consequences,
measurement and dietary influences, Int. J. Food Sci. Nutr. (1996),http://dx. doi.org/10.3109/09637489609012586.
[45] C. Yao, X. Li, K. Murdiastuti, C. Kosugi-Tanaka, T. Akamatsu, N. Kanamori, K. Hosoi, Lipopolysaccharide-induced elevation and secretion of interleukin-1beta in the submandibular gland of male mice, Immunology (2005),http:// dx.doi.org/10.1111/j.1365-2567.2005.02212.x.
[46] M. Kamachi, A. Kawakami, S. Yamasaki, A. Hida, T. Nakashima, H. Nakamura, H. Ida, M. Furuyama, K. Nakashima, K. Shibatomi, T. Miyashita, K. Migita, K. Eguchi, Regulation of apoptotic cell death by cytokines in a human salivary gland cell line: Distinct and synergistic mechanisms in apoptosis induced by tumor necrosis factor ?? and interferon ?? J. Lab. Clin. Med. (2002),http://dx. doi.org/10.1067/mlc.2002.120648.
[47] M. Amer, J.C. Elverdin, J. Fernández-Solari, V.A. Medina, A.P. Chiarenza, M.I. Vacas, Reduced methacholine-induced submandibular salivary secretion in rats with experimental periodontitis, Arch. Oral Biol. (2011),http://dx.doi.org/ 10.1016/j.archoralbio.2010.11.004.
[48] B. Xiang, X. Li, F. Zhang, Underlying protective mechanism ofa1-adrenoceptor activation against irradiation-induced damage in rat submandibular gland, Arch. Oral Biol. (2013),http://dx.doi.org/10.1016/j.archoralbio.2013.03.014. [49]M. Bralic, M. Muhvic-Urek, V. Stemberga, M. Golemac, S. Jurkovic, J. Borcic, A.
Braut, J. Tomac, Cell death and cell proliferation in mouse submandibular gland during early post-irradiation phase, Acta Med. Okayama (2005). [50] A. Zalewska, M. Knä, M. Maciejczyk, N. Waszkiewicz, A. Klimiuk, M.
Choroman´ska, J. Matczuk, D. Waszkiel, H. Car, Antioxidant profile, carbonyl and lipid oxidation markers in the parotid and submandibular glands of rats in different periods of streptozotocin induced diabetes, Arch. Oral Biol. (2015),